Abstract
Recently considerable attention focused on the development of excimers and exciplexe lamp. These lamps are sources of spontaneous radiation based on the transitions of excited molecules called excimers when pure noble gas or a mixture of several noble gases is added. The excited molecules are considered to be exciplex when the gas mixture contains one or more halogens in addition to noble gases or mercury. The de-excitation of these molecules to their dissociative fundamental states induces a loss of energy in the form of UV, visible and IR radiation. The work is based on an experimental study of a pure krypton lamp and theoretical study of the Kr/Cl2 plasma chemistry in terms of the homogenous model. The lamp is excited by a dielectric barrier discharge, the gas breakdown is obtained between two flat and identical electrodes of 5 cm × 5 cm surface area, these electrodes are insulated and separated by two dielectric plates of permittivity 4 and thickness 2 mm. The space between the two dielectrics is 2 mm and is filled with gas at pressure of 129 to 460 mbar. In order to carry out a parametric study, the influence of several parameters such as applied voltage, frequency and gas pressure was studied. A spectroscopic and kinetic analysis of a pure krypton and Kr/Cl2 mixture excilamp excited by DBD is reported here. The interpretation of the experimental and theoretical results allowed to explain chemical processes responsible of the production of excimers inducing UV emission and to link these processes to the IR emissions that control the population of the excited metastable states of krypton. These states are indirectly responsible for UV emission.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
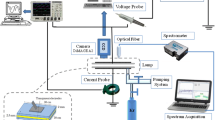
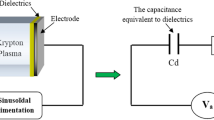
The lamp excited by DBD can have several types of configurations. In the literature, there are configurations of planar, coaxial or plan-plan geometry or other geometries that depend on the type of power supply used and the field of application required for these lamps [1, 2]. In our work we consider a flat discharge consisting of two metal electrode plates covered by a dielectric material. The insulating aspect of semiconductor materials introduced into a silent electrical discharge or dielectric barrier discharge requires a specific use of the applied voltage. In this type of discharge, it is not possible to supply the discharge with direct voltage because the insulating characteristic of the dielectrics leads the discharge to a total extinction just after the formation of the first avalanche and the accumulation of charges on the inner surface of the dielectrics, thus forming a wall that blocks the passage of the current [3]. The discharge will therefore eventually go out. However, the capacitive nature of dielectrics requires that these devices be powered by an alternative electrical source. In this paper we will study the kinetic parameters of the dielectric barrier discharge in a pure krypton discharge and in the Kr-Cl2 mixture under two kinds of excitation: rectangular pulsed excitation and sinusoidal excitation. In particular, due to the pulsed and repetitive form of dielectric barrier discharges, the profile of the applied voltage plays a crucial role in determining the current profile that is often used to characterize the discharge. The shape of the excitation used closely influences the UV emission efficiency of the lamp [4].
However, in recent years many researchers have shown a growing interest in studying this gases and this is mainly due to recent advances in the application of radiation emitted by pure Kr or mixtures Kr-Cl2 lamps in the medical field and in pollution control [5, 6]. The first work on gas discharges for mixing krypton and chlorine was thus carried out with the aim of developing UV/Visible lasers Green [7] was among the first to study the subject of mono-halide rare gas lasers and in his experimental work he evaluated some rate of reactions that may occur between rare gases such as Argon, Krypton and Xenon with halogens such as Fluore, Chlorine and Brome. Flannery’s [8, 9] work on the recombination of rare gas molecular ions with halogen atomic ions in a gas discharge has made it possible to make very important progress in the study of the chemical kinetics of the mixture. He thus estimated the rates of recombination reactions that form the basis of kinetics in this type of mixture. Thus Flannery’s work made it possible to understand the process of formation of the exciplex RgX* from the rare gas atom Rg and the negative ion of the halogen X− [10,11,12]. The three-body reaction rates given by Flannery are obtained after several experiments on several gas mixtures.
2 Kinetic Model
In a rare gas DBD lamp the electrical energy is efficiently converted into electro-magnetic energy by transforming the electrical energy of the discharge into the energy of movement of the electrons, which by colliding with heavy atoms contribute to chemical reactions [13].
2.1 Kinetic Model for Kr/Cl2 Gas Mixture
In our case of the gas mixture between krypton and chlorine the process of formation of the excited exciplex molecule begins with an excitation neutral atoms to metastable and resonat excited sate and ionization of the rare gas atoms:
The second step is the formation of rare gas molecular ions:
This process is often referred to as demirisation, which consists to formation of an ionic molecule of noble gas by reaction with three bodies. It is a fundamental process in the formation of the exciplex responsible for the characteristic UV emission of the lamp.
The formation of halogen ions occurs through the processes of the attachment reactions of the shape of:
For the formation of exciplex molecules, reaction processes are necessary and common to all types of rare gas and halogen mixtures: the three most important channels for exciplex formation are:
The formed exciplex is not really stable and quickly dissociates into a neutral atom typically in a few nanoseconds. The energy due to the de-excitation of the excited molecules is released in the form of photon radiation in the ultraviolet range:
Though, the efficient generation of UV photons depends on the form of excitation of the applied voltage, the pulse frequency, the concentration of chlorine in the gas and the gas pressure [14].
2.2 Kinetic Model for Pure Krypton
The kinetic model developed in case of pure Krypton excilamp describes the processes that accompany the excitation of a Kr gas. Since, the different excited and ionic atoms and molecules formed in inter-electrode space contribute to the formation of the excimer molecules responsible for emission in 148 nm range. The fundamental reactions which controls the radiation output of the dielectric barrier discharge for pure krypton gas. The chemical cycle leads to the creation of the excimers (Kr2 * (1Σu), (Kr2 * (3Σu)) whose de-excitation to the fundamental and dissociative state produces UV emissions in the 147 and 148 nm, respectively. the process begins with ionization and excitation of the Krypton atoms on different states of metastable, resonon and higher excited states. collisions between these different excited atoms and electrons as well as with other neutral atoms contribute to the formation of ionized krypton atoms and molecules which by three-body collisions induce a formation of the excited excimer molecules of krypton. The work in this article is a synthesis of several studies on excimer lamps for two types of gases (halogen-rare gas mixture and pure rare gas). The graphs presented in this section are the results of several studies on dielectric barrier discharges for the mixing of rare gases and halogen and work on pure rare gases. We present here a statistical, selective and comparative study in results of modeling work recognized and validated by several publications [15] and experimental work confirmed and recognized [16, 17].
3 Results
3.1 Efficiency Study for a Krypton-Chlorine Mixing Lamp
Where the lamp is alimented by a pulsed voltage, the results show a high efficacy for 222 nm, corresponding to a value of 8.4% for a total lamp efficacy of around 14.9%. Whereas, for sinusoidal excitation, the total efficiency of the discharge is around 9.6% and a partial efficiency for 222 nm is in the order of 6.4%. in contrast to sinusoidal excitation, under pulsed excitation the pulses are short with a rapidly increasing voltage over time, thus the atoms are rapidly excited and collide through harpoon processes faster for the formation of the KrCl * (B,C) exciplex. However, when the reaction rate is high the efficiency of the UV emission is high. Thus, the efficiency is in a maximum at a critical pressure of about 150 torr and then becomes low as the gas pressure increases. This is observed in both cases of pulsed excitation or sinusoidal excitation (Fig. 64.1).
3.2 Efficiency Study for a Pure Krypton Excilamp
The luminous efficacy behavior of the discharge in the case of pure Krypton discharge is completely different that in the krypton chlorine discharge. The lamp is in its maximum efficiency for a pressure of 450 torr (Fig. 64.2).
4 Conclusion
The work carried out in this article consists in identifying and analyzing the dielectric barrier discharge system for plasma lamps. In this work, we have addressed, using digital discharge models, the kinetics of a high-pressure electric discharge for a noble gas halogen lamp (Kr-Cl2) and for a pure krypton lamp. The use of two excitation modes, sinusoidal excitation and pulsed excitation, for the applied voltage was discussed to study the effect of the applied voltage waveform on the electrical and chemical parameters of the discharge. This allowed us to distinguish the best excitation mode to be used in the case of pure noble gas or rare gas and halogen mixture lamps in dielectric barrier discharges. A comparison between the efficiencies obtained for the different excitation modes: pulsed and sinusoidal shows that the use of pulsed voltage is the best way to improve the excilamps and this for critical and optimal working conditions on the gas pressure or on the voltage applied and the percentage of halogen in the case of gas mixtures in order to improve the radiative emission efficiency of the lamp.
References
K. Nassour, M. Brahami, S. Nemmich, N. Hammadi, N. Zouzou, A. Tilmatine, Comparative experimental study between surface and volume DBD ozone generator. Ozone-Sci. Eng. 38, 70–76 (2016)
M.S. Tyagi, B.L. Meena, A.K. Sharma, R. Prakash, Analysis of discharge parameters in xenon-filled coaxial DBD tube. IEEE Trans. Plasma Sci. 39(6), 1475–1481 (2011)
U.N. Pal, M. Kumar, H. Khatun, A.K. Sharma, Discharge characteristics of dielectric barrier discharge (DBD) based VUV/UV sources. in International Symposium on “Vacuum Science and Technology”, Journal of Physics: Conference Series, vol. 114 (2008), p. 012065
S. Beleznai, G. Mihajlik, I. Maros, L. Balázs, P. Richter, High frequency excitation waveform for efficient operation of a xenon excimer dielectric barrier discharge lamp. J. Phys. D: Appl. Phys. 43, 015203 (2010)
C. Heslin, D. Boehm, V. Milosavljevic, M. Laycock, P.J. Cullen, P. Bourke, Quantitative assessment of blood coagulation by cold atmospheric plasma. Plasma Med. 4, 153–163 (2014)
H.J. Kim, H.I. Yong, S. Park, K. Kim, T.H. Kim, W. Choe, C. Jo, Effect of atmospheric pressure dielectric barrier discharge plasma on the biological activity of naringin. Food Chem. 160, 241–245 (2014)
J.M. Green, A new generation of ultra-violet/visible gas lasers. Opt. Laser Technol. 10(6), 289–300 (1978)
M.R. Fannery, T.P. Yang, App. Phys. Lett. 32(5), 327 (1978)
M.R. Fannery, T.P. Yang, App. Phys. Lett. 33(7), 574 (1978)
J.-Y. Zhang, I.W. Boyd, Multi-wavelength excimer ultraviolet sources from a mixture of krypton and iodine in a dielectric barrier discharge. Appl. Phys. B Lasers Opt. 71, 177–179 (2000)
J.Y. Zhang, I.W. Boyd, Efficient excimer ultraviolet sources from a dielectric barrier discharge in raregas/halogen mixtures. Am. Inst. Phys. J. Appl. Phys. 80(2), 633–638 (1996)
L.C. Ciobotaru, Investigation of monochromatization light effect at molecular/atomic level in electronegative-electropositive gas mixtures plasma. Int. J. Math. Comput. Phys. Electr. Comput. Eng. 6, 1552–1556 (2012)
A. Belasri, N.L.D. Bachir, Z. Harrache, Plasma chemical and electrical modeling of a dielectric barrier discharge in Kr–Cl2 gas mixtures. Plasma Chem. Plasma Process. 33(1), 131–146 (2013)
N.L.D. Bachir, A. Belasri, A simplified numerical study of the Kr/Cl2 plasma chemistry in dielectric barrier discharge. Plasma Sci. Technol. 15(4), 343 (2013)
A. Belasri, Z. Harrache, Electrical and kinetical aspects of homogeneous dielectric-barrier discharge in xenon for excimer lamps. Am. Inst. Phys. Phys. Plasmas 17, 123501 (2010)
N.L.D. Bachir, A. Belasri, P. Guillot, B. Caillier, Radiative emissions in visible–IR of krypton excilamp: experimental and theoretical interpretations. Plasma Chem. Plasma Process. 39(5), 1243–1254 (2019)
B. Ren’an, S. Mingdong et al., Development of 146 nm vacuum UV light source. in 18th International Vacuum Congress, 2012 Published by Elsevier under responsibility of Chinese Vacuum Society (CVS). Phys. Procedia 32, 477–481(2012)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Larbi Daho Bachir, N., Belasri, A., Guillot, P., Caillier, B. (2020). Spectroscopic Study of a Kr and Kr/Cl2 Excilamps Under Sinusoidal and Pulsed Excitation. In: Belasri, A., Beldjilali, S. (eds) ICREEC 2019. Springer Proceedings in Energy. Springer, Singapore. https://doi.org/10.1007/978-981-15-5444-5_64
Download citation
DOI: https://doi.org/10.1007/978-981-15-5444-5_64
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-5443-8
Online ISBN: 978-981-15-5444-5
eBook Packages: EnergyEnergy (R0)