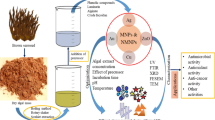
Abstract
In the recent past, there has been a marked increase in the research utilizing nature as a “bio-laboratory” for eco-friendly synthesis of nanoparticles with controlled morphologies and unique properties. Several biotemplates sourced from plants, microorganisms, marine ecosystem, etc., have been explored for the biosynthesis of metallic and metal oxide nanoparticles. These bionanoparticles have been utilized for a diverse range of applications including catalysis, solar energy, water treatment, nanotherapeutics, drug delivery, diagnostics, etc. This chapter deals with the use of marine resources for the development of bionanoparticles for anticancer applications. The marine ecosystem provides abundant bioactives that may have a significant impact in the development of pharmaceutical and nanotechnology products. Marine algae and microorganisms have been widely studied for their natural ability to sequester metal ions and synthesize inorganic nanoparticles either intracellularly or extracellularly. Marine polysaccharides represent a new class of biomaterials that is still underutilized and have the potential to act as reducing as well as stabilizing agents influencing the surface properties of the biosynthesized nanoparticles. They also have been explored for surface modification of prepared nanoparticles for conferring biocompatibility and surface functionality. The exact mechanism of biosynthesis of nanoparticles using different marine resources is yet to be elucidated. In addition, although these green nanoparticles have been reported to have inherent biocompatibility and selective toxicity to cancer cells, a mechanistic approach to unravel their exact mode of action, comprehensive toxicity studies, and clinical trials are warranted before they can be put to clinical use.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
7.1 Introduction
7.1.1 Why Green Synthesis?
The use of nanoparticles in healthcare has the potential to provide more efficient and affordable diagnosis, customized treatments, and follow-up of several debilitating diseases [1, 2]. Several techniques that have been reported for the synthesis of nanoparticles can be broadly classified as top-down and bottom-up approaches. The top-down methods for the synthesis of nanoparticles are ball milling, microcontact printing, lithography, etc., while the commonly used bottom-up approaches include chemical reduction, self-assembly, pyrolysis, sol–gel processing, etc. [3]. However, these methods involve costly, toxic, and nonbiodegradable reagents as stabilizing and reducing agents, which may accumulate in the environment in solid, liquid, or gaseous forms, thereby limiting the applications of these nanoparticles. The removal of residual organic solvents in the chemical synthesis methods from the final nanoparticle-based formulations can often be tedious. Further, majority of these methods are very expensive and cumbersome, have low material conversion rates, and involve the use of high energy, and therefore, an alternative method using biological agents for the synthesis of nanoparticles is the need of the hour. Further, the nanoparticles can easily enter the human body, cross natural barriers, and interact with biomolecules in the blood or within organs, tissues, or cells. Hence, great caution is to be exercized with regard to the accumulation and long-term adversities of these engineered nanomaterials to the environment and human beings [4, 5]. In this regard, the green synthesis of nanoparticles poses as a safer, eco-friendly, simple, energy-efficient, and low-cost alternative.
Green nanotechnology, which involves eco-friendly preparation of nanoparticulate systems by avoiding the use of hazardous chemicals and solvents, has been mostly explored for the synthesis of different metal and metal oxide nanoparticles. Environmentally safe reducing agents, stabilizing or capping agents, and solvents are among the principal criteria involved in the bottom-up green synthesis of nanoparticles. Several biomolecules sourced from plant extracts, seaweeds, algae, bacteria, viruses, fungi, and animal-derived polysaccharides, such as chitin and silk, have been explored for their natural potential to reduce metallic ions into neutral atoms without the use of hazardous chemicals. Biosynthesis of nanoparticles is an energy-efficient process and eliminates the need for high temperature, pressure, or energy. The pH, temperature, substrate concentration, enzyme activity, sonication, reaction time, and many other factors can be modified to control the nucleation and crystal growth as well as the final shape and size of the nanoparticles. Majority of the reducing agents used in the green synthesis of nanoparticles also act as capping agents, thereby rendering biocompatibility and stability to the metal nanoparticles. Biosynthesis of metal nanoparticles like gold, silver, copper, cobalt, palladium, platinum, etc., and metal oxide/salt nanoparticles like titanium oxide, copper oxide, zinc oxide, lead sulfide, iron oxide, nickel oxide, magnesium oxide, etc., have been reported so far. The size and shape of the synthesized nanoparticles determine their fundamental electronic, optical, magnetic, and catalytic properties [6,7,8,9,10].
The exact mechanism of biosynthesis of nanoparticles is yet to be elucidated. However, several compounds like terpenoids, phenolics, flavonoids, enzymes, sugars, proteins, pigments, alkaloids, and other reducing agents present in the plant extracts and microbial cells have been reported to trigger the reduction of metal ions to nanoparticles. In microbial-mediated nanoparticle synthesis, the electrostatic interaction between the bacterial cell wall and metal ions in addition to the intracellular or cell surface enzymes plays a role in the nanoparticle synthesis [11, 12]. In addition, peptides such as phytochelatins have been reported to be synthesized in response to heavy metal stress in plants [13], bacteria [14], and fungi [15]. They are involved in sequestering metal ions and stabilizing the synthesized nanoparticles [16]. Nevertheless, the major drawback for the biological synthesis of nanoparticle is that the exact mechanism of nanoparticle synthesis is to be elucidated to ensure scalability and reproducibility of the processes. Further, the slow microbial growth and biosynthesis under natural conditions may increase the duration of nanoparticle synthesis.
7.1.2 Marine-Based Biosynthesis of Nanoparticles
Marine bio-nanotechnology is a less explored but exciting area of research that holds great promise in the field of nanotechnology. Most of the research work on biosynthesis of metal, metal sulfide, and metal oxide nanoparticles has been based on terrestrial plants and organisms. The diverse marine environment is a rich source of bioactive reducing agents, precursor molecules (silica, calcium, chitosan), and stabilizing agents (polysaccharides, lipids, and peptides) that may find vast applications in the field of pharmaceutical and biotechnology product development. Owing to the fact that the marine ecosystem is totally different from terrestrial ecosystem, the biomolecules like polyphenols, flavonoids, alkaloids, tannins, enzymes, peptides, etc., sourced from marine ecosystem would differ in their capability to sequester metal ions, reduce them, and stabilize the formed nanoparticles. Several marine organisms including bacteria, cyanobacteria, yeasts, fungi, sponges, etc., in addition to algae, mangroves, and seagrasses have been studied for biosynthesis of nanoparticles. These marine-based biogenic nanoparticles were then investigated for their antimicrobial, anticancer, and antifungal properties, with the major chunk of the research being directed toward evaluation of antimicrobial properties [17,18,19,20,21,22].
Considering the enhanced utility of nanoparticles in anticancer applications, the antioxidant properties and the apoptotic potential of marine-based biogenic nanoparticles need to be explored in detail, and the exact mechanism of action needs to be discovered. It is believed that most of the biogenic nanoparticles cause generation of reactive oxygen species (ROS), which activate the caspase 3 enzyme that triggers apoptosis. Further, the increased oxidative stress can cause oxidation of glutathione, an antioxidant that prevents cell damages due to ROS [23]. However, exact mechanisms might change with respect to the different marine sources and the synthesis conditions. Some of the marine-based biogenic nanoparticles investigated for anticancer applications have been discussed in the following sections.
7.2 Biosynthesis of Anticancer Nanoparticles Using Marine Algae
Marine algae are eukaryotic, oxygenic photoautotrophs and are rich sources of biomolecules including polysaccharides, polyphenols, carotenoids, protein, and other phytochemicals, which act as effective metal-reducing agents and capping agents. They are broadly classified into microalgae (e.g., phytoplanktons that remain suspended in the water) and macroalgae or seaweeds (plant-like organisms with size ranging from a few centimeters to several meters). They are also classified based on the algal body or thallus pigmentation into brown algae, red algae (predominantly marine based), and green algae (marine and fresh water based). Algae-derived biomolecules are promising candidates for the biosynthesis of different metallic and metal oxide nanoparticles. Although several algae have been investigated for intracellular or extracellular biosynthesis of different nanoparticles, the exact mechanism of synthesis or the factors to control the size and shape of the products are not yet fully understood. Similar to microbial mediated biosynthesis, the electrostatic interaction between the metal ions and negatively charged cell surface leading to sequestering of the metal ions on the cell wall and further reduction by cellular enzymes/biocatalysts into nanoparticles is thought to be applicable to algae-mediated biosynthesis also. The synthesis method, in most cases, is a straightforward room temperature process involving mixing of a metal salt solution with an aqueous solution of algal extract. The mono or divalent metal ions get reduced to their zerovalent states and undergo subsequent nucleation and growth to form nanoparticles indicated by a color change in the reaction mixture. The algal biomolecules also act as surfactants on specific crystal facets and influence the size, morphology, and physicochemical properties of the final nanoparticles [24]. Currently, the role of experimental parameters like concentration of algal extract and metal salt, pH, reaction time, and temperature is being investigated; however, the role of specific biomolecules and their individual influence in nanoparticle growth and properties need to be studied further. Among the various metal nanoparticles, gold and silver nanoparticles have been extensively investigated for antibacterial, anticancer, and anti-fungicidal applications [24, 25]. The following section describes the studies exploring anticancer applications of algae-mediated biogenic nanoparticles.
The extract of an edible marine brown alga, Ecklonia cava, has been reported as an effective reducing and capping agent for biosynthesis of silver nanoparticles and has been widely investigated for its antimicrobial, antioxidant, and anticancer activities [26]. Biosynthesized, spherical silver nanoparticles having an average size of 43 nm were prepared using an aqueous extract of Ecklonia cava. They demonstrated a significant antibacterial activity against Escherichia coli and Staphylococcus aureus and a strong apoptotic activity against human cervical cancer cells, HeLa cells (IC50 value, 59 μg/mL) [27]. In another study, a wide range of seaweeds (Ulva fasciata, Corallina elongata, Gelidium crinale, Laurencia obtusa, Cystoseira myrica, and Turbinaria turbinata) were evaluated for their ability to produce silver nanoparticles, and the antitumor efficiency of different concentrations of such biosynthesized nanoparticles was evaluated on Ehrlich ascites carcinoma (EAC) cells. Silver nanoparticles biosynthesized by Turbinaria turbinata exhibited the highest cytotoxicity against EAC in vitro [28]. These nanoparticles of 8–16 nm size were then evaluated for their in vivo anticancer effect against Ehrlich cell carcinoma (ECC) in mice. A dose-dependent reduction in tumor size and reduction in elevated white blood cell count in tumor-bearing mice were observed when treated with these biogenic silver nanoparticles [29]. In a similar study, anticancer efficacy of different concentrations of silver nanoparticles biosynthesized by various blue green algae (Anabaena oryzae, Nostoc muscorum, and Calothrix marchica) was analyzed on EAC cells, and the nanoparticles biosynthesized using Calothrix marchica showed highest anticancer activity [30]. Padina tetrastromatica is another seaweed which was utilized for the green synthesis of near spherical silver nanoparticles of 30–40 nm for anticancer applications against breast cancer MCF-7 cells [31]. Biosynthesized silver nanoparticles prepared using red seaweed Pterocladiella capillacea showed bactericidal activity and concentration-dependent cytotoxicity in human hepatocellular cancer cell line, Hep G2, indicating that the alkaloids present in the algae may contribute to the cytotoxic effects [32]. Another red alga, Gracilaria corticata, was employed for the green synthesis of silver nanoparticles, which were then studied for antimicrobial (against Gram-positive and Gram-negative bacteria, fungal species) and anticancer (against HeLa cells) applications [33].
Sargassum is a genus of brown macroalgae that have been widely explored for the biosynthesis of silver nanoparticles. Govindaraju et al. employed Sargassum vulgare to synthesize 10 nm-sized silver nanoparticles and studied their anticancer efficacy against human myeloblastic leukemic cells (HL60) and cervical cancer cells (HeLa). The study established the potential of such nanoparticles as a prophylactic agent in cancer treatment along with the usual chemotherapy regime [34]. The same group also demonstrated the extracellular synthesis of silver nanoparticles of size 30–40 nm from Sargassum ilicifolium. Antibacterial activity against five clinical pathogens and the in vitro toxicity against a brine shrimp, Artemia salina, were established in the study [35]. In another study, the methanolic extract of the seaweed Sargassum polycystum was used for the synthesis of silver nanoparticles of size 5–7 nm, and their antimicrobial and anticancer effects were analyzed. The bioactive component in the crude extract was identified as fatty acids, which along with the nanoparticles exerted anticancer activity in breast cancer cell line, MCF-7 [36]. Devi et al. developed silver nanoparticles using aqueous extracts of the macroalga Sargassum longifolium and observed that these nanoparticles showed a dose-dependent cytotoxicity against Hep-2 cell line [37]. They also studied the cytotoxic activity of silver nanoparticles synthesized using the seaweed Hypnea sp. as a function of the nanoparticle dimensions. Smaller nanoparticles exerted higher cytotoxicity in human colon adenocarcinoma cells, HT-29 [38].
The possible mechanisms of cellular uptake and cytotoxicity induced by silver nanoparticles are depicted in Fig. 7.1.
Possible mechanisms of cellular uptake and cytotoxicity of silver nanoparticles. (Reprinted with permission from Akter M, Sikder MdT, Rahman MdM, Ullah AKMA, Hossain KFB, Banik S, et al. A systematic review on silver nanoparticles-induced cytotoxicity: Physicochemical properties and perspectives. Journal of Advanced Research. 2018 Jan 1;9:1–16. [23])
Apart from silver, majority of the research on green synthesis of metallic nanoparticles has been focused on the preparation of gold nanoparticles. Some of the challenges inherent to current cancer treatment modalities may be overcome using gold nanoparticles, for instance, they can be utilized for radiation dose enhancement or hyperthermia-induced radio-sensitization. Single-celled green and blue-green algae like Spirulina platensis and Tetraselmis kochinensis have been reported to exhibit strong binding toward tetra-chloro-aurate/silver ions, which get bound to the algal surface and subsequently reduced to gold/silver nanoparticles, respectively [39, 40]. Biosynthesized gold nanoparticles of size 15 nm were prepared using an aqueous extract of the red seaweed Corallina officinalis as the reducing and stabilizing agent. The polyphenol content of the algae along with the gold nanoparticles brought about dose-dependent cytotoxicity and necrosis in breast cancer cells, MCF-7 [41]. In another study, brown seaweed Padina gymnospora was used to fabricate gold nanoparticles of size 8–21 nm, and the cytotoxic efficacy of the prepared nanoparticles was studied in liver cancer (HepG2) and lung cancer (A549) cell line. A specific cell toxicity in liver cancer cells was observed via DNA fragmentation while the lung cancer cells were less affected. Thus, further studies are warranted to elucidate the exact mechanism by which gold nanoparticles inhibit specific cancer cell progression [42]. Another brown macroalga, Cystoseira baccata, was also used to synthesize spherical, stable, polycrystalline gold nanoparticles with a mean diameter of 8.4 ± 2.2 nm. In vitro cytotoxicity studies of these nanoparticles using colon cancer cell lines HT-29 and Caco-2, as well as normal fibroblast cell line PCS-201-010, revealed their potential to treat colon rectal cancer [43]. Several studies utilizing the Sargassum genus of brown algae for preparation of gold nanoparticles have also been reported. Stable nanoparticles of gold were prepared by treating aqueous gold precursor with the aqueous extract of Sargassum muticum that acts as both reducing and capping agent [44]. In another study, spherical gold nanoparticles of size 35 nm and zeta potential −27 mV were biosynthesized using Sargassum swartzii. These nanoparticles exhibited a dose-dependent apoptotic activity against human cervical carcinoma (HeLa) cells [45]. In yet another study, Sargassum glaucescens extract was used to prepare and stabilize gold nanoparticles of size 4 nm, and their cytotoxic activity was analyzed in cervical (HeLa), liver (HepG2), breast (MDA-MB-231), and leukemia (CEM-ss) cell lines. The nanoparticles showed dose- and time-dependent cytotoxicity via intrinsic apoptotic pathway in all cancer cell lines while remaining nontoxic to the normal human mammary epithelial cells (MCF-10A) [46].
Brown algae from Sargassum genus were also utilized for the biosynthesis of metal oxide nanoparticles. Different seaweeds such as Caulerpa peltata, Hypnea valencia, and Sargassum myriocystum were screened for their ability to synthesize zinc oxide nanoparticles extracellularly. Water-soluble fucoidan pigments present in Sargassum myriocystum extract were found to be responsible for reduction and stabilization of zinc oxide nanoparticles [47]. Namvar et al. identified the bioactive materials like amino, sulfate, carboxyl, and hydroxyl groups in extract of Sargassum muticum for the biosynthesis of zinc oxide nanoparticles. The particles with a hexagonal morphology with size ranging from 3 to 57 nm were analyzed for their cytotoxicity in murine cancer cells (CT-26, WEHI-3, 4T1, and CRL-1451) and normal fibroblast cells (3T3). Further, the nanoparticles had selective toxicity against leukemia cell line, WEHI-3, but no adverse effect on normal fibroblast cells [48]. The same group evaluated the antiangiogenic and apoptotic properties of these zinc oxide nanoparticles on human liver cancer cell line (HepG2), which were found to be efficient at all concentration and time of incubation. Hence, these nanoparticles may be proposed as a supplemental drug in cancer treatment for decreasing angiogenesis and inducing apoptosis after in-depth in vivo analysis [49]. They also attempted in situ biosynthesis of zinc oxide/hyaluronan nanocomposite using aqueous Sargassum muticum extract. The cytotoxic analysis of the nanocomposite carried out in pancreatic adenocarcinoma (PANC-1), ovarian adenocarcinoma (CaOV-3), colonic adenocarcinoma (COLO205), and acute promyelocytic leukemia (HL60) cells revealed that the composite was most toxic to the HL60 cells while it did not have any adverse effect in normal human lung fibroblast (MRC-5) cell line [50]. Ferric oxide nanoparticles having a cubic morphology of size 18 ± 4 nm were also prepared by a one-step biosynthetic method using Sargassum muticum extract. The authors identified the amino, carboxyl, and hydroxyl functional groups present in the polysaccharide algal walls to act as both reducing agent and capping agent. Subsequent in vitro studies revealed that these nanoparticles induced cell cycle arrest and apoptosis by activating caspase-3 and caspase-9 in a time-dependent fashion in human cell lines for leukemia (Jurkat), breast cancer (MCF-7), cervical cancer (HeLa), and liver cancer (HepG2) [51].
The different metal/metal oxide nanoparticles synthesized using marine algae and evaluated for their anticancer efficacy are summarized in Table 7.1.
7.3 Biosynthesis of Anticancer Nanoparticles Using Marine Microbes
Marine microorganisms, which account for 98% of ocean biomass, include bacteria, cyanobacteria, actinobacteria, yeast, and fungi. These tiny organisms are found abundantly in the marine ecosystem and easily adapt to extreme environments involving a range of acidity, alkalinity, temperatures, and salinity. Several metal-tolerant microorganisms have been known for their potential to synthesize metallic nanoparticles, either extracellularly or intracellularly. In intracellular biosynthesis, the microbial cell wall, owing to its negative charge, electrostatically interacts with the cationic metal ions. Further, the cell wall enzymes facilitate the reduction of these metal ions to nanoparticles, which then diffuse into the cell interior. Extracellular biosynthesis of metal nanoparticles involves a NADH-dependent reductase enzyme that supplies electrons and gets oxidized to NAD+. This transfer of electrons from NADH results in the bioreduction of metal ions into metal nanoparticles (Fig. 7.2) [19, 22, 52].
Possible mechanisms of biosynthesis of metal nanoparticles by microorganisms. (Reprinted with permission from Salunke BK, Sawant SS, Lee S-I, Kim BS. Microorganisms as efficient biosystem for the synthesis of metal nanoparticles: current scenario and future possibilities. World Journal of Microbiology and Biotechnology. 2016 Apr 2;32(5):88. [12])
Marine cyanobacteria are among the most primitive, photoautotrophic marine bacteria. They have been explored for the biosynthesis of metal nanoparticles owing to their ability to produce water-soluble fluorescent pigments and phycobiliproteins. Different functional groups, such as hydroxyl group, carboxyl anions, amino acids, and intracellular proteins, have been identified to be involved in the intracellular biosynthesis [53, 54]. Geetha et al. utilized marine cyanobacteria Gloeocapsa sp. for the intracellular synthesis of gold nanoparticles. The nanoparticles were of spherical and triangular shape having size less than 100 nm. The antitumor activity of the gold nanoparticles was studied in HeLa cells, and the IC50 value calculated was 250 mg [55]. Another cyanobacterium isolated from deep sea water, Pseudomonas aeruginosa (JQ989348), was used for synthesis of silver nanoparticles. These particles having size between 13 and 76 nm showed high antimicrobial activity against Escherichia coli, Vibrio cholerae, Aeromonas sp., and Corynebacterium sp. They also had notable activity against biofilm-forming bacteria like P. aeruginosa and Staphylococcus aureus. Further, in vitro toxicity assay using human cervical cancer cells revealed that these nanoparticles have excellent cytotoxicity [56]. In another study, 15 marine bacteria were evaluated for their potential for biosynthesis of silver nanoparticles, and the most promising Pseudomonas sp. was further investigated. The biosynthesized nanoparticles were analyzed for their antibacterial, antifungal, antifouling, antioxidant, and bioremediation activities. In vitro cytotoxicity studies in HepG-2, MCF-7, and CaCo-2 cell lines revealed that the nanoparticles had the highest inhibitory activity against MCF-7 and HepG-2 cells. The differences in cytotoxicity were attributed to the variation in nanoparticle–cell surface interaction and subsequent disturbances in cell composition with cell type [57].
Marine actinobacteria, which play a pivotal role in breaking down organic compounds and recycling the organic matter, have also been investigated for potential biosynthesis of metallic nanoparticles. Manivasagan et al. studied the biosynthesis and characterization of gold nanoparticles using Nocardiopsis sp. MBRC-1 isolated from the marine sediment samples. The prepared nanoparticles of size 45 nm showed excellent antimicrobial, antifungal, as well as dose-dependent anticancer activity against HeLa cells [58]. The same group also developed gold nanoparticles biosynthesized using another actinobacteria Nocardiopsis sp. MBRC-48 and evaluated its antimicrobial, antioxidant, and anticancer activity. The synthesized nanoparticles had an average size of 11.57 ± 1.24 nm and were spherical in shape. They exhibited good antimicrobial activity against pathogens like Bacillus subtilis, Pseudomonas aeruginosa, Escherichia coli, Staphylococcus aureus, Candida albicans, Aspergillus niger, A. fumigatus, and A. brasiliensis. Further, they showed strong antioxidant activity and dose-dependent cytotoxicity against cervical cancer cell line, HeLa, via apoptosis [59].
Hamed and group studied several actinomycetes isolates from sediment of the Suez Gulf, Egypt, for their ability for extracellular biosynthesis of gold nanoparticles. In one study, isolate M8 out of nine isolates was selected and identified as Streptomyces griseus and utilized for the biosynthesis of gold nanoparticles. These nanoparticles possessed significant antimicrobial activity against Gram-positive and Gram-negative bacteria. Further in vitro cytotoxicity studies revealed that the bionanoparticles exhibited a significant degree of anticancer activity against colon carcinoma cells (HCT-116) and breast carcinoma cells (MCF-7) [60]. In another study, the most potent isolate among 41 actinomycetes tested was identified as Streptomyces rochei MHM13 and used for the biosynthesis of silver nanoparticles. These biosynthesized nanoparticles of size 18–27 nm significantly inhibited the growth of several pathogenic bacteria and also possessed antifouling properties. The in vitro cytotoxicity studies were conducted in different cell lines, viz., hepatocellular carcinoma (HepG-2), breast carcinoma (MCF-7), colon carcinoma (HCT-116), prostate carcinoma (PC-3), lung carcinoma (A-549), intestinal carcinoma (CaCo), larynx carcinoma (HEP-2), and cervical carcinoma (HeLa). The nanoparticles had reasonable anticancer activity against Hep-G2, HCT-116, A-549, MCF-7, and PC-3 cell lines, while the CACO, HEP-2, and HeLa cells were resistant toward the cytotoxic activity [61]. In yet another work, the fermentation parameters controlling the production of bioactive metabolites by marine Streptomyces cyaneus strain Alex-SK121 were optimized, and the cell-free supernatant was used for the biosynthesis of spherical gold nanoparticles having size 6.5–20 nm. Antibacterial studies of the nanoparticles exhibited good activity against Gram-positive and Gram-negative pathogenic bacteria. Further studies in human breast and liver carcinoma cells showed that the biosynthesized particles had excellent antioxidant and antitumor activity [62]. Subbaiya et al. synthesized silver nanoparticles using actinomycetes isolated from marine soil sample and identified as Streptomyces atrovirens. These bionanoparticles showed profound anticancer activity in MCF-7 breast cancer cells in a dose-dependent manner and induced cell morphological changes as revealed by acridine orange and ethidium bromide double staining methods [63]. Streptomyces sp., well-known for its ability to produce several novel secondary metabolites, was studied for optimization of the use of a novel fucoidanase for the green synthesis of gold nanoparticles. The biosynthesized nanoparticles exhibited a dose-dependent cytotoxicity against HeLa cells with an IC50 value of 350 μg/mL at 24 h and 250 μg/mL at 48 h [64]. Streptomyces sp. was also used as a reducing agent for the biosynthesis of zinc oxide nanoparticles. These nanoparticles were evaluated for their antibacterial activity against E. coli and Bacillus subtilis as well as cytotoxicity against A549 lung cancer cells [65].
Enterococcus sp. is another marine bacterium which was studied for the green synthesis of gold nanoparticles. These nanoparticles were spherical in shape having size 6–13 nm and exhibited significant anticancer activity against liver cancer (HepG2) and lung cancer (A549) cells at 100-μg concentration [66]. Extracellular biosynthesis of silver nanoparticles has also been reported using culture supernatant of Enterococcus sp. The developed spherical nanoparticles in the size range of 10–80 nm showed enhanced antimicrobial activity when used in combination with marketed antibiotics. Further in vitro cytotoxicity analysis in liver cancer (HepG2) and lung cancer (A549) cells revealed the potential of these nanoparticles to inhibit cell growth [67]. The bacterium E. coli (VM1), isolated from marine sediments, was also exploited for its capability of biosynthesis of silver nanoparticles. The in vitro anticancer assay done in human lung cancer cells (A549), human cervical cancer cells (HeLa), and normal (Vero) cells showed dose-dependent cytotoxicity of silver nanoparticles. Loss of membrane integrity, cell shrinkage, and reduced cell concentrations, typical of apoptosis, were observed in all cells; however, the extent of cellular toxicity was more in cancer cells compared to normal cells [68].
Marine fungi have also been explored for green synthesis of metal nanoparticles, and the fungal proteins have been reported to play a vital role in the synthesis and stability of the nanoparticles. The cell-free filtrates of different fungal species (Aspergillus flavus SP-3, Trichoderma gamsii SP, Trichoderma gamsii SP-4, Talaromyces flavus SP-5, and Aspergillus oryzae SP-6) isolated from marine sediments were used for the biosynthesis of silver nanoparticles. The silver nanoparticles synthesized using Trichoderma gamsii SP-4 had the maximum antibacterial, antifungal, and antioxidant activity. It was observed that the biosynthesized nanoparticles had a concentration-dependent activity when tested in Hep2 cell line [69].
Table 7.2 summarizes the wide variety of marine microbes that were used for the biosynthesis of metal/metal oxide nanoparticles investigated for anticancer applications.
7.4 Biosynthesis of Anticancer Nanoparticles Using Other Marine Sources
The callus extract of the sand dune plant, Citrullus colocynthis, was used to synthesize silver nanoparticles, which when tested on human epidermoid larynx carcinoma cells (HEp-2) showed anticancer property with an IC50 value of 500 nM [70]. In another study, gold nanoparticles were synthesized using an extract derived from upcycling sea wastes by jellyfish as the reducing agent. The nanoparticles were spherical and triangular in shape and exhibited significant cytotoxicity against HeLa cancer cells through AKT and ERK downregulation but not against normal cells like NIH-3T3 and Raw 264.7 cells [71].
7.5 Surface Modification and Biocompatibility of Biosynthesized Nanoparticles
Most of the metal/metal oxide nanoparticles developed via green synthesis have been reported to be nontoxic and biocompatible in normal cell lines, but highly toxic in cancer cell lines. However, a few studies noted an interesting observation that both cancerous and normal cells did not show metal nanoparticle-mediated cytotoxicity. For instance, Patra et al. reported that the silver and gold nanoparticles synthesized using leaf extracts did not show any cytotoxic effect in the range of 0.3–2.5 μM on different cancer and normal cell lines unless conjugated or loaded with an anticancer drug [72]. In another study, the gold nanoparticles synthesized using microbial filtrates were found to be biocompatible when tested for in vitro cytotoxicity on normal 3T3-L1, H9c2, and cancerous HepG2 cell lines in the concentration range of 0.01–1000 μg/mL [73]. Thus, it is possible that the cytotoxicity of biosynthesized nanoparticles would not only depend on the particle size, surface area, and reactivity but also on the source and mechanism of biosynthesis of nanoparticles.
In order to avoid aggregation and impart stability in addition to ensuring biocompatibility of green nanoparticles, it is important to select environment-friendly stabilizing agents and functionalization pathways. Biosynthesis of metallic nanoparticles using natural polysaccharides as stabilizing and reducing agents can be considered as a promising approach in this regard. Metallic precursors are reduced to zerovalent state, followed by the nucleation and nanocrystals growth in a typical bottom-up synthesis. Polysaccharides can be employed as the hosts that combine with guest metallic precursor ions/nanoparticles through noncovalent bonding causing a change in the order of free energy which enables stabilization, morphological control, and kinetic growth of the nanoparticles. The stereogenic centers of polysaccharides also aid in the anchoring resulting in homogeneous and biocompatible nanoparticles [74].
Marine polysaccharides including sulfated polysaccharides from algae, polysaccharides derived from the exoskeleton of marine crustaceans like chitin and chitosan, and exopolysaccharides produced by marine microorganisms have been explored for biosynthesis of nanoparticles as well as imparting biocompatibility, stability, and surface functionality. Chitosan has been widely explored as a reducing agent and a stabilizer in the biosynthesis of metallic nanoparticles, facilitating surface modification and thus improving stability. Chitosan exhibits high biocompatibility and acts as an effective adjuvant permitting efficient interaction and permeation across the cellular membranes. The charge transfer from polar groups present in chitosan aids in the reduction of metal ions, whereas the electrostatic forces between metal ions and the amino groups in chitosan play a vital role in the formation and stabilization of metal nanoparticles [21]. Chitosan-derived polysaccharide solution was used for biosynthesis of silver nanoparticles, which exhibited high antibacterial activities toward both Gram-negative and Gram-positive bacteria and in vitro cytotoxicity via apoptosis against HepG2 (hepatocellular carcinoma), Lu (lung carcinoma), KB (epidermic carcinoma), and MCF-7 (breast carcinoma) cancer cells [75]. In another study, chitosan–silver bionanocomposite was prepared, and it was observed that chitosan augmented the antimicrobial efficacy of silver nanoparticles [76]. Highly stable cationic chitosan-stabilized gold nanoparticles were also prepared, and the effect of adding tripolyphosphate into chitosan solution on the size and shape of nanoparticles was also studied. Addition of tripolyphosphate resulted in spherical and polygonal gold nanoparticles with a bimodal size distribution [77].
Polysaccharides extracted from marine algae have also been used as biocompatible reducing and stabilizing agents. Laminarin, fucoidan, and alginates are extracted from marine brown algae; carrageenans, agarose, and porphyran are sulfated polysaccharides extracted from red algae, while ulvan is isolated from green algae [21]. Fucoidans have been used for the biosynthesis of metal nanoparticles and utilized for anticancer, antiangiogenic, anti-inflammatory, and drug delivery applications. For instance, biosynthesized silver nanoparticles using seaweed polysaccharide fucoidan were developed and further coated with chitosan to form an electrolyte complex on the surface conferring stability. The developed nanoparticles showed significant microbial inhibition and anticancer activity in human cervical cancer cells (HeLa) [78]. Similarly, fucoidan-mimetic glycopolymer was used for surface modification of gold nanoparticles which conferred selective toxicity of these nanoparticles against human colon cancer cell lines (HCT116) in comparison with mouse fibroblast cell lines (NIH3T3) [79]. Fucoidan was also used as a coating material to prepare stable formulations of poly(isobutylcyanoacrylate) nanoparticles, which were found to be cytotoxic to J774 macrophage and NIH3T3 fibroblast cell lines. Similar fucoidan-coated poly(isobutylcyanoacrylate) core-shell nanoparticles were also used for loading miRNA against cardiovascular diseases [80]. Fucoidan being an anticancer agent itself was reported to show synergistic anticancer and photothermal effects in HeLa, A549, and K562 cells when coated onto copper sulfate nanoparticles via layer-by-layer method [81]. Multifunctional doxorubicin-loaded fucoidan capped gold nanoparticles were also developed for drug delivery and photoacoustic imaging [82].
Carrageenan is another biopolymer obtained from marine red algae that was investigated for the biosynthesis of metal/metal oxide nanoparticles. In situ synthesis and stabilization of magnetite nanoparticles attempted using κ, ι, and λ carrageenans have been reported. The presence of carrageenan resulted in smaller particles and prevented oxidation of the magnetite nanoparticles by determining oxygen diffusion rates through the medium [83]. Carrageenan oligosaccharides have also been employed as a reducing and capping agent to prepare gold nanoparticles, which exhibited significant cytotoxicity in colon (HCT-116) and breast cancer (MDA-MB-231) cells [84]. Porphyran extracted from algae was also used as a reducing agent for one pot, green synthesis of gold nanoparticles, which were then used as a carrier for the delivery of an anticancer drug [85]. In another study, in vitro cytotoxicity and in vivo subacute oral toxicity of gold nanoparticles biosynthesized using the sulfated polysaccharide porphyran were studied. The in vitro cytotoxicity analysis of porphyran-reduced gold nanoparticles in normal monkey kidney cell line revealed their nontoxic nature [86]. Another polysaccharide that was explored for green synthesis is laminarin, a storage compound obtained from the brown alga Turbinaria ornata. Silver nanoparticles biosynthesized using laminarin were analyzed for their free radical scavenging activities and cytotoxicity against retinoblastoma Y79 cell lines. These nanoparticles were found to induce apoptosis as evidenced by flow cytometry and DNA fragmentation study [87]. Thus, marine polysaccharides comprise a new class of materials that are highly stable, biocompatible, biodegradable, economical, and abundant. Most of the research involving these polysaccharides is at the laboratory level. After detailed in vivo and clinical evaluations, they can be put to use for large-scale production of bionanoparticles for a host of applications in the biomedical, food, and pharmaceutical industries.
7.6 Conclusions
Research in the field of green synthesis of nanomaterials is in a highly investigative phase for a wide variety of applications including therapeutics, drug delivery, biosensors, catalysis, etc. Owing to their inherent biocompatibility and selective toxicity to cancer cells, they have been widely explored for the treatment and diagnosis of cancer; however, their clinical applications are yet to be realized via clinical trials. Marine ecosystem offers abundant resources in terms of marine algae, microorganisms, and polysaccharides, which can be utilized for biosynthesis of nanomaterials. A thorough understanding of the mechanism of biosynthesis and their mode of action in addition to systematic comparison with conventional chemical counterparts is necessary to tailor the size and shape of nanoparticles as well as their intended application. In addition, surface modifications in overcoming key physiological barriers in vivo are also vital in the effective targeting of these nanoparticles to cancer cells. Furthermore, a comprehensive acute and chronic toxicity analysis of these nanoparticles is the need of the hour to establish their safety and rule out any long-term hazards associated with their use.
Currently, green synthesis possesses some limitations in terms of residues of polysaccharides, flavonoids, alkaloids, enzymes, etc., being attached to the nanoparticle surface even after purification. Similarly, contamination by pathogens in case of biosynthesis involving microorganisms could be a concern, and hence, the downstream processing of these nanoparticles also demands attention. Another limitation is the adsorption of protein on the nanoparticle surface or the protein corona effect in biological fluids that defines the nanoparticle–cell interaction. Considering these factors, proper optimization for large-scale industrial biosynthesis of nanoparticles and economic analysis in comparison with the conventionally used chemical methods will result in great strides toward sustainability and environment-friendly research.
References
Pelaz B, Alexiou C, Alvarez-Puebla RA, Alves F, Andrews AM, Ashraf S et al (2017) Diverse applications of nanomedicine. ACS Nano 11(3):2313–2381
Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MDP, Acosta-Torres LS et al (2018) Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnol 16:71
Paliwal R, Babu RJ, Palakurthi S (2014) Nanomedicine scale-up technologies: feasibilities and challenges. AAPS PharmSciTech 15(6):1527–1534
Patel P, Shah J (2017) Safety and toxicological considerations of nanomedicines: the future directions. Curr Clin Pharmacol 12(2):73–82
Viswanath B, Kim S (2017) Influence of nanotoxicity on human health and environment: the alternative strategies. In: de Voogt P (ed) Reviews of environmental contamination and toxicology. Springer, Cham, pp 61–104. https://doi.org/10.1007/398_2016_12
Das RK, Pachapur VL, Lonappan L, Naghdi M, Pulicharla R, Maiti S et al (2017) Biological synthesis of metallic nanoparticles: plants, animals and microbial aspects. Nanotechnol Environ Eng 2:18
Kumar S, Lather V, Pandita D (2015) Green synthesis of therapeutic nanoparticles: an expanding horizon. Nanomedicine 10(15):2451–2471
Kharissova OV, Dias HVR, Kharisov BI, Pérez BO, Pérez VMJ (2013) The greener synthesis of nanoparticles. Trends Biotechnol 31(4):240–248
Thakkar KN, Mhatre SS, Parikh RY (2010) Biological synthesis of metallic nanoparticles. Nanomedicine 6(2):257–262
Kulkarni N, Muddapur U (2014) Biosynthesis of metal nanoparticles: a review. J Nanotechnol 15:2014
Mukherjee P, Ahmad A, Mandal D, Senapati S, Sainkar SR, Khan MI et al (2001) Fungus-mediated synthesis of silver nanoparticles and their immobilization in the mycelial matrix: a novel biological approach to nanoparticle synthesis. Nano Lett 1(10):515–519
Salunke BK, Sawant SS, Lee S-I, Kim BS (2016) Microorganisms as efficient biosystem for the synthesis of metal nanoparticles: current scenario and future possibilities. World J Microbiol Biotechnol 32(5):88
Cobbett CS (2000) Phytochelatin biosynthesis and function in heavy-metal detoxification. Curr Opin Plant Biol 3(3):211–216
Pages D, Rose J, Conrod S, Cuine S, Carrier P, Heulin T et al (2008) Heavy metal tolerance in Stenotrophomonas maltophilia. PLoS One 3(2):e1539
Guimarães-Soares L, Pascoal C, Cássio F (2007) Effects of heavy metals on the production of thiol compounds by the aquatic fungi Fontanospora fusiramosa and Flagellospora curta. Ecotoxicol Environ Saf 66(1):36–43
Liu F, Kang SH, Lee Y-I, Choa Y, Mulchandani A, Myung NV et al (2010) Enzyme mediated synthesis of phytochelatin-capped CdS nanocrystals. Appl Phys Lett 97(12):123703
Asmathunisha N, Kathiresan K (2013) A review on biosynthesis of nanoparticles by marine organisms. Colloids Surf B Biointerfaces 103:283–287
Baker S, Harini BP, Rakshith D, Satish S (2013) Marine microbes: invisible nanofactories. J Pharm Res 6(3):383–388
Manivasagan P, Nam SY, Oh J (2016) Marine microorganisms as potential biofactories for synthesis of metallic nanoparticles. Crit Rev Microbiol 42(6):1007–1019
Vijayan SR, Santhiyagu P, Ramasamy R, Arivalagan P, Kumar G, Ethiraj K et al (2016) Seaweeds: a resource for marine bionanotechnology. Enzyme Microb Technol 95:45–57
Manivasagan P, Oh J (2016) Marine polysaccharide-based nanomaterials as a novel source of nanobiotechnological applications. Int J Biol Macromol 82:315–327
Patil MP, Kim G-D (2018) Marine microorganisms for synthesis of metallic nanoparticles and their biomedical applications. Colloids Surf B Biointerfaces 172:487–495
Akter M, Sikder MT, Rahman MM, Ullah AKMA, Hossain KFB, Banik S et al (2018) A systematic review on silver nanoparticles-induced cytotoxicity: physicochemical properties and perspectives. J Adv Res 9:1–16
Fawcett D, Verduin JJ, Shah M, Sharma SB, Poinern GEJ (2017) A review of current research into the biogenic synthesis of metal and metal oxide nanoparticles via marine algae and seagrasses. J Nanosci 2017:15
El-Sheekh MM, El-Kassas HY (2016) Algal production of nano-silver and gold: their antimicrobial and cytotoxic activities: a review. J Genet Eng Biotechnol 14(2):299–310
Athukorala Y, Kim K-N, Jeon Y-J (2006) Antiproliferative and antioxidant properties of an enzymatic hydrolysate from brown alga, Ecklonia cava. Food Chem Toxicol 44(7):1065–1074
Venkatesan J, Kim S-K, Shim MS (2016) Antimicrobial, antioxidant, and anticancer activities of biosynthesized silver nanoparticles using marine algae Ecklonia cava. Nanomaterials (Basel) 6(12):235
Khalifa KS, Hamouda RA, Hamza DHA (2016) In vitro antitumor activity of silver nanoparticles biosynthesized by marine algae. Dig J Nanomater Bios 11(1):213–221
El Bialy EB, Hamouda RA, Khalifa KS, Hamza DHA (2017) Cytotoxic effect of biosynthesized silver nanoparticles on ehrlich ascites tumor cells in mice. Int J Pharmacol 13(2):134–144
Khalifa KS, Hamouda RA, Hamza HA (2016) Antitumor activity of silver nanoparticles biosynthesized by micro algae. J Chem Pharm Res 8(3):1–6
Selvi BCG, Madhavan J, Santhanam A (2016) Cytotoxic effect of silver nanoparticles synthesized from Padina tetrastromaticaon breast cancer cell line. Adv Nat Sci Nanosci 7(3):035015
El Kassas HY, Attia AA (2014) Bactericidal application and cytotoxic activity of biosynthesized silver nanoparticles with an extract of the red seaweed Pterocladiella capillacea on the HepG2 cell line. Asian Pac J Cancer Prev 15(3):1299–1306
Poornima S, Valivittan K (2015) Synthesis, characterization, antimicrobial activity and anticancerous efficacy (HeLa cell lines) by Gracilaria corticata mediated synthesized silver nanoparticles. Int J Curr Res Multidiscip 1(6):1–18
Govindaraju K, Krishnamoorthy K, Alsagaby SA, Singaravelu G, Premanathan M (2015) Green synthesis of silver nanoparticles for selective toxicity towards cancer cells. IET Nanobiotechnol 9(6):325–330
Kumar P, Selvi SS, Prabha AL, Rani LM, Suganthi P, Devi BS et al (2012) Antibacterial activity and in vitro cytotoxicity assay against brine shrimp using silver nanoparticles synthesized from Sargassum ilicifolium. Dig J Nanomater Bios 7940:1447–1455
Thangaraju N, Venkatalakshmi RP, Chinnasamy A, Kannaiyan P (2012) Synthesis of silver nanoparticles and the antibacterial and anticancer activities of the crude extract of Sargassum polycystum C. Agardh Nano Biomed Eng 4(2):89–94
Devi JS, Bhimba BV, Peter DM (2013) Production of biogenic silver nanoparticles using Sargassum longifolium and its applications. Indian J Mar Sci 42(1):125–130
Devi JS, Bhimba BV (2012) Silver nanoparticles: antibacterial activity against wound isolates & in vitro cytotoxic activity on Human Caucasian colon adenocarcinoma. Asian Pac J Trop Dis 2:S87–S93
Govindaraju K, Basha SK, Kumar VG, Singaravelu G (2008) Silver, gold and bimetallic nanoparticles production using single-cell protein (Spirulina platensis) Geitler. J Mater Sci 43(15):5115–5122
Senapati S, Syed A, Moeez S, Kumar A, Ahmad A (2012) Intracellular synthesis of gold nanoparticles using alga Tetraselmis kochinensis. Mater Lett 79:116–118
El-Kassas HY, El-Sheekh MM (2014) Cytotoxic activity of biosynthesized gold nanoparticles with an extract of the red seaweed Corallina officinalis on the MCF-7 human breast cancer cell line. Asian Pac J Cancer Prev 15(10):4311–4317
Singh M, Kumar M, Manikandan S, Chandrasekaran N, Mukherjee A, Kumaraguru AK (2014) Drug delivery system for controlled cancer therapy using physico-chemically stabilized bioconjugated gold nanoparticles synthesized from marine macroalgae, Padina gymnospora. J Nanomed Nanotechnol S5:009
González-Ballesteros N, Prado-López S, Rodríguez-González JB, Lastra M, Rodríguez-Argüelles MC (2017) Green synthesis of gold nanoparticles using brown algae Cystoseira baccata: its activity in colon cancer cells. Colloids Surf B Biointerfaces 153:190–198
Namvar F, Azizi S, Ahmad MB, Shameli K, Mohamad R, Mahdavi M et al (2015) Green synthesis and characterization of gold nanoparticles using the marine macroalgae Sargassum muticum. Res Chem Intermediat 41(8):5723–5730
Dhas TS, Kumar VG, Karthick V, Govindaraju K, Shankara Narayana T (2014) Biosynthesis of gold nanoparticles using Sargassum swartzii and its cytotoxicity effect on HeLa cells. Spectrochim Acta A 133:102–106
Ajdari Z, Rahman H, Shameli K, Abdullah R, Abd Ghani M, Yeap S et al (2016) Novel gold nanoparticles reduced by Sargassum glaucescens: preparation, characterization and anticancer activity. Molecules 21(3):123–123
Nagarajan S, Arumugam Kuppusamy K (2013) Extracellular synthesis of zinc oxide nanoparticle using seaweeds of gulf of Mannar, India. J Nanobiotechnol 11(1):39
Namvar F, Rahman HS, Mohamad R, Azizi S, Tahir PM, Chartrand MS et al (2015) Cytotoxic effects of biosynthesized zinc oxide nanoparticles on murine cell lines. Evid Based Complement Alternat Med 2015:593014
Sanaeimehr Z, Javadi I, Namvar F (2018) Antiangiogenic and antiapoptotic effects of green-synthesized zinc oxide nanoparticles using Sargassum muticum algae extraction. Cancer Nanotechnol 9(1):3
Namvar F, Azizi S, Rahman HS, Mohamad R, Rasedee A, Soltani M et al (2016) Green synthesis, characterization, and anticancer activity of hyaluronan/zinc oxide nanocomposite. Onco Targets Ther 9:4549–4559
Namvar F, Rahman HS, Mohamad R, Baharara J, Mahdavi M, Amini E et al (2014) Cytotoxic effect of magnetic iron oxide nanoparticles synthesized via seaweed aqueous extract. Int J Nanomedicine 9:2479–2488
Gahlawat G, Choudhury AR (2019) A review on the biosynthesis of metal and metal salt nanoparticles by microbes. RSC Adv 9(23):12944–12967
Gunasekaran M (2011) Biosynthesis and characterization of silver nanoparticles using marine cyanobacterium, Oscillatoria willei NTDM01. Dig J Nanomater Biostruct 6(2):385–390
Xie J, Lee JY, Wang DIC, Ting YP (2007) Silver nanoplates: from biological to biomimetic synthesis. ACS Nano 1(5):429–439
Geetha S, SathakkathulZariya J, Aarthi R, Blessie H (2014) Green synthesis of gold nanoparticle using marine cyanobacteria Gloeocapsa sp and the antitumor potential. J Chem Pharm Sci 4:172–174
Ramalingam V, Rajaram R, PremKumar C, Santhanam P, Dhinesh P, Vinothkumar S et al (2014) Biosynthesis of silver nanoparticles from deep sea bacterium Pseudomonas aeruginosa JQ989348 for antimicrobial, antibiofilm, and cytotoxic activity. J Basic Microbiol 54(9):928–936
Hassan WMS, Abd El-latif HH (2018) Characterization and applications of the biosynthesized silver nanoparticles by marine Pseudomonas sp. H64. J Pure Appl Microbiol 12(3):1289–1299
Manivasagan P, Venkatesan J, Senthilkumar K, Sivakumar K, Kim S-K (2013) Biosynthesis, antimicrobial and cytotoxic effect of silver nanoparticles using a novel Nocardiopsis sp. MBRC-1. BioMed Res Int 2013:287638
Manivasagan P, Alam MS, Kang K-H, Kwak M, Kim S-K (2015) Extracellular synthesis of gold bionanoparticles by Nocardiopsis sp. and evaluation of its antimicrobial, antioxidant and cytotoxic activities. Bioproc Biosys Eng 38(6):1167–1177
Hamed M, Abdelftah LS (2019) Biosynthesis of gold nanoparticles using marine Streptomyces griseus isolate (M8) and evaluating its antimicrobial and anticancer activity. Egypt J Aquat Biol Fish 23(1):173–184
Abd-Elnaby HM, Abo-Elala GM, Abdel-Raouf UM, Hamed MM (2016) Antibacterial and anticancer activity of extracellular synthesized silver nanoparticles from marine Streptomyces rochei MHM13. Egypt J Aquat Res 42(3):301–312
El-Batal AI, Al Tamie MSS (2015) Biosynthesis of gold nanoparticles using Marine Streptomyces cyaneus and their antimicrobial, antioxidant and antitumor (in vitro) activities. J Chem Pharm Res 7(7):1020–1036
Subbaiya R, Saravanan M, Priya AR, Shankar KR, Selvam M, Ovais M et al (2017) Biomimetic synthesis of silver nanoparticles from Streptomyces atrovirens and their potential anticancer activity against human breast cancer cells. IET Nanobiotechnol 11(8):965–972
Manivasagan P, Oh J (2015) Production of a novel fucoidanase for the green synthesis of gold nanoparticles by Streptomyces sp. and its cytotoxic effect on HeLa cells. Mar Drugs 13(11):6818–6837
Balraj B, Senthilkumar N, Siva C, Krithikadevi R, Julie A, Potheher IV et al (2017) Synthesis and characterization of zinc oxide nanoparticles using marine Streptomyces sp. with its investigations on anticancer and antibacterial activity. Res Chem Intermediat 43(4):2367–2376
Rajeshkumar S (2016) Anticancer activity of eco-friendly gold nanoparticles against lung and liver cancer cells. J Genet Eng Biotechnol 14(1):195–202
Rajeshkumar S, Malarkodi C, Vanaja M, Annadurai G (2016) Anticancer and enhanced antimicrobial activity of biosynthesizd silver nanoparticles against clinical pathogens. J Mol Struct 1116:165–173
Maharani V, Sundaramanickam A, Balasubramanian T (2016) In vitro anticancer activity of silver nanoparticle synthesized by Escherichia coli VM1 isolated from marine sediments of Ennore southeast coast of India. Enzyme Microb Technol 95:146–154
Anand Bibin G, Thomas CKN, Prakash S, Kumar CS (2015) Biosynthesis of silver nano-particles by marine sediment fungi for a dose dependent cytotoxicity against HEp2 cell lines. Biocatal Agric Biotechnol 4(2):150–157
Satyavani K, Gurudeeban S, Ramanathan T, Balasubramanian T (2012) Toxicity study of silver nanoparticles synthesized from Suaeda monoica on Hep-2 cell line. Avicenna J Med Biotechnol 4(1):35–39
Ahn E-Y, Hwang SJ, Choi M-J, Cho S, Lee H-J, Park Y (2018) Upcycling of jellyfish (Nemopilema nomurai) sea wastes as highly valuable reducing agents for green synthesis of gold nanoparticles and their antitumor and anti-inflammatory activity. Artif Cells Nanomed Biotechnol 46(sup2):1127–1136
Patra S, Mukherjee S, Barui AK, Ganguly A, Sreedhar B, Patra CR (2015) Green synthesis, characterization of gold and silver nanoparticles and their potential application for cancer therapeutics. Mater Sci Eng C 53:298–309
Kalpana D, Srikanth K, Tirupathi Pichiah PB, Cha YS, Lee YS (2014) Synthesis, characterization and in vitro cytotoxicity of gold nanoparticles using cultural filtrate of low shear modeled microgravity and normal gravity cultured K. pneumoniae. Macromol Res 22(5):487–493
Wang C, Gao X, Chen Z, Chen Y, Chen H (2017) Preparation, characterization and application of polysaccharide-based metallic nanoparticles: a review. Polymers 9(12):689
Tran HV, Tran LD, Ba CT, Vu HD, Nguyen TN, Pham DG et al (2010) Synthesis, characterization, antibacterial and antiproliferative activities of monodisperse chitosan-based silver nanoparticles. Colloids Surf A Physicochem Eng Asp 360(1):32–40
Paulkumar K, Gnanajobitha G, Vanaja M, Pavunraj M, Annadurai G (2017) Green synthesis of silver nanoparticle and silver based chitosan bionanocomposite using stem extract of Saccharum officinarum and assessment of its antibacterial activity. Adv Nat Sci Nanosci 8(3):035019
Huang H, Yang X (2004) Synthesis of chitosan-stabilized gold nanoparticles in the absence/presence of tripolyphosphate. Biomacromolecules 5(6):2340–2346
Venkatesan J, Singh SK, Anil S, Kim S-K, Shim MS (2018) Preparation, characterization and biological applications of biosynthesized silver nanoparticles with chitosan-fucoidan coating. Molecules 23(6):1429
Tengdelius M, Gurav D, Konradsson P, Påhlsson P, Griffith M, Oommen OP (2015) Synthesis and anticancer properties of fucoidan-mimetic glycopolymer coated gold nanoparticles. Chem Commun 51(40):8532–8535
Antunes JC, Benarroch L, Moraes FC, Juenet M, Gross M-S, Aubart M et al (2019) Core-shell polymer-based nanoparticles to deliver MiR-155-5p to endothelial cells. Mol Ther Nucleic Acids 17:210–222. https://doi.org/10.1016/j.omtn.2019.05.016
Jang B, Moorthy MS, Manivasagan P, Xu L, Song K, Lee KD et al (2018) Fucoidan-coated CuS nanoparticles for chemo-and photothermal therapy against cancer. Oncotarget 9(16):12649–12661
Manivasagan P, Bharathiraja S, Bui NQ, Jang B, Oh Y-O, Lim IG et al (2016) Doxorubicin-loaded fucoidan capped gold nanoparticles for drug delivery and photoacoustic imaging. Int J Biol Macromol 91:578–588
Daniel-da-Silva AL, Trindade T, Goodfellow BJ, Costa BFO, Correia RN, Gil AM (2007) In situ synthesis of magnetite nanoparticles in carrageenan gels. Biomacromolecules 8(8):2350–2357
Chen X, Zhao X, Gao Y, Yin J, Bai M, Wang F (2018) Green synthesis of gold nanoparticles using carrageenan oligosaccharide and their in vitro antitumor activity. Mar Drugs 16(8):277
Venkatpurwar V, Shiras A, Pokharkar V (2011) Porphyran capped gold nanoparticles as a novel carrier for delivery of anticancer drug: in vitro cytotoxicity study. Int J Pharm 409(1):314–320
Venkatpurwar V, Mali V, Bodhankar S, Pokharkar V (2012) In vitro cytotoxicity and in vivo sub-acute oral toxicity assessment of porphyran reduced gold nanoparticles. Toxicol Environ Chem 94(7):1357–1367
Remya RR, Rajasree SRR, Suman TY, Aranganathan L, Gayathri S, Gobalakrishnan M et al (2018) Laminarin based AgNPs using brown seaweed Turbinaria ornata and its induction of apoptosis in human retinoblastoma Y79 cancer cell lines. Mater Res Express 5(3):035403
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Pulakkat, S., Patravale, V.B. (2020). Marine Resources for Biosynthesis and Surface Modification of Anticancer Nanoparticles. In: Saquib, Q., Faisal, M., Al-Khedhairy, A.A., Alatar, A.A. (eds) Green Synthesis of Nanoparticles: Applications and Prospects. Springer, Singapore. https://doi.org/10.1007/978-981-15-5179-6_7
Download citation
DOI: https://doi.org/10.1007/978-981-15-5179-6_7
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-5178-9
Online ISBN: 978-981-15-5179-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)