Abstract
Portland cement plays a very important part in construction, but the manufacturing process emits almost 5–7% of the CO2 in the world and it is one of the main causes of global warming. This paper discusses about the alternative material to Portland cement and compares between their strength and durability. The main material in this study is fly ash which is an industrial waste and easily available. In India, every year almost 120 million tons of fly ash is produced in the power plants and fly ash is very rich in silicon and aluminium; that is why it is a very good replacement for cement and this way we can recycle the waste also. Geopolymer is a mixture of fly ash with sodium hydroxide (NaOH) and sodium silicate (Na2SiO3). In this paper, geopolymer is made using the combination of M25-grade concrete. The ratio between NaOH and Na2SiO3 is kept constant which is 1:2 and the morality of NaOH varied from 10 M to 16 M. In geopolymer concrete, cement is 100% replaced by fly ash. The compressive strength and durability parameter is compared between geopolymer concrete cubes (which is kept at different curing conditions) and concrete cubes in which Portland cement is replaced by 25% of fly ash.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Portland cement is the principal material used in construction but the manufacturing process is very harmful for environment as it releases 1 tonne of CO2 per 1 tonne of cement [1]. It is one of the green house gases responsible for global warming and is a great threat to mankind. Nowadays, it is an important issue to develop environmentally friendly and sustainable construction material [2]. The only way to reduce the effect alternative material to Portland cement are blended cement and geopolymers [3]. Geopolymer proves to be the best which completely replaces the Portland cement by aluminosilicate materials like fly ash, blast furnace slag, metakoline, silica fume, etc., which are rich in alumina (Al) and silica (Si) with alkali activators like NaOH, Na2SiO3 KOH, K2SiO3, etc. [4]. On curing at higher temperature, polymerization takes place and products are calcium aluminates silicate hydrate(C–A–S–H) and sodium aluminate silicate hydrate(N–A–S–H) which bind the inert materials to form geopolymer concrete [4,5,6,7]. One of the drawback is the heat curing which is not suitable for sight conditions. Normal curing provides lower strength. On other hand, the alkali activator NaOH of higher molar value provides higher strength.
1.1 Effect of Curing on Strength
It was observed that high-temperature cured GPC with rest period about 3 days after casting gains higher strength than GPC cured immediately after casting with low temperature and OPC concrete at 28 days. GPC samples displayed little gain of strength after steam curing was over up to 28 days [8]. As curing temperature increases the strength of the GP mortar increases and optimum temperature is found to be 75 °C. Also the duration of heat curing enhances the strength and optimum heat curing duration is predicted as 2 days. Microstructure of the mortar get weakened at elevated curing temperature and therefore strength get reduced [9]. Compressive strength increases up to 100 °C curing temperature and at 120 °C started decreasing. This loss of strength attributed to the formation of crack due to the loss of moisture at high temperature. Compressive strength of the OPC found to be more than GPC. This is due to heterogeneous microstructure which is due to the disruption of packing of binder at the presence of air void [10]. In comparison to KOH, NaOH activator provides better compressive strength because higher amount of silicate and aluminates monomers are found in case of NaOH. It is attributed that the sodium cations of NaOH provide better geopolymerization due to smaller in size as compared to potassium cation and can easily migrate through the network of moist gel [10] reported that the naphalene-based superplasticizer provides better strength than melamine formaldehyde and polycarboxilate ether-based superplasticizer. Noushini and Castel [11] reported that compressive strength of geoplymer concrete cured at 75 °C for 18 h is found to be about 20 and 15% more than that of heat-cured and normal curing OPC concrete, respectively. Curing at 75 °C for 18 h may be considered as the optimum temperature and duration of curing. Normal curing of GPC at an ambient temperature produces much lower strength and are not suitable for practical purpose. Gunasekara et al. [12] reported that the Geopolymerization continues beyond 90 days up to 365 days and the compressive strength is at per with cement concrete. Compressive strength of GPC and OPC concrete increases with curing period and strength of GPC is higher than that of OPC concrete. Also compressive strength for both the cases are higher for high-temperature curing because the increase in temperature accelerates the geopolymerization in GPC and hydration in OPC concrete [×6]. AdbElaty et al. [2] reported that at higher alkali solution/binder (AS/B) ratio, there is noticeable amount of compressive strength, split tensile strength and modulus of elasticity enhancement and best result is found for 30–40% of NaOH. Also found (AS/B) plays a rule as that of W/C ratio on OPC concrete. Increase in liquid to binder ratio (L/B) in alkali solution reduces the mechanical properties and vice versa [2]. Chemical activator of multi-compound Na2SiO3/NaOH) are found to be most effective for the enhancement of compressive strength [7]. Compressive strength of geopolymer mix is effected by chemical reactivity between the amorphous AlO3, CaO and Na2O present in fly ash and alkali activators and this chemical reactivity depends on the median particle size and contents of amorphous SiO2. Finer is the fly ash particles better is the blending in geopolymer mix due to smaller friction between its components and also increase in exposure area of amorphous SiO2 to alkali activators. Thus, as a result, there is better geopolymerization [13]. Hadi et al. [13] also reported that fly ash with finer particles has greater coverage of aggregates and forms a dense intertransition zone on aggregate surface which results in higher binding strength. Thus, the optimum value of alkaline to fly ash ratio (AL/F) to achieve optimum vale of compressive strength are different for different fly ash sources depending on particle size and contents of amorphous SiO2, CaO and Na2O. Geopolymerization is controlled by chemical equilibrium achieved between amorphous Al2O3 and SiO2. Thus, the optimum value of Na2SiO/NaOH ratio is dominated by characteristic of fly ash. High amount of Si4+ Na+ is liberated from Na2SiO3 may congested and inhibits the geopolymerization [13]. Fly ash having higher contents of SiO2 necessitates higher amount of NaOH to release SiO2 and other oxides from fly ash to initiate geopolymerization. Larger sized particles in fly ash reduces the surface area exposure of amorphous SiO2 and Al2O3 to alkali activator. On the other hand, unreacted particles exhibits week in geopolymerization and results in strength degradation [13]. It is observed that increase in temperature in hydrothermal curing, enhances the compressive strength. It is attributed to the acceleration of geopolymerization reaction due to the increase in liberation of more reactive alkali at higher temperature [14]. On normal curing at 95% humidity, compressive strength increases with time due to the increase in reactive alkali which accelerates the geopolymerization [14].
1.2 Workability
Particle size and shape have dominant influence on the workability of GP mortar. Finer is the fly ash higher is the flow value. On the other hand, if fly ash particles of are spherical and smooth higher is the flow value [9]. Workability of geopolymer concrete increases due to lubricating effect of Na2SiO3 on spherical fly ash particles [12]. But high (Na2SiO3/NaOH) ratio reduces the workability due to high viscosity of Na2SiO3. On the other hand, high-liquid alkaline/fly ash ratio increases the workability. High concentration of NaOH increases the setting time [7]. N-based superplasticizer is most effective in geopolymer matrix activated by NaOH only but PC-based superplasticizer is most effective if the activator is the mixture of Na2SiO3 and NaOH. Flow value was tested with slump test and found that the OPC concrete exhibits more slump value than GPC and it is attributed to the viscosity of Na2SiO3 GPC increases the cohesiveness. [15]. Polycarboxylate (PC)-based sperplasticizer is found to be more effective to class C fly ash than class F fly ash. It is because negatively charged particles of PC-based superplasticizer get strongly bonded with Ca+ ion liberated from Class C fly ash providing negative ion which repulse each other and increases the dispersion capacity. Thus, flow value increases [16]. On the contrary, napthalene-based superplasticizer is more effective for class F fly ash. It is attributed to better chemical stability of the naphalene-based superplasticizer in alkaline environment science the pH value of this superplasticizer ranges from 6 to 9 [16].
1.3 Porosity and Microstructure
Porosity of fly ash-based GPC is found to be more than that of OPC counterpart. It is because Na2–AlO3–SiO2–H2O gel is less denser than C–S–H gel in OPC concrete. Both heat as well as ambient cured GPC exhibit lower water absorption value than that of OPC concrete. But inappropriate curing condition increases the voids and thus water absorption increases. [11]. In case of fly ash geopolymer concrete, uneven material distribution due to more quantity of coarse particles leads high microporosity [12]. X-Ray tomography test reveals that the voids in Portland pozzolana cement concrete (PPCC) are less than that of GPC but size of the voids in GPC are smaller [15]. Fly ash-based GPC is more porous because the principal reaction product the sodium alumino silicate gel (N–A–S–H) is three-dimensional network product attributes higher porosity. On he other hand, presence of un-reacted fly ash particles also causes high porosity [15, 17]. Sorpitivity coefficient decreases for fly ash-based GPC cured at 75 °C and curing duration 24 h. It is attributed to the formation of denser geopolymer net work which reduces the void and increases the tortuosity. However, increase in heat curing temperature and duration increases the serpitivity coefficient. It is attributed to the elevated temperature which extends the capillary pore net work and thus effects the tortuosity characteristic [11]. GPC specimen displays the high sorptivity value due to large pore structure [17].
1.4 Durability
Geopolymerization continues and corresponding increase in alumino silicate gel fills the cracks and voids. Thus, the durability properties improve with time [12]. Geopolymer concrete exhibits better resistance to aggressive environment laden with acid and sulphate [7]. In some environments, carbonation depth is found to be more in GPC than that in OPC counterpart. In case of fly ash-based GPC, NaCO3 is formed as a primary carbonation product and it would dissolve in water when exposed to weather. Thus, leaching out of carbonation product causes GPC more porous and allow more CO2 diffusion through the concrete surface [17]. Chloride penetration and free chloride contents in GPC are found to be more compared to OPC counterpart because of high porosity and thus the GPC concrete are more prone to reinforcement corrosion. In case of OPC concrete, the presence of C3AF and C–S–H gel binds the chloride contents to form Fidel’s salt and thus the rate of chloride penetration diminishes. Also amount of free chloride is found to be less than GPC. High porosity of GPC results in high chloride penetration [17]. Objective of present study to compare the flow value and compressive strength of fly ash-based geopolymer concrete cured at normal temperature and at high temperature in oven, with normal OPC concrete and fly ash concrete with 25% of fly ash replacement. Also comparison of durability parameters such as acid resistance, chloride resistance and sorptivity. Therefore, in this research work, class F fly ash is used as aminosilicate material and mixture of NaOH and Na2SiO3 is used as alkaline solution. The ratio between NaOH and Na2SiO3 is kept fixed which is 1:2 throughout the experiment but four types of molarities of NaOH is taken 10, 12, 14 and 16 M molar, respectively, to study the effect of molarity on strength and flow value. The change in molarity shows a difference in adhesiveness, flow, strength prominently. Durability and mechanical properties of geopolymer concrete specimen cured in two different curing conditions are compared with that of the OPC concrete and fly ash concrete counterparts. Different curing conditions show a significant difference in strength in geopolymer concrete.
2 Experimental Programme
To fulfil the research objective, experimental programme under taken are discussed in this part. Details about the material properties, mixing, preparation, curing and testing for strength and durability requirements are discussed.
3 Materials
Fly ash used in this study is class F fly ash obtained from NALCO, Angul power plant, Orissa. The physical properties are given as: Specific gravity = 2.9, Water absorption = 15%, Colour—Tan to dark grey. Chemical compositions are given in Table 1 and Fig. 1 for fly ash.
NaOH or caustic soda of make Adity Birla were used in the experiment is procured from Shivam Chemicals, Bhubaneswar. The properties specified by the manufacture are given as chemical formula = NaOH, Melting point = 318 °C, Appearance—White, waxy, opaque crystals, Odour—odourless and Density = 2.13 g/cm3. Figure 2 shows the flakes of NaOH.
Industrial-grade Na2SiO3 was procured from Surendra chemicals, Kolkata. The properties provided by the manufacture are as follows: Chemical formula = Na2SiO3, Appearance—White to greenish opaque crystals or liquid, Density = 2.61 g/cm3 and Melting point = 1,088 °C. Figure 3 shows the liquid Na2SiO3.
OPC 53 grade cement from RAMCO Cement Limited was used and physical properties obtained from laboratory test are given as follows: Consistency = 28%, Initial and final setting times = 100 min and 230 min, respectively, Compressive strength after 3, 7 and 28 days are 35.5 Mpa, 47 Mpa and 55.5 Mpa, respectively, Specific gravity = 3.15. All physical properties satisfy the requirements of IS 12269-1987 [18].
Crushed granite sized 10 mm and 20 mm are used as coarse aggregate. The physical properties are given as Specific Gravity = 2.7, Fineness Modulus = 6.2, Water Absorption = 0.4%. All above physical properties and gradations confirms to the specifications of IS: 383-1970 [19].
Fine aggregates obtained from River Mahanadi conforms to zone III. The physical properties are given as Specific Gravity = 2.65, Fineness Modulus = 2.47, Water Absorption = 0.85% and are conforming to the specification of IS: 383-1970 [19].
Clean potable tap water obtained from the Laboratory of Civil Engineering Department of KIIT, Deemed-to-be-University was used for mixing and curing of concrete.
3.1 Preparation of Concrete Specimens and Details
Three types of cube specimens of size 150 mm × 150 mm × 150 mm [20] were used in the study. Those are (1) normal OPC concrete specimen, (2) fly ash concrete(cement replaced by 25% fly ash and (3) geopolymer concrete specimens (GPC) and detailed in Table 2.
3.2 Mix Proportions
Mix proportion by weight = 1:1.25:2.24 was selected as found from mix design for M 25 using OPC 53 grade cement as per IS:10262-2008. Ingredients for normal concrete and fly ash concrete are given in Table 3, numbers of cube samples were casted 12 for each categories of concrete.
For geopolymer concrete, same the mix proportion as above was used (1:1.25:2.24) by replacing cement by fly ash by 100%. Binder/alkaline ratio was taken 0.45. The ratio of NaOH to Na2SiO3 was taken 1:2 which remains constant for all samples. Constituents of different samples are tabulated in Table 4. Numbers of cube samples were casted 27 for each categories of geopolymer concrete.
3.3 Mixing Procedure
For normal concrete and fly ash concrete, normal mixing procedure was followed. Cube specimens were casted by placing fresh concrete in three layers and tamping with 25 blows with tamping bar and compaction with vibrating table for 30 s and finally finished with trawling (Figs. 4, 5 and 6).
For geopolymer concrete, samples of alkali solution to total alkali ratio as 1:2 were used. The blending of Na2SiO3 and NaOH exerts some amount of heat. To take it down to the surrounding temperature, the arrangement was kept for 24 h before they were blended with fly ash and aggregates. In concrete mixture machine, the aggregates and fly ash were mixed dry for around 5 min. Then mixing was done with alkali blend and requisite amount of water for 5 min. Cube specimens were casted by placing fresh concrete in three layers and tamping with 25 blows with tamping bar and compaction with vibrating table for 30 s and finally finished with trawling.
3.4 Casting of Cube Specimens
The steel moulds were covered with oil on their inside surface and concrete mix was poured into the moulds in three layers. Each layer was consistently compacted by a tamping bar with 25 numbers of blows and by vibrating table for 1 min. Finally, the top surface was finished utilizing a trowel.
3.5 Curing Process
The ordinary concrete cubes and the fly ash concrete cubes were kept in curing tank up to 7, 28 and 56 days after de-moulding. Half of the geopolymer concrete cubes were placed in the curing tank after de-moulding and another half were kept in the oven for 24 h at 75 °C and after that those were kept in ambient temperature.
4 Testing
4.1 Workability of Fresh Concrete
See Table 5.
4.2 Testing of Hardened Concrete for Compressive Strength
Testing of CTM machine of 2000 KN from AIMIL LTD is available in the Department of Civil Engineering Laboratory of KIIT, Deemed-to-be-University, is shown in Fig. 7. For compressive strength, the specimens were tested for 7, 28 and 56 days and following the test procedure in accordance to IS:516-1959. [21]. Test results in average are summarized in Table 6.
4.3 Test for Durability Parameters
In durability parameters, acid resistance, chloride resistance and sorptivity were tested on the specimens after 28 days of curing drying to constant weight oven at 100 °C.
4.3.1 Acid Attack on Concrete
To study the results of acid resistance and its effect on all categories of specimen, those were immersed in 1% solution on sulphuric acid (H2SO4) as described above for 28 days. Cube specimens were taken out one day before test and dried completely up to constant weight by oven at 100 °C. And then the weight was taken and tested for compressive strength including the reference samples which were kept for comparison purpose. Details of test results on weight difference and strength changes are given in Table 7.
4.3.2 Chloride Attack on Concrete
To study the results of chloride attack and its effect on geopolymer concrete specimen and MOF specimen 1% solution on sodium chloride (NaCl) is used. After 28 days of curing and the weight was taken both geopolymer concrete and MOF specimens were immersed entirely into the acid solution and kept for more 28 days. Cube specimens were taken out one day before test and dried completely. And then the weight was taken and tested. Some reference sample was kept for comparison purpose. The weight difference and strength changes are given in Table 8.
4.3.3 Sorptivity
Geopolymer cubes (16 molar) were cured for 28 days and after that dried in oven at 100 °C. After that the cubes were taken out and three side of the cubes were coated with colour and epoxy to restrict the flow to one side only. The specimens were then immersed in water not more than 5 mm depth. The water absorption were measured for a span of 30 min in an interval provided in the Table 9. Each time the specimen were taken out and excess water was wiped off with the help of a damped cloth. Those were weighted in weight machine.
I = S.t½, therefore S = I/t½; Where S = sorptivity in mm, t = elapsed time in mint. I (cumulative infiltration) = Δw/Ad Δw = change in weight = W2–W1, where W1 = Oven dry weight of cube in grams, W2 = Weight of cube after 30 min capillary suction of water in grams, A = surface area of the specimen through which water penetrated and d = density of water.
5 Result and Discussion
The previous chapter was about the test done and the results. This chapter discuss about the comparison result and the reason.
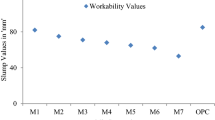
5.1 Workability
Change of workability in term of slump values are represented in the Fig. 8. Slump value of OPC concrete is 120 mm, whereas fly ash concrete displays 125 mm slump. It is due to the spherical particle size of fly ash. On the contrary, the slump value of G10 samples reduces to 90 mm and decreases as molarities of Na2SiO3 in GPC increases. It is attributed to the increase in viscosity of Na2SiO3 increases the cohesiveness of the GPC mix [15].
5.2 Compressive Strength Results
The compressive strength of all mixes at the age of 7, 28 and 56 days are provided in Table 5 and represented in Fig. 9.
OPC concrete and fly ash concrete are found to achieve much better strength up to 56 days. Seven days strength of fly ash concrete is lower than that of OPC concrete due to replacement of 25% fly ash which are not involved in pozzolanic action in early ages. Strength enhancement between 7 days to 56 days in OPC concrete is found to be 41% which is lower than that for fly ash concrete (76%). Fly ash concrete presents higher strength than OPC concrete both at the age of 28 and 56 days and it is due to the additional C–S–H gel produced from pozzolinic reaction [1]. Compressive strength of GPC samples increases with the increase in molarities of NaOH for both the curing conditions. It is observed that the compressive strength of 16 M oven-cured GPC cubes were highest. High-temperature cured GPC specimens show comparatively much more strength as compared to the counterparts which were cured in normal curing tank. Strength enhancement in all GPC sample of both curing conditions between 7 and 56 days are small and about 12%. Strength of temperature-cured GPC with 16 M NaOH is observed to be as per the OPC and fly ash concrete both at the age of 28 and 56 days. Normally cured 16 M GPC samples gains strength up to 30 N/mm2 which satisfy the requirement of M20 concrete.
The change in compressive strength with the change in molarities of NaOH for both the curing conditions is represented in Fig. 10. The reason for the enhancement of strength in both the oven-cured and normal cured specimen with increase in the molarity of NaOH on GPC may be attributed to the availability of more alkalis for geopolymerization. Higher strength in temperature-cured GPC sample than the normal cured counterparts may be attributed to the availability of more reactive alkalis at higher temperature.
5.3 Acid Attack Results
The test result of acid resistance was given in previous Table 7. The test results shows that the 25% fly ash replacement specimen (MOF) had a drastic fall in strength, at about 48%, whereas the strength loss for 10 M, 12 M, 14 M and 16 M specimen are 12%,11.5% 10.2% and 8%, respectively.
Figure 11 shows the change in strength after acid attack and from the graph it is clearly visible that the G16MA shows greater acid resistivity as compared to the other specimens. In Fig. 12, we can see the weight change of the specimen due to the leaching effect of acid. Weight loss in OPC is 5.0%. The graph clearly shows that the weight loss in geopolymer specimens were very less about 0.35% as compared to the MOF specimen. So it may be concluded that geopolymer concrete poses very good resistance against H2SO4 attack.
5.4 Chloride Attack Results
Table 7 shows the test results of chloride attack on different specimen. The test result shows that MOF specimen lost only 1% of its strength where as strength loss of geopolymer concrete cube of 10 M, 12 M, 14 M and 16 M was 5.5%, 3.7%, 3.69% and 3%, respectively. Figure 12 represents nominal changes in strength in both geopolymer specimens as well as MOF specimen.
Figure 11 presents that there is a loss of weight in MPF sample, whereas there is an increase of weight after the immersion into the NaCl solution. It is due to some amount of salt get deposited into pore spaces the specimens. Percentage gain of weight of GPC samples decreases with an increase in the molarity of NaOH due to pore refinement.
5.5 Sorptivity Results
The test results are provided in Table 8 and Fig. 13 represents the variation of soptivity (s)with respect to an increase in time. Sorptivity increases in a rapid manner up to 5 min. It may be due to initial dryness of the specimen and surface porosity. Then the values decreases in irregular manner. It may be attributed to increase in capillary distance towards interior and tortiocity of capillary path.
6 Conclusion
Normal cured GPC displays lower strength in comparison to high-temperature cured GPC and normal cured OPC counterpart and strength increase with the increase in molarities of NaOH. Therefore, it may be recommended for the situations where there is requirement of low strength. Geopolymer concrete shows a very good compressive strength with compare to normal OPC concrete with 25% fly ash replacement. Increase in molarities of NaOH in geopolymer concrete enhances the compressive strength. GPC displays better resistance against acid attack but weak in chloride attack in comparison to OPC counterpart. Increase in molarities of NaOH in geopolymer concrete also enhances the resistance against in acid attack and chloride attack in term of weight and strength loss.
References
Nevelle AM (2012) Properties of concrete, text book on concrete tecnology, 4th Edn. Pearsion Education, p 63
AdbElaty MAA, Ghazy MF, El Hameed MFA (2017) Optimization of geopolymer concrete by principal component analysis. ACI Mater J Techn Pap 253–264
Aslani F (2016) Thermal performance modeling of geopolymer concrete. J Mater Civil Eng 28(1):04015062(1–12)
Mehta A et al. (2017) Influence of various parameters on strength and absorption properties of fly ash based geopolymer concrete designed by Taguchi method. Constr Build Mater 150:817–824
Albitar M, Mohamed MS, Vasintin P, Drechsler M (2017) Durability evaluation of geopolymer and conventional concretes. Constr Build Mater 136:174–185
Singh B, Ishwarya G, Gupta M, Bhattacharyya SK (2015) Geopolymer concrete: a review of some recent developments. Constr Build Mater 85:78–90
Ken PW, Ramli M, Ban CC (2015) An overview on the influence of various factors on the properties of geopolymer concrete derived from industrial by products. Constr Build Mater 77:370–395
Sarkar PK, Kelly S, Yao Z (2014) Effect of fire exposure on cracking, spalling and residual strength of fly ash geopolymer concrete. Mater Des 63(2014):584–592
Chindaprasirt P, Chareerat T, Hatanaka S, Cao T (2011) High strength geopolymer using Fine High calcium Fly ash. J Mater Civ Eng 23(3):264–270
Okoye FN, durgaprasad J, Singh NB (2015) Mechanical properties of alkali activated fly ash/Kaolin based geopolymer Concrete. Constr Build Mater 98:685–691
Noushini A, Castel A (2016) Effect of heat curing on transport properties of low-calcium fly ash based geo-polymer concrete. Constr Build Mater 112:464–477
Gunasekara.C, Law DW, Setunge S (2016) Long term permeation properties of different fly ash geo polymer concretes. Constr Build Mater 124:352–362
Hadi MNS, Al-Azzawi M, Yu T Effect of fly ash characteristic and alkaline activator components on compressive strength of fly ash based geo-polymer mortar
Allahverdi A, Vafaei M, Maghsoodloorad H (2017) Quality control and assessment of geopolymer cements based on reacted and free alkali. Constr Build Mater 153:274–283
Pilehvar S, Cao VD, Szczotok AM, Valentini L, Salvioni D,Magistri M, Pamies R, Kjoniksen AL (2017) Mechanical properties and micro scale changes of geopolymer concrete and Portland cement concrete containing micro-encapsulated phase change. Cement Concrete Res 100:341–349
Xie J, Kayali O (2016) Effector super plasticizer on workability enhancement of Class F and Class C fly ash based geopolymers. Constr Build Mater 122:36–46
Pasupati K,Berndt M,Sanjayan J, Rajeev P (2017) Durability of low-calcium fly ash based geopolymer concrete culvert in a saline environment. Cement Concrete Res 100:297–310
IS 12269-1987, Specification for 53 grade ordinary Portland cement. BIS, New Delhi
IS: 383-1970, Specificationfor coarse and fine aggregates from natural sources for concrete, reaff 1993. BIS, New Delhi
IS:456-2000 Plain and reinforced concrete code of practice. BIS, New Delhi
IS:516-1959 Methods of testing strength of concrete Reaff 1999. BIS, New Delhi
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Dutta, A., Moharana, N.C. (2021). Mechanical and Durability Properties of Fly Ash-Based Geopolymer Concrete. In: Das, B., Barbhuiya, S., Gupta, R., Saha, P. (eds) Recent Developments in Sustainable Infrastructure . Lecture Notes in Civil Engineering, vol 75. Springer, Singapore. https://doi.org/10.1007/978-981-15-4577-1_61
Download citation
DOI: https://doi.org/10.1007/978-981-15-4577-1_61
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-4576-4
Online ISBN: 978-981-15-4577-1
eBook Packages: EngineeringEngineering (R0)