Abstract
Water is one of the fundamental needs for the life on earth. However, the wastewater released from the industries consists of dyes and other organic molecules, which become the serious issue for the water pollution. Among all water remediation techniques, heterogeneous photocatalysis has gained scientific attention for the water purification in terms of degradation of dyes and other organic pollutants. Heterogeneous photocatalysis is a very robust, low cost method and can provide complete mineralization of the pollutants. In this context, this chapter deals with the basic principle and mechanism of heterogeneous photocatalysis; and the parameters affecting the degradation kinetics. Furthermore, the different functional photocatalyst material, their limitation and the modification in the structure of semiconductor catalyst to absorb visible light are discussed in this chapter.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
15.1 Introduction
The rapid population growth and global warming are comprehensively affecting our ecosystem, which reducing its capability to provide with sufficient quantity of food, drinkable water and good environment to live a healthy life (Pimental 1991). Water is one of the essence requirements for the human kind to survive on earth. To feed large population, there is exponential increase in the industrialization, agricultural activities from which many hazardous chemicals as pollutants are flowing through the wastewater without any pre-treatment, which is adversely affecting the water resources. As per World Health Organization (WHO) report, more than five million peoples die annually due to diseases associated with unsafe drinking water and inadequate sanitation (Gleick 2015). The major sources of water adulteration are industrial and agricultural waste which deprive the quality of water sources by contaminating it with the hazardous wastes like textile dyes, paints, pesticides, herbicides, heavy metals (such as mercury (Hg), lead (Pb), etc.), benzene, and phenolic compounds (Singh et al. 2019). By keeping in mind about the steady decrease in the clean and safe ground water level, there is an urgent requirement for developing a low cost and high competent water treatment technology to manage the wastewater efficiently and to mitigate its harsh effects on the environment.
To save the clean water for drinkable and other important purposes, the recycling of wastewater effluent is one of the possible solutions by treating it to reuse in the agricultural and industrial consumption (Chong et al. 2010). As mentioned earlier, wastewater effluent contains hazardous chemicals like heavy metals and organic compounds; the efficient treatment of these is a challenging task. There are different wastewater treatment processes, which can be combined together in three types: primary, secondary and tertiary treatment process (Gupta et al. 2012). Primary treatment processes include filtration, centrifugation, sedimentation, coagulation and flotation method. Filtration and centrifugation are generally used to remove the suspended solid particles in wastewater physically; coagulation and flotation methods are used to remove the suspended solids, oil and grease by adding certain chemicals like activated silica (Gupta et al. 2012). However, these methods do not mineralize the pollutants completely. Furthermore, these methods have some working difficulties such as sludge generation and phase change of the pollutants (Saggioro et al. 2016). Secondary water treatment process includes the removal of pollutants by using biological route in which certain microbes like bacteria are used to degrade the pollutants in simple molecules like water, carbon dioxide and ammonia gas (Gupta et al. 2012; Goswami et al. 2017; Kushwaha et al. 2017). However, most of the dyes effluent in wastewater are obstinate towards the biological degradation, which ultimately decrease the efficiency of degradation through this treatment method (Saggioro et al. 2016). Tertiary wastewater treatment processes are mainly responsible to provide clean and safe water for consumption purposes by treating the wastewater upto 99% which include the processes like distillation, crystallization, oxidation, solvent extraction and electro-dialysis (Gupta et al. 2012). By considering the essential factors for adopting a technology like cost effectiveness, eco-friendliness, recyclability and overall efficiency of wastewater remediation, the complete oxidation of harmful compounds of wastewater effluent into non-hazardous products such as H2O and CO2 is attracting as a most reliable technology (Serpone et al. 2010; Oller et al. 2011).
Among all the available wastewater treatment techniques, the advanced oxidative processes (AOP) are the most attractive, which can be highly efficient for the degradation of dyes and other organic pollutants. These processes involve the in-situ generation of highly reactive and non-selective hydroxide radical (OH˙) oxidant that promote the complete mineralization or endorse the production of biodegradable by-products like water (H2O) and carbon dioxide (CO2) (Yoon et al. 2001). Advanced oxidation processes (AOP) include a variety of methods like Fenton process, ozonation, ozone with peroxide are chemical based methods to degrade the organic pollutants (Balciolu and Otker 2003; Khataee et al. 2009), whereas UV/fenton process, UV/ozone, UV/peroxide, homo and heterogeneous photocatalysis use photon to induce the degradation processes, thus called as photochemical processes. (Fujishima et al. 2000; Ghaly et al. 2001; Gernjak et al. 2003). Light induced methods increase the efficiency of the degradation process.
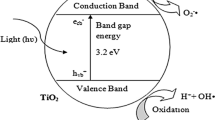
Among all light induced processes, the heterogeneous photocatalysis is the most attractive and efficient method for the degradation of wide range of hazardous organic pollutants into biodegradable compounds and ultimately complete mineralization by producing water (H2O) and carbon dioxide (CO2) gas. In the heterogeneous photocatalysis, light is incident on the surface of catalyst, which further induces the charge separation between the valence band and conduction band of the catalyst. This charge separation is the key of the photocatalysis for the organic/dyes pollutants mineralization without producing any secondary pollutants. This chapter presents the mechanistic understanding of photocatalytic degradation, operating parameters, photocatalytic materials and modification in the semiconductor photocatalyst to absorb visible light in detail (Table 15.1).
15.2 Mechanism of Heterogeneous Photocatalysis
Heterogeneous photocatalysis can be defined as the process in which light is used to increase the efficiency of the reaction in the presence of catalyst. In 1972, Fujishima and Honda were the first to describe the process of heterogeneous photocatalysis by demonstrating the photochemical splitting of water into hydrogen and oxygen under UV light by using TiO2 as the catalyst (Fujishima and Honda 1972). By considering the importance of this viable process, many researchers have carried out extensive research for different application like water splitting into hydrogen and oxygen, degradation of organic and inorganic effluent in water and air, dehalogenation and many others related to energy and environment (Ollis 1985; Teoh et al. 2012; Serpone et al. 2012).
The fundamental processes involved in the heterogeneous photocatalysis are as follows (Saravanan et al. 2017; Ahmed et al. 2011):
-
When the light (photon) incident on the surface of semiconductor having incident energy equal or greater than the bandgap energy of semiconductor, the electrons of the valence band adsorbs the energy and gets excited to the conduction band.
-
This excitation process of electron into conduction band leaves a hole in the valence band. This charge separation is the fundamental of photocatalysis, which induces the degradation of harmful chemicals at the surface of semiconductor.
-
The hole in the valence band can either oxidize the adsorbed harmful organic pollutants on the surface of semiconductor directly or react with the water (H2O) to produce hydroxide radical. The reaction with water is the predominant process as generally the concentration of water molecules would be more abundant relative to pollutant(s). The generated hydroxide radical has high oxidizing character, which is mainly responsible for the degradation of the organic compounds.
-
The electron in the conduction band further reacts with the adsorbed oxygen to reduce it into superoxide radical, which can further degrade the adsorbed pollutants.
This synergetic effect of charge separation into hole in valence band and electron in conduction band is responsible for the complete mineralization of harmful organic pollutants. A schematic representation of the photocatalytic mechanism of dye degradation on semiconductor photocatalyst is shown in Fig. 15.1. An important point for consideration is that the presence of oxygen or air is favourable to the heterogeneous photocatalysis as it prevents the electron–hole recombination process by reacting with electron in conduction band.
15.3 Operation and Influence of Parameters on Heterogeneous Photocatalysis
The degradation of organic compounds by using heterogeneous photocatalysis process mainly depends upon the factors such as catalyst physical parameters (structure, size, shape and surface area), pH of solution, light intensity, impurity concentration in wastewater, catalyst concentration and oxidizing agent (Bahnemann et al. 2007; Cassano and Alfano 2000; Rajeshwar et al. 2008).
15.3.1 Effect of Catalyst Physical Parameters
The photocatalytic activity of the photocatalyst is dependent upon the catalyst physical properties such as its crystal size, composition, surface area, porosity, bandgap and hydroxyl functionalities on catalyst surface. Crystal size can be the most important parameter since its direct relation with the surface area of catalyst. In general, the catalyst particles with relatively high surface area shows better photocatalytic activity due to availability of large number of sites for the dye adsorption. Lu et al. (2007) use natural rutile (rutile ~90%) and P25 (80% anatase and 20% rutile) type of TiO2 as catalytic surface to compare its efficiency for the photocatalytic degradation of methyl orange (MO). After 2 h illumination, the photocatalytic efficiency of the above two catalyst was found to be 82.3% and 94.8%, respectively. A possible reason was the particle size of P25 (∼30 nm) which is significantly smaller compared with the natural rutile sample (70–80 μm). Another possible reason for the higher photocatalytic activity of P25 would be due to the slow electron–hole recombination process as compared to the case of natural rutile. As mentioned, P25 is composed of 80% anatase and 20% rutile phase in which nano-crystals of rutile are dispersed in the matrix of anatase. Due to lower bandgap of rutile (3.0 eV) compared to anatase (3.2 eV), rutile absorbs the energy of illuminated light, which separates the electron–hole pair. Then, the electron transfer from the conduction band of rutile to anatase inhibits the electron–hole recombination process, which can further move to the surface of the catalyst and increase the degradation process of dyes (Bahnemann et al. 2007). While in the case of natural rutile, the photocatalytic efficiency is low due to having more defects in its crystal structure, which serves as the electron–hole recombination centre (Lu et al. 2007). Besides these, other commercially available TiO2 powders, namely Hombikat UV100 and PC500 have also been used for the photocatalytic degradation of organic dyes (Bahnemann et al. 2007; Saquib et al. 2008). Hombikat UV100 is composed of 100% anatase with a high specific BET surface area of >250 m2/g and primary particle size of 5 nm. The photocatalyst PC 500 is also composed of 100% anatase having a specific BET surface area of 287 m2/g and primary particle size of 5–10 nm, while Degussa P25 have a specific BET-surface area of 50 m2/g and primary particle size of 20 nm. It has been found that the activity of these photocatalysts is not only depend upon their physical properties such as BET surface area, defects and density of surface hydroxyl groups but also depend on the type of the model pollutant for the degradation. Saquib et al. (2008) have reported that the Hombikat UV100 have high photocatalytic activity for the degradation of Fast Green FCF, whereas Degussa P25 have shown relatively higher activity for the Patent Blue VF. As discussed earlier, Degussa P25 shows the higher activity due to slow electron–hole recombination process, whereas Hombikat UV100 exhibit high photocatalytic activity due to fast interfacial electron transfer process (Martin et al. 1994; Saquib et al. 2008). Lindner et al. (1995) have reported that the UV100 have almost four times more photocatalytic activity as compared to P25 for the degradation of dichloroacetic acid. It is observed that Hombikat UV100 can be a better catalyst for the degradation of benzidine and 1,2-diphenylhydrazine (Muneer et al. 2002). Recently, Flores et al. (2014) have synthesized ZnO nanostructures with different morphology such as hexagonal disks, dumb bell shaped, rice and rod like structures for the photocatalytic degradation of methylene blue (MB) in aqueous solution. This study reported that the activity of these nanostructures is directly related with the surface area and defect content in the nanostructure. It has been suggested that the ZnO nanostructure might be a low cost alternative to the TiO2 for the degradation of organic pollutants due to similar electronic bandgap (3.2 eV) and relatively higher photocatalytic activity especially for azo dyes (Flores et al. 2014). Akyol et al. (2004) have carried out photocatalytic decolourization of an azo dye Remazol red (RR), which shows that ZnO catalyst has much higher activity as compared to TiO2 for the degradation process. Lee et al. (2009) have carried out the study using ZnO and TiO2 for the photocatalytic degradation of total organic carbon (TOC) from aqueous solution of phenol which establishes that the ZnO nanopowder has 1.6 fold higher photocatalytic activity as compared to Degussa P-25 that is known as a standard photocatalyst.
15.3.2 Effect of pH of Solution
The photocatalytic degradation of organic dyes is strongly influenced by the pH of the solution as the charge on the surface of catalyst alters with the change in the pH of the solution. In addition, the physio-chemical properties of organic compound in the wastewater can also change with the change in the pH. As a result, the electrostatic interaction between the catalyst surface and organic dye is effected with the change in pH of the solution which will change the adsorption affinity of the organic pollutant on the surface of the catalyst thus would alter the degradation rate (Reza et al. 2017; Kazeminezhad and Sadollahkhani 2016). TiO2 as a photocatalyst has an amphoteric character, i.e. the surface of TiO2 can be charged positive or negative in acidic and basic pH, respectively, which will affect the degradation kinetics of the dyes on the surface of TiO2 (Poulios et al. 2000; Reza et al. 2017). Moreover, it has been found that the pH of the solution affects the formation of hydroxide radical by the reaction between light induced holes on the surface of catalyst and hydroxide ions. At low pH, the holes are considered as the major participant during the oxidation step in photocatalytic degradation, whereas at neutral and high pH, the hydroxide radicals are considered to be the predominant species for the degradation of organic dyes (Shifu and Gengyu 2005). As the presence of hydroxide ions are high on the surface of TiO2, which would increase the generation of hydroxide radical at high pH. Thus, the degradation efficiency would be high at higher pH in the case of TiO2, which have been indicated through the literature. Bubacz et al. (2010) have reported an increase in the rate of the photocatalytic degradation of methylene blue on the surface of TiO2 with the increase in pH. Su et al. (2009) have reported the degradation of acid blue 80 dye at different pH (2–10) on the surface of TiO2 and found the highest activity at pH 10, which can be attributed to the high adsorption of cationic acid blue 80 dye on the anionic TiO2 surface at pH 10. Ling et al. (2004) have reported the degradation of methylene blue at the surface of TiO2 at different pH and found highest activity at pH 12. Aly and Abd-Elhamid (2018) have reported the degradation of methylene blue on the surface of SiO2 nanoparticles at different pH and found that discolouration increases at very low pH of 1 and high pH of 11. As stated above, these results can be attributed to the fact that the positive holes are predominant oxidation species at low pH. On the contrary, at high pH, OH˙ radical is more easily generated by oxidizing more hydroxide ions present on the surface of SiO2, which increase the efficiency of degradation (Goncalves et al. 1999). Chen et al. (2017) reported the degradation of azo dyes such as methyl orange (MO), direct black 38(DB38) and Congo red (CR) on the surface of ZnO at different pH condition. They found that the degradation of these dyes is more efficient at low pH (acidic) in contrast to high basic pH. This can be understood in terms of the charge on the surface of ZnO and the properties of dyes at different pH condition. The ZnO surface becomes positively at acidic pH and the above dyes are anionic in nature. Thus, at lower pH, the high degradation of these dyes was attained due to the high electrostatic attraction between anionic dyes and positively charged ZnO catalytic surface, which results in the increase in the adsorption of dyes. On the contrary, at higher pH, there would be electrostatic repulsion between the anionic dyes and negatively charged ZnO surface, which results in negligible adsorption.
15.3.3 Effect of Light Intensity
The wavelength and the intensity of the incident light affect the amount of light absorption by the semiconductor photocatalyst. The electron–hole separation in photochemical reaction is initiated by the incident light with a particular wavelength and intensity on the surface of photocatalyst, which eventually monitor the degradation rate of the pollutant on the catalyst surface. It has been reported that the rate of photocatalytic degradation is better when the artificial UV-Vis light source is used as compared to solar irradiation (Viswanathan 2018). Ollis et al. (1992) have reported that the kinetics of the photocatalytic degradation is dependent on the intensity of incident light.
Accordingly,
-
1.
At low intensity of light (0–20 mW/cm2), the kinetics of photodegradation would follow first order, i.e. the rate would increase linearly with increasing intensity of light;
-
2.
At the intermediate light intensities, the kinetics follow half order, i.e. the rate of the photochemical reaction would depend on the square root of the intensity of incident light.
-
3.
At high intensities of incident light, the kinetics follow zero order w.r.t light intensity, i.e. the rate of photochemical reaction is independent of the intensity of light.
With the increase in light intensity, there would be more photons per unit time and unit area. Which increase the electron–hole separation on semiconductor catalytic surface, which eventually enhance the photocatalytic activity. However, at high light intensity, the number of activation sites on catalytic surface remains the same beside the increase in photon concentration. Thereby, the photochemical reaction rate would become independent of the light intensity.
15.3.4 Effect of Pollutant Concentration
The pollutant type and its concentration in the solution also affect the photocatalytic degradation process. In the solution containing pollutant and the catalyst, the pollutant molecules get adsorb on the surface of the catalyst. When there is increase in the concentration of the pollutant substrate, there will be subsequently more demand of the catalyst surface to adsorb the large number of pollutant molecules, however with the same amount of catalyst, the pollutant molecules would cover all active sites. Therefore, the demand of reactive radicals (OH˙ and ˙O2) for the degradation of pollutant would increase; however, the formation of these radicals on the catalyst surface would remain constant for a given catalyst amount, light intensity and irradiation time. Therefore, the available reactive radicals (OH˙ and ˙O2) would not be sufficient for the degradation of pollutant at its higher concentration (Bahnemann et al. 2007). Consequently, degradation rate of pollutant decreases with the increase in its concentration. Qamar and Muneer (2009) have reported that with the increase in concentration of the vanillin from 0.35 mM to 0.5 mM, the degradation rate increases, but with further increase in concentration from 0.5 mM to 1 mM, the rate of degradation decreases.
15.3.5 Effect of Catalyst Concentration
The amount of the photocatalyst also influences the kinetics of photocatalytic degradation. In general, with the increase in the quantity of semiconductor catalyst in photochemical reactor, the number of active sites on the semiconductor photocatalyst increases, which in turn would produce more number of reactive radicals (OH˙ and ˙O2) (Ahmed et al. 2011). Hence, there will be increase in the degradation rate of pollutants. However, after an optimum concentration, the further increment in catalyst loading may adversely affect the kinetics of degradation. With the further increase in catalyst concentration, there will be decrease in the penetration depth of light into the solution, which would diminish the light scattering. In addition, the agglomeration of catalyst particles could increase with the increment in catalyst concentration, which decreases the overall surface area for light absorption. Due to these combined effect, the degradation rate decreases with the increase in catalyst concentration beyond optimum limit. However, optimum limit could be different with different type of catalyst and design of photochemical reactor. Sharma et al. (2008) reported the degradation of isoproturon on TiO2/SBA-15 substrate with different loading of TiO2 catalyst from 0.5 to 2 g/L. They found that there is increase in the degradation rate when catalyst concentration increases from 0.5 to 1 g/L and thereafter, the degradation rate decreases slightly.
15.3.6 Effect of Oxidizing Agent
In the photocatalysis process using conventional catalyst like TiO2, electron–hole recombination process is the major drawback, which decreases the efficiency of photochemical degradation of dyes. The presence of molecular oxygen traps the excited electron of conduction band, thus inhibit the electron–hole recombination process to some extent. However, the presence of oxidizing agent(s) such as ozone (O3), hydrogen peroxide (H2O2), bromate (BrO3−) and peroxodisulphate (S2O82−) can further increase the kinetics of photochemical degradation of dyes (Wang et al. 2002; Saquib et al. 2008; Chen et al. 2018; Rehman et al. 2018; Feilizadeh et al. 2019). This is primarily due to their role in trapping more numbers of electrons from conduction band and thus further preventing the electron–hole recombination process, which may generate more oxidizing radicals (OH˙) and thus leads to increase in the photochemical degradation of dyes. It has been found that the H2O2 influences the kinetics of photochemical degradation by producing more hydroxide radical (OH˙) in two ways either by reduction of H2O2 by trapping electron at the conduction band or by self-decomposition of H2O2 into OH˙ when light falls on it, thereby increase the rate of dye degradation. Mahmoodi et al. (2006) have reported the effect of H2O2 on the photochemical degradation of the Reactive Blue 8 (RB 8) and Reactive Blue 220 (RB 220) and found increase in the degradation rate when concentration of H2O2 increases from 0 to its optimal concentration (300 mg/L for RB8 and 450 mg/L for RB220).
The effect of various parameters on the photocatalytic degradation of pollutants shown in literature has been summarized in Table 15.2.
15.4 Photocatalyst Material(S)
In recent times, several semiconducting material such as metal oxide(s) like TiO2, ZnO, ZrO2, WO3, CeO2, Fe2O3, Bi2O3, V2O5 and metal sulphide(s) like CdS and ZnS are used as catalyst surface in heterogeneous photocatalysis (Saravanan et al. 2017; Viswanathan 2018). Among all these, TiO2 has received the great attention in the photocatalysis industry due to its low cost and toxicity, chemical inertness, high photochemical activity, and non-specific oxidizing character (Akpan and Hameed 2009). TiO2 comprises three phases: anatase (3,2 eV), rutile (3.0 eV) and brookite (3–3.6 eV). Among these, anatase is the most effective phase for the dye and other organic pollutant degradation due to its constituent favourable position to adsorb the pollutant. The oxygen ions on the anatase are in the triangular position, which efficiently increase the absorption of organic molecules, whereas the titanium ions in the anatase phase are oriented in such a way to favour the reaction with the adsorbed species. Among others, ZnO (3.3 eV), SnO2 (3.57 eV) and CeO2 (3.19 eV) can be considered as alternative for TiO2 for the degradation of organic pollutants due to their high adsorbing capacity (Shinde et al. 2017).
However, due to large bandgap of these metal oxide catalyst(s), it can only absorb UV light which limits the efficient use of its applicability in degradation of organic pollutants and dyes since only about 10% of the solar spectrum consists of ultraviolet light (< 400 nm). In the rest of the radiant energy emitted from the sun, about 50% lies in the infrared (IR) region (> 700 nm) and about 40% in the visible region (400–700 nm) (Qiang and James 2003). On the earth crust, sunlight consists of only 3–5% of UV light, which makes it insufficient for the efficient degradation of dyes (Wang et al. 2019). To make use of enormous potential of sunlight, researchers are concentrating on to develop materials, which can absorb lower energy photon especially of visible light to make the degradation of dyes more efficient (Nakata and Fujishima 2012; Saravanan et al. 2017). Although there are certain metal sulphides like CdS (2.42 eV) and PbS (0.37 eV) which can absorb visible light photon due to their relatively lower bandgap; however, these metal sulphides are not stable, toxic in nature and composed of rare earth element which limits its application for the degradation of dyes (Boldish and White 1998; Shiga et al. 2016). It has been suggested that the bandgap of the above metal oxide(s) can be tuned in the visible light wavelength by adopting some techniques, which may improve the structure for high photocatalytic degradation of dyes. The next section will discuss about those improvement methods.
15.5 Modification of Catalyst
In spite of the extensive R&D on TiO2 as a photocatalyst for degradation of dyes and other organic pollutants, the low photo-quantum efficiency of TiO2 is still a major problem. This may be due to the rapid recombination of photo-generated electron and hole. In order to inhibit the electron–hole recombination process and to tune the bandgap of catalyst to absorb the visible energy for high photocatalytic activity, the surface of the semiconductor catalyst can be modified in the following ways: (1) Making a composite with metal/metal oxides or with carbon based materials like activated carbon, graphite, graphene and carbon nanotubes (CNTs); (2) Doping of certain metal and non-metal in the structure of semiconductor photocatalyst; and (3) Surface sensitization by dye/polymer.
15.5.1 Modification by Making Composite
One of the possible approaches for inhibiting the electron–hole recombination and modifying the bandgap of catalyst to work in visible region of solar radiation is to make coupled semiconductor photocatalyst(s). Recently, many semiconductor composites like Ag2O/TiO2, CuO@ZnO core shell, CdS/TiO2, PbS/TiO2, CdS/ZnO, and so on have been reported (Liu et al. 2017; Mansournia and Ghaderi 2017; Aziz et al. 2019; Jana et al. 2016). It has been found that the photocatalytic activity can be enhanced by making composite system as compared to single component photocatalyst. This is because the composition of two-semiconductor photocatalyst having different bandgaps can inhibit the electron–hole recombination. Recently, Aziz et al. (2019) have reported the composites PbS/TiO2 and CdS/TiO2 for enhancing the photocatalytic degradation of azo-based dye (acid orange-56) in the visible light by using the above principle. It has been shown that the activity of composite(s) PbS/TiO2 and CdS/TiO2 is significantly enhanced towards acid orange-56 in the visible light, while anatase-TiO2 alone did not show photocatalytic activity with the same conditions. This is because in the presence of visible light, there is rapid generation of electron–hole in the PbS and CdS due to their harmonized bandgap energy with visible light as compared to anatase-TiO2. Since the conduction band of TiO2 is situated at lower position to the conduction band of PbS and CdS, the photo-generated electron in PbS and CdS transfer to the conduction band of TiO2. In this way, the electron–hole recombination process inhibits and thus the photochemical activity toward the acid orange-56 degradation increases (Aziz et al. 2019).
In another approach, the composite of semiconductor with carbon based materials like activated carbon, graphite, graphene and CNTs and graphitic C3N4 have been explored. These heterostructure(s) enhance the photocatalytic activity because of high electrical conductivity, high surface area, high adsorption capacity and increase in charge separation and transfer at the heterojunction. Recently, Lin et al. (2018) have reported the hybrid structure of reduced graphene oxide/TiO2/graphitic carbon nitride (g-C3N4) for the enhanced photocatalytic degradation of methylene blue (MB) under solar light irradiation. In this heterostructure, the porous structure of reduced graphene oxide (rGO) and g-C3N4 increase the adsorption and photocatalytic reaction sites by making excellent dispersion of TiO2 nanoparticles on the heterostructure, while g-C3N4 increases the visible light absorption due to its lower bandgap. Furthermore, rGO helps to improve the photo-generated charge separation due to its high electronic conductivity. This ternary heterostructure has shown significant enhancement in the photocatalytic activity, which is about 17.2, 8.6 and 2.7 times more than that of g-C3N4, rGO/TiO2 and TiO2/g-C3N4. Similar reports comprising effect of composite materials with metal oxide(s) and carbon materials on the photocatalytic degradation of dyes and other pollutants are presented in Table 15.3.
15.5.2 Modification by Doping of Metal/Non-metal
The doping of certain metal/metal ion(s) into the semiconductor lattice is another approach to inhibit the electron–hole recombination and to extend the absorption of light in visible region. Many transition metals such as V, Cr, Fe, Ni, Co, Zn, W, Mo; and noble metals such as Pt, Au, Ag and Pd have been used as a dopant in the lattice of semiconductor to increase the charge separation (Garcia et al. 2017; Liu et al. 2013; Inturi et al. 2014; Sowmya et al. 2018). Inturi et al. (2014) have reported the doping of many transition metals (M = V, Cr, Fe, Co, Mn, Mo, Ni, Cu, Y, Ce and Zr) in the lattice of TiO2 for the visible light photocatalytic degradation of acetonitrile. In their study, Cr doped TiO2 showed the superior photocatalytic activity, which is 8–19 times higher than other metal-doped catalyst. It has been observed that during doing of Cr in TiO2 lattice, Ti3+ species originate in the structure. The Ti3+ energy level lies in between the valence and conduction band of TiO2, which helps to absorb the photon in visible region. On the other side, Cr nanoparticles may serve as trapping centre for the photo-generated electron in the conduction band of TiO2. In this way, the doping of Cr in the TiO2 inhibits the electron–hole recombination and increases the overall efficiency toward the acetonitrile degradation.
Doping/co-doping with non-metal(s) such as B, C, N and S in the semiconductor lattice can also increase the photocatalytic efficiency in visible light by red shifting the bandgap of semiconductor (Giannakas et al. 2016; Helmy et al. 2018). Jin et al. (2018) reported the efficient photocatalytic degradation of methyl orange (MO) on N doped anatase TiO2 catalyst and with increasing concentration of nitrogen, the activity toward degradation increases, whereas bare anatase TiO2 have no catalytic activity in visible light. Koltsakidou et al. (2018) reported the photocatalytic degradation of fluorouracil using TiO2-P25 and N/S doped TiO2 catalyst under visible light and found the best activity with codoped catalyst at the optimum molar concentration ratio of one.
15.5.3 Modification by Dye/Polymer Sensitizer
The photocatalytic activity of semiconductor in the visible light can also be influenced by using certain dye/polymer molecules such as reactive red dye 198 (RR 198), eosin-Y, merbromine, 2,7-dichlorofluorescein, curcumin, cobalt(II)phthalocyanine-tetrasulfonate, chrysoidine G, tolylene diisocyanate and polymer such as poly(fluorine-co-thiophene) (PFT) as the sensitizers (Rehman et al. 2009; Buddee et al. 2014; Behjou et al. 2013). When visible light is incident, dye/polymer molecule get excited and the excited electron moves to the conduction band of the semiconductor where these electrons are trapped by molecular oxygen to form superoxide radical (O2−) which can further increase the rate of degradation of organic pollutants. The literature reports comprising effect of doping of various metals, non-metals and surface sensitizers (dyes/polymers) on the photocatalytic degradation of organic pollutants are presented in Table 15.4.
The above modification(s) in the structure of semiconductor catalyst especially TiO2 have efficiently enhanced its activity in visible light. However, to apply a particular photocatalyst at the industrial scale, long-term stability and large-scale production have to be considered. Black TiO2 having high visible light response and thus high photocatalytic activity can be another interesting alternative to be used in the industry. Black TiO2 can be synthesized via hydrogenation of P25 TiO2 and can have a very low bandgap of about 1.0 eV which is lower than the P25 itself. However, the method used for the hydrogenation of P25 generally requires hydrogen atmosphere at high temperature and pressure, which impose a safety issue during its preparation. However, recently, Wang et al. (2019) introduced a hydrothermal method to overcome the shortcomings of hydrogenation method to reduce the P25 TiO2 with reducing agent like zinc (Zn) and aluminium (Al). The processed black TiO2 from this method have very low band in the visible region.
15.6 Conclusion
Based on recent studies, this chapter dealt with the mechanistic understanding of various operating parameters such as catalyst physical parameters, pH, light intensity, catalyst concentration, pollutant concentration and presence of oxidizing agent, which effect the photocatalytic degradation of various dyes and other organic pollutants. The studies exhibit that these parameters can significantly influence the photocatalytic degradation process. Photocatalyst degradation process has emerged as an excellent method for the mineralization of wastewater pollutants. However, the finding of an appropriate low cost, scalable photocatalyst, which can absorb visible light or sunlight, is still a challenge. The future work should concentrate on this issue in a very specific mode to provide a low cost, non-toxic, scalable, durable and reproducible photocatalyst, which can increase the efficiency of photocatalytic degradation by working in sunlight as the light source.
References
Ahmed S, Rasul MG, Martens WN, Brown R, Hashib MA (2011) Advances in heterogeneous photocatalytic degradation of phenols and dyes in wastewater: a review. Water Air Soil Pollut 215:3–29
Ahmed MA, Abou-Gamra ZM, Medien HAA, Hamza MA (2017) Effect of porphyrin on photocatalytic activity of TiO2 nanoparticles toward rhodamine B photodegradation. J Photochem Photobiol B Biol 176:25–35
Akpan UG, Hameed BH (2009) Parameters affecting the photocatalytic degradation of dyes using TiO2-based photocatalysts: a review. J Hazard Mater 170:520–529
Akyol A, Yatmaz HC, Bayramoglu M (2004) Photocatalytic decolorization of Remazol Red RR in aqueous ZnO suspensions. Appl Catal B 54:19–24
Aly HF, Abd-Elhamid AI (2018) Photocatalytic degradation of methylene blue dye using silica oxide nanoparticles as a catalyst. Water Environ Res 90(9):807–817. https://doi.org/10.2175/106143017X15131012187953
An L, Wang G, Cheng Y, Zhao L, Gao F, Cheng Y (2015) Synthesis of CdS/ZnO Nanocomposite and its enhanced photocatalytic activity in degradation of methyl orange. Russ J Phys Chem A 89(10):1878–1883
Aziz MI, Mughal F, Naeem HF, Zeb A, Tahir MA, Basit MA (2019) Evolution of photovoltaic and photocatalytic activity in anatase-TiO2 under visible light via simplistic deposition of CdS and PbS quantum-dots. Mater Chem Phys 229:508–513
Bahnemann W, Muneer M, Haque MM (2007) Titanium dioxide-mediated photocatalysed degradation off selected organic pollutants in aqueous suspensions. Catal Today 124:133–148
Balciolu IA, Otker M (2003) Treatment of pharmaceutical wastewater containing antibiotics by O3 and O3/H2O2 processes. Chemosphere 50:85–95
Behjou A, Aghaie H, Zare K, Aghaie M (2013) Synthesis and investigation of visible-light-activated rutile phase modified TiO2. Asian J Chem 25(2):880–882
Bendjabeur S, Zouaghi R, Kaabeche ONH, Sehili T (2017) Parameter affecting adsorption and photocatalytic degradation behavior of gentian violet under UV irradiation with several kinds of TiO2 as a photocatalyst. Int J Chem React Eng 15(4):20160206. https://doi.org/10.1515/ijcre-2016-0206
Boldish SI, White WB (1998) Optical band gaps of selected ternary sulfides minerals. Am Mineral 83:865–871
Bubacz K, Choina J, Dolat D, Morawski AW (2010) Methylene blue and phenol photocatalytic degradation on nanoparticles of anatase TiO2. Pol J Environ Stud 19:685–691
Buddee S, Wongnawa S (2015) Removal of dyes by photocatalytically active curcumin-sensitized amorphous TiO2 under visible light irradiation. J Sol-Gel Sci Technol 75:152–163
Buddee S, Wongnawa S, Sriprang P, Sriwong C (2014) Curcumin-sensitized TiO2 for enhanced photodegradation of dyes under visible light. J Nanopart Res 16:2336
Cassano AE, Alfano OM (2000) Reaction engineering of suspended solid heterogeneous photocatalytic reactors. Catal Today 58:167–197
Chen X, Wu Z, Liu D, Gao Z (2017) Preparation of ZnO photocatalyst for the efficient and rapid photocatalytic degradation of Azo dyes. Nanoscale Res Lett 12:143
Chen L, Cai T, Cheng C, Xiong Z, Ding D (2018) Degradation of acetamiprid in UV/H2O2 and UV/persulfate systems: a comparative study. Chem Eng J 351:1137–1146
Chong MN, Jin B, Chow CWK, Saint C (2010) Recent developments in photocatalytic water treatment technology: a review. Water Res 44:2997–3027
Ertis IF, Boz I (2017) Synthesis and characterization of metal-doped (Ni, co, Ce, Sb) CdS catalysts and their use in methylene blue degradation under visible light irradiation. Modern Research in Catalysis 6:1–14
Feilizadeh M, Attar F, Mahinpey N (2019) Hydrogen peroxide-assisted photocatalysis under solar light irradiation: interpretation of interaction effects between an active photocatalyst and H2O2. Can J Chem Eng 97(7):2009–2014
Flores NM, Pal U, Galeazzia R, Sandovalb A (2014) Effects of morphology, surface area, and defect content on the photocatalytic dye degradation performance of ZnO nanostructures. RSC Adv 4:41099
Fujishima A, Honda K (1972) Electrochemical photolysis of water at a semiconductor electrode. Nature 238(5358):37–38
Fujishima A, Rao TN, Tryk DA (2000) Titanium dioxide photocatalysis. J Photochem Photobiol C 1(1):1–21
Garcia OA, Valencia JE, Romero R, Rico-Cerda JL, Albiter MA, Natividad R (2017) W and Mo doped TiO2: synthesis, characterization and photocatalytic activity. Fuel 198:31–41
Gernjak W, Krutzler T, Glaser A, Malato S, Caceres J, Bauer R, Fernandez-Alba AR (2003) Photo-Fenton treatment of water containing natural phenolic pollutants. Chemosphere 50:71–78
Ghaly MY, Hartel G, Mayer R, Haseneder R (2001) Photochemical oxidation of p-chlorophenol by UV/H2O2 and photo-Fenton process. A comparative study. Waste Manag 21:41–47
Giannakas AE, Antonopouloua M, Daikopoulos C, Deligiannakis Y, Konstantinoua I (2016) Characterization and catalytic performance of B-doped, B–N co-doped and B–N–F tri-doped TiO2 towards simultaneous Cr(VI) reduction and benzoic acid oxidation. Appl Catal B Environ 184:44–54
Gleick PH (2015) Dirty water: estimated death from water-related diseases 2000-2020. Pacific Institute for Studies in Development, Environment, and Security, Oakland, pp 1–12. www.pacinst.org
Goncalves MST, Oliveira-Campos AMF, Pinto EMMS, Plasencia PMS, Queiroz MJRP (1999) Photochemical treatment of solutions of Azo dyes containing TiO2. Chemosphere 39:781–786
Goswami L, Manikandan NA, Pakshirajan K, Pugazhenthi G (2017) Simultaneous heavy metal removal and anthracene biodegradation by the oleaginous bacteria Rhodococcus opacus. 3 Biotech 7:37
Gupta VK, Ali I, Saleh TA, Nayak A, Agarwal S (2012) Chemical treatment technologies for waste-water recycling-an overview. RSC Adv 2:6380–6388
He R, Zhou J, Fu H, Zhang S, Jiang C (2018) Room-temperature in situ fabrication of Bi2O3/g-C3N4 direct Z-scheme photocatalyst with enhanced photocatalytic activity. Appl Surf Sci 430:273–282
Helmy AT, Nemr AE, Mousa M, Arafa E, Eldafrawy S (2018) Photocatalytic degradation of organic dyes pollutants in the industrial textile wastewater by using synthesized TiO2, C-doped TiO2, S-doped TiO2 and C, S co-doped TiO2 nanoparticles. J Water Environ Nanotechnol 3(2):116–127
Hoshiyama N, Dabwan AHA, Katsumata H, Suzuki T, Furukawa M, Kaneco S (2016) Enhanced photocatalytic degradation of bisphenol A in aqueous solution by Ag-doping ZnO. Open J Inorg Non-Met Mater 6:13–17. https://doi.org/10.4236/ojinm.2016.63003
Inturi SNR, Boningari T, Suidanb M, Smirniotis PG (2014) Visible-light-induced photodegradation of gas phase acetonitrile using aerosol-made transition metal (V, Cr, Fe, Co, Mn, Mo, Ni, Cu, Y, Ce, and Zr) doped TiO2. Appl Catal B Environ 144:333–342
Jana TK, Maji SK, Pal A, Maiti RP, Dolai TK, Chatterjee K (2016) Photocatalytic and antibacterial activity of cadmium sulphide/zinc oxide nanocomposite with varied morphology. J Collids Interface Sci 480:9–16
Jin YJ, Linghu J, Chai J, Chua CS, Wong LM, Feng YP, Yang M, Wang S (2018) Defect evolution enhanced visible-light photocatalytic activity in nitrogen-doped anatase TiO2 thin films. J Phys Chem C 122:16600–16606
Kang X, Han Y, Song X, Tan Z (2018) A facile photo assisted route to synthesis N, F-codoped oxygen-deficient TiO2 with enhanced photocatalytic performance under visible light irradiation. Appl Surf Sci 434:725–734
Kazeminezhad I, Sadollahkhani A (2016) Influence of pH on the photocatalytic activity of ZnO nanoparticles. J Mater Sci Mater Electron 27(5):4206–4215
Khataee V, Vatanpour V, Amani AR (2009) Decolorization of C.I. acid blue 9 solution by UV/nano -TiO2, Fenton, Fenton-like, electro-Fenton and electrocoagulation processes: a comparative study. J Hazard Mater 161:1225–1233. https://doi.org/10.1016/j.jhazmat.2008.04.075
Koltsakidou A, Antonopoulou M, Evgenidou E, Konstantinou I, Lannakas AE, Papadaki M, Bikiaris D, Lambropoulou DA (2018) Photocatalytical removal of fluorouracil using TIO2-P25 and N/S doped TiO2 catalysts: a kinetic and mechanistic study. Sci Total Environ 578:257–267
Kushwaha A, Rani R, Kumar S, Thomas T, David AA, Ahmed M (2017) A new insight to adsorption and accumulation of high lead concentration by exopolymer and whole cells of lead-resistant bacterium Acinetobacter junii L. Pb1 isolated from coal mine dump. Environ Sci Pollut Res 24:10652–10661
Lee JC, Park H-J, Lee J-H, Kim H-S, Chung Y-J (2009) Photocatalytic degradation of TOC from aqueous phenol solution using combusted ZnO nanopowder. J Electroceram 22:110–113
Liang B, Zhang W, Zhang Y, Zhang R, Liu Y (2019) Efficient visible-light photocatalyst synthesized by modifying SnO with activated carbon. Mater Res Express 6:015603
Lin HH-H, Lin AY-C, Hung C-L (2015) Photocatalytic oxidation of cytostatic drugs by microwave-treated N-doped TiO2 under visible light. J Chem Technol Biotechnol 90:1345–1354
Lin P, Hu H, Lv H, Ding Z, Xu L, Qian D, Wang P, Pan J, Li C, Cui C (2018) Hybrid reduced graphene oxide/TiO2/graphitic carbon nitride composites with improved photocatalytic activity for organic pollutant degradation. Appl Phys A 124:510
Lindner M, Bahnemann DW, Hirthe B, Griebler WD (1995) Novel TiO2 powders as highly active photocatalysts. In: Stine WB, Tanaka T, Claridge DE (eds) Solar water detoxification; solar engineering. ASME, New York, pp 339–408
Ling CM, Mohamed AR, Bhatia S (2004) Performance of photocatalytic reactors using immobilized TiO2 film for the degradation of phenol and methylene blue dye present in water stream. Chemosphere 57:547–554
Liu M, Qiu X, Miyauchi M, Hashimoto K (2013) Energy-level matching of Fe(III) ions grafted at surface and doped in bulk for efficient visible-light photocatalysts. J Am Chem Soc 135(27):10064–10072
Liu H, Hu Y, He X, Jia H, Liu X, Xu B (2015) In-situ anion exchange fabrication of porous ZnO/ZnSe heterostructural microspheres with enhanced visible light photocatalytic activity. J Alloys Compd 650:633–640
Liu B, Mu L, Han B, Zhang J, Shi H (2017) Fabrication of TiO2/Ag2O heterostructure with enhanced photocatalytic and antibacterial activities under visible light irradiation. Appl Surf Sci 396:1596–1603
Lu A, Li Y, Lv M, Wang C, Yang L, Liu J (2007) Photocatalytic oxidation of methyl orange by natural V bearing rutile under visible light. Sol Energy Mater Sol Cells 91:849–1855
Lu C, Zhang L, Zhang Y, Liu S (2016) Electrodeposition of TiO2/CdSe heterostructure films and photocatalytic degradation of methylene blue. Mater Lett 185:342–345
Mahmoodi NM, Arami M, Limaee NY, Tabrizi NS (2006) Kinetics of heterogeneous photocatalytic degradation of reactive dyes in an immobilized TiO2 photocatalytic reactor. J Colloid Interface Sci 295:159–164
Mansournia M, Ghaderi L (2017) CuO@ZnO core-shell nanocomposites: novel hydrothermal synthesis and enhancement in photocatalytic property. J Alloys Compd 691:171–177
Martin ST, Herrmann H, Choi W, Hoffmann MR (1994) Time-resolved microwave conductivity. Part 1.-TiO2 photoreactivity and size quantization. J Chem Soc Faraday Trans 90:3315–3322
Muneer M, Singh HK, Bagnemann D (2002) Semiconductor-mediated photocatalysed degradation of two selected priority organic pollutants, benzidine and 1, 2-diphenylhydrazine, in aqueous suspension. Chemosphere 49:193–203
Nakata K, Fujishima A (2012) TiO2 photocatalysis: design and applications. J Photochem Photobiol C 13(3):169–189
Oller I, Malato S, Sanchez-Perez JA (2011) Combination of advanced oxidation processes and biological treatments for wastewater decontamination-a review. Sci Total Environ 409(20):4141–4166
Ollis DF (1985) Contaminant degradation in water. Environ Sci Technol 19(6):480–484
Ollis DF, Pelizzetti E, Serpone N (1992) Photocatalyzed destruction of water contaminants. Environ Sci Technol 25:1522–1529
Osin OA, Yu T, Cai X, Jiang Y, Peng G, Cheng X, Li R, Qin Y, Lin S (2018) Photocatalytic degradation of 4-nitrophenol by C, N-TiO2: degradation efficiency vs. embryonic toxicity of the resulting compounds. Front Chem 6:192. https://doi.org/10.3389/fchem.2018.00192
Pimental D (1991) Global warming, population growth, and natural resources for food production. Soc Nat Resour 4:347–363. https://doi.org/10.1080/08941929109380766
Poulios I, Avranas A, Rekliti E, Zouboulis A (2000) Photocatalytic oxidation of Auramine O in the presence of semiconducting oxides. J Chem Technol Biotechnol 75:205–212
Qamar M, Muneer M (2009) A comparative photocatalytic activity of titanium dioxide and zinc oxide by investigating the degradation of vanillin. Desalination 249:535–540
Qiang F, James R (2003) Encyclopedia of atmospheric sciences. Academic Press, Amsterdam, pp 1859–1863
Rajeshwar K, Osugi ME, Chanmanee W, Chenthamarakshan CR, Zanoni M, Kajitvichyanukul P, Krishnan-Ayer R (2008) Heterogeneous photocatalytic treatment of organic dyes in air and aqueous media. J Photochem Photobiol C 9(4):171–192
Raza W, Faisal SM, Owais M, Bahnemann D, Muneer M (2016) Facile fabrication of highly efficient modified ZnO photocatalyst with enhanced photocatalytic, antibacterial and anticancer activity. RSC Adv 6:78335
Rehman S, Ullah R, Butt AM, Gohar ND (2009) Strategies of making TiO2 and ZnO visible light active. J Hazard Mater 170(2–3):560–569
Rehman F, Sayed M, Khan JV, Shah NS, Khan HM, Dionysioua DD (2018) Oxidative removal of brilliant green by UV/S2O82−, UV/HSO5− and UV/H2O2 processes in aqueous media: a comparative study. J Hazard Mater 357:506–514
Ren L, Li Y, Hou J, Zhao X, Pan C (2014) Preparation and enhanced photocatalytic activity of TiO2 nanocrystals with internal pores. ACS Appl Mater Interfaces 6:1608–1615
Reza KM, Kurny AS, Gulshan F (2017) Parameters affecting the photocatalytic degradation of dyes using TiO2: a review. Appl Water Sci 7:1569–1578
Saad ST, Al-Gubury HY, Alrazzak NA (2018) Photocatalytic degradation of monoazo dye in ethanol using zinc oxide in ultra-violet radiation. Asian J Chem 30(10):2334–2336
Saggioro EM, Oliveira AS, Moreira JC (2016) Heterogeneous photocatalysis remediation of wastewater polluted by indigoid dyes. In: Textile wastewater treatment. Intech Open, London, p 94. https://doi.org/10.5772/63790
Salehi M, Hashemipour H, Mirzaee M (2012) Experimental study of influencing factors and kinetics in catalytic removal of methylene blue with TiO2 nanopowder. Am J Environ Eng 2(1):1–7
Saquib M, Tariq MA, Faisal M, Muneer M (2008) Photocatalytic degradation of two selected dye derivatives in aqueous suspensions of titanium dioxide. Desalination 219:301–311
Saravanan R, Gracia F, Stephen A (2017) Basic principles, mechanism, and challenges of photocatalysis. In: Khan MM et al (eds) Nanocomposites for visible light-induced photocatalysis. Springer, Cham, pp 19–40. https://doi.org/10.1007/978-3-319-62446-4_2
Serpone N, Horikoshi S, Emeline AV (2010) Microwaves in advanced oxidation processes for environmental applications: a brief review. J Photochem Photobiol C 11:114–131
Serpone N, Emeline AV, Horikoshi S, Kuznetsov VN, Ryabchuk VK (2012) On the genesis of heterogeneous photocatalysis: a brief historical perspective in the period 1910 to the mid-1980s. Photochem Photobiol Sci 11:1121
Sharma MVP, Kumari VD, Subrahmanyam M (2008) TiO2 supported over SBA-15: An efficient photocatalyst for the pesticide degradation using solar light. Chemosphere 73:1562–1569
Shifu C, Gengyu C (2005) Photocatalytic degradation of pesticides using floating photocatalyst TiO2.SiO2 beads by sunlight. Sol Energy 79:1–9
Shiga Y, Umezawa N, Srinivasan N, Koyasu S, Sakai E, Miyauchi M (2016) A metal sulfide photocatalyst composed of ubiquitous elements for solar hydrogen production. Chem Commun 52:7470–7473
Shinde DR, Tambade PS, Chaskar MG, Gadave KM (2017) Photocatalytic degradation of dyes in water by analytical reagent grades ZnO, TiO2 and SnO2: a comparative study. Drink Water Eng Sci 10:109–117
Singh PK, Kushwaha A, Hans N, Gautam A, Rani R (2019) Evaluation of the cytotoxicity and interaction of lead with lead resistant bacterium Acinetobacter junii Pb1. Braz J Microbiol 50(1):223–230
Sowmya SR, Madhu GM, Hashir M (2018) Studies on nano-engineered TiO2 photo catalyst for effective degradation of dye. IOP Conf Ser Mater Sci Eng 310:012026. https://doi.org/10.1088/1757-899X/310/1/012026
Su Y, Deng L, Zhang N, Wang X, Zhu X (2009) Photocatalytic degradation of C.I. acid blue 80 in aqueous suspensions of titanium dioxide under sunlight. React Kinet Catal Lett 98(2):227–240. https://doi.org/10.1007/s11144-009-0059-4
Teoh WY, Scott JA, Amal R (2012) Progress in heterogeneous photocatalysis: from classical radical chemistry to engineering nanomaterials and solar reactors. J Phys Chem Lett 3:629–639
Thennarasu G, Sivasamy A (2019) Mn doped ZnO nano material: a highly visible light active photocatalyst for environmental abatment. Inorg Nano-Metal Chem 48(4–5):239–246
Viswanathan B (2018) Photocatalytic degradation of dyes: an overview. Current Catalysis 7(1):1–25
Wang S, Shiraishi F, Nakano K (2002) A synergistic effect of photocatalysis and ozonation on decomposition of formic acid in an aqueous solution. Chem Eng J 87:261–271
Wang F, Ma Z, Ban P, Xu X (2017) C, N and S codoped rutile TiO2 nanorods for enhanced visible-light photocatalytic activity. Mater Lett 195:143–146
Wang T, Zhang Y-L, Pan J-H, Li B-R, Wu L-G, Jiang B-Q (2019) Hydrothermal reduction of commercial P25 photocatalysts to expand their visible-light response and enhance their performance for photodegrading phenol in high-salinity wastewater. Appl Surf Sci 480:896–904
Yoon J, Lee Y, Kim S (2001) Investigation of the reaction pathway of OH radicals produced by Fenton oxidation in the conditions of wastewater treatment. Water Sci Technol 44(5):15–21
Zulmajdi SLN, Ajak SNFH, Hobley J, Duraman N, Harunsani MH, Yasin HM, Nur M, Usman A (2017) Kinetics of photocatalytic degradation of methylene blue in aqueous dispersions of TiO2 nanoparticles under UV-LED irradiation. Am J Nanomater 5(1):1–6
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Thareja, S. (2021). Mechanistic Understanding of Heterogeneous Photocatalysis for the Dye Degradation in Wastewater. In: Gupta, P.K., Bharagava, R.N. (eds) Fate and Transport of Subsurface Pollutants. Microorganisms for Sustainability, vol 24. Springer, Singapore. https://doi.org/10.1007/978-981-15-6564-9_15
Download citation
DOI: https://doi.org/10.1007/978-981-15-6564-9_15
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-6563-2
Online ISBN: 978-981-15-6564-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)