Abstract
Barium titanate (BaTiO3) and Ba1−xNdxTiO3 thin films used by chemical solution deposition method and prepared with spin-coating technique. The thin films of BaTiO3 and Ba1−xNdxTiO3 were grown on a quartz and silicon (Si) substrate. The research aim is to get the best of mole variation of Nd doping to ferroelectric material properties. Doping Nd was varied at 5, 10% in BaTiO3. The thin films were annealed at 850 °C with one hour holding time. The crystal structure, optical and electrical properties of BaTiO3 and Ba1−xNdxTiO3 have been investigated. The microstructure of BaTiO3 and Ba1−xNdxTiO3 were analyzed using XRD equipment, which showed that BaTiO3 and Ba1−xNdxTiO3 crystal had a perovskite shape. The addition mole dopant (x) neodymium (Nd3+), the diffraction angle of the XRD results were seen shifting to the right. As the mole dopant (x) Nd increased, so crystallinity from the Ba1−xNdxTiO3 thin films decreased. The crystallite size of BaTiO3, Ba0.95Nd0.05TiO3, and Ba0.9Nd0.1TiO3 were 70; 21; 28 nm. The addition mole dopant (x) Nd3+ resulted in smaller grain sizes but it did not significantly affect thickness. The greater the addition mole dopant (x) Nd, the smaller the band-gap produced. The results of the IV characterization showed that the Ba1−xNdxTiO3 thin films had a response to light and photovoltaic effects.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
The development of science and technology is increasingly advanced, particularly in Physics in the area of electronics. The growth of thin films is mostly done and applied in the electronics field such as solar cells. Solar cells are the use of solar energy as an alternative energy that is made by converting solar energy into electrical energy. Ferroelectric is a dielectric material that is able to change the electric polarization spontaneously due to the application of an external electric field [1]. The development of ferroelectric thin film research is increasingly becoming a great potential issue since it can be applied to solar cells [2]. The electric field in the ferroelectric solar cells can control and regulate the response of solar cells [3]. When ferroelectric material is applied to solar cells under ultraviolet lighting, this ferroelectric material will have a high output voltage i.e., above 1 kV [2].
BaTiO3 is one of the materials that attract the attention of many researchers because its ferroelectric nature has a high dielectric constant and a large electro-optical coefficient [4]. The BaTiO3 thin film has an ABO3 perovskite structure, where A is located at the corner of the cube, B is located on the diagonal of the cube space, and O is located at the diagonal plane of the cube [5]. Perovskite BaTiO3 has five types of crystal structure including hexagonal, cubic, tetragonal, orthorhombic, and rhombohedral. Each of which depends on the temperature of the phase [6]. BaTiO3 can be made in the form of thin films using several deposition methods such as vacuum and non-vacuum methods. The vacuum method consists of Laser Ablation, Chemical Vapor Deposition (CVD), PVD, Ion Plating [7]. The non-vacuum method consists of Screen Printing, Dip Coating, Electrophoresis, Electrodeposited, Spray Pyrolysis, and Spin Coating [5], sol-gel or Chemical Solution Deposition (CSD) [6]. CSD is a thin-film manufacturing method that combines physical and chemical methods. The steps of making thin films by using CSD method include making solutions, conducting spin coating process with a certain rotational speed, then conducting annealing process [8].
BaTiO3 has a wide gap energy (Eg > 3 eV) [3]. To use the BaTiO3 as solar cell material, it is necessary to reduce the energy gap by giving doping. Materials that can be used for doping administration such as Ni2+, Er3+, Mn4+, Zn2+, Hf4+, Fe2+, Ce4+, W6+, Gd3+, Nd3+, Sr2+ and Co2+ [3]. Atoms in perovskite materials can be doped using other atoms provided in which the radius of the atom is almost the same with the radius of the atom replaced [8]. The addition of neodymium (Nd3+) can replace Ba2+ in the position of perovskite A. This happens since the atomic radius Nd3+ (0.995 Å) is smaller than Ba2+ (1.35 Å) and the radius of Ti4+ (0.68 Å) [8]. The addition mol dopan neodymium (Nd3+) in the making process of BaTiO3 is ion donor (donor doping) that donates excess valence electrons to ion Ba2+ [8]. The doping material given can affect the crystal structure, microstructure, and optical properties of a material [3]. In this paper presented BaTiO3 substituted ionic Nd3+ [8].
In this research, BaTiO3 thin film is doped with (Nd3+) use the chemical solution deposition (CSD) method with the spin coating technique. The solution BaTiO3 and Ba1−xNdxTiO3 was grown on quartz and silicon (Si) substrates deposited using spin coating with a rotational speed of 5000 rpm. The samples annealing process used 850°C with a holding time of 1 h. Samples were characterized using X-Ray Diffraction (XRD) equipment used to determine the microstructure, Scanning Electron Microscopy (SEM) used to determine the grain size and the thickness of the film, UV-Vis used to determine the amount of absorbance and transmittance of thin films, and a Keithley I–V Meter used to determine the amount of current and voltage of thin films.
2 Experiment
The thin films of BaTiO3 and Ba1−xNdxTiO3 used by chemical solution deposition method (CSD). Doping Nd was varied at 5, 10 in BaTiO3. The material used in this research was Barium Acetate [Ba(CH3COO)2] 99.00%, Neodymium Acetate [Nd(CH3COO)3] 99.9%, Acetic Acid [(CH3COOH)] 100%, Titanium Isopropoxide [Ti(OC3H7)4] 97%, Ethylene Glycol [(HOCH2CH2OH)] 100%. There were several steps of making BaTiO3 and Ba1−xNdxTiO3 solutions including preparing tools and materials and weighing ingredients to mixing materials. The solution was deposited on the Quartz and silicon (Si) substrate, prepared with spin-coating technique at a rotational speed of 5000 rpm. The samples were annealed at 850 °C with a holding time of 1 h. The material properties of the samples were identified using XRD equipment for microstructure testing. The XRD equipment had a Lamda Cu (1.5406 Å). Samples were characterized using SEM to determine the morphology and the thickness of the samples. In addition, samples were characterized using UV-Vis spectrophotometer to determine the amount of absorbance and transmittance of thin films. Ultimately, samples were characterized using a Keithley I–V Meter to determine the amount of current and voltage of thin films.
3 Result and Discussion
3.1 Characterization of Microstructure
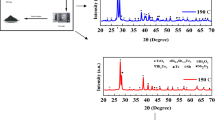
The XRD characterization results was show on Fig. 1. Based on Fig. 1, it can be seen that there are almost no other phases that affect the crystal structure of BaTiO3 thin film and Ba1−xNdxTiO3. In this experiment, increasing mole dopant Nd succeeded in replacing Ba, therefore the diffraction pattern produced by Ba1−xNdxTiO3 was similar to BaTiO3 pattern.
Based on the XRD results, with the addition mole dopant Nd, the diffraction angle is seen shifting to the right. These results are consistent with what has been done by Sandi et al. [9]. The ionic Nd3+ can replace Ba2+ in the perovskite structure. Along with the addition mole dopant Nd, it was also seen that the intensity value produced from XRD was getting higher. In accordance with the research of Iriani et al. the additional mole dopant Nd3+ on the BaTiO3 thin films can increase the intensity of value formed [8].
The calculation of the crystallinity samples can be seen in Table 1. The addition mole dopant Nd causes the crystallinity of the thin film decrease. The crystallinity of the samples is calculated from the magnitude of the intensity of the diffraction peaks formed. The crystallinity of the samples is a picture of the level of regularity of the crystals that are formed in each film.
The crystallite size of BaTiO3 and Ba1−xNdxTiO3 thin films can be calculated using the following Scherrer equation [3]:
where the value of \(\beta\) is the value of FWHM, \(\theta\) is the diffraction angle, \(k\) is the constant of Scherrer which is at 0.9, and \(\lambda\) is the wavelength of X-ray. The calculation of the crystallite size samples can be seen in Table 1.
Based on the additional mole dopant Nd, the result of crystallite size dwindled. In accordance with studies that have been carried out by Gaur et al. that the crystallite size decreases with the additional mole dopant Nd [8]. The result of the crystallite size ranges from 70 to 28 nm.
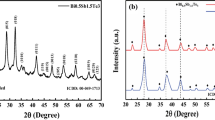
The refined XRD results using the GSAS software can be seen in Fig. 2. This software processes with a refinement process so that the crystal structure and lattice parameters a, b, and c of the thin film can be identified.
The lattice parameter values from the GSAS software refinement results can be seen in Table 3. The result shows that thin film BaTiO3 and Ba1−xNdxTiO3 has a tetragonal structure since it has a lattice parameter value a = b ≠ c and the angle α = β = γ = 90°. The results of refinement Ba1−xNdxTiO3 thin film diffraction pattern with a variation mole dopant Nd, show the alteration of lattice parameter because of dopant Nd3+ into BaTiO3. Decrease in the Ba1−xNdxTiO3 lattice parameter with respect to the initial lattice parameter BaTiO3 caused the diffraction angle of the Ba1−xNdxTiO3 thin film with a variation mole dopant Nd has shifted from the BaTiO3 diffraction angle on ICDD which has a certain field orientation (hkl). It can affect the distance between atoms in the crystal and cause changes in parameters including grid a, b, and c. These results are in accordance with research conducted by Iriani et al. [8]. The changing in lattice parameters in the results of the refinement, thin film diffraction patterns in this study were not very significant (Table 2).
3.2 Characterization of Morphology
The morphology of BaTiO3 and Ba1−xNdxTiO3 thin films with magnitude 80,000× are shown in Fig. 3. Based on the addition mole dopant Nd in the BaTiO3, the porosity of the thin film decreases. This happens since the granules in the thin film have experienced perfect diffusion.
Based on the result of SEM characterization, it is obtained the grain size and the thickness of BaTiO3 and Ba1−xNdxTiO3 that can be seen in Table 4. The grain size of BaTiO3 thin films cannot be calculated since the granules are completely diffused. When there is an increase in the concentration of mole dopant Nd in the thin film of BaTiO3, the grain size decreases. The results of this study are in accordance with the research conducted by Zhang et al. [10]. However, in the sample of Ba0.90Nd0.10TiO3, the grain size of the sample increased. The reason for the morphological change was not determined. It can only be assumed that the grain boundaries in the SEM results are clearly visible so that the number of grain size that can be calculated and the average is increasing.
The thickness was got from the results of cross-section measurements on the BaTiO3 and Ba1−xNdxTiO3 thin film, as presented in Table 4. The thickness of BaTiO3 and Ba1−xNdxTiO3 films affects the addition mole dopant Nd. When BaTiO3 was given an additional mole dopant Nd, the thickness of the film got thinner. However, the more mole concentrations dopant Nd was added, the thickness of the film got thicker. As the results of research from Iriani et al. [8]. The thickness of Ba0.95Nd0.05TiO3 is 300 nm, Ba0.90Nd0.10TiO3 is 335 nm, and the thickness of BaTiO3 pure is 633 nm.
3.3 Characterization of Optical Properties
The calculation of band-gap is using the method of tauch-plot with the following equation:
where \(h\upsilon\) is the photon energy, \(A\) is a constant, \(\alpha\) is the coefficient of absorption, and \(E_{g}\) is the optical band-gap [3].
The transmittance spectrum of quartz substrate grown by BaTiO3 and Ba1−xNdxTiO3 is presented in Fig. 4. It can be seen that the higher the concentration mole dopant Nd is given, the lower the peak transmittance value. Based on Fig. 4, it is found that the thin film of BaTiO3 reaches the highest transmittance value. The amount of this transmittance value is inversely proportional to the value of the absorbance value.
The transmittance value of BaTiO3 thin film in the visible light area is greater if mole dopant Nd is increased. The addition mole dopant Nd can increase the absorption of visible light in the thin film of BaTiO3. The transmittance of the Ba1−xNdxTiO3 film decreases with the decreasing wavelengths to a certain extent. Waves of absorption from thin films Ba1−xNdxTiO3 are bigger if it is compared to BaTiO3. This result is in accordance with the research by Fu et al. [11].
Based on UV-Vis and cross-section test results, the band-gap value is obtained. The band-gap values of the BaTiO3 and Ba1−xNdxTiO3 thin films are presented in Table 5. The results show that the band-gap of BaTiO3 thin films is greater than Ba1−xNdxTiO3. This happens since the addition mole dopant Nd can reduce the band-gap of the thin film. In this study, the Nd3+ ionic replaces the Ba2+ ion on the A-side so that it can cause the narrowing of the conduction band-gap. This is in accordance with the research of Jiang et al. [3]. In this study, the value of the band-gap of the thin film of BaTiO3 and Ba1−xNdxTiO3 is around 3.00–3.64 eV for the overall concentration of mole.
3.4 Characterization of Electrical Properties
The characterization of electrical properties (current–voltage) from BaTiO3 and (Ba1−xNdxTiO3) thin films was got by varying mole dopant Nd3+ using Keithley I–V meters. Figure 5 shows the current and voltage relationship curves of BaTiO3 and Ba1−xNdxTiO3 films in dark and light conditions which indicate that the thin film has diode characteristics. The curve shows that in the Ba1−xNdxTiO3 film with Si substrate (p-type semiconductor), a connection has occurred. This curve shows that there is an induced current flow (photocurrent) so that the Ba1−xNdxTiO3 film shows the response to the light. The Ba1−xNdxTiO3 film when it is irradiated (in light conditions) has more conductive properties if it is compared to that when it is in dark conditions due to the absorption of photon energy. The result of the photon energy charge can be separated and collected by the cathode and anode. The results of this study are in accordance with the research conducted by Wang et al. [12]. The amount of Isc and Voc value from BaTiO3 and Ba1−xNdxTiO3 film are shown in Table 6. Based on Table 6, it can be seen that the additional mole dopant Nd3+ can increase the value of Isc and Voc. The results are in accordance with the research conducted by Jiang et al. [3].
4 Conclusions
The addition of mole dopant Nd3+ on BaTiO3 caused the change of angel diffraction. The diffraction angle were seen shifting to the right. As the mole dopant Nd increased, so crystallinity from the Ba1−xNdxTiO3 thin films decreased. The crystallite size of BaTiO3, Ba0.95Nd0.05TiO3, and Ba0.9Nd0.1TiO3 were 70; 21; 28 nm. Result of refine with GSAS software show the dopan Nd have been included in the structure BaTiO3. The addition mole dopant Nd3+ resulted in smaller grain sizes but it did not significantly affect the thickness. The greater the addition mole dopant Nd, the smaller the band-gap produced. Moreover, the results of the IV characterization show that the Ba1−xNdxTiO3 thin films have a response to the light and the photovoltaic effects.
References
Iriani Y, Yasin SR (2018) Fabrication of barium titanate doped strontium using coprecipitation method. IOP Conf Ser: Mater Sci Eng 333:012047
Cai W, Fu CL, Lin ZB, Deng XL (2011) Vanadium doping effects on microstructure and dielectric properties of barium titanate. Ceram Int 37:3643–3650
Jiang W, Cai W, Lin Z, Fu C (2013) Effects of Nd-doping on optical and photovoltaic properties of barium titanate thin films prepared by sol–gel method. J Mater Res Bull 48:3092–3097
Kumbhar SS, Mahadik MA, Chougule PK, Mohite VS, Hunge YM, Rajpure KY, Moholkar AV, Bhosale CH (2015) Structural and electrical properties of barium titanate (BaTiO3) thin films obtained by spray pyrolysis method. Mater Sci Poland 33(4):852–861
Vijatovic MM, Bobic JD, Stojanovic BD (2008) History and challenges of barium titanate: Part II. Sci Sinter 40:155–165
Li W, Xu Z, Chu R, Fu P, Hao J (2009) Structure and electrical properties of BaTiO3 prepared by sol–gel process. J Alloy Compd 482:137–140
Irzaman PY, Apipah ER, Noor I, Alkadri A (2015) Characterization of optical and structural of lanthanum doped LiTaO3 thin films. Integr Ferroelectr 167:137–145
Iriani Y, Jamaludin A, Nurhadi N (2016) Deposition barium titanate (BaTiO3) doped lanthanum with chemical solution deposition. J Phys: Conf Ser 776:012064
Sandi DK, Supriyanto A, Jamaluddin A, Iriani Y (2016) The influences of mole composition of strontium (x) on properties of barium strontium titanate (Ba1-xSrxTiO3) prepared by solid state reaction method. Am Inst Phys Conf Proc 1710:030006.1–030006.4
Zhang W, Cao L, Wang W, Su G, Liu W (2013) Effects of neodymium doping on dielectric and optical properties of Ba(1-x)NdxTi1.005O3 ceramics. Ceram Silik 2:146–150
Fu C, Cai W, Lin Z, Jiang W (2014) Photovoltaic effects of bismuth ferrite and Nd-doped barium titanate thin films prepared by sol–gel method. Mater Sci Forum 787:347–351
Wang W, Liu F, Lau CM, Wang L, Yang G, Zheng D (2014) Field-effect BaTiO3-Si solar cells. Appl Phys Lett 104:123901
Acknowledgements
The authors wish to thank Kementrian Riset, Teknologi and Pendidikan Tinggi for financial support through Penelitian Dasar 2019 No 719/UN27.21/PP/2019.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Rini, R.P., Setyadi, A.U.L.S., Nurosyid, F., Iriani, Y. (2020). Microstructure, Optical, and Electrical Properties of Barium Titanate (BaTiO3) and Ba1−xNdxTiO3 Thin Films Deposited by Chemical Solution Deposition (CSD) Method. In: Sabino, U., Imaduddin, F., Prabowo, A. (eds) Proceedings of the 6th International Conference and Exhibition on Sustainable Energy and Advanced Materials. Lecture Notes in Mechanical Engineering. Springer, Singapore. https://doi.org/10.1007/978-981-15-4481-1_76
Download citation
DOI: https://doi.org/10.1007/978-981-15-4481-1_76
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-4480-4
Online ISBN: 978-981-15-4481-1
eBook Packages: EngineeringEngineering (R0)