Abstract
The purpose of this study is to provide the researchers with the bigger academic network for the understanding of the incorporated proof about current pharmacogenomics information on pediatric oncology and hematology. This primary assessment will help and guide the researchers and clinicians by giving a thought about the current situation of research, as far as customizing drug for kids with malignant growth (cancer). This study also describes the gene-drug associations with substantial and adequate power of association put forward by PharmGKB (Pharmacogenomics Knowledgebase). Some drugs with strong pharmacogenetic evidence like thiopurines and some with moderate pharmacogenetic evidence like vincristine are also discussed. Upcoming suggestions to achieve this objective will help to put forward the recommendations. Pharmacogenetic gene-drug link studies, thus, influence the reconsidering of study plans to produce sensible results. Genetic roots of oxidative force reaction or genome stability disturbing reaction were categorized as association with moderate evidence but have not yet been tested in children.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Pediatrics
- Pharmacogenomics
- PharmGKB
- Thiopurine
- Irinotecan
- Cisplatin
- Methotrexate
- Cyclophosphamide and vincristine
1 History of Drug Reaction in Children
People with similar sickness will show inverse respond frequently with identical medication. Some people will have the best response to medication, though some experience slight or no results. Few patients will have contrary drug reactions, while others don’t have. Some patients need a high or low dosage related to normal dose demarcated in medical tribunals to get optimal results from the drug. Pharmacogenomics finds the connection between genetic differences and drug responses. Single nucleotide polymorphisms can lead to fluctuation in function and quantity of proteins and so in drug response.
Maximum pharmacogenomic investigations have been done on adults. It is significant to understand that results in the mature population can’t be produced in the pediatric population. Processes or systems are under progress in children. Drugs might perform an inverse function in children as compared to adults. Though genetic differences persist, the influence of handling heterogeneity might be diverse at a young age. Pharmacogenomic studies in pediatric cancer focused on forecasting which patients will agonize from adverse ADRs [1].
About 20% of malignant growth in pediatric patients doesn’t react to usual treatment [2], and 22% of emergency clinic confirmations in common people are because of antagonistic drug responses [3]. The helpful healing mediators utilized in malignant growth chemotherapy are frequently managed at legal high dosages [4]; this is due to between patient inconstancy and tight remedial extent which ends up in a range of results from extreme toxicities to underexposure. Some portion of this fluctuation can be credited to innate hereditary varieties influencing the medication pharmacodynamics and pharmacokinetics. The investigation of the connection between hereditary qualities and medication work is most normally known as pharmacogenetics or pharmacogenomics. Pharmacogenomics is a valid, recognized, and personalized approach of treatment and has the potential to build up drug safety levels and efficiency [5, 6]. Most of the patients react diversely to a drug because of their constitutive genetic shuffling, yet also because of transformations or epigenetic marks gained among the procedure of neogenesis or treatment. This survey expects to concentrate on germ line varieties that may influence treatment adequacy and toxic quality [7, 8]. By October 2015, in Budapest (Hungary), during 3rd congress under (ESPT) European Society of Pharmaco-genomics and Personalized Therapy, a preface conference was held with an aim of establishing individualized pediatric treatment involved therapy in Hematology and Oncology sections and testing on children’s would be the goal. The fundamental reason for existing was to encourage the exchange and coordination of pharmacogenetic analysis from investigational study into applied clinics, to unite essential and translational research, and to teach well-being experts all through Europe as this information is essential to achieve the future goals in pharmacogenomics.
2 Ontogeny Role in Pharmacogenomics
Pharmacogenomics in youngsters, in contrast to adults, must be seen concerning body improvement apart from the physiological changes because of the disorder. When an infant becomes an adult, variations happen in physique structure. For example, an untimely newborn of about 1.5 kg has only 3% of physique fat, which prompts 12% with the aid of the 40th week development and farther 25% by the 4th month [9]. Correspondingly, the protein will increase from 25% at the beginning to 50% in a fully grown individual. These progressions need to be observed while examining the distinctions in pharmacokinetic information in connection to the genotypes. An additional issue of significance for the scientific efficacy of pharmacogenetic tests in youngsters is ontogeny of drug-metabolizing enzymes (DMEs), target proteins. One of the best examples is an expansion of drug metabolism capacity linked with expression of cytochrome P450 (CYP) [10]. These enzymes of the CYP3A family can make a change in their activity from fetus to adulthood which includes four types of members in humans that are 3A4, 3A5, 3A7, and 3A43, respectively. CYP3A4 is richly expressed CYP in a small digestive system and liver recovering 35–45% of CYP proteins. CYP3A4 has a very stumpy action during childbirth, coming to around 35–45% of grown-up movement by the principal month and adult action by the 6th month of an adult, surpassing grown-up action of an adult (120%) somewhere in the range of 1 and 4 years old, and diminishing to grown-up levels after adolescence [11]. CYP3A5 and CYP3A4 both are homologous about 83% and represent themselves in kidneys and the liver but at very low levels, whereas CYP3A5 and CYP2B6 are among two CYPs alongside with the phase II of enzyme N-acetyltransferase showing no change in appearance and the functioning of genetic variants in developmental stages from childhood to adulthood. In contrast, CYPs of family CYP3A7 and CYP3A4 which are mostly expressed in newborns, fetal, and in the embryonic liver, are 90% homologous [12]. Comparable inconstancy in articulation amid improvement is noted for CYP2D6. It was shown that embryos under 30 weeks old express under 5% of the CYP2D6 movement in contrast with the grown-ups. Once after birth, movement bit by bit increases, between days 8 and 28 the action is 30%, and between about a month and 5 years, the action is 70% in adults. Also, CYP articulation and movement can be influenced by basic medical issues, for example, nonalcoholic greasy liver ailment and neonatal diabetes.
Like DMEs, the ontogeny of medication targets is likewise significant in the assessment and performance of pharmacogenetics. For example, multi-drug resistant protein 1 and ATPs binding cassette, whereas G2 is articulated earlier in childhood, though different transporters like Organic anion transporter or multi-drug safe protein 2 (MRP-2) display delayed the development and decreased articulation levels with the main long stretches of adolescence contrasted with the adults [13].
The above-described cases are only a couple of instances of ontogeny commitment to quality control variances. A current publication demonstrates that up to 689 types of genes are otherwise articulated in developmental stages advancement just in lymphoblastic cells. Utilizing genetic articulation profiling of lymphoblast cells, scientists had the option to recognize three specific gatherings: prepubertal which is under 7, pubertal that is from 7 years to 17 years, and early adulthood gathering which is more than 17 [5] proposing a lot of formative genes which may express in a freeway.
The effects of drugs and their responses to children or adults may reveal the importance of ontogeny. More exposure to ototoxicity with cisplatin and its treatment [14, 15], consequences for neurological advancement connected to methotrexate [16], more consent of the tacrolimus or lethargy to codeine in newborn children are nevertheless a couple of cases [17]. Finally, few of related cancer diseases include acute lymphoblastic leukemia (ALL), osteosarcoma (OS), and neuroblastoma (NB) and may appear primarily in younger ones than adults so that’s why it is linked to ontogeny giving extra help that pediatric pharmacogenomics ought to be considered as an unmistakable field. The shortage of data and agreement on ontogeny is as yet one of the significant constraints for an unmistakable comprehension of the utility of hereditary variations [18].
The primary step to evaluating gene-drug associations is to highlight individual drugs widely used across European clinics to treat pediatric cancer patients for conditions like brain tumors, leukemia, lymphomas, and solid tumors [19]. Primary drugs were included only to simplify the search, whereas any auxiliary treatments such as prophylactics or co-medications were ruled out. Secondly, it was important to check whether these drugs had gene-drug associations that were enlisted in the Pharmacogenomics Knowledgebase and were integrated into clinical guidelines of the Clinical Pharmacogenetics. PharmGKB is an accessible complete resource that checks and integrates facts on the effect of the genetic differences in drug reactions for clinical application or research. It is programmed to systematically extract gene-drug associations from scientific databases and evaluate evidence. Since CPIC is responsible for the preparation of medical strategies for the gene or drug associations that mollify the utmost criteria of confirmation with special emphasis on clinical importance, PharmGKB beautifully collaborates with it to give stunning gene-drug pairs and evidence for clinicians and researchers worldwide.
The method used to extract data on pediatric oncology pharmacogenomics starts with identifying and defining of drugs used in Europe. CPIC and PharmGKB collectively function to search out drug-gene pairs and the strongest evidence for their application. In terms of strength of evidence, genes are grouped accordingly into four classes. Finally, the research found in the pediatric section in CPIC is reviewed and evaluated.
As previously mentioned, PharmGKB rates gene-drug associations into four comprehensive groups founded on power (with “1” being the resilient and “4” being the feeblest) of indication for the association. The first level contains a gene or drug relations that indicate important p values in more than one cohort and rather with a larger magnitude of consequence. The second level consists of suggestions that were reproducible with studies besides them that do not show worth with the association showing the smaller magnitude of the effect. The third level is built on solitary studies presenting substantial association with the indication not reproduced. The fourth level is centered on individualized reports and in vitro, molecular, or functional assays [20]. The PharmGKB approach was tailored for this review with a few amendments in group 3rd and 4th level and both groups were merged in a solo group of a gene-drug linkage having a fairly little possibility of the entering medical trial in a period whereas group 1 and 2 were kept segregated.
3 Drugs with Pharmacogenetic Evidence
By the early screening, we were capable to classify the following drug-gene pairs with pharmacogenetic evidence like thiopurines/thiopurine S-methyltransferases (TPMT) pair, and cisplatin, carboplatin, irinotecan, and vincristine have moderate pharmacogenetic evidence.
3.1 Thiopurines/Thiopurine S-Methyltransferases (TPMT) Pair
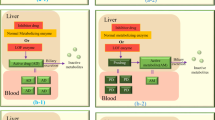
Pharmacogenomic pair of thiopurines with TPMT is perhaps the peak extensively deliberate drug-gene interface in medicine of pediatrics. Thiopurines are functional as prodrugs that transformed into thioguanine nucleotides by hypoxanthine-guanine phosphoribosyltransferase. TGNs are very cytotoxic mixtures that function by integrating into DNA or RNA producing damage to nucleic acids, finally leading to the expiry of cancerous cells. On the downside, TGNs can generate apoptosis in resistant cells producing ADRs mostly neutropenia, thrombocytopenia, as well as hepatotoxicity, commonly manifesting as a veno-occlusive disease [21]. TGNs inactivate through S-methylation by the cytosolic TPMT. TPMT action is affected by polymorphisms occurring in the gene [22, 23]. In medical determinations, the individuals are dispersed into three main groups: ordinary, intermediary, and reduced metabolizers created on the existence of one or two damages of functional alleles. Alleles 2, 3A, 3B, and 3C are by remote the most widely found irregular alleles and are expected to forecast up to 90% of the TPMT function [24]. Apart from the aforementioned, a total of 34 TPMT alleles have been discovered and termed in multiple inhabitants but with minor regularities [25] (Fig. 15.1).
Metabolism of TPMT thiopurine S-methyltransferase [26]
This drug-gene link takes significant medical effects because treatment consequences of childhood ALL with 6MP are very closely linked to maximum tolerable drug dose. The notion is supported by observations of concentration of TGNs and TPMT genotype, which collectively work in an inversely linked relationship to the capability of patients to tolerate full doses of 6MP. Subjects showing poor metabolism of TMPT were able to tolerate not more than 7% of 6MP dose in the children, with those with intermediate and normal metabolism ranging from 65% to 84% tolerance of the treatment in the treatment regime. TPMT metabolizers lost 2% of entire treatment weeks, the indifference of 16–76% of missed weeks for TPMT transitional and TPMT deprived metabolizers, respectively [27]. The normal metabolism of TMPT allowed lag of 2% in total handling weeks, whereas patients with intermediate and poor metabolism demonstrated a lack of response in 16% and 76% of total weeks, respectively. CPIC has developed guidelines that state dosage for patients with normal metabolism traits. A 30–70% decrease is optional for 6MP and 30–50% reduction for 6TG for the intermediate metabolizer class. Poor metabolism traits demanded to get 6MP or 6TG with a 90% decrease in dosage direction decreased to three times per week to avoid ADRs. Proactive patient testing has been actively called to reduce the chances of contrary drug responses in the situation of cancerous disease or to diminish time wanted for mounting titration of drug dose [28].
Identifying the significance of ontogeny, the indication on thiopurines and TPMT association assembled in pediatric cohorts. Childhood leukemia is flourishing childhood neoplastic disease, and a bulk amount of studies has been directed in the populace of pediatric in this regard [29].
3.2 Vincristine
Vincristine is extensively used as a mixture chemotherapeutic negotiator for handling brain tumors, leukemias, lymphomas, neuroblastoma, retinoblastoma, and rhabdomyosarcoma in pediatrics. Vincristine prevents microtubule formation by its toxic properties by ultimately leading to mitotic arrest and apoptosis. Twenty-five percent of both pediatric and adult patients grow medically important vincristine-induced peripheral neuropathy, influencing indirect activities that embrace reduction in dose or termination of cure [30]. CYP3A5 has mostly tangled vincristine metabolism and is articulated only in about 10–20% Caucasians and 80% of Americans [31]. Some contradictory rumors with most of the cases of lesser vincristine clearance in the patients holding deprived CYP3A5 metabolism are more susceptible to the development of VIPN [32]. Also, some populace transformations were detected for this association [33].
Fresh genome extensive suggestion research recognized an irregularity in the centrosomal protein 72 gene link with the VIPN throughout the extension phase of ALL handling with a large number of vincristine dosages [34]. Authors revealed in a similar study that a minor expression of CEP72 produced and enhanced the sensitivity to vincristine. These conclusions elevated confidence for safe vincristine dosage expectancy in ALL usage procedures. However, another reviewing study through the beginning stage of ALL conduct in children of Spanish could not support this link, due to alterations in the study project, populace, and phase of handling protocol [35].
4 Conclusion
The baseline recommendations for adults put forward by pediatric pharmacogenomics for thiopurines/TPMT association have shown consistency in results across multiple studies and depicted a profound effect on patients, and the study needs more attention. Thiopurines are one of the few drugs that were supported by strong evidence with its association with TPMT, but further research into the drug-gene relationship is needed before it can be put to practice in hospitals. Sensible indication gene-drug connections were typically formed for pharmacogenetics. Research turns up as either scarce or too complex to allow comparison due to variance created diagonally handling procedures, diseases, populaces, and assessable consequences. On the contrary, vincristine has been found sufficiently quoted in Dutch and French guidelines, suggesting a reduction of the drug dose in some cases of patients exhibiting low metabolism. CYP3A5 is a possible alternative to use lower doses while also implementing a protracted treatment regime. Lack of data available for this gene-drug pair in pediatric pharmacogenetics opens new doors to research on this gene. Pharmacogenetic gene-drug association studies, thereby urging vast study designs to produce reasonable results.
5 Future Directions
Before any extra gene-drug interaction-based trials can be initiated in hospitals, rigorous and in-depth analysis yielding strong evidence of gene-drug associations must be performed. PharmGKB provides a valuable platform for clinicians and scientists to analyze and interpret data from pharmacogenomics. Though it facilitates the end user, caution must be taken while interpreting lower-level gene-drug associations as statistics may vary in terms of gene-drug associations. A lacking element in pediatric pharmacogenomics deals with an ontology that requires proper comprehension of the impact of genetic variants. Multiple combinations of drugs used in the treatment of cancer also present a hurdle, which makes it difficult to identify the effect of a single drug component. Future research can be enhanced by creating a standardized treatment regimen spread over different institutions and pinpoint the exact genetic association with the overall treatment cycle.
References
McLeod HL, Evans WE (2001) Pharmacogenomics: unlocking the human genome for better drug therapy. Annu Rev Pharmacol Toxicol 41:101–121
Pritchard-Jones K, Dixon-Woods M, Naafs-Wilstra M, Valsecchi MG (2008) Improving recruitment to clinical trials for cancer in childhood. Lancet Oncol 9(4):392–399
Mitchell AA, Lacouture PG, Sheehan JE, Kauffman RE, Shapiro S (1988) Adverse drug reactions in children leading to hospital admission. Pediatrics 82(1):24–29
MacNeil M, Eisenhauer E (1999) High-dose chemotherapy: is it standard management for any common solid tumor? Ann Oncol 10(10):1145–1161
Nebert DW (1999) Pharmacogenetics and pharmacogenomics: why is this relevant to the clinical geneticist? Clin Genet 56(4):247–258
Stevens A, Hanson D, Whatmore A, Destenaves B, Chatelain P, Clayton P (2013) Human growth is associated with distinct patterns of gene expression in evolutionarily conserved networks. BMC Genomics 14(1):547
Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG (2013) Cancer drug resistance: an evolving paradigm. Nat Rev Cancer 13(10):714
Longley D, Johnston P (2005) Molecular mechanisms of drug resistance. J Pathol 205(2):275–292
Bar-Shalom D, Rose K (2014) Pediatric formulations: a roadmap, vol 11. Springer, New York
Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE (2003) Developmental pharmacology—drug disposition, action, and therapy in infants and children. N Engl J Med 349(12):1157–1167
Leeder JS, Kearns GL (1997) Pharmacogenetics in pediatrics: implications for practice. Pediatr Clin N Am 44(1):55–77
de Wildt SN, Kearns GL, Leeder JS, van den Anker JN (1999) Cytochrome P450 3A. Clin Pharmacokinet 37(6):485–505
Brouwer KL, Aleksunes LM, Brandys B, Giacoia GP, Knipp G, Lukacova V, Meibohm B, Nigam SK, Rieder M, de Wildt SN, Pediatric Transporter Working Group (2015) Human ontogeny of drug transporters: review and recommendations of the pediatric transporter working group. Clin Pharmacol Ther 98(3):266–287
Finkielstain GP, Forcinito P, Lui JC, Barnes KM, Marino R, Makaroun S, Nguyen V, Lazarus JE, Nilsson O, Baron J (2008) An extensive genetic program occurring during postnatal growth in multiple tissues. Endocrinology 150(4):1791–1800
Knight KRG, Kraemer DF, Neuwelt EA (2005) Ototoxicity in children receiving platinum chemotherapy: underestimating a commonly occurring toxicity that may influence academic and social development. J Clin Oncol 23(34):8588–8596
Kushner BH, Budnick A, Kramer K, Modak S, Cheung NKV (2006) Ototoxicity from high-dose use of platinum compounds in patients with neuroblastoma. Cancer 107(2):417–422
Bleyer W, Fallavollita J, Robison L, Balsom W, Meadows A, Heyn R, Sitarz A, Ortega J, Miller D, Constine L (1990) Influence of age, sex, and concurrent intrathecal methotrexate therapy on intellectual function after cranial irradiation during childhood: a report from the Children’s Cancer Study Group. Pediatr Hematol Oncol 7(4):329–338
Lazaryan M, Shasha-Zigelman C, Dagan Z, Berkovitch M (2015) Codeine should not be prescribed for breastfeeding mothers or children under the age of 12. Acta Paediatr 104(6):550–556
Uppugunduri RS, Ansari M (2016) Commentary: a myriad aberrations on information of ontogeny of drug metabolizing enzymes in the pediatric population: an obstacle for personalizing drug therapy in the pediatric population. Drug Metab Lett 10(2):72–74
Whirl-Carrillo M, McDonagh EM, Hebert J, Gong L, Sangkuhl K, Thorn C, Altman RB, Klein TE (2012) Pharmacogenomics knowledge for personalized medicine. Clin Pharmacol Ther 92(4):414–417
Szumlanski C, Otterness D, Her C, Lee D, Brandriff B, Kelsell D, Spurr N, Lennard L, Wieben E, Weinshilboum R (1996) Thiopurine methyltransferase pharmacogenetics: human gene cloning and characterization of a common polymorphism. DNA Cell Biol 15(1):17–30
Weinshilboum RM, Sladek SL (1980) Mercaptopurine pharmacogenetics: monogenic inheritance of erythrocyte thiopurine methyltransferase activity. Am J Hum Genet 32(5):651
Collie-Duguid E, Pritchard S, Powrie R, Sludden J, Collier D, Li T, McLeod H (1999) The frequency and distribution of thiopurine methyltransferase alleles in Caucasian and Asian populations. Pharmacogenetics 9(1):37–42
Appell ML, Berg J, Duley J, Evans WE, Kennedy MA, Lennard L, Marinaki T, McLeod HL, Relling MV, Schaeffeler E, Schwab M, Weinshilboum R, Yeoh AE, McDonagh EM, Hebert JM, Klein TE, Coulthard SA (2013) Nomenclature for alleles of the thiopurine methyltransferase gene. Pharmacogenet Genomics 23(4):242
Relling MV, Hancock ML, Rivera GK, Sandlund JT, Ribeiro RC, Krynetski EY, Pui CH, Evans WE (1999) Mercaptopurine therapy intolerance and heterozygosity at the thiopurine S-methyltransferase gene locus. J Natl Cancer Inst 91(23):2001–2008
Mlakar V, Huezo-Diaz Curtis P, Satyanarayana Uppugunduri C, Krajinovic M, Ansari M (2016) Pharmacogenomics in pediatric oncology: review of gene—drug associations for clinical use. Int J Mol Sci 17(9):1502
Relling M, Gardner E, Sandborn W, Schmiegelow K, Pui CH, Yee S, Stein CM, Carrillo M, Evans WE, Klein TE, Clinical Pharmacogenetics Implementation Consortium (2011) Clinical pharmacogenetics implementation consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing. Clin Pharmacol Ther 89(3):387–391
Swen J, Nijenhuis M, de Boer A, Grandia L, Maitland-van der Zee A-H, Mulder H, Rongen GA, van Schaik RH, Schalekamp T, Touw DJ, van der Weide J, Wilffert B, Deneer VH, Guchelaar HJ (2011) Pharmacogenetics: from bench to byte—an update of guidelines. Clin Pharmacol Ther 89(5):662–673
Pui C-H, Evans WE (2006) Treatment of acute lymphoblastic leukemia. N Engl J Med 354(2):166–178
Jordan MA, Toso RJ, Thrower D, Wilson L (1993) Mechanism of mitotic block and inhibition of cell proliferation by taxol at low concentrations. Proc Natl Acad Sci 90(20):9552–9556
Egbelakin A, Ferguson MJ, MacGill EA, Lehmann AS, Topletz AR, Quinney SK, Li L, McCammack KC, Hall SD, Renbarger JL (2011) Increased risk of vincristine neurotoxicity associated with low CYP3A5 expression genotype in children with acute lymphoblastic leukemia. Pediatr Blood Cancer 56(3):361–367
Xie H-G, Wood AJ, Kim RB, Stein CM, Wilkinson GR (2004) Genetic variability in CYP3A5 and its possible consequences. Pharmacogenomics 5(3):243–272
Moore AS, Norris R, Price G, Nguyen T, Ni M, George R, van Breda K, Duley J, Charles B, Pinkerton R (2011) Vincristine pharmacodynamics and pharmacogenetics in children with cancer: a limited-sampling, population modelling approach. J Paediatr Child Health 47(12):875–882
Sims RP (2016) The effect of race on the CYP3A-mediated metabolism of vincristine in pediatric patients with acute lymphoblastic leukemia. J Oncol Pharm Pract 22(1):76–81
Diouf B, Crews KR, Lew G, Pei D, Cheng C, Bao J, Wheeler HE (2015) Association of an inherited genetic variant with vincristine-related peripheral neuropathy in children with acute lymphoblastic leukemia. JAMA 313(8):815–823
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Zafar, B., Ghaffar, M., Salahuddin, H. (2020). History of Drug Reaction in Children Suffering from Cancer. In: Masood, N., Shakil Malik, S. (eds) 'Essentials of Cancer Genomic, Computational Approaches and Precision Medicine. Springer, Singapore. https://doi.org/10.1007/978-981-15-1067-0_15
Download citation
DOI: https://doi.org/10.1007/978-981-15-1067-0_15
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-1066-3
Online ISBN: 978-981-15-1067-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)