Abstract
Because of the draining petroleum combustible reservoirs like crude fuel, coke, and natural gasoline, the current pace of commercial development is indefensible. Accordingly, numerous sustainable power source has been employed; however, the potentials of a few different sources like plastics waste are still to wholly created as a business project. Along with age group of waste plastics expanding, current Indian enactment directs high recuperation rates, and rules favors waste management innovation decisions that possess a higher situation of a waste management progressive system. Pyrolysis is a procedure that changes over waste plastics in a relevant fluid product that can be accepted as a potential origin for several reasons such as automobile vehicles, power generators, and diesel engines, etc. Plastics pyrolysis depends on the thermal or occasionally reactant breakdown of the polymer composition. This examination aimed to develop the pyrolysis system model for the extraction of oil/diesel from plastic wastes that can be sold at extremely cheaper rates than those available. Developed pyrolysis system model has tested as alternative for the extraction of oil. Results shows, oil extraction of 10–20 ml could be obtained by burning 180–380 gm of plastic.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
The increase in plastic using up has been going on speedily since the last 5–6 decades because of lightweight and ability of plastic to form. Today, the principal interests are the requirement for energy and the degradation of the environment, which are because of expanding population and accelerated industrialization [1]. Actions are initiated to defeat the fossil fuel crisis by looking for options to replace gasoline and diesel. The development of alternative fuel technologies is created to give the alternative to fossil fuels [2]. The techniques focused are bioethanol, biodiesel, biodiesel derived from lipids, recycling the waste oils, pyrolysis, gasification, dimethyl ether, and biogas [3]. The use of plastic in a world was about 5 million tons annually in 1950s has enlarged to 20 times from about 100 million tons [4]. Currently, most plastic waste is disposed of in landfills or deposits, which involve our precious land spaces. The disposal methods, such as landfills, reuse, and combustion, can produce serious risks, particularly in human and environmental health [5]. Only a small percentage of plastic waste enters the reuse/recycling options, such as the use as a filler on asphalt roads or as a raw material for secondary product generation, such as recycled rubber, nonnatural barriers, or breakwaters [6].
Therefore, plastic waste can be deemed an energy resource. It has a sizeable calorific value, a high volatile content, and decreased ash content than coal and bio-ass [7]. Therefore, the residual plastic is the right suitor for the utilization of thermal elimination. These characteristics present it a perfect element for thermal processes like pyrolysis and gasification. Waste into the energy is designed to process potential materials into waste that are plastics, biomass, and rubber tires to oil [8]. The pyrolysis is growing as a substitute to give biofuel for compensation of the fossil fuel [9, 10]. Plastic waste is studied in the study as an accessible technology. The pre-treatment of the material is comfortable, as described in the article. The plastic is necessary to be classified and dried. Also, pyrolysis is neither toxic nor dangerous to an environment, unlike incineration [11]. Pyrolysis found enormously flexible procedure fit for large- and small-scale production [12].
2 Materials and Methods
2.1 Types of Plastics, Properties, and Its Uses
The different kinds of plastic have various features such as moisture content, heat resistant, chemical resistance, surface phenomenon, etc., which can be the prominent phenomenon for typical household uses [13]. The classification of plastic based on properties is listed in Table 1.
2.2 Principle of Pyrolysis
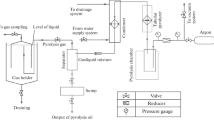
Pyrolysis is an endothermic process, an ecologically attractive method to treat plastic waste. The method practices average temperatures (300–700) °C and an oxygen-independent atmosphere for chemical decomposition of solid plastic waste into coal, oil, and gas, as per Fig. 3, which produces a minimal discharge of the nitrogen oxide and sulfur compared to incineration, the most popular method of industry [14]. Pyrolysis method consists of a collection of waste plastic, weighing and adding a waste plastic including catalyst into the reactor, pyrolysis of this waste plastic and collection, analyzing the extracted oil of a waste plastic [15]. The systematic flowchart of this process is presented within the Fig. 1.
The feedstocks utilized for the experiments were waste plastics having polyethylene terephthalate and high-density polyethylene and the same were obtained from the dumping place, and the little plastic recycling at Nagpur City, Maharashtra, India. The appearance of these feedstocks such as the collection of waste plastic, cleaning it, and shredding the waste plastics are shown in Fig. 2.
2.3 Experimentation on Pyrolysis Setup
Pyrolysis set-up as in Fig. 3 mainly comprises of reactor, GI coupling, GI pipe, condenser, water inlet, water outlet, RB flask, condenser, LPG gas cylinder, and iron stand.
The specification for material, top and bottom diameter, depth, volume, and weight of the reactor for pyrolysis setup is listed in Table 2.
In this process after the entire setup of pyrolysis was done, as it turned on and fired with the help of matchbox along with turning on the flow of cold water into a condenser and left for observation. The reactor gets consistently heated, which further raises a temperature of the reactor, causing the waste plastics to break and release vapors [16]. The air-packed manufacturing of reactor gives no leakage to the system and allows a vapor for flow through the outlet pipe, which then goes into the inner glass tube of the condenser [17, 18]. Meanwhile, the noncondensable vapors get out within the environment from the loosely tightened end of the condenser and the RB flask [19, 20]. The condensable vapor sticks to the inner wall of the tube and simultaneously forms droplets of oil as a result of heat exchanged with the cold water and get collected into the RB flask. After sometimes, it is seen that the flow of oil from the condenser stops, which shows that the number of plastics added into the reactor is pyrolyzed [21, 22]. Still to confirm that there are no leftover condensable vapors the reactor is heated for a few more minutes.
Subsequent cooling of reactor, remaining ash is there and plastic compressed like char, which needs to be separate using sieving. The char can be further utilized in road construction.
In the experimentation, 180–400 gm of the feedstock was supplied to a pyrolysis reactor. After this, pyrolyzer unit plus reformer was heated up to selected temperatures. Output obtained from a process in liquid form were got inside the RB flask
3 Results and Discussions
The amount of weight plastic 180 gm, 380 gm, 400 gm was tested for 1 h, 2 h, and 2 h 15 min, respectively. Amount of oil collected from waste plastic is, as presented in Table 3.
The amount of fuel getting collected in the RB flask and fuel collected at the end of the experimentation is as presented in Fig. 4.
Table 4 reflects the various properties of the liquid product for diesel grade.
4 Conclusions
The conclusions reached by experimentations are,
-
1.
Pyrolysis method looks an efficient waste-to-energy converter which is considered reasonable to turn plastic into liquefied outputs and to enhance the waste plastics.
-
2.
Pyrolysis could be carried under minimal expenses for small-scale waste plastic oil extraction, and 10–20 ml could be obtained by burning 180–380 gm of plastic.
-
3.
Rather than direct burning of plastic into the atmosphere, converting into fuel decreases 80 percent of CO2 emission in the atmosphere.
-
4.
Fewer emissions of unburned hydrocarbons in plastic pyrolysis waste oil as comparing to diesel.
-
5.
Obtained diesel oil or oil has better performance and, as compared, has 30–40% low production costs.
-
6.
The waste plastic recycling will perform a crucial task in the transformation of a newer era.
-
7.
The gas portion can be analyzed in the future with gas chromatography to know the contents of CO, CO2, H2, N2, and others.
References
Syamsiro, M., Saptoadi, H., Norsujianto, T., Noviasri, P.: Fuel oil production from municipal plastic wastes in sequential pyrolysis and catalytic reforming reactors. Energy Procedia. 47, 180–188 (2014)
Wong, S.L., Ngadi, N., Abdullah, T.A.T., Inuwa, I.M.: Current state and future prospects of plastic waste as source of fuel: a review. Renew. Sustain. Energy Rev. 50, 1167–1180 (2015)
Dogan, O., Celik, M.B., Ozdalyan, B.: The effect of plastic derived fuel/diesel fuel blends utilization on diesel engine performance and emissions. Fuel 95, 340–346 (2012)
UNEP. Converting waste plastics into resource: compendium of technologies. In: United Nations Environment Programme. Osaka (2009)
Siauw, H., Toc, C., Drive, O.P., Seoud, H., Stanciulescu, M.: Conversion of polyethylene to transportation fuels through pyrolysis and catalytic cracking, 30–33 (1995)
Kunwar, B., Cheng, H.N., Chandrashekaran, S.R., Sharma, B.K.: Plastics to fuel: a review. 54, 421–428 (2016)
Al-Salem, S.M., Lettieri, P., Baeyens, J.: The valorization of plastic solid waste (PSW) by primary to quaternary routes: from re-use to energy and chemicals. Prog. Energy Combust. Sci. 36(1), 103–129 (2010). https://doi.org/10.1016/j.pecs.2009.09.001
Murugan, S., Ramaswamy, M., Nagaranian, G.: The use of tyre pyrolysis oil in diesel engines. Waste Manag. 28(12), 2743–2749 (2008)
Williams, P.T.: Pyrolysis of waste tyres: a review. Waste Manag. 33(8), 1714–1728 (2013)
Leung, D., Wang, C.: Fluidized-bed gasification of waste plastic powders. Fuel Process. Technol. 84(1–3), 175–196 (2003)
Bernardo, M., Lapa, N., Goncalves, M.: Toxicity of char residues produced in the co-pyrolysis of different wastes. Waste Manage. 30, 628–635 (2010)
Chenier, P.J.: Survey of Industrial Chemistry, 3rd edn. Kluwer Academic/Plenum Publishers, New York (2002)
Thorat, P.V, Warulkar, S., Sathone, H.: Thermofuel—pyrolysis of waste plastic to produce liquid hydrocarbons. 3, 14–18 (2013)
Karatas, H., Olgun, H., Engin, B., Akgun, F.: Experimental results of gasification of waste plastic with air in a bubbling fluidized bed gasifier. Fuel 105, 566–571 (2013)
Nahid, M., Hamid, H.: Catalytic coprocessing of waste plastics and petroleum residue into liquid fuel oils. J. Anal. Appl. Pyrol. 86, 141–147 (2009)
Portofino, S., Donatelli, A., Iovane, P., Innella, C., Civita, R., Martino, M., Matera, D.A., Russo, A., Cornacchina, G., Galvango, S.: Steam gasification of waste tyre: influence of process temperature on yield and product composition. Waste Manag 33(3), 672–678 (2013)
Evans, A., Evans, R.: The composition of a plastic: typical components. The Waste Resour. Act. Programme, Banbury (2006)
Betancur, M., Martinez, J.D., Murillo, R.: Production of activated carbon by waste plastic thermochemical degradation with CO2. J. Hazard. Mater. 168(2–3), 882–887 (2009)
Malkow, T.: Novel and innovative pyrolysis and gasification technologies for energy efficient and environmentally sound MSW disposal. Waste Manag. 24(1), 53–79 (2004)
Galvango, S., Casciaro, G., Casu, S., Martino, M., Mingazzini, C., Russo, A., Portofino, S.: Steam gasification of tyre waste, poplar, and refuse-derived fuel: a comparative analysis. Waste Manag. 2, 678–689 (2009)
Islam, M.R., Tushar, M., Haniu, H.: Production of liquid fuels and chemicals from pyrolysis of Bangladeshi bicycle/rickshaw plastic wastes. J. Anal. Appl. Pyrol. 82(1) (2008)
Murugan, S., Ramaswamy, M., Nagarajan, G.: A comparative study on the performance, emission and combustion studies of a DI diesel engine using distilled tyre pyrolysis oil-diesel blends. Fuel 87(10–11), 2111–2121 (2008)
Acknowledgements
Authors are thankful to Municipal Corporation, Nagpur City, Maharashtra, India, for providing permission to utilize plastics waster such as polyethylene terephthalate and high-density polyethylene for experimental work.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Waghmare, S.N., Shelare, S.D., Tembhurkar, C.K., Jawalekar, S.B. (2020). Pyrolysis System for Environment-Friendly Conversion of Plastic Waste into Fuel. In: Singh, S., Prakash, C., Ramakrishna, S., Krolczyk, G. (eds) Advances in Materials Processing . Lecture Notes in Mechanical Engineering. Springer, Singapore. https://doi.org/10.1007/978-981-15-4748-5_13
Download citation
DOI: https://doi.org/10.1007/978-981-15-4748-5_13
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-4747-8
Online ISBN: 978-981-15-4748-5
eBook Packages: EngineeringEngineering (R0)