Abstract
Gastric cancer (GC) is the third most frequent cause of cancer-related deaths worldwide. The Molecular Mechanism of pathogenesis in GC is still unknown and unclear due to the Chemoresistance. Chemotherapy still remains only a single treatment for GC patients. Among those patients, most are becoming resisting to chemotherapeutic agents, nowadays chemoresistance causes recurrence and is a major challenge in the treatment of cancer because of the deregulations of numerous signaling pathways such as a tumor suppressor gene signaling, PI3K/Akt signaling, NF-кB signaling, Wnt/β-catenin signaling, mitogen-activated protein kinase (MAPK), Hedgehog signaling, Hippo signaling, Notch signaling pathways, and epidermal growth factor receptor (EGFR) have been found in GC. Epithelial-mesenchymal transition (EMT), as a major process during embryogenesis and tumor genesis, as well as is playing a vital role in chemoresistance of GC. In this chapter we summarize important molecular pathway aspects of multi-drug resistance (MDR). It is crucial for the identification of the new drug target, and combination therapy to clarify these complex molecular signaling mechanisms.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
8.1 Introduction
Remarkable progress has been made on the development and progression of human gastric cancer (GC) over the past decade. Gastric cancer represents the third leading cause of cancer deaths worldwide (Sitarz et al. 2018). The incidence rates of GC vary in different regions, with a higher incidence in Eastern Asia, European and South American countries and a lower incidence in North America and some parts of Africa (Marques-Lespier et al. 2016; Torre et al. 2016). In the management of unresectable tumors, several chemotherapeutic strategies have been used to relieve symptoms, to decrease the risk of recurrence and distant metastasis (Hamamoto 2015; Liu et al. 2016; Shin et al. 2016). The 5-year overall survival (OS) rate varies from 20% to 35% in these patients (Chon et al. 2017; Kuo et al. 2014). chemoresistance is a major hindrance to effective and successful cancer treatment in various cases. There are many reasons that lead to the failure of cancer chemotherapy (Fig. 8.1 and Table 8.1). Several lines of evidence report the involvement of tumor microenvironment (TME), Hedgehog (Hh), p53 oncogene, phosphatidylinositol-3 kinase (PI3K)/Akt, Notch signaling, mitogen-activated protein kinase (MAPK), Hippo signaling and WNT signaling pathways play role in GC chemoresistance (Gao et al. 2018; Martin et al. 2013). Hence, it is essential to understand its molecular mechanisms to identify a novel therapeutic target for cancer cell invasiveness and metastasis suppression. In this chapter, we summarized the major molecular signaling pathways that are involved in chemoresistance of GC.
8.2 Oncogenes p53
The p53 is one of the most well-known tumor suppressor genes involved in various important processes such as apoptosis, cell cycle regulation, and DNA repair. Hence p53 is also called as “guardian of the genome (Lane 1992). It has been observed that p53 universally mutated in all categories of cancer including GC. The cyclin-dependent kinase inhibitor P21 is a major target of p53 activity and these are associated with cell cycle arrest and tumor growth inhibition (TGI). About 60% of GC tissues showed a reduction in P21 expression and it significantly correlates with tumor metastasis, invasiveness and poor prognosis (Gamboa-Dominguez et al. 2007).
Further, p53 gene mutations rate was found to be 0–77% in GC (Fenoglio-Preiser et al. 2003). Moreover, the function of p53 alterations causes by loss of heterozygosity (LOH) including a high incidence of p53 mutations and infrequently by DNA methylation. Several mutations may exist in a one tumor causing in the heterogeneity of the p53 position, high-expression of the p53 protein, and the low level of p53 function which are initial events in GC (Bellini et al. 2012). Although the multifaceted relationship between p53 and chemoresistance in GC has been studied for several years, the outcomes are inconsistent. Currently, an epidemiological study was conducted to explain the associations between p53 mutations and the response to chemotherapy. The results indicated that p53 might be a good prognostic biomarker for early response to chemotherapy in GC (Xu et al. 2014).
A study has examined in GC cell lines for p53 mutational status and the results indicate that wild-type p53 protein expression increase during treatment with 5-fluorouracil (5-FU), mitomycin C, and cis-dichlorodiammin-e platinum (CDDP), In contrast, these consequences show that the mutation of p53 is predictive of chemosensitivity in GC (Nabeya et al. 1995). p53 induced apoptosis has been confirmed in GC cells by down-regulating of Bcl-2 protein and the up-regulating the expression of mutated p53 genes in MKN-74 cells after CDDP treatment (Ikeguchi et al. 1997). Additionally, Chen et al. showed that treatment with rAd-p53 significantly increased the sensitivity of the GC to chemotherapy by enhancing Bax expression and inhibits apoptosis (Chen et al. 2011). A study reported that miR-27b plays a major role in tumor development to chemotherapy in vitro and in vivo. Interestingly, up-regulation of miR-27b leads to enhanced miR-508-5p expression, mediated by mutant P53 in GC-associated MDR (Shang et al. 2016). Matsuhashi et al. demonstrate that combined administration of 5-FU and CDDP, induce apoptosis in MKN45, but not in MKN28 cell line these data indicated that the mutated p53 may deliver confirmation of the idea that p53 expression is related to MDR in GC (Matsuhashi et al. 2005). Hamada et al. analyzed and detected 4 GC patients with p53 mutations out of 24 patients with other cancer by immune-histochemical staining and found that p53-inducible WAF1/CIP1 protein in wild type p53 expression but not in mutant-p53 these results suggest that mutations in p53 are associated with lower response or chemosensitivity in GC (Hamada et al. 1996). In addition, it shows that microRNAs are involved in the up-regulation of MDR1 and overexpression of miR-19a/b confers resistance to doxorubicin on GC cells and decreasing the expression of Bcl-2 and Bax gene (Wang et al. 2013a).
8.3 Growth Factor Receptor and Signaling
Epidermal growth factor receptor (EGFR) is a transmembrane protein. Up-regulation of EGFR has been reported in 9–30% of GC cases (Terashima et al. 2012). It is a kind of glycoprotein receptor with HER family of tyrosine kinase activity, When the EGFR extracellular domain binds to its ligands proteins such as transforming growth factor-α (TGF-α), it promotes dimer formation with other EGFR family members which leads to high expression of EGFR and activate PI3K/Akt/mTOR, JAK/stat3, SOS/Grb2/Ras, and src/FAK/ROS pathways, as well as involved in differentiation, proliferation adhesion and metastasis in GC (Lee et al. 2015; Roskoski 2014).
Further, overexpression of EGFR can trigger STAT3 and NFκB, which leads to chemoresistance and poor prognosis in GC. Recently, Huafeng et al. identified that the up-regulation of integrin β4 expression was promoted gefitinib resistance and proliferation by inhibiting apoptosis and showed a negative correlation between integrin β4 and EGFR in GC patients (Huafeng et al. 2018).
An in vitro study on cell lines showed High EGFR expression with MET activation and Kirsten-Ras (KRAS) and CDH1 gene mutations was positively associated with cetuximab resistance in GC (Heindl et al. 2012). To complement this result one study also showed that, activation of KRAS mutation promotes cetuximab resistance in GC cell line (Kneissl et al. 2012). Zhu et al. reported that gefitinib resistance was associated with up-regulation of Dopamine and adenosine 3′,5′-cyclic monophosphate-regulated phosphoprotein, Mr 32,000 (DARPP-32) through EGFR mediated phosphatidylinositol-3-kinase–AKT signaling pathways in GC cells lines (Zhu et al. 2011).
Furthermore, Ye et al. indicated that autophagy plays a vital role in the resistance of HER2-positive in NCI-N87 cell lines to trastuzumab (Tzb) and showed that Tzb drug prevents cell apoptosis by autophagic flux inhibition. Which activate the Akt/mTOR pathway in GC (Ye et al. 2018). Additionally, Wang et al. found that patients with Tzb resistance existing high HER2 somatic copy number alterations (SCNA) during development. The PIK3CA mutations were significantly advanced in patients with innate resistance, compared with standard, as well as NF1 mutations also contributed a role in Tzb resistance in GC (Wang et al. 2018). Moreover, a current study showed that FOXO1 gene works as connective linker among HER2 and MET signaling pathways and play a key role in the regulation of the Apatinib resistance in HER2-positive GC cells. These findings suggest a novel strategy for treatment to overcome apatinib resistance in GC patients (Park et al. 2018). Very recent a study demonstrates that EGFR can stimulate ATXN2L gene expression and promotes cell invasiveness which leads to oxaliplatin resistance. This data indicates poor prognosis for overall survival and recurrence in GC tissue (Lin et al. 2019). Another study observed that Leucine-rich repeats and immunoglobulin-like domains 1 (LRIG1) was up-regulated in chemosensitive GC MDR cell lines via EGFR-mediated PI3K/AKT and MAPK/ERK signaling pathways and decreased expression of LRIG1 (Zhou et al. 2018).
8.4 PI3K/AKT Signaling Pathway Activation
The PI3K/Akt is a serine/threonine-specific kinase protein work as a key regulator of cell growth, proliferation, migration, and survival; it has been observed that is frequently active in GC. Overexpression of PIK3CA is frequently detected with a poor outcome in GC (Tsujitani et al. 2012) the triggering of TKI activates PI3K, which initiates AKT. Activated Akt can phosphorylate various Bcl-2-associated death promoter protein (BAD) on ser136 for the detach from Bcl-X/Bcl-2 gene family and overcome apoptosis initiating signals of BAD. Mutations in PIK3CA lead to activation of the PI3K signaling activity reported in GC confirmed by microarray analysis (Li et al. 2005). It has been reported the aberrant activation of the Akt also able to stimulate NFkB up-regulation, which helps in the transcription of pro-survival genes and overexpression of Akt1 outcomes in drug resistance of GC cells to chemotherapeutic agents. Nam et al. found that high expression of p-AKT and AKT was 78% and 74% in GC, respectively (Nam et al. 2003). Yu et al. observations have proven that high expression and phosphorylation of Akt could be deactivated by etoposide, doxorubicin and wortmannin and could increase the resistance of GC cells to chemotherapy through PI3K/Akt signaling pathway (Yu et al. 2008).
Moreover, a study has revealed that activated AKT and LOH of PTEN plays a major role in broad-spectrum resistance to adriamycin, mitomycin C, cis-platinum and 5-FU chemo drugs, mediated by AKT/PI3K pathways in GC patients (Oki et al. 2005). Another report also compliments this result adding with fluorouracil resistance treatment (Murakami et al. 2007). and Down-regulation of PTEN can lead to CDDP resistance, in GC cells (Byun et al. 2003) Liu et al. demonstrated that Etoposide can stimulate activation of the PI3K/AKT signaling pathway, which reduced the chemo drugs sensitivity of SGC7901 and BGC823 GC cell lines (Liu et al. 2006) Further, in one study, demonstrate that the overexpression of AKT at the molecular and cellular level is associated with CDDP resistance through the JAK2/STAT3 pathway and decreased the chemosensitivity of GC cells in vitro and in vivo (Zhang et al. 2013). And the down-regulation of AKT1 significantly increased cell sensitivity towards AGS cells to adriamycin, cisplatin, 5-fluorouracil, and vincristine chemotherapeutic drugs (Han et al. 2006).
It has been noticed that NF-kB work as a chemotherapeutic inducer of AKT activation, degradation, and phosphorylation also involved in the chemoresistance of GC cells (Yu et al. 2010). Recently Song et al. found that loss of CD133 stem cell biomarker significantly increased the growth inhibition of chemo agents and knockdown of CD133 significantly reduced the PI3K activity in the GC (Song et al. 2018). However, the PI3K/AKT signaling pathway plays a crucial role in drug resistance, the molecular mechanism of PI3K/AKT activation in chemoresistance is not completely understood. According to a study, Survivin plays an important role in downstream of AKT. higher levels of survivin and p-AKT have been detected In CDDP-resistant GC (Sun et al. 2014) Additionally, the overexpression of p-AKT could be responsible for MDR in AGS GC cell lines by the up-regulation of BCL-2 expression (Han et al. 2007).
8.5 Mitogen-Activated Protein Kinase Signaling Pathway
The mitogen-activated protein kinase (MAPK) including p38 and JNK kinase signaling pathway responds to extracellular stimulation and broadly expressed in eukaryotic organisms (Johnson and Lapadat 2002). which plays a crucial role in several biological processes, such as cell proliferation, differentiation, and survival of tumor cells. Deregulation of the MAPK signaling is associated with the progression of GC (Yang and Huang 2015). Besides, these numerous studies have confirmed that the MAPK pathway is also involved in chemotherapy resistance in GC. According to Atmaca et al. phosphorylated MAPK was positive in 59.6% of Cases with metastatic GC and Overall survival was found 8.5 months also, it has been observed that the expression of p-MAPK in primary and metastatic tumors was similar. These results directed that p-MAPK expression may be a probable predictive marker in metastatic GC who is ongoing treatment with chemotherapy (Atmaca et al. 2011). Further, the overexpression of the p38-MAPK signaling pathway was found in vincristine-resistant SGC7901/VCR GC cell lines and to be responsible for the MDR (Guo et al. 2008). Tan et al. demonstrates that the up-regulation of p38 MAPK pathway was involved in doxorubicin resistance in GC cells (SGC7901, BGC823) and xenograft model besides inhibition of p38 MAPK increased GC cell sensitivity to doxorubicin through the induction of the BAX and decrease in BCL-2 expression (Tan et al. 2014). Wang et al. found that etoposide and 5-FU could be activated miRNA-16 expression in vitro and in vivo, and the overexpression of miRNA-16 is mediated by p38MAPK in GC (Wang et al. 2013b). Recently, Teng, et al. was shown, that the expression of DUSP1 gene was significantly higher in the early stages of GC and associated with apatinib (Apa) resistance in GC cells through activation of MAPK signaling pathways in vitro (Teng et al. 2018).
Another current study reported that mammalian ste20-like kinase 1 (MST1) play a vital role in the progression of GC and the reduced sensitivity to CDDP in MKN45 cell lines. Down-regulation of miR-135b resulting in the reverse of CDDP resistance and increases the cells death via activation of MST1-mediated MAPK signaling pathway (Zhou and Chen 2019). along with, Chen et al. found that The down-regulation of miR206 is significantly associated with CDDP resistance of GC cells via induction of MAPK3 pathway (Chen et al. 2019).
8.6 NF-кB Signaling Pathways
Nuclear factor kappa B (NF-κB) constitutes a family of transcription factors and regulated by polyubiquitination, proteasomal degradation, and phosphorylation, by IκB protein. Which form homo and heterodimers and responsible for up-regulation or suppression of many genes involved in inflammation, cell proliferation, cell survival and immunity (Neumann and Naumann 2007). The activation of NF-κB RelA homology domain driven cytokines to include IL-1, IL-6, IL-8, MCP-1, TNF, pro- and anti-apoptotic factors. Especially, deregulation of NF-κB signaling pathway promotes tumor genesis which Associated with poor prognosis of GC and chemoresistance (Kinoshita et al. 2010; Kwon et al. 2012; Maeda and Omata 2008). Zhou et al. revealed that AKT1 expression was induced by doxorubicin. The activation of AKT can increase the binding of NF-kB on Notch1 promoter. Further up-regulation of PTEN by Notch-activated transcription factor (CBF1) in vitro and in vivo results, suggested that an AKT1/NF-kB/PTEN play an important role in the development of chemoresistance in GC (Zhou et al. 2013). Camp et al. found that NF-kB is activated in NCI-N87 and AGS human GC cells in response to 5-FU and SN-38 chemotherapeutic drugs, and May outcome in inducible chemoresistance (Camp et al. 2004). A another study demonstrated that ERas-overexpressing clones were significantly more resistant to CPT-11 after treatment of rapamycin than the control in GCIY cells via activation of NF-κB/mTOR pathway (Kubota et al. 2011) A recent study reported that conditioned medium (CM) made by the metabolism of SGC-7901 GC cell lines increased drug resistance by activating the ataxia-telangiectasia mutated (ATM) and NF-κB pathways in GC cells (Zhuang et al. 2018) Zhi et al. found that Up-regulation of APRIL in AGS cells significantly decreased the efficacy of CDDP in vitro and in vivo and data showed that NF-κB pathway involved in APRIL-mediated chemo-resistance in GC patients (Zhi et al. 2015). Further, a study revealed that IL-8 was overexpressed in GC drug-resistant patients, and increased the IC50 of CDDP in AGS cells, located in cancer-associated fibroblasts (CAFs). Instantaneously, IL-8 therapeutic enriched the expression of PI3K, p-AKT, p-IKb, p-p65 and ABCB1, besides promotes chemoresistance through NF-κB activation and up-regulation of ABCB1 (Zhai et al. 2019). In Additional, Xu et al. reported that drug-resistant GC cells secrete more chemokine C-C motif ligand 2 (CCL2) than drug-sensitive cells and decreased the drug-induced cytotoxicity by inhibiting autophagy and increase SQSTM1 expression. Besides, these enhanced the expression of SQSTM1 in turn, activated CCL2 transcription via the NF-κB signal pathway, demonstrating as a positive feedback drug resistance (Xu et al. 2018). Hypoxia is another well-recognized common feature in tumor biology to be a key point of treatment resistance and poor prognosis in several cancer patients. Hypoxia leads to the expression of many genetic factors that are involved in tumor progression and metastasis in GC (Griffiths et al. 2005). HIF-1α can induce the vascular endothelial growth factor (VEGF) expression and inflammatory state through NF-kB signaling pathway which leads to the suppression of p53 and promotes 5-FU and CDDP chemoresistant in human GC cells (Rohwer et al. 2010). Nakamura et al. has been reported that HIF-1α leads to drug resistance against adjuvant chemotherapy using 5-FU in advance gastric tumor patients (Nakamura et al. 2009). overexpression of HIF-1α increases the expression of Bcl-2 and reduces the expression of Bax protein outlining hypoxia-induced drug resistance in GC (Liu et al. 2008).
8.7 EMT
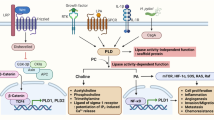
Epithelial-mesenchymal transition (EMT) is a multistage reprogramming process play a vital role in the development of homogenous adhesion that is essential for embryonic expansion and fibrotic disease (Peng et al. 2014). During EMT progression, there is a loose cell polarity in epithelial cell junctions and increase invasive properties of the mesenchymal stem cell (MSCs). Consequently, the expression of epithelial marker such as E-cadherin showed down-regulation and activates β-catenin, which translocate into the nucleus and modulates the expression of VEGF, CD44, cyclin D1kinase, and c-Myc which leads to tumor initiation and progression. it has been observed that the expression of mesenchymal markers such as Snail, Slug, Vimentin, ZEB1, ZEB2 is up-regulated in tumor cells (Thiery et al. 2009).
However, the ability to self-renewal, an overexpression of Drug resistance genes, have shown that the EMT is a major molecular mechanism linked with metastasis and provide resistance to chemotherapy (Mitra et al. 2015). Recent studies have proved that hyaluronan-mediated motility receptor (HMMR) was up-regulated in 5-Fu resistant GC cell line. Further, biopsies sample observed that HMMR increased the cancer stem cell (CSCs) properties and resistance to chemotherapy via TGF-beta/Smad2-induced EMT in GC (Zhang et al. 2019). Kim et al. described the Testican-1 are responsible for EMT mediated signaling and confers acquired resistance to apatinib in HER2-positive gastric cancer in in-vitro (Kim et al. 2014).
Similarly, Huang et al. found that up-regulation of HER2/Snail double-positive patients had poor survival and significantly associated with CDDP-resistant in GC cells mediated by EMT (Huang et al. 2016). Eukaryotic translation initiation factor 5A2 (eIF5A2) is an essential tumor-promoting function in GC. One report showed that the Silencing of eIF5A2 factor enhanced the sensitivity of GC cells to cisplatin by mediating EMT (Sun et al. 2018b). A current study found that phosphorylated p-HER4, HER4, YAP1, and Vimentin were significantly higher and HER2 and E-cadherin were found down-regulated in response to the trastuzumab in vivo. These results revealed that the major role of the HER4-YAP1 in trastuzumab resistance of HER2-positive GC cells via induction of EMT (Shi et al. 2018). Kang et al. demonstrated that up-regulation of DUSP4 can enhance doxorubicin resistance by stimulating EMT in GC cells (Kang et al. 2017).
A study Report proposed that depletion of TAZ (transcriptional co-activator) caused partial Transition of EMT to MET in CDDP resistant GC cells, which are negatively regulated by the Hippo pathway (Ge et al. 2017). Wang et al. also showed that the chemoresistance to cisplatin-induced EMT in human GC cells (Wang et al. 2016). Additionally, has been observed that Doxorubicin is able to induce EMT in GC patients through inhibition of the β-catenin signaling pathway by indomethacin and inhibition of p300 in BGC-823 GC cell (Han et al. 2013; Han et al. 2014). Moreover, Fas belongs to a member of the TNF family, which stimulate tumor cell motility inducing EMT, and support metastasis formation in GC. down-regulation of Snail and Twist expression significantly decreased Fas-induced motility, as well as the use of oxaliplatin chemo drug prompt to induce EMT moderately resulting in chemo-resistant through Fas signaling pathway (Zheng et al. 2013).
8.8 Conclusion
The chemoresistance of tumor cells to chemotherapy occurs from a reduction in drug availability and induction of several oncogenic signaling pathways. Due to the cell specificity to chemoresistance. Major chemoresistance-related proteins are localized in the cell membrane; these proteins are complex and highly versatile in various events, including apoptosis, proliferation, autophagy, and EMT. Now it is essential for the cancer patients receiving targeted combine therapy to increase therapeutic efficacy and reduced tissue toxicity. This chapter may deliver a further understanding of molecular signaling network in Gastric cancer chemoresistance, which facilitates the establishment of novel therapeutic targets and potential chemo sensitive biomarkers to decrease the cancer recurrence and improve the patient lifespan.
Abbreviations
- 5-FU:
-
Fluorouracil
- Apa:
-
Apatinib
- ATM:
-
Ataxia-telangiectasia mutated
- BAD:
-
Bcl-2-associated death promoter protein
- CCL2:
-
chemokine C-C motif ligand 2
- CDDP:
-
Cisplatin
- DARPP-32:
-
Dopamine and adenosine 3′, 5′-cyclic monophosphate-regulated phosphoprotein, Mr. 32000
- EGFR:
-
Epidermal growth factor receptor
- EMT:
-
Epithelial-mesenchymal transition
- GC:
-
Gastric cancer
- HMMR:
-
Hyaluronan-mediated motility receptor
- KRAS:
-
Kirsten-Ras
- LOH:
-
Loss of heterozygosity
- LRIG1:
-
Leucine-rich repeats and immunoglobulin-like domains 1
- MAPK:
-
Mitogen-activated protein kinase
- MDR:
-
Multidrug resistance
- MSC:
-
Mesenchymal stem cell
- MST1:
-
Mammalian ste20-like kinase 1
- NF-кB:
-
Nuclear factor-kappa B
- PI3K/Akt:
-
Phosphoinositide-3-kinase–protein kinase B
- TGI:
-
tumor growth inhibition
- Tzb:
-
trastuzumab
- VEGF:
-
Vascular endothelial growth factor
References
Atmaca A, Pauligk C, Steinmetz K, Altmannsberger HM, Jager E, Al-Batran SE (2011) Prognostic impact of phosphorylated mitogen-activated protein kinase expression in patients with metastatic gastric cancer. Oncology 80:130–134
Bellini MF, Cadamuro AC, Succi M, Proenca MA, Silva AE (2012) Alterations of the TP53 gene in gastric and esophageal carcinogenesis. J Biomed Biotechnol 2012:891961
Byun DS, Cho K, Ryu BK, Lee MG, Park JI, Chae KS, Kim HJ, Chi SG (2003) Frequent monoallelic deletion of PTEN and its reciprocal associatioin with PIK3CA amplification in gastric carcinoma. Int J Cancer 104:318–327
Camp ER, Li J, Minnich DJ, Brank A, Moldawer LL, MacKay SL, Hochwald SN (2004) Inducible nuclear factor-kappaB activation contributes to chemotherapy resistance in gastric cancer. J Am Coll Surg 199:249–258
Chen GX, Zheng LH, Liu SY, He XH (2011) rAd-p53 enhances the sensitivity of human gastric cancer cells to chemotherapy. World J Gastroenterol 17:4289–4297
Chen Z, Gao YJ, Hou RZ, Ding DY, Song DF, Wang DY, Feng Y (2019) MicroRNA-206 facilitates gastric cancer cell apoptosis and suppresses cisplatin resistance by targeting MAPK2 signaling pathway. Eur Rev Med Pharmacol Sci 23:171–180
Chon SH, Berlth F, Plum PS, Herbold T, Alakus H, Kleinert R, Moenig SP, Bruns CJ, Hoelscher AH, Meyer HJ (2017) Gastric cancer treatment in the world: Germany. Trans Gastroenterol Hepatol 2:53
Fenoglio-Preiser CM, Wang J, Stemmermann GN, Noffsinger A (2003) TP53 and gastric carcinoma: a review. Hum Mutat 21:258–270
Gamboa-Dominguez A, Seidl S, Reyes-Gutierrez E, Hermannstadter C, Quintanilla-Martinez L, Busch R, Hofler H, Fend F, Luber B (2007) Prognostic significance of p21WAF1/CIP1, p27Kip1, p53 and E-cadherin expression in gastric cancer. J Clin Pathol 60:756–761
Gao JP, Xu W, Liu WT, Yan M, Zhu ZG (2018) Tumor heterogeneity of gastric cancer: from the perspective of tumor-initiating cell. World J Gastroenterol 24:2567–2581
Ge L, Li DS, Chen F, Feng JD, Li B, Wang TJ (2017) TAZ overexpression is associated with epithelial-mesenchymal transition in cisplatin-resistant gastric cancer cells. Int J Oncol 51:307–315
Griffiths EA, Pritchard SA, Welch IM, Price PM, West CM (2005) Is the hypoxia-inducible factor pathway important in gastric cancer? Eur J Cancer 41:2792–2805
Guo X, Ma N, Wang J, Song J, Bu X, Cheng Y, Sun K, Xiong H, Jiang G, Zhang B, Wu M, Wei L (2008) Increased p38-MAPK is responsible for chemotherapy resistance in human gastric cancer cells. BMC Cancer 8:375
Hamada M, Fujiwara T, Hizuta A, Gochi A, Naomoto Y, Takakura N, Takahashi K, Roth JA, Tanaka N, Orita K (1996) The p53 gene is a potent determinant of chemosensitivity and radiosensitivity in gastric and colorectal cancers. J Cancer Res Clin Oncol 122:360–365
Hamamoto Y (2015) Complications in advanced or recurrent gastric cancer patients with peritoneal metastasis during and after palliative systemic chemotherapy. Mol Clin Oncol 3:539–542
Han Z, Hong L, Wu K, Han S, Shen H, Liu C, Han Y, Liu Z, Han Y, Fan D (2006) Reversal of multidrug resistance of gastric cancer cells by downregulation of Akt1 with Akt1 siRNA. J Exp Clin Cancer Res 25:601–606
Han Z, Hong L, Han Y, Wu K, Han S, Shen H, Li C, Yao L, Qiao T, Fan D (2007) Phospho Akt mediates multidrug resistance of gastric cancer cells through regulation of P-gp, Bcl-2 and Bax. J Exp Clin Cancer Res 26:261–268
Han R, Xiong J, Xiao R, Altaf E, Wang J, Liu Y, Xu H, Ding Q, Zhang Q (2013) Activation of beta-catenin signaling is critical for doxorubicin-induced epithelial-mesenchymal transition in BGC-823 gastric cancer cell line. Tumour biology: the journal of the international society for. Oncodev Biol Med 34:277–284
Han RF, Ji X, Dong XG, Xiao RJ, Liu YP, Xiong J, Zhang QP (2014) An epigenetic mechanism underlying doxorubicin induced EMT in the human BGC-823 gastric cancer cell. Asian Pac J Cancer Prev: APJCP 15:4271–4274
Heindl S, Eggenstein E, Keller S, Kneissl J, Keller G, Mutze K, Rauser S, Gasteiger G, Drexler I, Hapfelmeier A, Hofler H, Luber B (2012) Relevance of MET activation and genetic alterations of KRAS and E-cadherin for cetuximab sensitivity of gastric cancer cell lines. J Cancer Res Clin Oncol 138:843–858
Huafeng J, Deqing Z, Yong D, Yulian Z, Ailing H (2018) A cross-talk between integrin beta4 and epidermal growth factor receptor induces gefitinib chemoresistance to gastric cancer. Cancer Cell Int 18:50
Huang D, Duan H, Huang H, Tong X, Han Y, Ru G, Qu L, Shou C, Zhao Z (2016) Cisplatin resistance in gastric cancer cells is associated with HER2 upregulation-induced epithelial-mesenchymal transition. Sci Rep 6:20502
Ikeguchi M, Tatebe S, Kaibara N, Ito H (1997) Changes in levels of expression of p53 and the product of the bcl-2 in lines of gastric cancer cells during cisplatin-induced apoptosis. Eur Surg Res 29:396–402
Johnson GL, Lapadat R (2002) Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 298:1911–1912
Kang X, Li M, Zhu H, Lu X, Miao J, Du S, Xia X, Guan W (2017) DUSP4 promotes doxorubicin resistance in gastric cancer through epithelial-mesenchymal transition. Oncotarget 8:94028–94039
Kim HP, Han SW, Song SH, Jeong EG, Lee MY, Hwang D, Im SA, Bang YJ, Kim TY (2014) Testican-1-mediated epithelial-mesenchymal transition signaling confers acquired resistance to lapatinib in HER2-positive gastric cancer. Oncogene 33:3334–3341
Kinoshita J, Fushida S, Harada S, Makino I, Nakamura K, Oyama K, Fujita H, Ninomiya I, Fujimura T, Kayahara M, Ohta T (2010) PSK enhances the efficacy of docetaxel in human gastric cancer cells through inhibition of nuclear factor-kappaB activation and survivin expression. Int J Oncol 36:593–600
Kneissl J, Keller S, Lorber T, Heindl S, Keller G, Drexler I, Hapfelmeier A, Hofler H, Luber B (2012) Association of amphiregulin with the cetuximab sensitivity of gastric cancer cell lines. Int J Oncol 41:733–744
Kubota E, Kataoka H, Tanaka M, Okamoto Y, Ebi M, Hirata Y, Murakami K, Mizoshita T, Shimura T, Mori Y, Tanida S, Kamiya T, Aoyama M, Asai K, Joh T (2011) ERas enhances resistance to CPT-11 in gastric cancer. Anticancer Res 31:3353–3360
Kuo CY, Chao Y, Li CP (2014) Update on treatment of gastric cancer. J Chin Med Assoc 77:345–353
Kwon HC, Kim SH, Oh SY, Lee S, Lee JH, Jang JS, Kim MC, Kim KH, Kim SJ, Kim SG, Kim HJ (2012) Clinicopathologic significance of expression of nuclear factor-kappaB RelA and its target gene products in gastric cancer patients. World J Gastroenterol 18:4744–4750
Lane DP (1992) Cancer. p53, guardian of the genome. Nature 358:15–16
Lee HC, Su MY, Lo HC, Wu CC, Hu JR, Lo DM, Chao TY, Tsai HJ, Dai MS (2015) Cancer metastasis and EGFR signaling is suppressed by amiodarone-induced versican V2. Oncotarget 6:42976–42987
Li VS, Wong CW, Chan TL, Chan AS, Zhao W, Chu KM, So S, Chen X, Yuen ST, Leung SY (2005) Mutations of PIK3CA in gastric adenocarcinoma. BMC Cancer 5:29
Lin L, Li X, Pan C, Lin W, Shao R, Liu Y, Zhang J, Luo Y, Qian K, Shi M, Bin J, Liao Y, Liao W (2019) ATXN2L upregulated by epidermal growth factor promotes gastric cancer cell invasiveness and oxaliplatin resistance. Cell Death Dis 10:173
Liu SQ, Yu JP, Yu HG, Lv P, Chen HL (2006) Activation of Akt and ERK signalling pathways induced by etoposide confer chemoresistance in gastric cancer cells. Dig Liver Dis 38:310–318
Liu L, Ning X, Sun L, Zhang H, Shi Y, Guo C, Han S, Liu J, Sun S, Han Z, Wu K, Fan D (2008) Hypoxia-inducible factor-1 alpha contributes to hypoxia-induced chemoresistance in gastric cancer. Cancer Sci 99:121–128
Liu D, Lu M, Li J, Yang Z, Feng Q, Zhou M, Zhang Z, Shen L (2016) The patterns and timing of recurrence after curative resection for gastric cancer in China. World J Surg Oncol 14:305
Maeda S, Omata M (2008) Inflammation and cancer: role of nuclear factor-kappaB activation. Cancer Sci 99:836–842
Marques-Lespier JM, Gonzalez-Pons M, Cruz-Correa M (2016) Current perspectives on gastric Cancer. Gastroenterol Clin N Am 45:413–428
Martin TAYL, Sanders AJ et al (2013) Cancer invasion and metastasis: molecular and cellular perspective. Landes Biosci
Matsuhashi N, Saio M, Matsuo A, Sugiyama Y, Saji S (2005) The evaluation of gastric cancer sensitivity to 5-FU/CDDP in terms of induction of apoptosis: time- and p53 expression-dependency of anti-cancer drugs. Oncol Rep 14:609–615
Mitra A, Mishra L, Li S (2015) EMT, CTCs and CSCs in tumor relapse and drug-resistance. Oncotarget 6:10697–10711
Murakami D, Tsujitani S, Osaki T, Saito H, Katano K, Tatebe S, Ikeguchi M (2007) Expression of phosphorylated Akt (pAkt) in gastric carcinoma predicts prognosis and efficacy of chemotherapy. Gastric Cancer 10:45–51
Nabeya Y, Loganzo F Jr, Maslak P, Lai L, de Oliveira AR, Schwartz GK, Blundell ML, Altorki NK, Kelsen DP, Albino AP (1995) The mutational status of p53 protein in gastric and esophageal adenocarcinoma cell lines predicts sensitivity to chemotherapeutic agents. Int J Cancer 64:37–46
Nakamura J, Kitajima Y, Kai K, Mitsuno M, Ide T, Hashiguchi K, Hiraki M, Miyazaki K (2009) Hypoxia-inducible factor-1alpha expression predicts the response to 5-fluorouracil-based adjuvant chemotherapy in advanced gastric cancer. Oncol Rep 22:693–699
Nam SY, Lee HS, Jung GA, Choi J, Cho SJ, Kim MK, Kim WH, Lee BL (2003) Akt/PKB activation in gastric carcinomas correlates with clinicopathologic variables and prognosis. APMIS 111:1105–1113
Neumann M, Naumann M (2007) Beyond IkappaBs: alternative regulation of NF-kappaB activity. FASEB J 21:2642–2654
Oki E, Baba H, Tokunaga E, Nakamura T, Ueda N, Futatsugi M, Mashino K, Yamamoto M, Ikebe M, Kakeji Y, Maehara Y (2005) Akt phosphorylation associates with LOH of PTEN and leads to chemoresistance for gastric cancer. Int J Cancer 117:376–380
Park J, Choi Y, Ko YS, Kim Y, Pyo JS, Jang BG, Kim MA, Lee JS, Chang MS, Park JW, Lee BL (2018) FOXO1 suppression is a determinant of acquired Lapatinib-resistance in HER2-positive gastric Cancer cells through MET Upregulation. Cancer Res Treat 50:239–254
Peng Z, Wang CX, Fang EH, Wang GB, Tong Q (2014) Role of epithelial-mesenchymal transition in gastric cancer initiation and progression. World J Gastroenterol 20:5403–5410
Rohwer N, Dame C, Haugstetter A, Wiedenmann B, Detjen K, Schmitt CA, Cramer T (2010) Hypoxia-inducible factor 1alpha determines gastric cancer chemosensitivity via modulation of p53 and NF-kappaB. PLoS One 5:e12038
Roskoski R Jr (2014) The ErbB/HER family of protein-tyrosine kinases and cancer. Pharmacol Res 79:34–74
Shang Y, Feng B, Zhou L, Ren G, Zhang Z, Fan X, Sun Y, Luo G, Liang J, Wu K, Nie Y, Fan D (2016) The miR27b-CCNG1-P53-miR-508-5p axis regulates multidrug resistance of gastric cancer. Oncotarget 7:538–549
Shi J, Li F, Yao X, Mou T, Xu Z, Han Z, Chen S, Li W, Yu J, Qi X, Liu H, Li G (2018) The HER4-YAP1 axis promotes trastuzumab resistance in HER2-positive gastric cancer by inducing epithelial and mesenchymal transition. Oncogene 37:3022–3038
Shin CH, Lee WY, Hong SW, Chang YG (2016) Characteristics of gastric cancer recurrence five or more years after curative gastrectomy. Chin J Cancer Res 28:503–510
Sitarz R, Skierucha M, Mielko J, Offerhaus GJA, Maciejewski R, Polkowski WP (2018) Gastric cancer: epidemiology, prevention, classification, and treatment. Cancer Manag Res 10:239–248
Song S, Pei G, Du Y, Wu J, Ni X, Wang S, Jiang B, Luo M (2018) Yu J: interaction between CD133 and PI3K-p85 promotes chemoresistance in gastric cancer cells. Am J Transl Res 10:304–314
Sun XP, Dong X, Lin L, Jiang X, Wei Z, Zhai B, Sun B, Zhang Q, Wang X, Jiang H, Krissansen GW, Qiao H, Sun X (2014) Up-regulation of survivin by AKT and hypoxia-inducible factor 1alpha contributes to cisplatin resistance in gastric cancer. FEBS J 281:115–128
Sun J, Xu Z, Lv H, Wang Y, Wang L, Ni Y, Wang X, Hu C, Chen S, Teng F, Chen W (2018a) Cheng X: eIF5A2 regulates the resistance of gastric cancer cells to cisplatin via induction of EMT. Am J Transl Res 10:4269–4279
Sun J, Xu Z, Lv H, Wang Y, Wang L, Ni Y, Wang X, Hu C, Chen S, Teng F, Chen W (2018b) Cheng X: eIF5A2 regulates the resistance of gastric cancer cells to cisplatin via induction of EMT. Am J Transl Res 10:4269–4279
Tan W, Yu HG, Luo HS (2014) Inhibition of the p38 MAPK pathway sensitizes human gastric cells to doxorubicin treatment in vitro and in vivo. Mol Med Rep 10:3275–3281
Teng F, Xu Z, Chen J, Zheng G, Zheng G, Lv H, Wang Y, Wang L, Cheng X (2018) DUSP1 induces apatinib resistance by activating the MAPK pathway in gastric cancer. Oncol Rep 40:1203–1222
Terashima M, Kitada K, Ochiai A, Ichikawa W, Kurahashi I, Sakuramoto S, Katai H, Sano T, Imamura H, Sasako M, Group A-G (2012) Impact of expression of human epidermal growth factor receptors EGFR and ERBB2 on survival in stage II/III gastric cancer. Clin Cancer Res 18:5992–6000
Thiery JP, Acloque H, Huang RY, Nieto MA (2009) Epithelial-mesenchymal transitions in development and disease. Cell 139:871–890
Torre LA, Siegel RL, Ward EM, Jemal A (2016) Global Cancer incidence and mortality rates and trends--an update. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive. Oncology 25:16–27
Tsujitani S, Saito H, Wakatsuki T, Ikeguchi M, Shirabe K, Morita M, Kakeji Y, Yano T, Maehara Y (2012) Relationship between expression of apoptosis-related proteins and the efficacy of postoperative chemotherapy in patients with T3 gastric cancer. Surg Today 42:225–232
Wang F, Li T, Zhang B, Li H, Wu Q, Yang L, Nie Y, Wu K, Shi Y, Fan D (2013a) MicroRNA-19a/b regulates multidrug resistance in human gastric cancer cells by targeting PTEN. Biochem Biophys Res Commun 434:688–694
Wang F, Song X, Li X, Xin J, Wang S, Yang W, Wang J, Wu K, Chen X, Liang J, Tian J, Cao F (2013b) Noninvasive visualization of microRNA-16 in the chemoresistance of gastric cancer using a dual reporter gene imaging system. PLoS One 8:e61792
Wang LL, Zhang XH, Zhang X, Chu JK (2016) MiR-30a increases cisplatin sensitivity of gastric cancer cells through suppressing epithelial-to-mesenchymal transition (EMT). Eur Rev Med Pharmacol Sci 20:1733–1739
Wang L, Zhang Q, Ni S, Tan C, Cai X, Huang D, Sheng W (2018) Programmed death-ligand 1 expression in gastric cancer: correlation with mismatch repair deficiency and HER2-negative status. Cancer Med 7:2612–2620
Xu HY, Xu WL, Wang LQ, Chen MB, Shen HL (2014) Relationship between p53 status and response to chemotherapy in patients with gastric cancer: a meta-analysis. PLoS One 9:e95371
Xu W, Wei Q, Han M, Zhou B, Wang H, Zhang J, Wang Q, Sun J, Feng L, Wang S, Ye Y, Wang X, Zhou J, Jin H (2018) CCL2-SQSTM1 positive feedback loop suppresses autophagy to promote chemoresistance in gastric cancer. Int J Biol Sci 14:1054–1066
Yang M, Huang CZ (2015) Mitogen-activated protein kinase signaling pathway and invasion and metastasis of gastric cancer. World J Gastroenterol 21:11673–11679
Ye H, Chai X, Wang X, Zheng Q, Zheng D, Wu F, Zheng C, Chen P (2018) Autophagy flux inhibition augments gastric cancer resistance to the anti-human epidermal growth factor receptor 2 antibody trastuzumab. Oncol Lett 15:4143–4150
Yu HG, Ai YW, Yu LL, Zhou XD, Liu J, Li JH, Xu XM, Liu S, Chen J, Liu F, Qi YL, Deng Q, Cao J, Liu SQ, Luo HS, Yu JP (2008) Phosphoinositide 3-kinase/Akt pathway plays an important role in chemoresistance of gastric cancer cells against etoposide and doxorubicin induced cell death. Int J Cancer 122:433–443
Yu LL, Dai N, Yu HG, Sun LM, Si JM (2010) Akt associates with nuclear factor kappaB and plays an important role in chemoresistance of gastric cancer cells. Oncol Rep 24:113–119
Zhai J, Shen J, Xie G, Wu J, He M, Gao L, Zhang Y, Yao X, Shen L (2019) Cancer-associated fibroblasts-derived IL-8 mediates resistance to cisplatin in human gastric cancer. Cancer Lett 454:37–43
Zhang LL, Zhang J, Shen L, Xu XM, Yu HG (2013) Overexpression of AKT decreases the chemosensitivity of gastric cancer cells to cisplatin in vitro and in vivo. Mol Med Rep 7:1387–1390
Zhang H, Ren L, Ding Y, Li F, Chen X, Ouyang Y, Zhang Y, Zhang D (2019) Hyaluronan-mediated motility receptor confers resistance to chemotherapy via TGFbeta/Smad2-induced epithelial-mesenchymal transition in gastric cancer. FASEB J 33:6365–6377
Zheng HX, Cai YD, Wang YD, Cui XB, Xie TT, Li WJ, Peng L, Zhang Y, Wang ZQ, Wang J, Jiang B (2013) Fas signaling promotes motility and metastasis through epithelial-mesenchymal transition in gastrointestinal cancer. Oncogene 32:1183–1192
Zhi X, Tao J, Xiang G, Cao H, Liu Z, Yang K, Lv C, Ni S (2015) APRIL induces cisplatin resistance in gastric cancer cells via activation of the NF-kappaB pathway. Cell Physiol Biochem 35:571–585
Zhou J, Chen Q (2019) Poor expression of microRNA-135b results in the inhibition of cisplatin resistance and proliferation and induces the apoptosis of gastric cancer cells through MST1-mediated MAPK signaling pathway. FASEB J 33:3420–3436
Zhou W, Fu XQ, Zhang LL, Zhang J, Huang X, Lu XH, Shen L, Liu BN, Liu J, Luo HS, Yu JP, Yu HG (2013) The AKT1/NF-kappaB/Notch1/PTEN axis has an important role in chemoresistance of gastric cancer cells. Cell Death Dis 4:e847
Zhou L, Li X, Zhou F, Jin Z, Chen D, Wang P, Zhang S, Zhuge Y, Shang Y, Zou X (2018) Downregulation of leucine-rich repeats and immunoglobulin-like domains 1 by microRNA-20a modulates gastric cancer multidrug resistance. Cancer Sci 109:1044–1054
Zhu S, Belkhiri A, El-Rifai W (2011) DARPP-32 increases interactions between epidermal growth factor receptor and ERBB3 to promote tumor resistance to gefitinib. Gastroenterology 141:1738–1748. e1731–1732
Zhuang X, Li X, Zhang J, Hu Y, Hu B, Shi Y, Sun Y, Hong G (2018) Conditioned medium mimicking the tumor microenvironment augments chemotherapeutic resistance via ataxiatelangiectasia mutated and nuclear factorkappaB pathways in gastric cancer cells. Oncol Rep 40:2334–2342
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Verma, H.K., Falco, G., Bhaskar, L.V.K.S. (2020). Molecular Signaling Pathways Involved in Gastric Cancer Chemoresistance. In: Raju, G., Bhaskar, L. (eds) Theranostics Approaches to Gastric and Colon Cancer. Diagnostics and Therapeutic Advances in GI Malignancies. Springer, Singapore. https://doi.org/10.1007/978-981-15-2017-4_8
Download citation
DOI: https://doi.org/10.1007/978-981-15-2017-4_8
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-2016-7
Online ISBN: 978-981-15-2017-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)