Abstract
The dye C.I. Acid Blue 80 (AB80) was easily degraded by TiO2-P25 assisted photocatalysis in aqueous dispersion under irradiation of sunlight. The optimal reaction conditions were [TiO2] = 2.0 g/L, pH = 10, [H2O2] = 5 mmol/L. The photocatalytic reaction followed pseudo-first order kinetics. The adsorption of AB80 onto TiO2 was in accord with Langmuir equation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
More than 7 × 105 tons of synthetic dyes are produced every year worldwide. About 10–15% of these dyes are lost as waste pollutants entering the environment [1]. Synthetic dyes usually contain complex aromatic structures which are chemically stable and resistant to biodegradation in nature. Their toxicity and persistence in the environment have been a great concern to aquatic life and human health [2]. Treatment and clean-up of these pollutants from wastewater thus become an essential issue of environmental and health protection. In recent years, removal of synthetic dyes from wastewater using heterogeneous photocatalysis has received great attention [3, 4]. Photocatalytic treatment refers to the combination of certain semiconductors (such as TiO2, ZnO, CdS, SiO2, Fe2O3) and the light for the production of reactive species. The produced reactive species such as hydroxyl radicals can oxidize a broad range of organic pollutants effectively and non-selectively [5, 6]. Titanium dioxide (TiO2) is generally recognized as one of the most efficient, non-toxic, and inexpensive photocatalysts.
Solar energy is free, and its supplies are unlimited; the utilization of this vast and abundant source of energy is significant especially for the energy crisis of modern society. Many previous studies discussed the photocatalytic degradation of target organic pollutants used TiO2 under sunlight [7, 8]. AB80 is one of the important species of acid dyes used widely for wool and nylon dyeing [9]. Ao et al. discussed the decoloration of AB80 using sol–gel TiO2 thin film [10]. Bianco Prevot et al. examined the early steps of the degradation process of AB80 using TiO2 as photocatalyst [9]. However, sunlight photocatalytic degradation of AB80 with TiO2 was not reported in previous studies.
In this work, the photocatalytic degradation of AB80 with TiO2-P25 under sunlight was studied. The adsorption and desorption of AB80 and kinetics as well as the influencing factors such as catalyst loading, pH and electron acceptors were also discussed.
Experimental
Materials and reagents
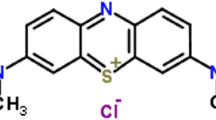
C.I. Acid Blue 80 (Fig. 1) was obtained from Qingdao Double-Peach Specialty Chemicals Co. Ltd (Qingdao, China), and was used without any further purification. The pH of the 0.1 mmol/L AB80 dye solution was 5.92. The water used in all experiments was double distilled. The photocatalyst TiO2 (Degussa P25) consists of 80% anatase and 20% rutile with a specific BET-surface area of 50 m2 g−1 and primary particle size of 21 nm. Analytical grade H2O2 (30% w/w), KBrO3 and (NH4)2S2O8 were obtained from Merck. The pH of the solution was adjusted using 0.1 M HNO3 and 0.1 M NaOH.
Apparatus
The sunlight photoreaction equipment was composed of ten open Pyrex test tubes (diameter of 23 mm and length of 21 cm) and a board. The test tubes were fixed on the board with an air tube extended to the bottom of each test tube to ensure the excess molecular oxygen required in the reaction and stirring the solution at the same time. The air tubes were adjusted to make them suitable for simultaneous air discharge. The slope angle of the board was adjusted to ensure the vertical irradiation of sunlight.
Procedure
Aqueous solutions of the dye with the desired amount of photocatalyst were added to reaction vessels, and then bubbled with air for at least 1 h in dark to allow equilibration of the system so the loss of compound due to adsorption can be taken into account. At regular time intervals, samples of 10 mL were taken and centrifuged to remove TiO2 particles. The experiment was carried out at noon of a sunny day. The intensity of the illumination was 108,000–124,000 lx and the temperature of the reaction solution was 30 ± 1 °C.
Analyses
The dye concentration was determined based on the absorbance at 626 nm measured by a DU 650 spectrophotometer (Beckman, America). The mineralization of the dye was monitored by measuring the total organic carbon (TOC) content with a TOC-VCPH (Shimadzu Company, Japan).
Adsorption and desorption test
The adsorption equilibrium experiments were performed with TiO2 dosage of 1.0 g/L and initial dye concentration of 0, 0.03, 0.05, 0.1, 0.15 and 0.2 mmol/L. The pH of the solutions was adjusted to certain values (pH 3.0, 6.0, 9.0), and then the solutions were shaken in the dark for 24 h at 30 °C in order to achieve adsorption equilibrium. The amount of dye adsorbed on the catalyst was calculated by mass balance.
The desorption experiments with 0.1 mmol/L AB80 solutions and 1.0 g/L TiO2 dosage were performed by adjusting the solutions to pH 11. Desorption of AB80 molecule from the photocatalyst after the photocatalytic reaction of 75 min under sunlight was also performed.
Results and discussion
Adsorption and desorption experiments
The adsorption of AB80 onto the surfaces of TiO2 was studied in our experiments. A blank test proved that part of dye molecules tended to flocculate when the solution was acidic. The flocculate part has been eliminated from the dye solution.
The adsorption of different concentrations of AB80 dye onto TiO2 at various pH is shown in Fig. 2. With increasing pH, the adsorption capacity (q) reduced significantly. When the initial dye concentration was 0.1 mmol/L, the adsorption capacities at pH 3, 6 and 9 were 0.0517, 0.022, 0.0048 mmol/g, respectively. For each adsorption curve, there was a similar tendency: with increasing equilibrium concentration (C e), the adsorption (q) tended to approach a constant value. When plotting 1/q versus 1/C e, a linear relationship was obtained that fitted to the rearranged Langmuir equation Eq. 1:
where q is the mass of dye adsorbed per unit weight of TiO2 (mmol/g), b is an affinity constant related to the energy of adsorption (L/mmol), q m is the maximum adsorption capacity (mmol/g) and C e is equilibrium concentration of the dye in solution (mmol/L). The values of b and q m were given by the linear expression (Table 1).
The adsorption varied with pH due to the surface charge property of TiO2 that changed with pH changes in the solution. The point of zero charge (PZC) for the used TiO2-P25 is close to 6.0 (the value was not uniform in different papers [11–13]). In acidic solution, the pH is lower than PZC and hence TiO2 surfaces are positively charged (Eq. 2) [6].
Reversely, in basic solution the surfaces are negatively charged as given in Eq. 3.
The AB80 dye in solution was negatively charged because of the sulfonate group. At pH < PZC, the electrostatic attraction between positively charged TiO2 surfaces and negatively charged AB80 molecule led to strong adsorption. At pH > PZC, TiO2 surfaces became negative and so it was electrostatically repulsive to the negatively charged dye molecules. Hence, the adsorption of dye was lower [14].
Desorption of AB80 from surfaces of TiO2 was studied in our experiment (Table 2). Before photodegradation, the concentration of AB80 was 0.0913–0.0917 mmol/L after desorption. This indicated that the majority of AB80 molecules desorbed from the surface of TiO2 when the pH of the solution was adjusted to 11. In order to clarify the contribution of adsorption and photodegradation, desorption experiment was performed after photocatalytic reaction. In acidic solution (pH 3.0), adsorption plays a major role, the concentration of AB80 increased from 0.00531 to 0.0389 mmol/L after desorption. When the pH of the solution was not adjusted (pH 6.2), most of the adsorbed AB80 molecules had photodegraded, the concentration of AB80 increased from 0 to 0.00432 mmol/L after desorption. The adsorbed AB80 molecule degraded completely in alkaline solution (pH 9.0).
Photocatalytic degradation of AB 80 under sunlight
The experiment of photocatalytic degradation dye AB80 under sunlight was carried out with initial AB80 concentration of 0.1 mmol/L and TiO2 dose of 1.0 g/L. Negligible decoloration of AB80 occurred for the solution with TiO2 in the dark, as shown in Fig. 3. A small amount of decoloration occurred with irradiation of sunlight in the absence of TiO2. A complete decoloration of AB80 could only be observed with the simultaneous presence of TiO2 and sunlight. The decoloration rate was much higher than the mineralization rate. This was because a large number of colorless intermediates still existed in the solution after decoloration. The removal of these intermediates from solution took longer time [9].
Photocatalytic degradation of AB80 under sunlight, [AB80] = 0.1 mmol/L, pH = 6.2, [TiO2] = 1 g/L, intensity of sunlight was 108,000–124,000 lx. (1) decoloration of AB80 with TiO2 in the dark, (2) mineralization of AB80 without TiO2 under sunlight, (3) decoloration of AB80 without TiO2 under sunlight, (4) mineralization of AB80 with TiO2 under sunlight, (5) decoloration of AB80 with TiO2 under sunlight
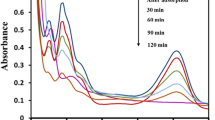
The UV–Vis spectra obtained from the dye solution as a function of irradiation time are shown in Fig. 4. The UV–Vis absorption of AB80 was characterized by two bands in the visible region (582 nm, 626 nm) and two bands in the ultraviolet region (202 nm, 260 nm). These different peaks could attribute to the n → π * transition and π → π * transition of anthraquinone and benzene rings [15]. The subsequent illumination of the aqueous AB80 suspension caused a continuous decrease of the intensities of the UV and Vis bands of AB80. The absorption peaks at 260 nm, 582 nm and 626 nm disappeared after the irradiation of 45 min. The concentration of AB80 became negligible compared to the concentrations of intermediate compounds. Complete decoloration was achieved at 75 min of irradiation when the absorbance in visible region reached to zero. Complete mineralization occurred in 135 min when the TOC of the solution decreased to zero.
The TiO2-P25 semiconductor absorbs light whose wavelength is less than or equal to 400 nm (band gap energy of TiO2-P25 is 3.2 eV), and only 5% of solar energy exist in this spectrum. However, in the sunlight photocatalysis experiment, the visible light could also participate in the reaction. In the case of dyes, due to their ability to absorb part of the visible light, a mechanism of degradation connected with visible light could occur as well. According to this mechanistic approach, the AB80 dye molecules adsorbed onto the TiO2 surface form appropriate excited states due to visible illumination and then these excited states mainly transfer electrons to the conduction band of TiO2 particles, as shown in Eqs. 4 and 5. The TiO2 (e−) could combine with O2, generate O2 •− radicals (Eq. 6) [16, 17].
Besides, singlet oxygen (1O2) has been found to be a much stronger oxidizing agent than ground state molecular oxygen. Formation of singlet oxygen via sensitization by AB80 can also play a considerable role in the visible light effect (Eq. 7).
Kinetics of the photocatalytic reaction
The kinetics of the photocatalytic degradation dye AB80 under sunlight was studied. The species that participated in the photocatalytic reaction were dye molecules and active radicals (OH•, O2 •−). The rate equation can be written as Eq. 8.
where C A is the concentration of dye, C B… is the concentration of radicals. In this way, the reaction orders depend on the numbers of reactive molecules. If the active radical concentration is constant and greater than the dye concentration, the above equation can be transformed to Eq. 9.
A plot of ln C 0/C versus irradiation time showed linear relationship (Fig. 5). This indicates that the photocatalytic degradation of AB80 dye with TiO2 obeys apparent pseudo-first order kinetics. The rate constants (k′) and correlation coefficients (R 2) are summarized in Table 3.
In our experiment, the initial rate (r 0) was employed to express the reaction ability, and r 0 was calculated from Eq. 10.
Effect of catalyst loading
The influence of the catalyst concentration on the initial reaction rate with 0.1 mmol/L of AB80 dye solutions and different concentrations of TiO2 varied from 0.3 to 4.0 g/L was studied in our experiment. The results are presented in Fig. 6. It is obvious that the rate increased with an increase of the concentration of catalyst up to [TiO2] = 2.0 g/L and then declined. The optimal TiO2 dosage was 2.0 g/L. A similar result was observed in previous studies [2, 18].
a Changes in AB80 concentration as a function of irradiation time with different catalyst loading. b Effect of catalyst loading on the initial reaction rate of AB80. Experimental condition: [AB80] = 0.1 mmol/L, pH = 6.2, [TiO2] = 0.3, 0.5, 1.0, 2.0, 4.0 g/L, intensity of sunlight was 108,000–124,000 lx
The increased degradation rate that followed the increase of catalyst loading could be attributed to the increase in the number of reactive radicals and absorbed dye molecules on the surfaces of TiO2. The decrease of initial rate could be due to an increasing opacity of the suspension and to an enhancement of the light reflectance caused by the excess of TiO2 particles. Moreover, particle–particle interaction became significant as the amount of particles in solution increased. The activated molecules tended to deactivate because of collision, thus reducing the site density for surface holes and electrons [19].
Effect of pH
The pH of the solution is an important factor affecting the degradation of dye during the course of photocatalysis because the surface charges property of the photocatalyst and the photo-oxidation process are all pH dependent [11]. We examined the photocatalytic degradation of AB80 under sunlight in aqueous solution with initial dye concentration of 0.1 mmol/L and TiO2 dose of 1.0 g/L between pH range of 2–10. Changes in dye concentration under different initial pH as a function of irradiation time and changes in initial rate of AB80 as a function of initial pH are plotted in Fig. 7a and b. Because of the strong adsorption in acidic solution, the initial dye concentration was low at pH 2 and 4. With the increase of pH from 2 to 6 and from 8 to 10, the initial reaction rate increased. However, in the pH range of 6–8, the initial rate was reduced slightly. Similar results were found by Prevot et al. [9], but they only tested three pH values (pH 3, 6.4 and 9). They found that the reaction process was the fastest at pH 6.4 and slightly slower at pH 9. In our experiment, we found that when the pH value increased to 10, the reaction rate increased again and the reaction rate was even faster.
The solution pH affected the generation of OH•, O2 •− radicals which are the primary oxidizing species for the photocatalytic oxidation. The generative process of OH•, radicals can be expressed by Eqs. 11 and 12. The generative process of O2 •− can be expressed by Eq. 7.
The point of zero charge for the used TiO2 is pH 6. When the pH of solution is lower than 6, the surface of the particles will be positively charged and a large quantity of dye molecules adsorb onto the TiO2 particles because of electrostatic attraction. It will be difficult for the electron (eCB −) and vacancy (hVB +) to contact with O2, H2O and OH− to produce radicals. Although the dye ions themselves adsorbed on the catalyst surface can react with both the electrons and the holes photochemically produced, the strong adsorption leads to a major decrease of the active centers on the catalyst surface, which means the absorption of the light quanta by the catalyst decreased as well. Moreover, the pH of the solution was adjusted using HNO3, and NO3 − could also adsorb on the TiO2 particles, as shown in Eq. 13. The adsorbed nitrates compete with the dye for the photo-oxidizing species on the surface and preventing the photocatalytic degradation of AB80 [20]. Besides, NO3 − as a well-known electron scavenger can also be reduced electrons on the surface of the catalyst particles.
On the contrary, when the pH of the solution is above 6, the surface of the particles will be negatively charged and it will be difficult for the dye molecules to approach the catalyst surfaces because of electrostatic repulsion. The reaction rate decreased in the range of pH 6–8. At higher pH values, the formation of active OH• species is favored, because the abundant OH− promoted the combination of hole (hVB +) and OH− to produce OH• [9].
Effect of electron acceptors
The electron–hole recombination during the course of photocatalysis was a major energy-wasting step. In order to inhibit the electron–hole recombination, a proper electron acceptor is usually added to the reaction system. Molecular oxygen has been the most universal electron acceptor in heterogeneous photocatalysis reactions. In addition to molecular oxygen, H2O2, KBrO3 and (NH4)2S2O8 were also added to the reaction solution to inhibit the electron–hole recombination [11]. The influence of electron acceptors such as H2O2, KBrO3 and (NH4)2S2O8 on the reaction rate is shown in Fig. 8.
It is clear that the addition of H2O2 enhanced the reaction rate significantly (Fig. 8). When the concentration of H2O2 was 5 mmol/L, the initial reaction rate was 2.78 times higher than the contrast rate. When the concentration of H2O2 was higher than 5 mmol/L, the initial rate began to fall. Hence 5 mmol/L H2O2 appeared to be optimal for the degradation. H2O2 can compensate for the lack of O2 and play a role as an external electron scavenger according to Eq. 14. It can trap the photogenerated conduction band electron (eCB −) and thus inhibiting the electron–hole recombination, at the same time produces hydroxyl radicals, as shown by the Eq. 14.
H2O2 also reacts with superoxide anion (O2 •−) to form hydroxyl radicals (Eq. 15).
In the presence of excess H2O2, it may react with OH• to form hydroperoxy radicals (HO2 •), which are detrimental to the potocatalytic action, as shown in Eqs. 16 and 17 [14].
The promotion of KBrO3 and (NH4)2S2O8 to the initial rate was low compared with H2O2 as shown in Fig. 8. The promotion of KBrO3 to the initial rate was higher than that of (NH4)2S2O8. Upon the addition of KBrO3 was 3 mmol/L, the initial rate improved by 48.6%. On further increase in the concentration of KBrO3, the initial rate is almost constant. Therefore, the optimal concentration of KBrO3 was 3 mmol/L. A blank test indicated that without the photocatalyst, the KBrO3 alone caused 29% decoloration after 75 min irradiation. This removal may be due to the excitation of dye molecule from S0 to S1 state by photo absorption followed by ionization (Eqs. 4 and 5) and the degradation of the ionized dye by BrO2 • (Eq. 18).
The enhancement of the reaction rate is due to its electron scavenging effect by the reaction between BrO3 − ion and conduction band electron (Eqs. 19 and 20). On further increase of KBrO3 addition only small enhancement was observed. This is due to adsorption effect of Br− ion on TiO2 surface, which affects the catalytic activity of TiO2 [14].
When the concentration of (NH4)2S2O8 was 2 mmol/L the initial rate improved by 29.9% (Fig. 8). On further concentration increase of (NH4)2S2O8, the initial rate didn’t increase any more. Therefore, the optimal concentration of (NH4)2S2O8 was 2 mmol/L. A blank test indicated that without the photocatalyst, the (NH4)2S2O8 alone caused 49.6% decoloration after 75 min irradiation. Therefore, the excited dye molecule followed by ionization and the degradation of the ionized dye by SO4 −•. Moreover, because of the oxidizability of S2O8 2−, part of dye molecule was oxidized directly. Our results are consistent with the conclusion of Prevot et al. in which they found the same effect of (NH4)2S2O8 for the photocatalysis of AB80 [9].
The enhancement of reaction rate is due to the inhibition of electron–hole recombination and the production of strong oxidant namely sulfate radical anion (SO4 −•). Peroxodisulfate ion scavenges the conduction band electron and promotes the charge separation, and at the same time produces SO4 −•, as shown in Eq. 21 [21].
The sulfate radical anion (SO4 −•) may react with water molecule producing hydroxyl radical (Eq. 22).
At high dosage of S2O8 2−, the inhibition of reaction occurs due to the increase in the concentration of SO4 2− ion (Eq. 21). The excess SO4 2− ions adsorb on the TiO2 surface and reduce the catalytic activity. The adsorbed SO4 2− ions also react with photogenerated holes (Eq. 23) and with hydroxyl radicals (Eq. 24).
Considering the environmental aspect, an aqueous solution of H2O2 can decompose to O2 and H2O with the increase of storage period at room temperature, so H2O2 is environmental friendly electron acceptor. An aqueous solution of (NH4)2S2O8 can also decompose at room temperature, but the product contains NH4 +, so (NH4)2S2O8 is not an environmental friendly electron acceptor. KBrO3 is not environmental friendly, either. In conclusion, H2O2 is the most efficient and environmental friendly among the three types of electron acceptors.
Conclusions
The dye AB 80 is easily degraded by TiO2-P25 assisted photocatalysis in aqueous dispersion under irradiation of sunlight. The adsorption of AB80 onto TiO2 was found favorable by the Langmuir approach, and with the increasing of pH the adsorption capacity reduced. Desorption experiments indicated that most of AB80 molecule adsorbed on the TiO2 can’t be degraded in acidic solution, but the adsorbed AB80 molecule degraded completely in alkaline solution. The dye solution with the concentration of 0.1 mmol/L was decolorized in 75 min and completely mineralized in 135 min with TiO2-P25 and sunlight. The photocatalytic decoloration of AB80 dye with TiO2 obeyed apparently pseudo-first order kinetics. The optimum catalyst loading for the degradation of 0.1 mmol/L AB80 solutions was 2.0 g/L. The pH value played an important role in the reaction; basic pH was more favorable for the reaction. The addition of electron acceptors such as H2O2, KBrO3 and (NH4)2S2O8 could enhance the reaction rate. H2O2 is the most efficient and environmental friendly among those three electron acceptors. When the concentration of H2O2 was 5 mmol/L, the initial reaction rate was 2.78 times higher than the comparable rate without it.
References
Al-Ghouti MA, Khraisheh MAM, Allen SJ, Ahmad MN (2003) J Environ Manage 69:229
Singh HK, Saquib M, Haque MM, Muneer M (2007) J Hazard Mater 142:374
Galindo C, Jacques P, Kalt A (2001) J Photochem Photobiol A Chem 141:47
Quici N, Morgada ME, Gettar RT, Bolte M, Litter MI (2007) Appl Catal B Environ 71:117
Damodar RA, Jagannathan K, Swaminathan T (2007) Sol Energy 81:1
Kansal SK, Singh M, Sud D (2007) J Hazard Mater 141:581
Xu J, Ao Y, Fu D, lin J, Lin Y, Shen X, Yuan C, Yin Z (2008) J Photochem Photobiol A Chem 199:165
Karunakaran C, Dhanalakshmi R (2008) Sol Energy Mater Sol Cells 92:1315
Bianco Prevot A, Baiocchi C, Brussino MC, Pramauro E, Savarino P, Augugliaro V, Marci G, Palmisano L (2001) J. Hazard Mater 35:971
Ao CH, Leung MKH, Lam RCW, Leung DYC, Vrijmoed LLP, Yam WC, Ng SP (2007) Chem Eng J 129:153
Bizani E, Fytianos K, Poulios I, Tsiridis V (2006) J Hazard Mater 136:85
Kaur S, Singh V (2007) J Hazard Mater 141:230
Li XZ, Fan CM, Sun YP (2002) Chemosphere 48:453
Muruganandham M, Swaminathan M (2006) Dyes Pigments 68:133
Etaiw SH, Abou Sekkina MM, El-Hefnawey GB, Assar SS (1982) Can J Chem 60:304
Epling GA, Lin C (2002) Chemosphere 46:561
Nasr C, Vinodgopal K, Fisher L, Hotchandani S, Chattopadhyay AK, Kamat PV (1996) J Phys Chem 100:8436
Saien J, Nejati H (2007) J Hazard Mater 148:491
Neppolian B, Choi HC, Sakthivel S, Arabindoo B, Murugesan V (2002) Chemosphere 46:1173
Sökmen M, Özkan A (2002) J Photochem Photobiol A Chem 147:77
Muruganandham M, Swaminathan M (2006) J Hazard Mater 135:78
Acknowledgements
This work was supported by Project 863 of China (No. 2006AA06Z362) and Innovation Key Project of the Chinese Academy of Sciences (No. KZCX2-YW-209).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Su, Y., Deng, L., Zhang, N. et al. Photocatalytic degradation of C.I. Acid Blue 80 in aqueous suspensions of titanium dioxide under sunlight. React Kinet Catal Lett 98, 227–240 (2009). https://doi.org/10.1007/s11144-009-0059-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-009-0059-4