Abstract
Supramolecular hybrid inorganic nanocomposites, as a burgeoning type of hybrid nanomaterials, have been prepared by anchoring macrocyclic organic molecules and supramolecules onto inorganic nanoscaffolds. Macrocyclic organic molecules, such as crown ethers, cryptands, calixarenes, cucurbiturils, pillararene, and cyclodextrins, have frequently been used as building blocks for supramolecular hybrid inorganic materials. These macrocyclic molecules anchoring onto the surface of inorganic nanomaterials particularly act as the valid host molecules that one or more “guest” molecules can bind to a “host” cavity reversibly. Among the various macrocyclic molecules, native and modified cyclodextrins (CDs) have long been recognized as the host molecules with inherent hydrophobic internal cavity and hydrophilic external surface in host-guest chemistry ; therefore, much attention of CDs has attracted in the construction of supramolecular hybrid inorganic nanomaterials.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
1 Introduction
Supramolecular hybrid inorganic nanocomposites, as a burgeoning type of hybrid nanomaterials, have been prepared by anchoring macrocyclic organic molecules and supramolecules onto inorganic nanoscaffolds. Macrocyclic organic molecules, such as crown ethers, cryptands, calixarenes, cucurbiturils, pillararenes, and cyclodextrins, have frequently been used as building blocks for supramolecular hybrid inorganic materials. These macrocyclic molecules anchoring onto the surface of inorganic nanomaterials particularly act as the valid host molecules that one or more “guest” molecules can bind to a “host” cavity reversibly. Among the various macrocyclic molecules, native and modified cyclodextrins (CDs) have long been recognized as the host molecules with inherent hydrophobic internal cavity and hydrophilic external surface in host-guest chemistry [1, 2]; therefore, much attention of CDs has attracted in the construction of supramolecular hybrid inorganic nanomaterials.
Cyclodextrins (CDs), readily available α-, β-, and γ-CDs, are cyclic oligosaccharides comprising six, seven, or eight D-(+)-glucopyranosyl units linked by α-1, 4-glycosidic bonds. α-, β-, and γ-CD molecules are shaped like cones with the primary hydroxyl groups from the narrow edge and secondary groups extending from the wider edge, which gives CD molecules a relatively hydrophobic central cavity and a hydrophilic outer surface. Moreover, CDs can be easily chemically modified to selectively introduce functionality on the primary and secondary faces [3]. Due to readily available, harmless, and capable to form sophisticated molecular and supramolecular structures, the CDs have been attracting immense attention from the scientific community for decades. This interest toward CDs is additionally strongly motivated by their numerous potential applications.
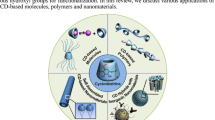
Cyclodextrin hybrid inorganic nanocomposites have made the combination of inorganic nanomaterials as solid supports and CDs used as the surface functional groups, leading the development of hybrid materials with improved functionalities and evolving many applications in chemical, environmental, and biological fields [4,5,6]. In this chapter, we shortly present the developments carried out in the preparation of cyclodextrin-contained carbon nanomaterials, magnetic nanomaterials, semiconductor quantum dots, and noble metal nanoparticles as well as their applications as molecular recognition sensor, adsorbent, and drug nanocarriers (Fig. 1).
2 Cyclodextrin-Functionalized Carbon Nanomaterials
Carbon nanomaterials (CNMs), with graphite being the parent structure, are found in different dimensionality including zero-dimensional fullerenes, one-dimensional carbon nanotubes (CNTs), two-dimensional graphene-family materials (GFMs) (i.e., graphene sheet, graphene oxide (GO), reduced graphene oxide (rGO), and graphene quantum dots (GQDs)), and three-dimensional graphene materials. Due to their unique electronic, optical, thermal, mechanical, and chemical properties, CNMs have attracted particular attention in many research fields. The properties of CNMs are strongly dependent on their atomic structures and interactions with other materials in the nanometer-scale dimensions. Therefore, the development of the CNMs’ chemical modification has led to the discovery of new applied materials. A wide variety of different chemical modification of CNMs, such as graphene, CNTs, and GQDs, were achieved via covalent and non-covalent approaches [7]. Through the optimization of chemical modifications with small molecules and biomolecules and the coupling with other nanomaterials (i.e., luminescent and magnetic), carbon nanomaterials have emerged as a platform for the preparation of sophisticated multifunctional smart materials capable of molecular recognition, adsorbents, and drug delivery, including cancer treatments.
Cyclodextrins (CDs), as the supramolecules with good water solubility and high biocompatibility, have been found to be ideal candidates to be conjugated to CNMs, toward combining their most promising features. Linking CDs to CNMs is considered to enable the introduction of some desired activity, and new features to the resultant nanostructures, as well as the improvement of the physicochemical or biological properties of pristine CNMs. The number of articles within this area of research is still growing, and new interesting materials are obtained. This part focuses on recent advances in the synthesis and application of the conjugates of CNMs and CDs in sensors, adsorption, and drug delivery.
2.1 Molecular Recognition by Electrochemical and Luminescent Sensors
CNMs can be considered as promising materials to be used for molecular recognition by electrochemical and luminescent sensors. A significant number of unsaturated π-bonds in their structure lead to a high electrical conductivity of CNMs. Enabling and enhancing the electron transfer process between an electrode and a given molecule is the principle of such applications of CNMs. It is considered that the enhancement in properties of CNMs toward electrochemical detection of the molecules can be achieved via incorporation of cyclodextrin units into the material. The CDs promote the supramolecular recognition of various molecules. Hence, the considered reason of improved performance of the CD-bearing CNM sensors is the synergistic effect between the adsorption of the detected molecules on the surface of the CNMs and the formation of the inclusion complexes with CDs.
Electrochemical sensing that uses conjugates of CNMs with CDs constitutes an emerging and widely explored area of molecular recognition research. Graphene oxide is a material characterized by a single graphene sheet, or “monolayer,” that displays unique electronic and mechanical properties that are advantageous for sensing applications. Fabrications of the sensors based on CD-modified two-dimensional GO or rGO covered both covalent and non-covalent approaches. From the synthetic point of view, the presence of oxygen groups on the surface of GO or rGO opens avenues for an enhanced hydrogen-bonding-dependent self-assembly phenomenon with the inclusion of CD units, as well as for covalent functionalization via, e.g., amide or ester bond formation. More works referred to the experimental procedure reported by Guo et al. (Fig. 2) [8] suggested the formation of the non-covalent β-CD/rGO material via the hydrogen-bonding-dependent adsorption of CDs on the surface of rGOs. Application of non-covalent CD/GO and CD/rGO materials as versatile electrochemical sensors can be clearly claimed, because these constructs were used for the detection of various molecules. Bioactive species [8, 9], drugs [8, 9], and organic pollutants [10, 11] were detected with excellent LOD values. Also the three-dimensional rGO as the bearing material has been used to conjugate with CD for fabricating an electrochemical sensor [9, 12].
The procedure for preparing CD-graphene organic-inorganic hybrid nanosheets and GNs, and sensing the guest molecules by an electrochemical strategy [8]. (Copyright 2010 American Chemical Society)
Meanwhile, carbon nanotubes, with unique tubular shape, possess encouraging electronic properties, such as good electrical conductivity and broad electrochemical potential window, and MWCNTs perform more excellent electronic properties in comparison to SWCNTs. CD/MWCNT sensors were successfully applied for the electrochemical determination of organic pollutants [13] and various biomolecules [14, 15]. Recently, construction of the hybrid carbon platforms composed of more than one graphene nanostructure, as cyclodextrin-bearing materials for electrochemical sensing, was also studied. Second carbon material [16], metallic nanoparticles [17] and metal oxide nanoparticles [18] have been introduced to the carbon platform for electrochemical sensing. Introduction of the second carbon material to a carbon platform was found to increase the number of CD units in the nanostructure, because of enhancing hydrogen-bonding interactions. And the hybrid with metallic nanoparticles and metal oxide nanoparticles improved the electrical conductivity and catalytic performance of CD-conjugated CNMs electrochemical sensor.
Due to the CD cavity’s unique hydrophobic character and inherent chirality, electrochemical sensing of CD-conjugated carbon nanomaterials for chiral recognition has been widely investigated [12, 19,20,21,22,23]. The cyclodextrin-conjugated graphene quantum dots β-CD/GQDs have been applied for the electrochemical detection of amino enantiomers [19, 20]. In another case, the graphene-β-cyclodextrin-nanocomposite-modified carbon paste electrode (GNS-β-CD-CPE) was developed to detect moxifloxacin hydrochloride (MOX) enantiomers by adsorptive stripping differential pulse voltammetric (AdSDPV) technique [23]. In very recent works, self-assemblies of Cu2+-modified β-cyclodextrin on poly-L-arginine/multi-walled carbon nanotubes (Cu-β-CD/PLA/MWCNTs) [21] and three-dimensional graphene with hydroxypropyl- β-cyclodextrin (3D-G/HP-β-CD) [12] have been constructed as the effective sensors for chiral recognition of tryptophan (Trp) enantiomers (Fig. 3). Both two developed sensors demonstrate excellent selectivity and applicability for the quantification of Trp in real blood samples.
Luminescence spectrometry is another valid method to explore the molecular recognition by CD-conjugated GNMs. Graphene has a higher quenching efficiency compared to other quenching agents, due to its enhanced electrical conductivity and two-dimensional planar structure, and therefore is a great energy transfer acceptor for FRET-based luminescent sensors [24, 25]. In one example, graphene oxide was used as a sensing platform for the detection of amantadine as demonstrated by Li et al. [24]. This probe was based on the host-guest interaction of mono-[6-(2-aminoethylamino)-6-deoxy]-β-cyclodextrin (EDA-CD)-functionalized graphene oxide with amantadine and rhodamine. In the absence of analyte, the emission of Rhodamine 6G was quenched by addition to the cyclodextrin-modified graphene oxide layer; however, introduction of amantadine, a pharmaceutical agent, displaced Rhodamine 6G from the surface and led to significant fluorescence increases. Furthermore, Mondal et al. also used a graphene-bound β-cyclodextrin as a cholesterol sensor based on the competitive host-guest interaction between Rhodamine 6G and cholesterol [26]. The emission of Rhodamine 6G was quenched by encapsulation in the cavity of the graphene-bound β-cyclodextrin. Cholesterol selectively displaced the Rhodamine 6G, freeing it from the quenching agent and turning the fluorescence back on. Carbon dots (CDots) or graphene quantum dots (GQDs), which are quasi one-dimensional graphene particles, have also been widely used for molecular recognition by luminescent sensors [27,28,29]. For example, the α-CD-modified CDots have been used in molecular recognition of derivatives of methyl viologen (MV2+), due to the MV2+-induced assembly of CDots via the host-guest approach [28]. The electron transfer processes between CDots and MV2+ have been explored carefully by time resolved fluorescence decay and transient absorption spectroscopy.
2.2 Carbon Materials Functionalized with Cyclodextrins as Adsorbents
The high surface area of the carbon nanomaterials as well as their ability to bind various organics via π-π stacking not only can be utilized toward the above-discussed electrochemical and luminescent detection but also can be played in adsorption. The main examples of such application of CNMs, primarily for graphene-family nanomaterials and carbon nanotubes, are in water treatment, which is associated with removing organic pollutants from aqueous solutions. Moreover, it has been well established that because of the unique properties of CDs toward the formation of inclusion complexes, these molecules can be employed to further enhance the sorption capacity of the resultant CNMs adsorbent. Therefore, a new trend to fabricate CD-decorated CNMs adsorbents can be regarded as the promising way in the development of applied materials for water treatment.
In general, the chemical energy of the covalent bond is higher in comparison to non-covalent interactions, and the stabilization of material structure could be ensured by covalent bonds during the adsorption process. Therefore, in contrast to electrochemical sensors of the conjugates of CDs and CNMs, covalent approaches were mainly employed for preparing CD-CNMs as the adsorbents. β-Cyclodextrin grafted on the surface of multi-walled carbon nanotubes exhibits a high adsorption in the removal of polychlorinated biphenyls (PCBs) from aqueous solution [30]. A cross-linked nanoporous polymer containing CD and carbon nanotube (CNT) has shown excellent adsorption capacity for p-nitrophenol which can be removed as much as 99% from a 10 mg/L spiked water sample; in contrast, activated carbon removed only 47% [31].
Recently, construction of the magnetic hybrid carbon platforms as cyclodextrin-bearing materials for adsorption was studied widely. Because of the superparamagnetism of magnetic nanoparticles, the magnetic adsorbents (containing Fe3O4 [32, 33] or Fe NPs [34] in the structure) were found to increase recycling ability with facilitating separation by external magnetic field. The presence of CD units in the resultant adsorbent can be considered as a crucial key point to enhance the stability of the material and to reduce the rate of the aggregation/agglomeration process, together with triggering some desired effects toward binding the adsorbate, such as inclusion complex formation.
2.3 Cyclodextrin-Functionalized Carbon Materials for Drug Delivery
Carbon nanomaterials have also been studied as drug delivery systems (DDS) . The properties of CNMs, such as low toxicity, high surface area, and unique mechanical and electrical properties, make CNMs promising drug or gene delivery vectors. Incorporation of CD units into a CNMs nanoplatform is considered to enhance the nanomaterial’s biological features, increase the number of drug molecules in the structure, and improve the stability and hydrophilicity of the material. All these features lay the basis for the design of effective DDS based on CD-conjugated CNMs.
In recent years, CNM-based targeted DDS with the inclusion of CDs has been explored primarily for GO [35] and MWCNTs [36] via covalent or non-covalent approaches. Their biocompatibility, dispersion stability, drug loading, and releasing properties were mainly studied. It is noteworthy that several drugs as the model drugs, like doxorubicin, [35] camptothecin [35], and guanine-based drugs [36], have been investigated to explore the possibility of drug loading and releasing. This means that the CD units incorporated into the CNMs retain their unique properties toward the formation of inclusion complexes with therapeutics. Also, the release profile of the drug was also studied, and controlled drug release at different pH values was presented. These studies can be considered as the initial research works on such systems toward their further bio-applications.
An emerging and still developing area of research in the field of nanomedicine is the creation of targeted and control released DDS. This so-called active targeting is achieved via incorporation of targeting ligands (TLs), such as antibodies or appropriate vitamins, into the structure of the nanomaterial [37]. The principle of targeted delivery involves the selective binding of the therapeutic system to a specific molecular target, such as tumor receptors. Active targeting was achieved via linking the nanoplatform with folic acid (FA) [38, 39], hyaluronic acid (HA) [40, 41], biotin [42], monoclonal antibodies (MoAb) [43], or nucleic acids [44]. In most cases, doxorubicin (DOX) [38, 41, 45] was used as a model drug; however, the systems for the delivery of camptothecin (CPT) [40], epirubicin (EPI) [45], or 5-fluorouracil (5-FU) [46] were also studied. In general, for CD-conjugated carbon nanomaterials DDS, the drug release behavior could be controlled by pH-dependent and photothermal stimulation. The host-guest interaction and hydrogen bonding between cyclodextrin and drug molecules dominate the pH-responsive drug release from the DDS. Because of the environment of cancer cells is more acidic than that for healthy cells, the pH-responsive drug release phenomenon together with the active targeting process enables an increase in the therapeutic’s pharmaceutical availability and the reduction of its toxicity. For example, the release ability of doxorubicin (DOX) from a β-CD-modified rGO nanosheet does not exceed 20% under physiological conditions (pH 7.4, 37 °C), but over 60% of DOX is released in an acidic environment (pH 5.3, 37 °C) [45]. In addition, it is noteworthy that the photothermal-stimulated drug releases that utilize the wonderful photothermal conversion performance of carbon nanomaterial have been provided a valid approach to improve the effect of photothermal therapy [39, 41].
Liu and co-workers constructed a tumor-targeted delivery system for CPT based on the inclusion complexation of hyaluronated adamantane (HA-ADA) with β-CD-functionalized graphene oxide (GO-CD) (Fig. 4) [40]. The ternary supramolecular nanomedicine (CPT@GO-CD–HA-ADA) exhibited a higher curative effect and a lower cytotoxicity than free CPT. The β-CD/ADA inclusion complex prevented the GO skeletons from intermolecular aggregation, and the resultant uniform and small-sized GO nanosheets promoted the targeted receptor-mediated internalization of the biocompatible supramolecular complex by cells. Another multifunctional-targeted drug delivery platform formed from CDHA-MGO (Fe3O4-reduced graphene oxide covalently modified with β-cyclodextrin-hyaluronic acid polymers) was contributed by Shuang and co-workers [41]. The obtained CDHA-MGO nanocomposite has good water dispersibility, easy magnetic separation, high near-infrared (NIR) photothermal heating, and excellent biocompatibility. The β-cyclodextrin-hyaluronic acid polymers efficaciously enhance the doxorubicin (DOX) loading amount up to 485.43 mg/g. Meanwhile, the Fe3O4-graphene oxide provides a facile photothermal response mechanism to handle the NIR-triggered release of DOX in weak acidic solvent environments. All the mentioned results clearly demonstrate the applicable potential of the developed nanoplatforms and lay the basis to further exploit these nanoconstructs as novel systems dedicated to targeted anticancer therapies in humans.
Construction of CPT@GO-CD-HA-ADA supramolecular assembly [40]. (Copyright 2014 Royal Society of Chemistry)
3 Cyclodextrin-Functionalized Magnetic Nanoparticles
In the last decades, many types of magnetic nanoparticles (MNPs) can be synthesized, including iron oxides and iron sulfide (Fe2O3, Fe3O4, and Fe3S4), as well as cobalt, manganese, nickel, and magnesium ferrites. Among them, Fe3O4, which is a ferromagnetic black color iron oxide of both Fe(II) and Fe(III), has been the most extensively studied. Many applications of magnetic nanoparticles rely on the use of magnetic fields to manipulate their properties, which depends on the effectiveness of the particle magnetic moment and the field gradient. However, most of these nanomaterials easily oxidize in the air atmosphere and show low stability in acidic media, which may lead to changes in their magnetic properties. Therefore, the synthesized MNPs are typically coated to improve their stability and dispersibility in water and to provide chemical functionality for the addition of bioactive molecules. Using proper coating can also minimize precipitation and the formation of agglomerates and prolong the circulation time in further applications.
Cyclodextrins (CDs) and their derivatives are well-known to form complexes with a large variety of organic molecules in aqueous solution by host-guest interaction, and thereby increasing both the water solubility and the stability of such molecules. The attachment to MNPs of CDs, able to form inclusion complexations with guest molecules, gives rise to MNPs with host-guest abilities without altering their original properties. Furthermore, the modification of CDs and their derivatives has dramatically enhanced the solubility, stability, and biocompatibility of the MNPs. Among the most used CD coating methods are in situ coating, post-synthesis adsorption, or post-synthesis grafting. Covalent anchoring was found to enhance the stability of CD-MNPs compared with adsorption coating [47, 48]. Vast cases in modification of CD in the surface of MNPs followed the layer-by-layer (LBL) technique [49,50,51,52,53,54,55]. For example, the surface of magnetic particles was modified with 3-aminpropyltriethoxysilane (APTES) firstly. The next step was the covalent bonding of mono-tosyl-CD onto the surface of modified iron NPs by means of layer-by-layer process [49,50,51,52]. Also, the one-pot route has been exploited to the preparation of CD-stabilized MNPs [56, 57]. Functionalized MNPs hold great potential in environmental, biomedical, and clinical applications owing to their many unique properties, such as larger surface area, and high permeability. In this part, we focus on the recent advances in the construction of CD-functionalized MNPs and their applications in identification-detection, adsorption-separation, and drug delivery.
3.1 Molecular Identification-Detection by Electrochemical and Luminescent Sensors
MNPs have captured many interests in chemosensors for identification and detection in terms of their high surface area, strong magnetic responsivity, electrical properties, and high adsorption ability [58]. Cyclodextrins (CDs), as a group of naturally cyclic oligosaccharides with a hydrophobic inner cavity and a hydrophilic exterior, may bind selectively various organic, inorganic, and biological molecules to form complexes. Therefore, CD-coated MNPs have been investigated as promising method for ion and molecular recognition by electrochemical sensing and spectral analysis [59,60,61,62].
Electrochemical sensing that uses conjugates of MNPs with CDs constitutes an emerging and widely explored area of molecular recognition research [52, 59,60,61]. Some bioactive species, like uric acid [49], dopamine [59], DNA [60], and amino acids [52, 61], have been detected with excellent LOD values. Furthermore, due to the CD cavity’s inherent chirality, electrochemical sensing of CD-MNPs for chiral recognition has been also investigated. For instance, Muñoz et al. synthesized a novel and specific material based on thiolated β-cyclodextrin-coated gold nanoparticle functionalized with cobalt ferrite magnetic nanoparticles (β-CDSH/Au/CoFe2O4-NPs) for modifying on graphene-paste electrode (Fig. 5) [61]. The β-CD-SH/Au/CoFe2O4-NP-modified graphene-paste electrode as a chiral sensor has been validated by chirally recognizing tryptophan (TRP) enantiomers, showing a good selectivity and sensitivity.
Schematic illustration of (a) the preparation of chiral mNBF; (b) the enantiorecognition assay for L- and D-TRP discrimination at the electrochemical cell via cyclic voltammetry. (Reproduced from Ref. 61, 2018 Elsevier B.V)
Spectrometry is another valid method to investigate the ion recognition by CD-functionalized MNPs [53, 62]. Through the combination of the host-guest interaction and sol-gel grafting approach, a fluorescent TSRh6G-β-cyclodextrin fluorophore/adamantane-functionalized magnetic nanoparticles (TFIC MNPs) has been constructed as a fluorescence sensing platform for Hg2+ recognition (Fig. 6) [62]. The addition of Hg2+ induced a remarkable emission enhancement of TFIC MNPs as a “turn-on” type fluorescence sensor and resulted in an obvious color change of the sample solution from light brown to pink. Due to the larger surface area and high permeability, the CD hybrid nanomaterial TFIC MNPs displayed significant selectivity and sensitivity for Hg2+ over other metal ions tested in aqueous solution. Otherwise, the TFIC MNPs could be separated and collected Hg2+ via external magnetic field, which can be used as an efficient adsorbent for the removal of trace Hg2+ from the aqueous environments.
The illustration of the synthesis of TFIC MNP fluorescent sensors for Hg2+ [62]. (Copyright 2013, Royal Society of Chemistry)
3.2 Cyclodextrin-Functionalized Magnetic Materials for Adsorption and Separation
CD-MNP-based nano-adsorbents have played an important role in the adsorption and separation of bioactive species, drugs, and inorganic or organic pollutants from several solution media. There are several advantages of CD-MNP adsorbents, such as (I) easy preparation and eco-friendliness, (II) low-cost and low-reagent consumption, (III) high selectivity and easy manipulation with an external magnetic field, (IV) being reusable adsorbents, and (V) high pre-concentration factors and combination with other modern detection techniques in online or offline mode. Therefore, a new trend to fabricate CD-decorated MNPs adsorbents can be regarded as the promising way in the development of applied materials for water treatment and biomedicine.
For CD-functionalized MNPs, the use of these materials for the abatement of environmental toxicants has been increasing continuously. The heavy metal ions [56, 63,64,65], radionuclides [54, 66], and organic molecules [51, 67,68,69] have been adsorbed and separated from the aqueous solutions. For example, the carboxymethyl-β-cyclodextrin (CM-β-CD)-modified Fe3O4 nanoparticles (CMCD-MNPs) for removal of copper ions from aqueous solution have been constructed by grafting CM-β-CD onto the magnetite surface using the carbodiimide method [63]. The results showed that the grafted CM-β-CD on the Fe3O4 nanoparticles enhanced the particle adsorption capacity owing to the strong ability of the multiple hydroxyl and carboxyl groups in CM-β-CD to adsorb metal ions. Another work revealed that β-CD-Fe3S4 NPs exhibited an enhanced and selective removal capacity toward Pb(II) in comparison with bare Fe3S4 NPs [56]. This research demonstrated that β-CD-stabilized Fe3S4 NPs can be a potential material for Pb(II) removal from wastewater. In another two works, the Fe3O4@CD MCs [66] and succinyl-β-cyclodextrin-APTES@Fe2O3 [54] were synthesized for the removal of Eu(III) and U(VI) from aqueous phase, respectively. In comparison to naked MNPs, the CD-modified MNPs demonstrated a higher adsorption capacity toward Eu(III) and U(VI), and these two adsorbents exhibit the pH dependence to radionuclides adsorption. In our recent work, the β-CD-modified Fe3O4 nanoparticles have been synthesized by layer-by-layer approach and used to adsorb methylene blue in aqueous solution [51]. In addition, carboxymethyl-β-cyclodextrin polymer-coated Fe3O4 nanoparticles (CDP-MNPs) have been used to remove the phenolic pollutants from water [68]. These researches demonstrated that the CDs and CD polymer-coated Fe3O4 nanoparticles could be used as the promising adsorbents for the elimination of organic pollutants from wastewater by magnetic separation technology.
The high surface area of the CD-coated magnetic nanomaterials (CD-MNMs) as well as their ability to separate with an external magnetic field not only can be utilized toward the above-discussed water treatment, but also CD-MNMs can act as the adsorbents for drugs [55] and bioactive species [70, 71] separation. Due to the CD cavity’s unique hydrophobic character and inherent chirality, chiral separation of CD-conjugated MNPs for drugs and bioactive species has been widely investigated [33, 57, 72, 73]. For instance, the β-CD-functionalized Fe3O4 nanospheres have been provided the ability to chirally discriminate amino acids enantiomers while serving as magnetic separators for effectively separating the isomers of different amino acids, especially for the tryptophan (Fig. 7) [57].
The illustration of preparation procedures and separation mechanism of β-CD-modified Fe3O4 nanoparticles for amino acids isomers [57]. (Copyright 2011, Royal Society of Chemistry)
3.3 Cyclodextrin-Functionalized Magnetic Materials for Drug Delivery
Magnetic nanoparticles are promising supramolecular chemotherapeutic drug carriers because they can deliver anticancer drugs more selectively to the target site under the guidance of an external magnetic field and hence abate the lesions in tissues precisely. The CD-modified iron oxide nanoparticles are attractive drug carriers by virtue of their biocompatibility, biodegradability, aqueous dispersibility, and magnetizability. The ability of CD-coated MNPs to function as nanocarriers has been demonstrated in several cases [50, 74,75,76].
A size-controllable supramolecular magnetic nanoparticles (SMNPs) have been prepared by utilizing a supramolecular system based on the host-guest interaction between β-CD and Ad with magnetic nanoparticles [75]. The DOX encapsulated on the DOX@SMNPs can be controlled release by quickly generating thermal energy when applying an external alternative magnetic field (AMF). The approximately 50% of DOX was released by applying an AMF only 2 min and effectively inhibited the tumor cell, which significantly decreasing the side effects compared to normal protocols. This result indicated that the drug dosage for cancer treatment can be significantly reduced by a magnetic field-controlled accurate. Level of drug concentration delivery to a tumor.
Shuang and co-workers constructed a pH-responsive controlled release system based on β-cyclodextrin-assembled magnetic Fe3O4 nanoparticles (β-CD-MNPs) for stereoisomeric doxorubicin (DOX) and epirubicin (EPI) delivery [50]. The loading behaviors of β-CD-MNPs for both DOX and EPI followed multilayer Freundlich isotherm adsorption, and the loading amounts are 70.27 mg/g (DOX) and 39.46 mg/g (EPI), respectively, owing to the different conformation of inclusion complexes. The β-CD-MNPs/DOX reveals higher and faster release in vitro and stronger resolution in cell than that of β-CD-MNPs/EPI. These findings could be extended to the design of the delivery of other stereoisomeric drugs, which may afford further guidance for their loading and release in biomedical applications.
4 Cyclodextrin-Functionalized Semiconductor Quantum Dots
Semiconductor quantum dots (QDs) exhibit unique optical and photophysical properties and represent one of the major advances in materials science in the last two decades. Because of QDs offer some advantages compared with conventional chromophores, such as broad absorption, narrow emission lines, low photobleaching, long lifetimes, and high quantum yields, QDs have been widely used as fluorescence probes in analytical chemistry, biology, and medicine. For application of QDs in sensor or in biological imaging, the QDs must be dispersible in aqueous solution and luminous with higher fluorescence efficiency. Thus, the synthesis and application of water-soluble QDs with controlled size and surface functionality should be explored and developed [77]. Cyclodextrins (CDs), as a representative supermolecule host, can form a size-modulated host-guest inclusion complex in the cavity and provide an external selectivity tuned by the functionalities on the rim. The CDs can be used as an ideal functional molecule to improve the solubility, stability, and biocompatibility of QDs. The combination of the luminescence properties of QDs and the molecular recognition ability of CDs has provided highly sensitive and selective sensing platforms for various targets. In this part, we focus on the recent advances in the construction of CD-functionalized QDs and their applications in molecular recognition [78, 79].
4.1 CD-Functionalized QDs as an Achiral Recognition Sensing Platforms
Luminescence sensing that uses conjugates of QDs with CDs constitutes an emerging and widely explored area of general ion or molecular recognition research [80,81,82,83,84]. Li et al. reported a kind of CD-modified CdSe/ZnS QDs, which have been prepared by using ultrasonic irradiation of a mixture of TOPO-coated CdSe/ZnS QDs and α-, β-, or γ-CD in anhydrous ethanol [80]. A host-guest interaction between TOPO ligand and CD resulted in the CD coating on the surface of QDs. Furthermore, the CD-modified CdSe/ZnS QDs have been explored to detect the phenol isomers by quenching fluorescence intensity. The quenching luminescence of the CD-coated QDs was attributed to the fact that the phenol molecules entered the cavity of the coating CD and competed with the TOPO to form an inclusion complex. Specifically, it was found that the luminescence of α-CD or β-CD-modified QDs can be quenched by all of the phenols, but only a little effect on γ-CD-modified QDs.
Li et al. prepared a kind of CD-CdSe QDs by directly replacing the oleic acid (Ole) on the CdSe QDs surface with β-CD in an alkaline aqueous solution [81]. CDs are modified on QDs’ surface via covalent binding, and the resulted β-CD-CdSe QDs exhibited high photoluminescence efficiency and stability in the water environment. Moreover, the several transition-metal ions (Ag+, Hg2+, Co2+, Zn2+) have efficiently quenched the restored fluorescence of β-CD-CdSe QDs. The results demonstrated the potential utility of β-CD-CdSe QDs for the detection of these ions with wide linear range.
Another sensing system based on β-CD and CdSe/ZnS QDs was developed for determination of ascorbic acid [82]. In this case, the fluorescence of the QDs was quenched by the oxidized ascorbic acid. Moreover, the β-CD-CuInS2 QDs have attracted attention as a new class of sensor for detection of adenosine-5-triphosphate (ATP) [83]. A slight enhancement of fluorescence emission from β-CD-CuInS2 QDs was observed upon addition of ATP-binding aptamer due to the host-guest interaction between aptamer and β-CD. The addition of ATP to β-CD-CuInS2 QDs/aptamer system further enhanced the fluorescence emission, which due to the aptamer-ATP complexes formed and included into the cavity of β-CD.
4.2 CD-Functionalized QDs as a Chiral Recognition Sensing Platform
Due to the CD cavity’s inherent chirality, luminescence sensing of CD-conjugated QDs for chiral recognition has been widely investigated [85,86,87,88,89]. In one example, Willner et al. have designed β-CD-modified CdSe/ZnS QDs for optical sensing and chiroselective sensing of different organic substrates based on a fluorescence resonance energy transfer (FRET) or an electron transfer (ET) mechanism [85]. The β-CD coated on the surface of CdSe/ZnS QDs was used to accommodate rhodamine B dye that acted as an optical label. Such systems were utilized for direct sensing of organic substrates exhibiting electron-acceptor or electron-donor properties via electron transfer quenching of the luminescence of the QDs. Moreover, the β-CD-functionalized QD/dye system was used for the chiroselective optical discrimination between D, L-phenylalanine and D, L-tyrosine enantiomers. The specific selection of aromatic chiral amino acid was explained by favored interactions of the phenylring with the β-CD receptor. The same β-CD-functionalized QDs were also used as direct luminescence sensors for the optical detection of p-nitrophenol using an ET quenching route. In a similar manner, water-soluble CD-modified CdSe/ZnS QDs were utilized as selective fluorescent assays for the recognition of amino acid enantiomers [86, 87]. The selective enantiorecognition of L-penicillamine and D-penicillamine was accomplished by host-guest interaction between the penicillamines and the β-CD pockets on the QDs.
Wei et al. reported a photoluminescence chiral assay for tryptophan enantiomers based on mono-6-SH-β-cyclodextrin capped Mn-doped ZnS quantum dots (β-CD-Mn-ZnS QDs) (Fig. 8) [88]. The β-CD-Mn-ZnS QDs have been prepared by hydrothermal process and exhibited dual photoluminescence (PL) at 430 nm and 598 nm. The PL intensity of β-CD-Mn-ZnS QDs responded to tryptophan enantiomers differently: L-Tryptophan enhances the PL intensity of β-CD-Mn-ZnS QDs drastically, whereas the D-isomer barely affects it. In addition, L-tryptophan can be detected in the presence of its stereoisomer with a detection limit of 5.4 nM in a linear range of 0–6.0 mM.
Detection of L/D tryptophan enantiomers by mono-6-SH-β-cyclodextrin capped Mn-doped ZnS quantum dots via photoluminescence intensity enhancing [88]. (Copyright 2015 Royal Society of Chemistry)
5 Cyclodextrin-Functionalized Noble Metal Nanoparticles
Noble metal nanoparticles (NPs) , such as Au NPs, Ag NPs, and Pd NPs, have attracted a growing interest of researchers due to their wide spectrum of potential applications in fields such as sensing, catalysis, or nanomedicine. The utility of metal NPs for any particular application strongly depends upon their physicochemical properties. Thus, a variety of synthetic strategies have been adopted to synthesize metal NPs with size, shape, and composition control. For application in biological sensing or in cell imaging, the corresponding nanoparticles must be soluble in water, and much attention has been paid to the development of green synthetic approaches leading to aqueous suspensions of nanoparticles. Therefore, CDs have appeared to be very eco-friendly capping agents for the metal nanoparticles synthesis. The role of CDs is diverse, ranging from stabilization of the NPs by providing a protective layer that prevents aggregation to increasing solubility in aqueous media. α-, β-, and γ-CDs, and their derivatives, have been widely employed to modify metal nanoparticles by interacting with proper guest molecules [90, 91]. In this part, we focus on the recent advances in the construction of CD-functionalized noble metal nanoparticles and their applications in identification-detection sensing platforms.
CD-coated Au and Ag NPs provide a kind of hybrid nanomaterials which can pave the way toward novel biological tracers as well as optoelectronic nanosensor. Generally, a distance-dependent surface plasmon resonance (SPR) band of AuNPs and AgNPs has been extensively employed for designing assembly-/disassembly-modulated colorimetric sensors. In this case, the high extinction coefficient of AuNPs and AgNPs permits that they can act as the ideal energy acceptors in constructing fluorescence resonance energy transfer (FRET) system for sensing and biosensing [92,93,94,95,96,97]. For example, a (RB-β-CD@Au NP) composite can be employed as an energy acceptor for turn-on fluorescent sensing of cholesterol based on the guest replacement reaction as shown in Fig. 9 [92]. In addition, β-CD-stabilized Au NPs revealed a unique ability to detect micromolar quantities of Pb2+ in the presence of other interfering metal cations, resulting in a visual color change from red to blue [93]. On the other hand, many systems were demonstrated as very effective substrates for the detection of various organic compounds using surface-enhanced Raman spectroscopy (SERS). The SERS technique is a very effective tool to analyze molecules by highly increasing the Raman signal intensity coming from molecules, which have been adsorbed on nanosized metallic surfaces [98]. Several organic moleculars, such as 1,10-phenanthroline, dopamine, aminopyrene, crystal violet, rhodamine B (RB), 4-aminothiophenol, have been demonstrated that CD-capped Au and Ag NPs exhibit a significant SERS effect to these molecular probes [99,100,101].
Schematic illustration of fluorescent turn-on detection of cholesterol using the (RB-β-CD@Au NP) composite [92]. (Copyright 2016 American Chemical Society)
6 Conclusions and Outlooks
The correlative research and developments in the area of functionalized inorganic nanomaterials, such as carbon, magnetic, semiconductor, and metal nanoparticles, have emerged into a cutting edge multidisciplinary nanotechnology. Notably, CDs have been widely used as ideal functional molecules to improve the stability, solubility, and bioavailability of these inorganic nanoparticles, based on their unique properties as supermolecular hosts. The remarkable optical, electronic, and magnetic properties of these inorganic nanoparticles can be combined with the recognition and inclusion ability of cyclodextrins for the development of multifunctional nanodevices. Such all-in-one nanodevices can bring together all the assets of materials involved in constructing nanohybrids, in a comprehensive manner, which may lead to a synergistic effect for sensing, removal or purification, and drug delivery. Thus, the CD hybrid inorganic nanocomposites offer fast, sensitive, and selective molecular recognition sensors; inexpensive, eco-friendly, high efficient, and recyclable adsorption-separation system; as well as new nanocomposites designed specifically to carry anticancer drugs and to treat cancer in virtue of high targeting and multiple stimuli-responsive brought by synergetic effect of all materials.
Although the construction and application of CD hybrid inorganic nanomaterials have been widely developed in recent years and achieved some charming advances, there are still many challenges for scientists. Firstly, the intelligent molecular recognition platform with more sensitivity and selectivity should be developed, of which the binding affinities can be adjusted according to response microenvironments for real-time monitoring in vivo. Secondly, the degradation function should be integrated into the CD hybrid inorganic nano-adsorbents. For the environment pollution treatment, perfect nano-adsorbents are extremely stable during the adsorption process, while they validly decompose the adsorbed substance by a specific catalyst, resulting in the pollutant getting rid of the environment effectively. Undoubtedly, the steadiness and reproducibility of the fabrication of chemical sensors and adsorbents should be further explored. And the employment of CD hybrid inorganic nanomaterials as both chiral sensors for enantiomeric recognition and chiral receptors for enantioselective separation of small chiral molecular guests still awaits exploration in the near future.
Furthermore, multifunctional CD hybrid inorganic nanomaterials should be advocated in nanomedicine, which integrated with diagnostic, imaging, and therapy into one nanosystem. The diagnostic and imaging played the important role in reporting the presence and location of the tumor, its status and its response to a specific treatment. And the synergistic combinations of multiple therapeutic modalities with controlled therapeutic process reduced the side effects and enhanced the effectiveness of cancer treatment. All of these provide the possibility of precision therapy. In particular, as we all know, the application of CD hybrid inorganic nanomaterials in nanomedicine should focus on an in-depth insight into the safety issues in the further, if starting the clinical trials. The development and commercial applications in the further should be sufficiently considered the abovementioned correlative fields.
References
Rekharsky MV, Inoue Y (1998) Complexation thermodynamics of cyclodextrins. Chem Rev 98(5):1875–1918
Szejtli J (1998) Introduction and general overview of cyclodextrin chemistry. Chem Rev 98(5):1743–1754
Khan AR, Forgo P, Stine KJ, D'Souza VT (1998) Methods for selective modifications of cyclodextrins. Chem Rev 98(5):1977–1996
Prochowicz D, Kornowicz A, Lewiński J (2017) Interactions of native cyclodextrins with metal ions and inorganic nanoparticles: fertile landscape for chemistry and materials science. Chem Rev 11(7):13461–13501
Kasprzak A, Poplawska M (2018) Recent developments in the synthesis and applications of graphene-family materials functionalized with cyclodextrins. Chem Commun 54(62):8547–8562
Zhou J, Yu GC, Huang FH (2017) Supramolecular chemotherapy based on host-guest molecular recognition: a novel strategy in the battle against cancer with a bright future. Chem Soc Rev 46(22):7021–7053
Georgakilas V, Otyepka M, Bourlinos AB, Chandra V, Kim N, Kemp KC, Hobza P, Zboril R, Kim KS (2012) Functionalization of graphene: covalent and non-covalent approaches, derivatives and applications. Chem Rev 112(11):6156–6214
Guo YJ, Guo SJ, Ren JT, Zhai YM, Dong SJ, Wang EK (2010) Cyclodextrin functionalized graphene nanosheets with high supramolecular recognition capability: synthesis and host-guest inclusion for enhanced electrochemical performance. ACS Nano 4(7):4001–4010
Liu JL, Leng XY, Xiao Y, Hu CG, Fu L (2015) 3D nitrogen-doped graphene/β-cyclodextrin: host-guest interactions for electrochemical sensing. Nanoscale 7(28):11922–11927
Guo YJ, Guo SJ, Li J, Wang EK, Dong SJ (2011) Cyclodextrin-graphene hybrid nanosheets as enhanced sensing platform for ultrasensitive determination of carbendazim. Talanta 84(1):60–64
Zhang M, Zhang H, Zhai XC, Yang X, Zhao HT, Wang J, Dong AJ, Wang ZY (2017) Application of beta-cyclodextrin-reduced graphene oxide nanosheets for enhanced electrochemical sensing of the nitenpyram residue in real samples. New J Chem 41(5):2169–2177
Liang WT, Rong YQ, Fan LF, Dong WJ, Dong QC, Yang C, Zhong ZH, Dong C, Shuang SM, Wong WY (2018) 3D graphene/hydroxypropyl-β-cyclodextrin nanocomposite as an electrochemical chiral sensor for the recognition of tryptophan enantiomers. J Mater Chem C 6(47):12822–12829
Yang L, Fan S, Deng G, Li Y, Ran X, Zhao H, Li CP (2015) Bridged β-cyclodextrin-functionalized MWCNT with higher supramolecular recognition capability: the simultaneous electrochemical determination of three phenols. Biosens Bioelectron 68:617–625
Ul AA, Qin YH, Howlader MMR, Hu NX, Deen MJ (2018) Electrochemical sensing of acetaminophen using multi-walled carbon nanotube and β-cyclodextrin. Sens Actuator B-Chem 254:896–909
Zarei K, Fatemi L, Kor K (2015) Stripping voltammetric determination of nicardipine using β-cyclodextrin incorporated carbon nanotube-modified glassy carbon electrode. J Anal Chem 70(5):615–620
Le HTN, Jeong HK (2017) Enhanced supramolecular recognition capability of γ cyclodextrin-graphite oxide-carbon nanotube composite. Electrochim Acta 250:259–266
Liu ZG, Xue Q, Guo YJ (2017) Sensitive electrochemical detection of rutin and isoquercitrin based on SH-β-cyclodextrin functionalized graphene-palladium nanoparticles. Biosens Bioelectron 89:444–452
Wang C, Li T, Liu Z, Guo Y, Li C, Dong C, Shuang S (2016) An ultra-sensitive sensor based on β-cyclodextrin modified magnetic graphene oxide for detection of tryptophan. J Electroanal Chem 781:363–370
Dong SQ, Bi Q, Qiao CD, Sun YM, Zhang X, Lu XQ, Zhao L (2017) Electrochemical sensor for discrimination tyrosine enantiomers using graphene quantum dots and β-cyclodextrins composites. Talanta 173:94–100
Jie O, Zhu Y, Yong K, Ma J (2015) Graphene quantum dots/β-cyclodextrin nanocomposites: a novel electrochemical chiral interface for tryptophan isomer recognition. Electrochem Commun 60:60–63
Lei P, Zhou Y, Zhang G, Zhang Y, Zhang C, Hong S, Yang Y, Dong C, Shuang S (2019) A highly efficient chiral sensing platform for tryptophan isomers based on a coordination self-assembly. Talanta 195:306–312
Zaidi SA (2017) Facile and efficient electrochemical enantiomer recognition of phenylalanine using β-Cyclodextrin immobilized on reduced graphene oxide. Biosens Bioelectron 94:714–718
Upadhyay SS, Kalambate PK, Srivastava AK (2017) Enantioselective analysis of moxifloxacin hydrochloride enantiomers with graphene-β-cyclodextrin-nanocomposite modified carbon paste electrode using adsorptive stripping differential pulse Voltammetry. Electrochim Acta 248:258–269
Li Y, Gao Y, Li YA, Liu SY, Zhang H, Su XG (2014) A novel fluorescence probing strategy based on mono-6-(2-aminoethylamino)-6-deoxy-β-cyclodextrin functionalized graphene oxide for the detection of amantadine. Sens Actuator B-Chem 202:323–329
Zor E, Saglam ME, Alpaydin S, Bingol H (2014) A reduced graphene oxide/α-cyclodextrin hybrid for the detection of methionine: electrochemical, fluorometric and computational studies. Anal Methods 6(16):6522–6530
Mondal A, Jana NR (2012) Fluorescent detection of cholesterol using β-cyclodextrin functionalized graphene. Chem Commun 48(58):7316–7318
Zhou SH, Xu HB, Gan W, Yuan QH (2016) Graphene quantum dots: recent progress in preparation and fluorescence sensing applications. RSC Adv 6(112):110775–110788
Mondal S, Purkayastha P (2016) α-Cyclodextrin functionalized carbon dots: pronounced photoinduced electron transfer by aggregated nanostructures. J Phys Chem C 120(26):14365–14371
Lin ZY, Kuo YC, Chang CJ, Lin YS, Chiu TC, Hu CC (2018) Highly sensitive sensing of hydroquinone and catechol based on β-cyclodextrin-modified carbon dots. RSC Adv 8(35):19381–19388
Shao D, Sheng G, Chen C, Wang X, Nagatsu M (2010) Removal of polychlorinated biphenyls from aqueous solutions using β-cyclodextrin grafted multiwalled carbon nanotubes. Chemosphere 79(7):679–685
Salipira KL, Mamba BB, Krause RW, Malefetse TJ, Durbach SH (2008) Cyclodextrin polyurethanes polymerised with carbon nanotubes for the removal of organic pollutants in water. Water SA 34(1):113–118
Wei L, Jiang X, Chen X (2014) A novel method of synthesizing cyclodextrin grafted multiwall carbon nanotubes/iron oxides and its adsorption of organic pollutant. Appl Surf Sci 320:764–771
Sinha A, Jana NR (2015) Separation of microcystin-LR by cyclodextrin-functionalized magnetic composite of colloidal graphene and porous silica. ACS Appl Mater Interfaces 7(18):9911–9919
Mphahlele K, Onyango MS, Mhlanga SD (2015) Adsorption of aspirin and paracetamol from aqueous solution using Fe/N-CNT/β-cyclodextrin nanocomopsites synthesized via a benign microwave assisted method. J Environ Chem Eng 3(4):2619–2630
Siriviriyanun A, Tsai YJ, Voon SH, Kiew SF, Imae T, Kiew LV, Looi CY, Wong WF, Lee HB, Chung LY (2018) Cyclodextrin- and dendrimer-conjugated graphene oxide as a nanocarrier for the delivery of selected chemotherapeutic and photosensitizing agents. Mater Sci Eng C-Mater Biol Appl 89:307–315
Iannazzo D, Mazzaglia A, Scala A, Pistone A, Galvagno S, Lanza M, Riccucci C, Ingo GM, Colao I, Sciortino MT, Valle F, Piperno A, Grassi G (2014) β-Cyclodextrin-grafted on multiwalled carbon nanotubes as versatile nanoplatform for entrapment of guanine-based drugs. Colloid Surf B-Biointerfaces 123:264–270
Srinivasarao M, Low PS (2017) Ligand-targeted drug delivery. Chem Rev 117(19):12133–12164
Yang Y, Zhang YM, Chen Y, Zhao D, Chen JT, Liu Y (2012) Construction of a graphene oxide based noncovalent multiple nanosupramolecular assembly as a scaffold for drug delivery. Chem Eur J 18(14):4208–4215
Hu Z, Wang C, Zhao F, Xu XR, Wang SH, Yu L, Zhang D, Huang YD (2017) Fabrication of a graphene/C60 nanohybrid via γ-cyclodextrin host-guest chemistry for photodynamic and photothermal therapy. Nanoscale 9(25):8825–8833
Zhang YM, Cao Y, Yang Y, Chen JT, Liu Y (2014) A small-sized graphene oxide supramolecular assembly for targeted delivery of camptothecin. Chem Commun 50(86):13066–13069
Liang WT, Huang Y, Lu DT, Ma XW, Gong T, Cui XD, Yu BF, Yang C, Dong C, Shuang SM (2019) β-Cyclodextrin–hyaluronic acid polymer functionalized magnetic graphene oxide nanocomposites for targeted photo-chemotherapy of tumor cells. Polymers 11(1):133–148
Wei GC, Dong RH, Wang D, Feng L, Dong SL, Song AX, Hao JC (2014) Functional materials from the covalent modification of reduced graphene oxide and β-cyclodextrin as a drug delivery carrier. New J Chem 38(1):140–145
Ko NR, Nafiujjaman M, Lee JS, Lim HN, Lee YK, Kwon IK (2017) Graphene quantum dot-based theranostic agents for active targeting of breast cancer. RSC Adv 7(19):11420–11427
Dong HQ, Li YY, Yu JH, Song YY, Cai XJ, Liu JQ, Zhang JM, Ewing RC, Shi DL (2013) A versatile multicomponent assembly via β-cyclodextrin host-guest chemistry on graphene for biomedical applications. Small 9(3):446–456
Wang CL, Li B, Niu WF, Hong SS, Saif B, Wang SB, Dong C, Shuang SM (2015) β-Cyclodextrin modified graphene oxide-magnetic nanocomposite for targeted delivery and pH-sensitive release of stereoisomeric anti-cancer drugs. RSC Adv 5(108):89299–89308
Boncel S, Herman AP, Budniok S, Jedrysiak RG, Jakobik-Kolon A, Skepper JN, Mueller KH (2016) In vitro targeting and selective killing of T47D breast cancer cells by purpurin and 5-fluorouracil anchored to magnetic CNTs: nitrene-based functionalization versus uptake, cytotoxicity, and intracellular fate. ACS Biomater Sci Eng 2(8):1273–1285
An-Hui L, Salabas EL, Ferdi S (2010) Magnetic nanoparticles: synthesis, protection, functionalization, and application. Angew Chem Int Ed 46(8):1222–1244
Sophie L, Delphine F, Marc P, Alain R, Caroline R, Luce VE, Muller RN (2008) Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem Rev 108(6):2064–2110
Zhu J, Jiang HE, Xiaoyan DU, Ruihua LU, Huang L, Xia GE (2011) A facile and flexible process of β-cyclodextrin grafted on Fe3O4 magnetic nanoparticles and host-guest inclusion studies. Appl Surf Sci 257(21):9056–9062
Wang CL, Huang LZ, Song SM, Saif B, Zhou YH, Dong C, Shuang SM (2015) Targeted delivery and pH-responsive release of stereoisomeric anti-cancer drugs using β-cyclodextrin assembled Fe3O4 nanoparticles. Appl Surf Sci 357:2077–2086
Zhou YH, Sun LL, Wang HX, Liang WT, Yang J, Wang L, Shuang SM (2016) Investigation on the uptake and release ability of β-cyclodextrin functionalized Fe3O4 magnetic nanoparticles by methylene blue. Mat Chem Phys 170:83–89
Wang HX, Zhou YH, Guo YJ, Liu WJ, Dong C, Wu YH, Li SD, Shuang SM (2012) β-Cyclodextrin/Fe3O4 hybrid magnetic nano-composite modified glassy carbon electrode for tryptophan sensing. Sensors Actuators B Chem 163(1):171–178
Zhang Y, Wang W, Li Q, Yang QB, Li YX, Du JS (2015) Colorimetric magnetic microspheres as chemosensor for Cu2+ prepared from adamantane-modified rhodamine and β-cyclodextrin-modified Fe3O4@SiO2 via host-guest interaction. Talanta 141:33–40
Helal AS, Mazario E, Mayoral A, Decorse P, Losno R, Lion C, Ammar S, Hemadi M (2018) Highly efficient and selective extraction of uranium from aqueous solution using a magnetic device: succinyl-β-cyclodextrin-APTES@maghemite nanoparticles. Environ Sci Nano 5(1):158–168
Cai KY, Li JH, Luo Z, Hu Y, Hou YH, Ding XW (2011) β-Cyclodextrin conjugated magnetic nanoparticles for diazepam removal from blood. Chem Commun 47(27):7719–7721
Kong L, Yan LL, Qu Z, Yan NQ, Li L (2015) β-Cyclodextrin stabilized magnetic Fe3S4 nanoparticles for efficient removal of Pb(II). J Mater Chem A 3(30):15755–15763
Chen X, Rao JA, Wang J, Gooding JJ, Zou G, Zhang QJ (2011) A facile enantioseparation for amino acids enantiomers using β-cyclodextrins functionalized Fe3O4 nanospheres. Chem Commun 47(37):10317–10319
Sun ZB, Cui GJ, Li HZ, Liu Y, Tian YX, Yan SQ (2016) Multifunctional optical sensing probes based on organic-inorganic hybrid composites. J Mat Chem B 4(31):5194–5216
Wu YP, Zuo F, Zheng ZH, Ding XB, Peng YX (2009) A novel approach to molecular recognition surface of magnetic nanoparticles based on host-guest effect. Nanoscale Res Lett 4(7):738–747
Jiang JJ, Lin XY, Ding D, Diao GW (2018) Enzyme-free homogeneous electrochemical biosensor for DNA assay using toehold-triggered strand displacement reaction coupled with host-guest recognition of Fe3O4@SiO2@ β-CD nanocomposites. Biosens Bioelectron 114:37–43
Munoz J, Gonzalez-Campo A, Riba-Moliner M, Baeza M, Mas-Torrent M (2018) Chiral magnetic-nanobiofluids for rapid electrochemical screening of enantiomers at a magneto nanocomposite graphene-paste electrode. Biosens Bioelectron 105:95–102
Wang W, Zhang Y, Yang QB, Sun MD, Fei XL, Song Y, Zhang YM, Li YX (2013) Fluorescent and colorimetric magnetic microspheres as nanosensors for Hg2+ in aqueous solution prepared by a sol-gel grafting reaction and host-guest interaction. Nanoscale 5(11):4958–4965
Badruddoza AZM, Tay ASH, Tan PY, Hidajat K, Uddin MS (2011) Carboxymethyl-β-cyclodextrin conjugated magnetic nanoparticles as nano-adsorbents for removal of copper ions: synthesis and adsorption studies. J Hazard Mater 185(2–3):1177–1186
Badruddoza AM, Bhattarai B, Suri RPS (2017) Environmentally friendly β-cyclodextrin-ionic liquid polyurethane-modified magnetic sorbent for the removal of PFOA, PFOS, and Cr(VI) from water. ACS Sustain Chem Eng 5(10):9223–9232
Mu B, Kang YR, Wang AQ (2013) Preparation of a polyelectrolyte-coated magnetic attapulgite composite for the adsorption of precious metals. J Mater Chem A 1(15):4804–4811
Guo ZQ, Li Y, Pan SH, Xu JZ (2015) Fabrication of Fe3O4@cyclodextrin magnetic composite for the high-efficient removal of Eu(III). J Mol Liq 206:272–277
Fuhrer R, Herrmann IK, Athanassiou EK, Grass RN, Stark WJ (2011) Immobilized β-Cyclodextrin on surface-modified carbon-coated cobalt nanomagnets: reversible organic contaminant adsorption and enrichment from water. Langmuir 27(5):1924–1929
Gong T, Zhou YH, Sun LL, Liang WT, Yang J, Shuang SM, Dong C (2016) Effective adsorption of phenolic pollutants from water using β-cyclodextrin polymer functionalized Fe3O4 magnetic nanoparticles. RSC Adv 6(84):80955–80963
Duan ZB, Ding XC, Wang Y, Zhu LJ, Xia DH (2018) A new strategy for fuel desulfurization by molecular inclusion with copper(II)-β-cyclodextrin@SiO2@Fe3O4 for removing thiophenic sulfides. Energy Fuel 32(11):11421–11431
Sinha A, Basiruddin SK, Chakraborty A, Jana NR (2015) β-Cyclodextrin functionalized magnetic mesoporous silica colloid for cholesterol separation. ACS Appl Mater Interfaces 7(2):1340–1347
Guo J, Wang NJ, Peng L, Wu JJ, Ye QQ, Feng AC, Wang ZP, Zhang C, Xing XH, Yuan JY (2016) Electrochemically-responsive magnetic nanoparticles for reversible protein adsorption. J Mat Chem B 4(22):4009–4016
Deng XJ, Li WB, Ding GS, Chen XP (2018) Enantioselective separation of RS-mandelic acid using β-cyclodextrin modified Fe3O4@SiO2/Au microspheres. Analyst 143(11):2665–2673
Yang X, Song X, Zhu H, Cheng C, Yu H, Zhang H (2018) Novel smart polymer brushes modified magnetic graphene oxide for highly efficient chiral recognition and enantioseparation of tryptophan enantiomers. ACS Appl Bio Mat 1(4):1074–1083
Luo Z, Cai K, Hu Y, Li J, Ding X, Zhang B, Xu D, Yang W, Liu P (2012) Redox-responsive molecular nanoreservoirs for controlled intracellular anticancer drug delivery based on magnetic nanoparticles. Adv Mater 24(3):431–435
Lee JH, Chen KJ, Noh SH, Garcia MA, Wang H, Lin WY, Jeong H, Kong BJ, Stout DB, Cheon J, Tseng HR (2013) On-demand drug release system for in vivo cancer treatment through self-assembled magnetic nanoparticles. Angew Chem Int Edit 52(16):4384–4388
Hong SS, Li ZB, Li CZ, Dong CA, Shuang SM (2018) β-Cyclodextrin grafted polypyrrole magnetic nanocomposites toward the targeted delivery and controlled release of doxorubicin. Appl Surf Sci 427:1189–1198
Zhou J, Yang Y, Zhang CY (2015) Toward biocompatible semiconductor quantum dots: from biosynthesis and bioconjugation to biomedical application. Chem Rev 115(21):11669–11717
Denis D, Shu-Han H, Nikodem T, Reinhoudt DN, Jurriaan H, Velders AH, Julius Vancso G (2010) Fabrication and luminescence of designer surface patterns with β-cyclodextrin functionalized quantum dots via multivalent supramolecular coupling. ACS Nano 4(1):137–142
Palaniappan K, Hackney SA, Liu J (2004) Supramolecular control of complexation-induced fluorescence change of water-soluble, β-cyclodextrin-modified CdS quantum dots. Chem Commun 23(23):2704–2705
Li H, Han C (2008) Sonochemical synthesis of cyclodextrin-coated quantum dots for optical detection of pollutant phenols in water. Chem Mater 20(19):6053–6059
Shang ZB, Hu S, Wang Y, Jin WJ (2011) Interaction of β-cyclodextrin-capped CdSe quantum dots with inorganic anions and cations. Luminescence 26(6):585–591
Duran GM, Contento AM, Rios A (2015) Continuous method incorporating β-cyclodextrin modified CdSe/ZnS quantum dots for determination of ascorbic acid. Anal Methods 7(8):3472–3479
Hu T, Na W, Xu Y, Su X (2017) Sensitive fluorescence detection of ATP based on host-guest recognition between near-infrared β-Cyclodextrin-CuInS2 QDs and aptamer. Talanta 165:194–200
Geng S, Lin SM, Liu SG, Li NB, Luo HQ (2016) A new fluorescent sensor for detecting p-nitrophenol based on β-cyclodextrin-capped ZnO quantum dots. RSC Adv 6(89):86061–86067
Freeman R, Finder T, Bahshi L, Willner I (2009) β-cyclodextrin-modified CdSe/ZnS quantum dots for sensing and chiroselective analysis. Nano Lett 9(5):2073–2076
Durán GM, Abellán C, Contento AM, Ríos Á (2017) Discrimination of penicillamine enantiomers using β-cyclodextrin modified CdSe/ZnS quantum dots. Microchim Acta 184(3):815–824
Han C, Li H (2010) Chiral recognition of amino acids based on cyclodextrin-capped quantum dots. Small 4(9):1344–1350
Wei YL, Li HH, Hao HY, Chen YX, Dong C, Wang GF (2015) β-Cyclodextrin functionalized Mn-doped ZnSquantum dots for the chiral sensing of tryptophan enantiomers. Polym Chem 6(4):591–598
Jie Z, Yun L, Zhang Z, Sha Y, Jian T, Wei L, Tang W (2016) Cyclodextrin-clicked silica/CdTe fluorescent nanoparticles for enantioselective recognition of amino acids. Nanoscale 8(10):5621–5626
Dreaden EC, Alkilany AM, Huang X, Murphy CJ, El-Sayed MA (2012) The golden age: gold nanoparticles for biomedicine. Chem Soc Rev 41(7):2740–2779
Alvarez J, Liu J, Román E, Kaifer AE (2000) Water-soluble platinum and palladium nanoparticles modified with thiolated β-cyclodextrin. Chem Commun 13:1151–1152
Zhao Y, Huang Y, Zhu H, Zhu Q, Xia Y (2016) Three-in-one: sensing, self-assembly, and cascade catalysis of cyclodextrin modified gold nanoparticles. J Am Chem Soc 138(51):16645–16654
Aswathy B, Avadhani GS, Suji S, Sony G (2012) Synthesis of β-cyclodextrin functionalized gold nanoparticles for the selective detection of Pb2+ ions from aqueous solution. Front Mater Sci 6(2):168–175
Wang J, Kong LT, Guo Z, Xu JY, Liu JH (2010) Synthesis of novel decorated one-dimensional gold nanoparticle and its application in ultrasensitive detection of insecticide. J Mater Chem 20(25):5271–5279
Wang M, Su K, Cao J, She Y, Wang Y (2018) “Off-On” non-enzymatic sensor for malathion detection based on fluorescence resonance energy transfer between β-cyclodextrin@Ag and fluorescent probe. Talanta 192:295–300
Qi M, Song JP, Zhang SF, Wang MF, Yong G, Dong C (2016) Colorimetric detection of riboflavin by silver nanoparticles capped with β-cyclodextrin-grafted citrate. Colloids Surf B Biointerfaces 148:66–72
Liu C, Lian J, Liu Q, Xu C, Li B (2016) β-Cyclodextrin-modified silver nanoparticles as colorimetric probes for the direct visual enantioselective recognition of aromatic α-amino acids. Anal Methods 8(29):5794–5800
Stiles PL, Dieringer JA, Shah NC, Van Duyne RP (2008) Surface-enhanced raman spectroscopy. Annu Rev Anal Chem (Palo Alto, Calif) 1:601–626
Teng H, Fei M, Qi L (2009) Facile synthesis and one-dimensional assembly of cyclodextrin-capped gold nanoparticles and their applications in catalysis and surface-enhanced raman scattering. J Phys Chem C 113(31):13636–13642
Dickson J, Geckeler KE (2009) Surfactant-directed multiple anisotropic gold nanostructures: synthesis and surface-enhanced Raman scattering. Langmuir the Acs J Surfaces Colloids 25(22):13224–13231
Teng H, Fei M, Limin Q (2010) Controlled synthesis of dendritic gold nanostructures assisted by supramolecular complexes of surfactant with cyclodextrin. Langmuir 26(10):7582–7589
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this entry
Cite this entry
Liang, W., Shuang, S. (2020). Cyclodextrin Hybrid Inorganic Nanocomposites for Molecular Recognition, Selective Adsorption, and Drug Delivery. In: Liu, Y., Chen, Y., Zhang, HY. (eds) Handbook of Macrocyclic Supramolecular Assembly . Springer, Singapore. https://doi.org/10.1007/978-981-15-2686-2_17
Download citation
DOI: https://doi.org/10.1007/978-981-15-2686-2_17
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-2685-5
Online ISBN: 978-981-15-2686-2
eBook Packages: Chemistry and Materials ScienceReference Module Physical and Materials ScienceReference Module Chemistry, Materials and Physics