Abstract
Tumours are groups of cells, consisting of heterogeneous types of cells that exhibit abnormal cellular characteristics and behaviours. The molecular characteristics of tumour cells can be used to classify the tumour types. In a tumour, the complexity of the population of cell types involved and their diverse gene expression patterns, contribute significantly to tumour heterogeneity, growth, metastasis and aggressiveness. Cancer stem cells (CSCs) are a small population of cells in a tumour that are highly plastic in nature and possess self-renewing capacity. The CSCs can differentiate into different cell types, and play crucial roles in tumour initiation, growth and progression. CSCs drive metastasis, therapeutic resistance and recurrence of cancers, and thus act as the key regulators of tumour aggressiveness. The CSCs trigger the epithelial to mesenchymal transition (EMT) of cells in the tumour, which leads to increased invasiveness of these cells. These unique subpopulations of cells can communicate with their tumour microenvironment (TME) or niche, and stimulate their niche to secrete several intrinsic factors, which triggers neoangiogenesis to promote metastasis. The multipotent and tumour-initiating abilities of CSCs stimulate or alter various signalling networks to cause extravasation of primary cancer cells that result in cancer metastasis. Consequently, the CSCs promote tumour aggressiveness, which can lead to relapse of cancers after various treatments, and thus, pose critical problems in designing novel therapeutics to specifically target and eliminate CSCs. Therefore, CSCs and tumour aggressiveness still remain as one of the major challenges in curing cancer, despite recent advancements in therapeutic approaches to treat various cancers. Here, we discuss the key roles of CSCs in the regulation of EMT, metastasis, cancer metabolism and critical signalling pathways that influences tumour aggressiveness.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Cancer stem cells
- Self-renewal
- Tumour initiation
- Epithelial to mesenchymal transition
- Invasiveness
- Angiogenesis
- Metastasis
- Tumour aggressiveness
8.1 Introduction
Cancer is a major lethal and universal ailment that kills millions of people every year throughout the world. In cancer, there is alteration of cell and tissue architecture [1], and if cancer is not treated at an early stage, it spreads from the primary site of tumour to various organs, which acts as its secondary site [2, 3]. This progression by which cancer cells disseminate to other parts of the body is called metastasis. There are various factors and processes involved in metastasis.
Cancer cells can spread to different parts of the body through various steps such as by simple invasion into neighbouring normal tissues or migration via the lymphatic or blood vessels from the primary site of tumour to distant secondary sites (i.e. different organs or tissues) for colonization. The formation of new blood vessels (neoangiogenesis) provides additional blood supply for more nutrients and growth factors, which would help in tumour growth or metastasis [4, 5]. The tumour is complex in nature as it contains a heterogeneous population of cells encapsulated inside the tumour that is surrounded by blood vessels, extracellular matrix (ECM), fibroblasts, immune cells, etc., which forms a distinct tumour microenvironment (TME), called niche [6]. The emergence of the existence of the cancer stem cells (CSCs) and recent evidence have widely opened a whole new approach in the understanding of this heterogeneous disease. The initiation and the development of the tumour are driven by these CSCs, the specialised cell subtypes, which are multipotent in nature and also possess self-renewing capacity [7]. CSCs are responsible for the differentiation and migration of the cancer cells along with the tumorigenicity. The CSC subpopulations are protected inside their niche, which acquires the necessary growth factors, and derive energy in the form of ATP from the glycolytic pathway and oxidative phosphorylation (OXPHOS) depending on their necessity.
In most cancer types, the unifying process that accounts for increased mortality is metastasis. Metastasis is a multi-step process. It involves different phases of cells, and it initially starts with mutation at a molecular level by an oncogenic hit due to genetic or environmental cause [4]. Recent insights in the field of cancer biology highlight the complex nature of this biological process, and helped in organising the intricacy based on defined principles called cancer hallmarks [8]. The hallmarks of cancer are depicted in Fig. 8.1.
Cancer cells rarely metastasise to skeletal muscle, and specific factors encourage comprehensive muscle wasting, which results in a condition known as cachexia, wherein the zinc gets hoarded in skeletal muscles through abnormal upregulation of the metal ion transporter ZRT- and IRT-like protein 14 (ZIP14). This phenomenon is the critical mediator of metastatic cancer-induced muscle wasting [9]. Epithelial to mesenchymal transition (EMT) is a process by which the CSCs obtain their mesenchymal phenotype, which favours the progression and aggressiveness of the tumours, resulting in metastasis.
Most of the tumours are comprised of normal cells along with other different cell types which help in driving the phenotypic heterogeneity and malignancy of cancer. An important part of the cancer cells and the so-called metastasis inducers are characterised by CSCs [10]. In the tumour mass, these cells are capable of self-renewal and differentiation. CSCs are also responsible for the metastatic properties of cancer cells [11]. The CSCs secrete cytokines which are important in preparing the tumour microenvironment (TME) and employing the myeloid cells to strengthen the cancer progression. Cancer-associated fibroblasts (CAFs) and activated tumour-associated macrophages (TAMs) release high amounts of matrix metalloproteinases (MMPs), growth factors and cytokines to withstand angiogenesis and to encourage the CSC invasion [12,13,14].
The cytokines, chemokines and growth factors secreted in the TME boost the migration capability of cancer cells and enhance the angiogenesis [15]. Also, CSCs can easily escape from the immune system [16] by altering the protein expression of intrinsic molecules including programmed cell death ligand 1 (PD-L1) [17], which further ensures the formation of the immunosuppressive microenvironment [18]. Also, most importantly, the cell surface markers of CSCs, like CD44 and CD133, are very vital molecules that confer the specificity in CSCs’ binding and targeting properties on the other tumour cells [19]. CSCs share genetic and epigenetic intricacy along with other cancer cells but adapt to survival challenges which is a vital property of these cells in escaping the cancer chemotherapy. Moreover, with their slow cell proliferation rate, when compared to other cancer cells, CSCs are believed to gain survival tactics, while the fast-growing cancer cells are destroyed by the various therapeutic treatments [20].
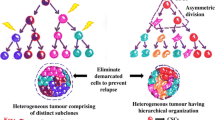
CSCs possess the ability to hijack the intrinsic signalling pathways such as Wnt/β-catenin, Hedgehog and Notch pathways [21]. These pathways are the key regulators of the adult stem cell homeostasis, and the CSCs impede the normal mechanism of the pathway and exploit its function over maintaining its own plasticity [22]. Interestingly, these subpopulations of cells evade apoptosis and take control of the immune system by inhibiting the function of the immune cells. Although it is potentially possible to treat cancers in its early stages, the metastasis and the cancer relapse are still the major issues even with all the cutting-edge state-of-the-art technology. CSCs undergo dormancy after they form a colony at a distant site, and are well protected inside their TME. Hence, the current therapies that target only actively proliferating cancer cells are unable to target and eliminate CSCs [23]. The tumour has heterogeneous populations of cells, and therefore, the treatment regimes fail to target CSCs. Eventually, the tumour regresses responding to treatment, but it relapses with high metastatic ability along with resistance towards the therapies, which leads to the high mortality rate in cancer patients. CSCs are resistant to many therapies because of their chemoresistance, radioresistance and immunosuppressive properties. Numerous laboratories and pharmaceutical companies are currently focusing on developing therapies to specifically target CSCs. Significant ways aimed at eliminating CSCs would impact ominously on cancer treatment, by countering metastasis and cancer relapse [24]. This chapter discusses some of the important roles of CSCs in modulating the metastatic potential, metabolic alterations and key signalling pathways and thus, tumour aggressiveness.
8.2 Cellular Behaviour and Niche
Aberrant activation in the cell signalling networks along with the production of different essential growth factors are necessary for the initiation of tumour. Furthermore, synthesis of angiogenic proteins by the tumour itself decides the fate of aggressiveness of the tumour [25]. Not all subtypes of tumours are highly metastatic in nature. The behaviour of the cells depends on the habitat where the tumour exists. The niche provides an enriched environment with the supply of all the intrinsic factors to the TME that drives the progression of the tumour in its growth and adversity [26]. The principal characteristics of the CSCs along with its phenotypic plasticity are well protected within the niche against the classical immune system and therefore promote the metastasis. Stem cell markers are functionally specific proteins that help in the process of identification of different tumour subtypes. There are various stem cell markers such as CD44 [27], CD133 [28], CD49f [29], ALDH [30], etc., which have specific roles in the development of different tumours. The characteristics of these stem cell markers are represented in Table 8.1.
In the primary carcinoma, the lack of oxygen in tumour cells increases the production of reactive oxygen species (ROS) due to impaired vascularisation inside the TME. The cellular stress due to hypoxia and the increased ROS levels initiate the CSC’s stress signalling pathway to boost the cancer cell survival and also, to maintain its stemness [31]. The mesenchymal stem cells (MSCs) along with the CSCs produce intrinsic chemokines, cytokines and other angiogenic growth factors to initiate neoangiogenesis, thereby, directing more blood supply to the tumour and increasing the plasticity of CSCs. Furthermore, it compromises the immune system by suppressing the cytotoxic functions of the immune cells [32].
In the normal cellular physiology, the MSCs establish a normal stem cell niche with various stemness factors to support stem cell survival. But the oncogenic hit transforms the normal stem cell niche to a CSC niche that triggers the initiation of the tumour. In the TME, the CSCs can activate the EMT pathway in the adjacent normal tissue and transform them into tumour cells to invade further and to grow at a new site with a separate niche to support the CSCs at that distant site [33]. Also, the primary CSCs can control the potential metastatic sites by releasing exosomes to facilitate the arrival of invading tumour cells. The exosomes along with other necessary growth factors prepare the metastatic niche and favour CSCs to establish a new colony at a distant site through the process of extravasation [34].
8.3 Conjunction Between CSCs and Tumour Aggressiveness
CSCs share the common characteristics of normal stem cells such as self-renewability and multi-lineage differentiation that have the capacity to drive tumour growth. The role of CSCs starts early during tumour initiation and sustains throughout the progression of the carcinoma. CSCs play a critical role in the metastasis and tumour relapse due to its high plasticity. The CSCs utilise various signalling pathways to evade immune cells, and also impede immune cell function against cancer cells. However, during normal metabolism, the systemic immune cells target cancer cells exhibiting high levels of cellular stress and ROS production [35].
In most of the currently available cancer therapies, the treatment regime is designed to usually target the whole organ for radiotherapy or the whole body for chemotherapy. But in advanced treatment programme such as the proton beam therapy, the tumour is targeted specifically [36]. But even with this treatment, the normal tissues are affected in an insignificant manner, whereas in other treatments, the damage done by the therapy is far worse causing overall deterioration of the body. Since the present therapies follow a holistic approach in their treatment regime and the CSCs are encapsulated and well protected within their niche, it becomes difficult to target the CSCs [37]. On the contrary, when the treatment kills cancer cells and the tumour shrinks eventually, the CSCs may go to a dormant state, and develop resistance towards that drug. In a few years, the tumour may relapse with enhanced resistance towards that drug treatment and exhibits aggressive metastatic potential.
CSCs play an integral role in oncogenesis by promoting tumour initiation, invasion and metastasis. CSCs initiate cancer due to their stemness which is acquired due to accumulation of genetic or epigenetic alterations and oxidative stress. CSCs establish their communication network with the TME, which favour their survival and migration, and also promote angiogenesis for metastasis [38].
8.4 Impact of Metastatic Potential on Tumour Aggressiveness
Metastatic dissemination of cancer cells is the ability of the malignant cells to initiate the invasion from the primary site to form a colony at a distant site. This invasiveness of the cancer cells involves various cascades of events such as intravasation, EMT and hijacking of different signalling pathways. These events enable the CSCs to gain their self-renewing property, which under normal physiological condition is primarily used for the maintenance of adult stem cell homeostasis, wherein the epithelial cells acquire mesenchymal characteristics [39]. While the tumour develops, it is intrinsic for the tumour cells to sustain growth and function in the hypoxic environment with the recruitment of the cellular components and modulation of their extracellular matrix.
The tumour acquires its vasculature through angiogenic factors and retains its blood supply for the growth of tumour. The tumour cells invade the normal tissues, and upon reaching the blood vessels, the tumour cells infiltrate through the endothelial barrier of the blood vessel, and enter into the circulation by the process called intravasation. After intravasation, the blood vessels in the tumour establish connections between the tumour and other organs, thereby providing a route for the circulating tumour cells (CTCs) to extravasate into the circulation for metastasis [40] (Fig. 8.2). CTCs possess epithelial properties, and cannot escape the microenvironment until they gain mesenchymal properties. EMT is one of the key events necessary for the extravasation of the CTCs into the vascular system. While the CTCs in circulation adhere and secure themselves in the adjacent organs, the entire process of tumour development at primary tumour sites are driven by CSCs, and the release of the CTCs is an active process that supports metastasis to distant secondary sites. Also, the CSCs prepare the metastatic niche at distant secondary sites with the help of necessary growth factors, before the arrival of the CTCs and its development to form secondary tumours [41].
CSCs promote neoangiogenesis and metastasis. The active proliferation of cancer cells at the site of tumour creates cellular stress due to the hypoxia and lack of necessary nutrients. This hypoxia condition results in the release of different growth factors, chemokines, cytokines and angiogenic factors resulting in the formation of new vasculature in the tumour to supply nutrients required for the tumour growth. The cancer cells start to invade the normal tissue and reach near the blood vessels. Intravasation is a crucial event in the dissemination of cancer, and it requires the cancer cells to enter the circulation to form a colony at a different secondary site. The circulating tumour cells (CTCs) travel in the circulation and extravasate at the distant tissue and undergo dormancy, preparing their metastatic niche at the secondary site. Later, they undergo accelerated proliferation and form a colony which quickly progresses to a full-blown tumour at the secondary organ site. Cancer cells are represented with serrated margins. Red coloured cells with serrated margins are CSCs. Green and yellow coloured cells with serrated margins are different tumour cells, which are represented to show the tumour heterogeneity. The blue or black coloured nucleus represents heterogeneity in the cancer genomes. MMPs matrix metalloproteinases, CTCs circulating tumour cells
The growth of the tumour after colony formation at the distant site is one of the crucial events in the process of metastasis. But in most cases, the CTCs after adhering at a distant organ enter a state of dormancy (Fig. 8.2). Metastatic dormancy is one of the major reasons for the tumour to relapse after cancer therapy [42]. Cancer treatments are often developed to target the tumour at the primary site. Though, the treatment kills most of the tumour cells and the tumour regress, it does not kill all the CSCs in the tumour. Few of the CSCs at the primary tumour often escape death from the treatment or enter a dormant state. Also, some tumour containing CSCs can often escape even from a successful precision surgery. The dormant tumour cells at a distant site or CSCs at a primary site can remain resilient towards a treatment regime, and can relapse to a full-blown tumour again in a few years. In relapsed cancers, the metastatic cells are dominant and are more fatal with mesenchymal properties, and their aggressiveness is doubled due to their acquired resistance to the therapies [43].
8.5 Factors Influencing Tumour Aggressiveness
The CSCs are influenced by various components such as hormones, enzymes, cytokines and growth factors (Fig. 8.3). Several studies have shown that hormones hasten the process of oncogenesis in different tumour conditions. The hormones, oestrogen and progesterone influence the growth of breast cancer cells by binding to their specific receptors in the cells. These hormones increase cancer cell division and rapidly promote tumour growth in a very short period of time, making it one of the aggressive forms of tumour. Furthermore, hormones such as testosterone, oestrogen and progesterone enhance drug resistance and metastasis of hormone-dependent tumours [44].
Various factors that influence tumour aggressiveness. Several factors influence the initiation and progression of tumour. These include both intrinsic and extrinsic factors that drive tumour growth and aggressiveness. The extrinsic (modifiable) factors include smoking, consumption of alcohol, unhealthy diet and lifestyle, which can be modified. The intrinsic factors include enzymes, hormones, growth factors, nutrients, chemokines, cytokines and tumour niche, which contribute to the rapid progression and dissemination of the tumour. Cancer cells are represented with serrated margins. Red coloured cell with serrated margins is CSC. Green and yellow coloured cells with serrated margins are different tumour cells, which are represented to show the tumour heterogeneity. The blue or black coloured nucleus represents heterogeneity in the cancer genomes. TGF-β transforming growth factor-beta, PDGF platelet-derived growth factor, VEGF vascular endothelial growth factor, MMPs matrix metalloproteinases, PGK1 phosphoglycerate kinase 1, PKM2 pyruvate kinase M2
Along with hormones, growth factors, cytokines and chemokines play a crucial role in the progression of tumours. The TME contains different types of cells that undergo hypoxia and cellular stress as the tumour grows, and so the tumour itself releases the necessary growth factors such as transforming growth factor-beta (TGF-β), platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), etc., in order to form new vasculatures (neoangiogenesis) for the tumour to supply blood and nutrients [45]. Additionally, there are few enzymes such as matrix metalloproteinases (MMPs), phosphoglycerate kinase 1 (PGK1) and pyruvate kinase M2 (PKM2), which affect the tumour cell behaviour and accelerate their progression by breakdown of the extracellular matrix (ECM), and fuelling the tumour cell metabolism to aid the invasion of cancer cells, by rapidly promoting peritoneal dissemination to the distant sites [46].
The crucial factor that is involved in tumour progression and development is the hypoxia caused due to increased proliferation, and thus, elevated cellular stress. The uncontrolled cell division creates an unfavourable environment inside the TME, which eventually leads to poor nourishment of cells. This initiates the need for excess growth factors and nutrients to cells, and therefore, the tumour itself secretes necessary angiogenic factors to construct new vasculatures (neoangiogenesis) inside the tumour, which enables blood supply to the tumour. Also, alteration in tumour metabolism is a key factor that determines the tumour growth and aggressiveness [47].
The CSCs control the switch between using the glycolytic and OXPHOS pathways to extract ATP. It is also based on the niche, which partly chooses the pathway for the ATP production. During the tumour initiation, the CSCs use the glycolytic pathway, but they switch to the OXPHOS pathway during tumour progression. Metastasis requires elevated energy expenditure as cells have to migrate to a distant site, and so, it depends on both the pathways [48]. Also, in few carcinomas, hormones act as critical factors, which double the invasiveness of tumour. Although several molecular mechanisms play vital roles in the initiation and progression of tumour, the environmental carcinogens influence the growth of tumour in the form of DNA damage due to higher ROS production and increased cellular stress. More importantly, modifiable risk factors including smoking, alcohol consumption, eating junk food and unhealthy lifestyle (e.g. obesity) increase the risk of cancers (Fig. 8.3) as these factors can also promote metastasis [49].
8.6 Molecular Mechanisms Involved in EMT
EMT is a process by which epithelial cells acquire mesenchymal characteristics by undergoing several changes including the loss of cell polarity. The acquisition of mesenchymal phenotype is influenced by different signalling factors, which are necessary to evade apoptosis and increase the migration potential of the cells. It also increases the secretion of degrading enzymes to penetrate the ECM, and this process promotes the invasiveness of cells [50]. EMT activates and maintains the stemness of the cells, and it plays a key role in the transition of normal stem cells to CSCs, by acquiring the invasive mesenchymal characteristics.
The EMT is a two-way process, and it can be reversed. EMT can change in either direction, and the conversion from mesenchymal phenotype to epithelial phenotype is called mesenchymal to epithelial transition (MET) (Fig. 8.4). It involves multiple signalling pathways such as Wnt/β-catenin, Hedgehog (Hh) and Notch signalling. The interaction between these signalling pathway and the immune cells, enables the normal stem cells to develop and maintain their plasticity. Various transcriptional factors such as Slug, Snail, Twist, Sox-9, etc. are involved in the transition from epithelial to mesenchymal cell characteristics [51]. The epithelial cells lose certain important characteristics, while acquiring invasiveness and metastatic properties. The epithelial phenotype markers [including E-cadherin, desmoplakin, Muc-1, cytokeratin-18, occludins, claudins and zonula occludens] are lost, while mesenchymal markers [including N-cadherin, vimentin, fibronectin, vitronectin, α-smooth muscle actin (α-SMA) and fibroblast-specific protein 1 (FSP1)] are acquired during EMT [52].
Effect of current therapies on CSCs and future treatment perspectives. The normal stem cells undergo oncogenesis due to accumulation of mutations, and the dysregulation of oncogenes and tumour suppressor genes of the cell cycle, which lead to active proliferation of the mutated cells and development of tumour. The current therapeutics do not specifically target the CSCs, and therefore, tumour relapses with multidrug resistance. The CSCs are very plastic in nature, and they possess the characteristics of self-renewability due to their control over key signalling pathways such as Wnt/β-catenin, Notch and Hedgehog. Ongoing research specifically target CSCs, and have shown some promising results in the eradication of tumour. Cancer cells are represented with serrated margins. Red coloured cells with serrated margins are CSCs. Green and yellow coloured cells with serrated margins are different tumour cells, which are represented to show the tumour heterogeneity. Pink coloured cell with serrated margins depicts the dead targeted CSC. The blue or black coloured nucleus represents heterogeneity in the cancer genomes. CSCs cancer stem cells
Importantly, growth factors and inflammatory cytokines, including interleukin (IL)-8 [an activator of the Janus-activated kinase/signal transducer and activator of transcription 3 (JAK/STAT3) pathway] are secreted by the stromal fibroblasts, and they promote tumour progression and account for the aggressiveness of cancers [53]. Nevertheless, the aberrant activation of the signalling pathways varies with cancer types. Few proteins, which have tumour suppressor property in one type of cancer are known to initiate and cause invasiveness in a different type of cancer. But, genetic instability or mutations and epigenetic alterations can activate oncogenes or inactivate tumour suppressor genes via metabolic reprogramming, which in turn can lead to increased cell proliferation, malignant transformation, metastasis or cancer relapse. Furthermore, CSC’s heterogeneity and the process of EMT are considered to largely contribute to the complexity and organ-specific metastasis of cancers [54].
8.7 Metabolic Alterations in Tumour Survival
Due to the complex nature of various TMEs, the CSCs tend to depend on different sources for energy. It has been identified that glucose- and oxidative-based metabolisms, feed the CSCs with energy derived from vital nutrients, along with amino acids like glutamine and lysine, which act as alternative fuel sources. In a normal cell, mitochondria produce ATP using their tricarboxylic acid (TCA) cycle coupled with OXPHOS, to catabolise acetyl-CoA produced from glycolysis and fatty acid oxidation [55]. But, CSCs tend to increase the glycolytic flux in aerobic condition, thereby increasing the production of ATP multifold to feed the anabolic demands of cancer cells (Fig. 8.5). The supply of essential growth factors and nutrients from the energy provided by the oxidative and glycolytic metabolisms, favour cancer cells to survive in unfavourable hypoxia condition, and helps them to proliferate, differentiate and evade apoptosis more efficiently [56].
Molecular mechanisms and metabolic alterations that drive CSCs in tumour development. The tumour cells in the primary site invade the normal tissues using various enzymes and cross the endothelial barrier, before entering into blood vessels for circulation. The tumour cells undergo EMT to gain mesenchymal properties, which aid in their rapid invasion. The circulating tumour cells (CTCs) intravasate to enter into blood vessels, then extravasate and undergo MET, which are required for dissemination and colonization of cancers at distant secondary sites in other tissues or organs. The tumour at the primary site uses the glycolytic pathway for the production of ATP, which is used as energy by cancer cells for their rapid growth. But, the tumour at the metastatic site uses both glycolytic and OXPHOS metabolisms for the production of ATP, and the tumour cells can switch the pathways depending on their need. Cancer cells are represented with serrated margins. Red coloured cells with serrated margins are CSCs. Green and yellow coloured cells with serrated margins are different tumour cells, which are represented to show the tumour heterogeneity. Orange coloured cells with serrated margins represent tumour cells with altered metabolism (i.e. using both glycolytic and OXPHOS pathways), whereas yellow coloured cells represent tumour cells using only glycolytic pathway. The blue or black coloured nucleus represents heterogeneity in the cancer genomes. ATP adenosine triphosphate, EMT epithelial to mesenchymal transition, CTCs circulating tumour cells, MET mesenchymal to epithelial transition, OXPHOS oxidative phosphorylation
Metabolic alterations in the cells are considered as one of the hallmarks of cancer. Glucose is a key nutrient that is necessary for the CSCs to survive in their microenvironment. It also favours the active proliferation of CSC populations as glucose induces the transcription of specific genes associated with the pathways of glucose metabolism (c-MYC, Glut, HK-1, HK-2 and PDK-1) in CSCs [57]. Growing evidence suggest that the mitochondrial oxidative metabolism is highly favoured for energy production in CSC populations. CSCs are metabolically plastic in nature as they can switch between either glycolytic or OXPHOS pathway, depending on their anabolic needs (Fig. 8.5).
Most of the quiescent CSCs, during the initial stages of tumour development, use the oxidative metabolism to match the demands of ATP requirement by the tumour. The higher rate of oxidative metabolism results in increased oxygen consumption, along with higher mitochondrial mass and increased ROS production in the mitochondria, but with lower glycolytic rate. On the contrary, the proliferative cancer cells (i.e. non-stem cells) use the glycolytic pathway which results in higher glucose uptake, but lower oxygen consumption with the least mitochondrial mass and ROS production. This helps in efficient cell differentiation, leading to increased invasiveness [58]. Interestingly, the proliferative CSCs during metastasis get their energy from both the pathways, as more energy is required for the cancer cells to migrate and colonize a new site to form a secondary tumour. The aggressiveness of the carcinoma during metastatic dissemination or cancer relapse is doubled, due to energy availability from both the glycolytic and OXPHOS pathways.
8.8 Signalling Pathways that Drive CSCs
CSCs and MSCs employ common mechanisms, which enable them to retain their stemness and plasticity. The most crucial mechanisms that are involved in CSC self-renewal are Wnt/β-catenin, Notch and Hedgehog (Hh) signalling pathways. Wnt/β-catenin signalling is a conserved pathway present in different organisms, and it plays a key role during development. It is an essential pathway that is required for the maintenance of adult stem cell homeostasis. The intercellular Wnt signalling molecules that are usually involved in the embryonic development, are altered during EMT, which leads to continuous activation of Wnt signalling and persistent synthesis of oncogenic proteins, thereby enhancing CSC’s self-renewal potential [59].
β-Catenin is the key molecule, which regulates the activation of the Wnt signalling pathway. Importantly, aberrant activation of Wnt/β-catenin pathway plays a crucial role in cancer growth, invasion, stemness and angiogenesis [60, 61]. In the absence of Wnt ligand, the multi-protein destruction complex containing Axin, adenomatous polyposis coli (APC) and glycogen synthase kinase-3β (GSK-3β) phosphorylates β-catenin, which is followed by ubiquitination and proteasomal degradation of β-catenin. However, when Wnt ligand bind to its receptor, conformational change occuring at its binding site activates downstream signalling, and β-catenin molecules are released from its destruction complex and accumulates in the cytoplasm. Later, the accumulated cytoplasmic β-catenin is imported into the nucleus, where it binds to the transcription factor Tcf/Lef, and activates Wnt target genes transcription. The target genes of Wnt/β-catenin signalling such as c-Myc, cyclin D1, MMP-7, CD44, COX-2, Axin2, etc. upon expression promote the adhesion and migration of CSCs. Furthermore, Wnt target gene expression also promotes the cellular differentiation and proliferation of CSCs, which lead to increased invasiveness of CSCs [62].
Notch signalling pathway plays a key role in cell-to-cell communication during the developmental stages. It also regulates cellular proliferation and differentiation, and apoptosis. It is essential for neural stem cell maintenance, immune regulation and normal haematopoiesis. The Notch signalling pathway is activated during cell-to-cell communication, when membrane-bound Jagged or Delta ligand bind to its specific receptor. The Notch receptor is a heterodimer, which is composed of non-covalently bound extracellular and transmembrane domains. After the binding of ligand to Notch receptor, the receptor undergoes a conformational change, which leads to its proteolytic cleavage by the metalloproteinase and γ-secretase, and the release of extracellular and intracellular fragments. The proteolytic cleavage of the heterodimeric Notch receptor releases the Notch intracellular domain (NICD) into the cytoplasm, which upon translocation into the nucleus activates transcription factors, resulting in the upregulated expression of Notch target genes such as c-Myc and HES-family members [63].
The Hedgehog (Hh) signalling pathway is intrinsic for stem cell maintenance as it controls tissue polarity and maintains patterning during embryogenesis. The genetic or epigenetic alterations in cells can generate CSCs, which hijacks the Hh signalling pathway for the maintenance of its plasticity or tumour growth. In the inactive state, the absence of Hh ligand results in the inhibition of Smoothened (Smo) receptor by the transmembrane receptor Patched (Ptch). This in turn activates a series of events in the cytoplasm, which subsequently phosphorylates and degrades Gli1/2 through proteasomal degradation. When adjacent cells secrete Hh, it binds to its receptor Ptch and activates Smo. Then, Gli1/2 molecules, which are bound to the complex in the cytoplasm are released from the Smo protein complex and translocate into the nucleus. This, results in the activation of transcription factors and the expression of Hh-associated genes [64]. The activated genes also include various genes that are directly and indirectly involved in the maintenance of the CSCs. Hh signalling upregulates JAG2, and also indirectly upregulates bone morphogenic protein 4 (BMP4) via FOXF1. Hh pathway can crosstalk with Wnt pathway, and can upregulate crucial Wnt proteins such as Wnt2B and Wnt5A. Furthermore, Hh signalling can induce stem cell markers such as LGR5, CD44 and CD133, upon interaction with Wnt and other signalling pathways [65].
In most types of cancers, CSCs hijack all the signalling pathways, which are functionally related to the maintenance of the adult stem cells, thereby promoting the growth and invasiveness of tumour. Recent studies suggest that CSCs are mostly maintained and driven in the TME with the help of these signalling pathways. Therefore, targeted inactivation of these pathways in most carcinomas may have clinical implications, as it would inhibit the self-renewability of CSCs. This approach could be used to target both CSCs and tumour to inhibit metastasis and tumour relapse, and therefore, would enable effective future treatments and ultimately cure for cancers [66].
8.9 Conclusions
CSCs possess the plasticity and self-sustaining ability to survive longer in dormant state within the TME, by evading cell death and remaining refractory to cell signalling or communication mechanisms. Importantly, CSCs can initiate tumours and cause relapse of cancers, which subsequently could lead to more aggressive carcinomas with increasing resistance to treatments. Cancer patients show poor survival rate, when they have metastatic cancer and cancer relapse, because these cancers possess CSCs. Hence, patients with metastatic and relapsed cancers cannot be cured with currently available cancer therapies. Therefore, developing advanced novel therapeutics, which can specifically target and eliminate CSCs in tumours, is an urgently required endeavour to treat, cure and eradicate cancers in the future.
References
Saito Y, Desai RR, Muthuswamy SK (2018) Reinterpreting polarity and cancer: the changing landscape from tumor suppression to tumor promotion. Biochim Biophys Acta Rev Cancer 1869(2):103–116
Becker A, Thakur BK, Weiss JM, Kim HS, Peinado H, Lyden D (2016) Extracellular vesicles in cancer: cell-to-cell mediators of metastasis. Cancer Cell 30(6):836–848
McAllister SS, Weinberg RA (2014) The tumour-induced systemic environment as a critical regulator of cancer progression and metastasis. Nat Cell Biol 16(8):717–727
Lambert AW, Pattabiraman DR, Weinberg RA (2017) Emerging biological principles of metastasis. Cell 168(4):670–691
Massague J, Obenauf AC (2016) Metastatic colonization by circulating tumour cells. Nature 529(7586):298–306
He J, Xiong L, Li Q, Lin L, Miao X, Yan S, Hong Z, Yang L, Wen Y, Deng X (2018) 3D modeling of cancer stem cell niche. Oncotarget 9(1):1326–1345
Prager BC, Xie Q, Bao S, Rich JN (2019) Cancer stem cells: the architects of the tumor ecosystem. Cell Stem Cell 24(1):41–53
van Zijl F, Krupitza G, Mikulits W (2011) Initial steps of metastasis: cell invasion and endothelial transmigration. Mutat Res 728(1–2):23–34
Wang G, Biswas AK, Ma W, Kandpal M, Coker C, Grandgenett PM, Hollingsworth MA, Jain R, Tanji K, Lomicronpez-Pintado S, Borczuk A, Hebert D, Jenkitkasemwong S, Hojyo S, Davuluri RV, Knutson MD, Fukada T, Acharyya S (2018) Metastatic cancers promote cachexia through ZIP14 upregulation in skeletal muscle. Nat Med 24(6):770–781
Turdo A, Veschi V, Gaggianesi M, Chinnici A, Bianca P, Todaro M, Stassi G (2019) Meeting the challenge of targeting cancer stem cells. Front Cell Dev Biol 7:16
Valent P, Bonnet D, De Maria R, Lapidot T, Copland M, Melo JV, Chomienne C, Ishikawa F, Schuringa JJ, Stassi G, Huntly B, Herrmann H, Soulier J, Roesch A, Schuurhuis GJ, Wohrer S, Arock M, Zuber J, Cerny-Reiterer S, Johnsen HE, Andreeff M, Eaves C (2012) Cancer stem cell definitions and terminology: the devil is in the details. Nat Rev Cancer 12(11):767–775
Bhowmick NA, Neilson EG, Moses HL (2004) Stromal fibroblasts in cancer initiation and progression. Nature 432(7015):332–337
Crawford Y, Ferrara N (2009) Tumor and stromal pathways mediating refractoriness/resistance to anti-angiogenic therapies. Trends Pharmacol Sci 30(12):624–630
Owen JL, Mohamadzadeh M (2013) Macrophages and chemokines as mediators of angiogenesis. Front Physiol 4:159
Gaggianesi M, Turdo A, Chinnici A, Lipari E, Apuzzo T, Benfante A, Sperduti I, Di Franco S, Meraviglia S, Lo Presti E, Dieli F, Caputo V, Militello G, Vieni S, Stassi G, Todaro M (2017) IL4 primes the dynamics of breast cancer progression via DUSP4 inhibition. Cancer Res 77(12):3268–3279
Lee Y, Shin JH, Longmire M, Wang H, Kohrt HE, Chang HY, Sunwoo JB (2016) CD44+ cells in head and neck squamous cell carcinoma suppress T-cell-mediated immunity by selective constitutive and inducible expression of PD-L1. Clin Cancer Res 22(14):3571–3581
Wu Y, Chen M, Wu P, Chen C, Xu ZP, Gu W (2017) Increased PD-L1 expression in breast and colon cancer stem cells. Clin Exp Pharmacol Physiol 44(5):602–604
Jachetti E, Caputo S, Mazzoleni S, Brambillasca CS, Parigi SM, Grioni M, Piras IS, Restuccia U, Calcinotto A, Freschi M, Bachi A, Galli R, Bellone M (2015) Tenascin-C protects cancer stem-like cells from immune surveillance by arresting T-cell activation. Cancer Res 75(10):2095–2108
Yao HJ, Zhang YG, Sun L, Liu Y (2014) The effect of hyaluronic acid functionalized carbon nanotubes loaded with salinomycin on gastric cancer stem cells. Biomaterials 35(33):9208–9223
Shen MM, Abate-Shen C (2010) Molecular genetics of prostate cancer: new prospects for old challenges. Genes Dev 24(18):1967–2000
Pastushenko I, Blanpain C (2019) EMT transition states during tumor progression and metastasis. Trends Cell Biol 29(3):212–226
Fouad YA, Aanei C (2017) Revisiting the hallmarks of cancer. Am J Cancer Res 7(5):1016–1036
Demaria O, Cornen S, Daeron M, Morel Y, Medzhitov R, Vivier E (2019) Publisher correction: harnessing innate immunity in cancer therapy. Nature 576(7785):E3
Sell S (2004) Stem cell origin of cancer and differentiation therapy. Crit Rev Oncol Hematol 51(1):1–28
Aslan C, Maralbashi S, Salari F, Kahroba H, Sigaroodi F, Kazemi T, Kharaziha P (2019) Tumor-derived exosomes: implication in angiogenesis and antiangiogenesis cancer therapy. J Cell Physiol 234(10):16885–16903
Plaks V, Kong N, Werb Z (2015) The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells? Cell Stem Cell 16(3):225–238
Paulis YW, Huijbers EJ, van der Schaft DW, Soetekouw PM, Pauwels P, Tjan-Heijnen VC, Griffioen AW (2015) CD44 enhances tumor aggressiveness by promoting tumor cell plasticity. Oncotarget 6(23):19634–19646
Glumac PM, LeBeau AM (2018) The role of CD133 in cancer: a concise review. Clin Transl Med 7(1):18
Yu KR, Yang SR, Jung JW, Kim H, Ko K, Han DW, Park SB, Choi SW, Kang SK, Scholer H, Kang KS (2012) CD49f enhances multipotency and maintains stemness through the direct regulation of OCT4 and SOX2. Stem Cells 30(5):876–887
Clark DW, Palle K (2016) Aldehyde dehydrogenases in cancer stem cells: potential as therapeutic targets. Ann Transl Med 4(24):518
Saikolappan S, Kumar B, Shishodia G, Koul S, Koul HK (2019) Reactive oxygen species and cancer: a complex interaction. Cancer Lett 452:132–143
McGranahan N, Swanton C (2017) Clonal heterogeneity and tumor evolution: past, present, and the future. Cell 168(4):613–628
Malanchi I, Santamaria-Martinez A, Susanto E, Peng H, Lehr HA, Delaloye JF, Huelsken J (2011) Interactions between cancer stem cells and their niche govern metastatic colonization. Nature 481(7379):85–89
Steinbichler TB, Dudas J, Skvortsov S, Ganswindt U, Riechelmann H, Skvortsova II (2019) Therapy resistance mediated by exosomes. Mol Cancer 18(1):58
Cubillos-Ruiz JR, Bettigole SE, Glimcher LH (2017) Tumorigenic and immunosuppressive effects of endoplasmic reticulum stress in cancer. Cell 168(4):692–706
Tsang DS, Patel S (2019) Proton beam therapy for cancer. CMAJ 191(24):E664–e666
Murgai M, Giles A, Kaplan R (2015) Physiological, tumor, and metastatic niches: opportunities and challenges for targeting the tumor microenvironment. Crit Rev Oncog 20(3–4):301–314
Bao B, Azmi AS, Ali S, Ahmad A, Li Y, Banerjee S, Kong D, Sarkar FH (2012) The biological kinship of hypoxia with CSC and EMT and their relationship with deregulated expression of miRNAs and tumor aggressiveness. Biochim Biophys Acta 1826(2):272–296
Shiozawa Y, Nie B, Pienta KJ, Morgan TM, Taichman RS (2013) Cancer stem cells and their role in metastasis. Pharmacol Ther 138(2):285–293
Kim MY, Oskarsson T, Acharyya S, Nguyen DX, Zhang XH, Norton L, Massague J (2009) Tumor self-seeding by circulating cancer cells. Cell 139(7):1315–1326
Ayob AZ, Ramasamy TS (2018) Cancer stem cells as key drivers of tumour progression. J Biomed Sci 25(1):20
Lee SY, Jeong EK, Ju MK, Jeon HM, Kim MY, Kim CH, Park HG, Han SI, Kang HS (2017) Induction of metastasis, cancer stem cell phenotype, and oncogenic metabolism in cancer cells by ionizing radiation. Mol Cancer 16(1):10
Desai A, Yan Y, Gerson SL (2019) Concise reviews: Cancer stem cell targeted therapies: toward clinical success. Stem Cells Transl Med 8(1):75–81
Folkerd EJ, Dowsett M (2010) Influence of sex hormones on cancer progression. J Clin Oncol 28(26):4038–4044
Aaronson SA (1991) Growth factors and cancer. Science 254(5035):1146–1153
Shay G, Lynch CC, Fingleton B (2015) Moving targets: emerging roles for MMPs in cancer progression and metastasis. Matrix Biol 44–46:200–206
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–674
Lasorella A, Benezra R, Iavarone A (2014) The ID proteins: master regulators of cancer stem cells and tumour aggressiveness. Nat Rev Cancer 14(2):77–91
Robichaud N, Sonenberg N, Ruggero D, Schneider RJ (2019) Translational control in cancer. Cold Spring Harb Perspect Biol 11(7):a032896
Mitra A, Mishra L, Li S (2015) EMT, CTCs and CSCs in tumor relapse and drug-resistance. Oncotarget 6(13):10697–10711
Shibue T, Weinberg RA (2017) EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat Rev Clin Oncol 14(10):611–629
Kong D, Li Y, Wang Z, Sarkar FH (2011) Cancer stem cells and epithelial-to-mesenchymal transition (EMT)-phenotypic cells: are they cousins or twins? Cancers (Basel) 3(1):716–729
Du B, Shim JS (2016) Targeting epithelial-mesenchymal transition (EMT) to overcome drug resistance in cancer. Molecules 21(7):E965
El-Kenawi A, Hanggi K, Ruffell B (2019) The immune microenvironment and cancer metastasis. Cold Spring Harb Perspect Med 10:a037424
Snyder V, Reed-Newman TC, Arnold L, Thomas SM, Anant S (2018) Cancer stem cell metabolism and potential therapeutic targets. Front Oncol 8:203
Al Tameemi W, Dale TP, Al-Jumaily RMK, Forsyth NR (2019) Hypoxia-modified cancer cell metabolism. Front Cell Dev Biol 7:4
De Francesco EM, Sotgia F, Lisanti MP (2018) Cancer stem cells (CSCs): metabolic strategies for their identification and eradication. Biochem J 475(9):1611–1634
Chae YC, Kim JH (2018) Cancer stem cell metabolism: target for cancer therapy. BMB Rep 51(7):319–326
Nusse R (2012) Wnt signaling. Cold Spring Harb Perspect Biol 4(5):a011163
Ramachandran I, Thavathiru E, Ramalingam S, Natarajan G, Mills WK, Benbrook DM, Zuna R, Lightfoot S, Reis A, Anant S, Queimado L (2012) Wnt inhibitory factor 1 induces apoptosis and inhibits cervical cancer growth, invasion and angiogenesis in vivo. Oncogene 31(22):2725–2737
Ramachandran I, Ganapathy V, Gillies E, Fonseca I, Sureban SM, Houchen CW, Reis A, Queimado L (2014) Wnt inhibitory factor 1 suppresses cancer stemness and induces cellular senescence. Cell Death Dis 5(5):e1246
Takebe N, Harris PJ, Warren RQ, Ivy SP (2011) Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog pathways. Nat Rev Clin Oncol 8(2):97–106
Shukla S, Meeran SM (2014) Epigenetics of cancer stem cells: pathways and therapeutics. Biochim Biophys Acta 1840(12):3494–3502
Venkatesh V, Nataraj R, Thangaraj GS, Karthikeyan M, Gnanasekaran A, Kaginelli SB, Kuppanna G, Kallappa CG, Basalingappa KM (2018) Targeting Notch signalling pathway of cancer stem cells. Stem Cell Investig 5:5
Katoh Y, Katoh M (2009) Hedgehog target genes: mechanisms of carcinogenesis induced by aberrant hedgehog signaling activation. Curr Mol Med 9(7):873–886
Niehrs C (2012) The complex world of WNT receptor signalling. Nat Rev Mol Cell Biol 13(12):767–779
Acknowledgements
We express our sincere appreciation to Dr. Yuvaraj Sambandam (Immune Monitoring Core, Comprehensive Transplant Center, Feinberg School of Medicine, Northwestern University, Chicago, IL, USA) for thoroughly reading this manuscript and providing critical suggestions.
Conflict of Interest: The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Chengizkhan, G., Bhaskaran, N., Kumaran, R.I., Ramachandran, I. (2020). Cancer Stem Cells and Tumour Aggressiveness. In: Pathak, S., Banerjee, A. (eds) Cancer Stem Cells: New Horizons in Cancer Therapies. Springer, Singapore. https://doi.org/10.1007/978-981-15-5120-8_8
Download citation
DOI: https://doi.org/10.1007/978-981-15-5120-8_8
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-5119-2
Online ISBN: 978-981-15-5120-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)