Abstract
Superhydrophobic materials have attracted wide attention due to their special wettability on the surface. In this paper, the basic definition, principle, and several common wetting models of superhydrophobic are summarized firstly. Then, the advantages and disadvantages of commonly used methods for preparing superhydrophobic materials are briefly analyzed, including dip coating method, spraying method, template method, chemical vapor deposition method, etching method, electrospinning method, phase separation method, and layer-by-layer assembly method. Finally, the application of superhydrophobic materials in oil-water separation, moisture-proof packaging, self-cleaning, and other fields is summarized and elaborated. The application of smart superhydrophobic materials which can respond to external stimuli such as pH, light, and temperature is also introduced. The purpose is to provide reference for the development of superhydrophobic materials. Preparing excellent superhydrophobic materials in a simple and environmentally friendly way is the key to meet the requirements of current industrial production.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
With the development of industrialization, superhydrophobic surfaces have attracted extensive attention and research. In nature, many plant leaves and animal feathers are superhydrophobic, the most classic of which is the lotus leaf effect. Inspired by the lotus leaf effect, superhydrophobic surfaces are usually constructed by combining micro and nano multilevel structures with low surface energy chemical composition. The superhydrophobic material generally refers to the material whose water contact angle on static surface is greater than 150° and rolling angle is less than 10° [1,2,3,4]. Superhydrophobic materials generally have excellent self-cleaning and anti-pollution properties due to their good non-wettability to water. Therefore, they have broad application prospects in daily life and many industrial fields [5,6,7].
In this paper, the current research status of superhydrophobic materials is briefly described, and some problems that need to be solved are also put forward. It aims to bring help to the future research and promote the further research of superhydrophobic materials.
1.1 Definition of Superhydrophobic
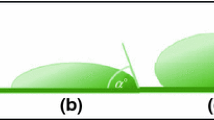
The wettability of liquid on solid surface is characterized by contact angle. The contact angle is the angle between the solid-liquid interface and the tangent of the gas-liquid interface along the solid-liquid-gas three-phase contact point. It can be expressed by Young’s equation, which describes the wettability of liquids on smooth solid surfaces. The contact angle of 90° is the critical angle for judging the wettability between droplets and solid surfaces. When theta is greater than 90°, it shows that the surface is hydrophobic and vice versa. In fact, there is almost no absolutely smooth surface in nature. The wetting state of liquid on rough solid surface is characterized by Wenzel model and Cassie-Baster model. Wenzel model considers that the apparent contact angle between droplet and solid surface is larger than the intrinsic contact angle due to the increase of surface roughness. While the Cassie-Baster model considers that the droplets are suspended on the concave and convex grooves on the solid surface, and the droplets fall on the solid-liquid-gas three-phase composite interface (Fig. 1).
2 Preparation Method of Superhydrophobic Surface
It has been shown that the superhydrophobic property of material surface is determined by its surface energy and surface roughness. Therefore, the superhydrophobic surface can be constructed by using the materials with low surface energy and constructing rough structure. At present, the methods used to construct superhydrophobic surface mainly include dip coating method, spraying method, template method, chemical vapor deposition method, etching method, electrostatic spinning method, phase separation method, and layer by layer self-assembly method. The related methods will be summarized below.
Dip Coating Method. At present, the dip coating method is considered to be the most commonly used method to construct the superhydrophobic surface. This method can be applied to the preparation of superhydrophobic surfaces with large substrates and does not require much time. In general, the substrate needs to be impregnated in the prepared superhydrophobic coating for treatment, and then it should be dried and cured. Superhydrophobic coatings contain solid particles, adhesives, and hydrophobic components that increase surface roughness. Wang et al. [8] firstly prepared silica nanoparticles by sol-gel method, and then the cotton fabric was soaked into a mixture of hydrophobic particles and dopamine hydrochloride for superhydrophobic treatment. The strongly adhered micro/nanoscale roughness with low surface energy imparted the fabric with excellent hydrophobicity and durability. Wang et al. [9] formed a superhydrophobic surface by soaking the paper into the prepared silica sol. The superhydrophobic paper had excellent mechanical properties and the functions of acid and moisture resistance.
Spraying Method. Superhydrophobic coating can be constructed on the surface of the substrate by spraying method. Hydrophobic nanoparticles are usually mixed with organic reagents and the resulting suspension is sprayed onto the substrate surface. This method is simple, efficient, and has a good application prospect. Lu et al. [10] prepared a coating by mixing two different size ranges of TiO2 nanoparticles in an ethanol solution containing perfluorooctyltriethoxysilane. And then the coating was sprayed onto the filter paper or glass surface. The modified filter paper or glass obtained good hydrophobicity and self-cleaning ability.
Template Method. Template method is to fabricate superhydrophobic surface quickly by self-made template or duplicating existing micro-and nano-structures. This method has the advantages of simplicity, efficiency, and large area replication, but the service life of the template is not long. Liu et al. [11] fabricated a transparent superhydrophobic surfaces by calcining candle-soot-coated polydimethylsiloxane (PDMS) films. The network structures were created on glass. The substrate exhibited a water contact angle of 163° and a high thermal stability.
Chemical Vapor Deposition. Chemical vapor deposition involves the chemical reaction of two or more gaseous materials and then the resulting new material is deposited onto the surface of the substrate. Bao et al. [12] proposed a method for fabricating superhydrophobic surfaces, which was developed by coating metal oxide nanoparticles, including ZnO, Al2O3 and Fe3O4, on various substrates followed by treatment with PDMS via chemical vapor deposition (CVD) method. Lau et al. [13] prepared hydrophobic vertical array carbon nanotubes (VACNTs) by chemical vapor deposition technology.
Etching Method. The etching method is to change the structure of solid surface by physical or chemical methods to form regular or irregular micro-nano size roughness. Then the wetting state of solid surface will be changed. The surface hydrophobicity can be regulated by the method through the operation and design of the surface structure, but the cost is high and it is not suitable for large area preparation. Sung-Woon et al. [14] obtained a superhydrophobic Si surface with a contact angle of 165° via plasma etching by combining the fabrication of microscale rod structures and the deposition of fluorocarbon films on the Si surface.
Electrostatic Spinning Method. Electrostatic spinning is a unique fiber manufacturing process in which polymer solution or its melt is placed in a high-voltage electrostatic field and doped with nanoparticles to form micro-nanofibers under the action of electric field force. It has the advantages of simple manufacturing device, various spinnable materials, and controllable process, which can be used alone or in combination with other methods. Lim et al. [15] used methyltriethoxysilane (MTES) as an ORMOSIL precursor to prepare silica (SiO2) sol containing methyl groups by sol-gel method. And then the superhydrophobic surface with multifunctionalities and thermal stability was obtained by creating hierarchically electrospun fibrous structures.
Phase Separation Method. Phase separation process usually utilizes the different solubility or thermal properties of mixtures to initiate the separation of two or more phases in the system, resulting in micron or nanometer rough structure. The method can be used to prepare uniform and large area superhydrophobic films due to the easy control of experimental conditions and simple operation. It has great practical value, but volatilization of solvents will cause certain environmental pollution. Zhang et al. [16] obtained the poly(vinylidene fluoride) (PVDF) membrane with thermal stability and mechanical stability by using phase separation method and adding ammonia water into the N-methyl-2-pyrrolidone (NMP) solution of PVDF. Obeso et al. [17] precipitated poly(hydroxybutyrate) (PHB) on the surface of cellulose fibers of papers using a phase separation process to obtain a superhydrophobic surface with rough texture at both micro and nano length scales.
Layer-by-layer Assembly Method. Layer-by-layer (LBL) assembly method is to assemble nanoparticles or polymer electrolytes onto the substrate by layer-by-layer deposition under the action of electrostatic, coordination bond. This method has the advantages of simple operation, easy control of assembly degree and structure. Shang et al. [18] fabricated highly transparent porous silica coatings on glass substrate through LBL assembly of raspberry-like polystyrene@silica (PS@SiO2) microparticles. Under the action of low surface energy materials, the prepared coatings showed superhydrophilic and superhydrophobicity properties. Gomes et al. [19] proposed a simple technique of LBL of applying polycation chitosan (CH) and polyanion alginates (ALG) to prepare CH/ALG multilayers on cotton samples.
3 Application of Superhydrophobic Materials
The special wettability of superhydrophobic materials can make them combine with different substrates so that they can be widely used in oil-water separation, self-cleaning, moisture-proof packaging, and responsive materials.
Oil-Water Separation. More and more oily wastewater is produced by modern industrial activities. The consequent environmental problems and the waste of water resources have aroused wide attention. At present, there are many methods for separating oil-water mixture, such as gravity method, centrifugal method, ultrasonic method, adsorption method, and filtration method. But low efficiency and poor stability are the biggest drawbacks of these methods. Oil-water separation is essentially an interfacial problem, so materials with special wettability have been extensively studied. Xu et al. [20] prepared hydrofluoric acid-titanium dioxide (HFA-TiO2) sol by mixing silica nanoparticles (SiO2) with ethanol solution. Then the porous substrates including polyester fabric and polyurethane sponge were coated with the coating solution by a simple dip coating method. The modified coatings showed unusual capabilities for controllable filtration of an oil–water mixture and selective removal of water from bulk oil. Dang et al. [21] fabricated a superhydrophobic polymer that can be applied to cotton fabric, filter paper, and PU by a simple random copolymerization. The wetting state of the coating can be changed by in situ and ex situ treatment. Thus, the coating can be applied in highly controllable oil/water separation such as continuous separation of oil/water/oil three phase mixtures (Fig. 2).
Responsive Function. In order to expand the application field of superhydrophobic materials, more and more researches have been conducted on smart materials that can respond to external stimuli such as temperature, pH, and light [22,23,24,25]. Xu et al. [26] prepared superhydrophobic coatings by mixing SiO2 with TiO2 modified by decanoic acid. The superhydrophobic coating presented hydrophobic properties in air and neutral water environment and hydrophilic properties in alkaline solution or ammonia vapor. Xu et al. [27] reported a template lamination method to fabricate multifunctional TiO2-high-density polyethylene (HDPE) nanocomposite surfaces with superhydrophobicity, UV-induced reversible wettability, and selfcleaning properties. Geissler et al. [28] prepared superhydrophobic paper with cellulose stearyl ester (CSE) solution and nano-particles, which could be responded to temperature. The paper would become hydrophilic after heat treatment at 70 °C for 5 min. Cao et al. [29] fabricated thermo and pH dual-controllable mesh by photo initiated free radical polymerization. The coated mesh showed superhydrophilicity and underwater superoleophobicity at certain temperature and pH. While the water resistance of poly(2-(dimethylamino)ethyl methacrylate) (PDMAEMA) hydrogel decreased when the temperature reached above 55 °C or pH was larger than 13 (Fig. 3).
Moisture-Proof Packaging. The mechanical properties of paper will be degraded due to the easy absorption of moisture by its fibers when it is used for storage and transportation. In order to widen the application field of paper, the superhydrophobic coating can be combined with paper, so that paper can be used in the field of moisture-proof packaging. A superhydrophobic paper was fabricated by precipitating TiO2 nanoparticles/sodium alginate (ALG) multilayers on paper surface layer-by-layer followed by an adsorption treatment of colloidal carnauba wax. The prepared superhydrophobic paper with excellent moisture-proofing property and high strength stability had a great potential in moisture-proof paper packaging [30]. Li et al. [31] developed a simple method to fabricate superhydrophobic paper which had excellent moisture resistance by depositing carnaubawax onto the surface of cellulose fibers via a phase separation method (Fig. 4).
Self Cleaning. The water droplets can roll freely on the surface of the substrate when the contact angle between water droplets and superhydrophobic surface is greater than 150° and the rolling angle is less than 10°, which can realize the self-cleaning characteristics of the material surface. Fu et al. [32] synthesized the quaternary ammonium salts (QAS) functionalized fluorinated copolymer tethered hydroxyl groups. And then the superhydrophobic nanocomposite coatings with self-cleaning properties were prepared by crosslinking fluorine copolymers and poly(urea-formaldehyde) nanoparticles (PUF NPs) nanoparticles with hexamethylene diisocyanate. The self-cleaning properties of nano-composite coatings were studied by using copper chloride dihydrate powder as pollutant. Excellent self-cleaning performance was achieved by adding PUF NPs into the copolymer. Chen et al. [33] prepared self-repairing superhydrophobic coatings based on TiO2 with photocatalysis and microcapsules, which was against UV irradiation and chemically stable. The coatings showed good self-cleaning property to the powder dirt (Fig. 5).
4 Conclusions
In this paper, the preparation methods and applications of superhydrophobic materials are reviewed in order to provide some reference for the future research of superhydrophobic materials. At present, superhydrophobic materials have been extensively studied, and some achievements have been achieved. However, there are few superhydrophobic materials in the actual production. Actually, there are still some problems to be solved urgently. For example, the preparation of superhydrophobic materials mostly involves fluorine-containing materials, which does not meet the current needs of green development. Although fluorine-containing substances can promote the hydrophobicity, the pollution to the environment and the harm to human health can not be ignored. Therefore, it is a challenge to develop economically and environmentally friendly superhydrophobic materials with excellent superhydrophobic performance, and it is also of great significance for the advancement of superhydrophobic research.
References
Wang B, Liang WX, Guo ZG, Liu WM (2015) Biomimetic super-lyophobic and super-lyophilic materials applied for oil/water separation: a new strategy beyond nature. Chem Soc Rev 44(1):336–361
Wang ST, Liu KS, Yao X, Jiang L (2015) Bioinspired surfaces with superwettability: new insight on theory, design, and applications. Chem Rev 115:8230–8293
Chu ZL, Feng YJ, Seeger S (2014) Oil/water separation with selective superantiwetting/superwetting surface materials. Angew Chem Int Ed 54(8):2328–2338
Xue ZX, Cao YZ, Liu N et al (2014) Special wettable materials for oil/water separation. J Mater Chem A 2:2445–2460
Yang C, Wang FJ, Li W et al (2016) Anti-icing properties of superhydrophobic ZnO/PDMS composite coating. Appl Phys A 122(1):1
Songok J, Toivakka M (2016) Enhancing capillary driven flow for paper-based microfluidic channels. ACS Appl Mater Interfaces 8(44):30523–30530
Zhou X, Kong JH, Sun JT et al (2017) Stable superhydrophobic porous coatings from hybrid ABC triblock copolymers and their anti-corrosive performance. ACS Appl Mater Interfaces 9(35):30056–30063
Wang JT, Chen YH (2015) Oil-water separation capability of superhydrophobic fabrics fabricated via combining polydopamine adhesion with lotus-leaf-like structure. J Appl Polym Sci 132(39):42614
Wang N, Xiong DS, Pan S et al (2016) Superhydrophobic paper with superior stability against deformations and humidity. Appl Surf Sci 389(32):354–360
Lu Y, Sathasivam S, Song JL et al (2015) Robust self-cleaning surfaces that function when exposed to either air or oil. Science 347(6226):1132–1135
Liu XJ, Xu Y, Ben KY et al (2015) Transparent, durable and thermally stable PDMS-derived superhydrophobic surfaces. Appl Surf Sci 339:94–101
Bao XM, Cui JF, Sun HX et al (2014) Facile preparation of superhydrophobic surfaces based on metal oxide nanoparticles. Appl Surf Sci 303:473–480
Lau KKS, Bico J, Teo KBK et al (2003) Superhydrophobic carbon nanotube forests. Nano Lett 3(12):1701–1705
Cho SW, Kim JH, Lee HM et al (2016) Superhydrophobic Si surfaces having microscale rod structures prepared in a plasma etching system. Surf Coat Technol 306:82–86
Lim HS, Baek JH, Park K et al (2010) Multifunctional hybrid fabrics with thermally stable superhydrophobicity. Adv Mater 22(19):2138–2141
Zhang WB, Shi Z, Zhang F et al (2013) Superhydrophobic and superoleophilic PVDF membranes for effective separation of water-in-oil emulsions with high flux. Adv Mater 25(14):2071–2076
Obeso CG, Sousa MP, Song WL et al (2013) Modification of paper using polyhydroxybutyrate to obtain biomimetic superhydrophobic substrates. Colloids Surf A 416(1):51–55
Shang QQ, Zhou YH (2016) Fabrication of transparent superhydrophobic porous silica coating for self-cleaning and anti-fogging. Ceramics Int 42:8706–8712
Gomes AP, Mano JF, Queiroz JA et al (2013) Layer-by-layer deposition of antimicrobial polymers on cellulosic fibers: a new strategy to develop bioactive textiles. Polym Adv Technol 24(11):1005–1010
Xu ZG, Zhao Y, Wang HX et al (2015) A superamphiphobic coating with an ammonia-triggered transition to superhydrophilic and superoleophobic for oil-water separation. Angew Chem 127(15):4610–4613
Dang Z, Liu LB, Li Y et al (2016) In situ and ex situ pH-responsive coatings with switchable wettability for controllable oil/water separation. ACS Appl Mater Interfaces 8(45):31281–31288
Xue BL, Gao LC, Hou YP et al (2013) Temperature controlled water/oil wettability of a surface fabricated by a block copolymer: application as a dual water/oil on-off switch. Adv Mater 25:273–277
Lee CH, Kang SK, Lim JA et al (2012) Electrospun smart fabrics that display pH-responsive tunable wettability. Soft Matt 8(40):10238
Tian DL, Zhang XF, Tian Y et al (2012) Photo-induced water-oil separation based on switchable superhydrophobicity-superhydrophilicity and underwater superoleophobicity of the aligned zno nanorod array-coated mesh films. J Mater Chem 22(37):19652–19657
Yin YJ, Guo N, Wang CX, Rao QQ (2014) AlterableSuperhydrophobic–superhydrophilicwettability of fabric substrates decorated with ion–TiO2 coating via ultraviolet radiation. Ind Eng Chem Res 53(37):14322–14328
Xu ZG, Zhao Y, Wang HX et al (2016) Fluorine-free superhydrophobic coatings with pH-induced wettability transition for controllable oil-water separation. ACS Appl Mater Interfaces 8(8):5661–5667
Xu QF, Liu Y, Lin FJ et al (2013) Superhydrophobic TiO2-polymer nanocomposite surface with UV-induced reversible wettability and self-cleaning properties. Appl Mater Interfaces 5(18):8915–8924
Geissler A, Loyal F, Biesalski M, Zhang K (2014) Thermo-responsive superhydrophobic paper using nanostructured cellulose stearoyl ester. Cellulose 21(1):357–366
Cao YZ, Liu N, Fu CK et al (2014) Thermo and pH dual-responsive materials for controllable oil/water separation. ACS Appl Mater Interfaces 6(3):2026–2030
Li P, Li H, Yang J, Meng YH (2016) Facile fabrication of superhydrophobic paper with excellent water repellency and moisture resistance by phase separation. BioResources 11(3):6552–6565
Li H, Yang J, Li P, Lan TQ, Peng LC (2017) A facile method for preparation superhydrophobic paper with enhanced physical strength and moisture-proofing property. Carbohyd Polym 160:9–17
Fu YC, Jiang JX, Zhang QH et al (2017) Robust liquid-repellent coatings based on polymer nanoparticles with excellent self-cleaning and antibacterial performances. J Mater Chem A 5:275–284
Chen KL, Gu K, Qiang SY, Wang CX (2017) Environmental stimuli-responsive self-repairing waterbased superhydrophobic coatings. RSC Adv 7:543–550
Acknowledgements
This work was supported in part by NSF of the Science and Technology Department of Shaanxi Province under Grant Nos. 2019JM-122, NSF of the Science and Technology Department of Shaanxi Province under Grant Nos. 2018JQ5100, Doctoral Research Initiation Fund of Xi’an University of Technology under Grant Nos. 108-451119007, NSF of the Key Laboratory of Shaanxi Provincial Department of Education under Grant Nos. 15JS075 and Shaanxi Collaborative Innovation Center of Green Intelligent Printing and Packaging.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Deng, B., Zhou, S., Chen, F., Du, B., Luo, R., Li, H. (2020). Preparation and Application of Superhydrophobic Surface Materials. In: Zhao, P., Ye, Z., Xu, M., Yang, L. (eds) Advanced Graphic Communication, Printing and Packaging Technology. Lecture Notes in Electrical Engineering, vol 600. Springer, Singapore. https://doi.org/10.1007/978-981-15-1864-5_113
Download citation
DOI: https://doi.org/10.1007/978-981-15-1864-5_113
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-1863-8
Online ISBN: 978-981-15-1864-5
eBook Packages: EngineeringEngineering (R0)