Abstract
Wax and paraffin precipitation is a major problem around the world, costing the petroleum industry billions of dollars yearly. As temperature drops below the Wax Appearance or Wax Precipitation Temperature (WAT/WPT) of crudes, paraffin starts to precipitate out and restrict or block the effective flow. There are different methods, such as mechanical and chemical remediation to deal with wax issues. Among the latter ones, the use of surfactants is favorably looked upon since they are small molecules with surface activity properties. This study aims to introduce novel aliphatic non-ionic surfactants with different chain length and degree of ethoxylation. In addition to chain length, the impact of branching on the hydrophobic part of the surfactants was also studied. A waxy crude oil from Brazil was characterized through determining its carbon distribution, WAT, viscosity and density based on industry standard methods. Several surfactants with different combinations of chain length/ethoxylation number were then selected for screening. The performance of surfactants was evaluated based on data obtained from treated crude versus the control sample through different experiments. Rheology studies were conducted at 50 to −10 °C and at shear rates of 5 and 300 s−1. The cold finger instrument was utilized to determine paraffin content of the untreated and treated crude. Finally, the paraffin crystal size was analyzed through microscopic studies. The results showed that shear rate can affect the wax treatment outcome as well as the effective concentration of surfactant. Therefore, it is important to assess the rheology at high and low shear rates. Some surfactants in the present study performed great at both low and high shear rates and were able to reduce the viscosity by 80% at temperatures well below WAT of the crude oil. The microscopy results confirmed that wax crystals were reduced in size and were more dispersed after treating the crude with these surfactants. The current study addresses the wax precipitation/deposition challenges of heavy crudes and proposes mitigating them through the use of some new non-aromatic non-ionic surfactants. The chemistries and findings of this research help the oil and gas industry to save money and time by mitigating flow assurance problems.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Crude oil is a commodity that is being actively traded and affects the economy and politics of the whole world. Crude oils are categorized and characterized based on physical and chemical properties. Asphaltene and resins are important components of the complex dispersive system of oil. Asphaltene in a colloidal state can be the attraction and precipitation focal points for the resins molecules that are dissolved in the oil (Can attract the resins and act as nucleation sites for them). Asphaltene-resin complexes can react with liquid hydrocarbons and these tiny aggregates can coagulate with Asphaltene-resin complexes below the crystallization temperature of hard paraffin. Therefore, Asphaltene-resin-paraffin complexes are created (Turbakov and Riabokon 2014; Kumar et al. 2016). Waxes are formed from living and fossilized sources or through a synthetic process and they are polydisperse association of polymethylene chain compounds. Although the petroleum wax that is separated in the refineries is very useful in other consumer products, it is very problematic in some petroleum production operations (Jennings and Weispfennig 2006). In particular, crude oils with high concentrations of high molecular weight paraffin are more susceptible to wax problems (Jennings and Weispfennig 2006). There is a significant difference in chemical composition of waxes; however, thermo plasticity appears to be their universal physical characteristic (Struchkov and Rogachev 2017). Wax is soluble in oil reservoirs under high pressure high temperature (HPHT) conditions. As the temperature is decreased below the wax appearance temperature (WAT), the wax crystallization process is started.
Organic deposition is mainly the result of continuous three-dimensional macro-molecular structure formation that is stabilized by Asphaltene and resin in the crude oil (Struchkov and Rogachev 2017). The precipitated wax crystal can deposit on the internal surface of pipeline throughout different phases of production either in offshore or onshore environment. Furthermore, wax deposition during oil flow causes reduction in effective flow rate or can completely block the flow (Labes-Carrier et al. 2002; Harun et al. 2016).
There are three different methods to deal with wax issues such as mechanical, thermal, and chemical, which each have their own advantages and drawbacks. Traditional treatments such as hot oiling and hot watering still are used to address wax deposition and precipitation that is a significant problem for oil industry. The biggest advantages of these methods are simplicity, cost, and immediate results. However, one of the important considerations in hot oiling/watering treatment is their effectiveness since the heat capacity of injected fluid is much lower than the heat capacity of the well, so at higher depths this practice is questionable (White et al. 2017). Moreover, a lot of hot oiling treatments are conducted using the same oil caused the wax deposition to reoccur and this can be problematic since the wax carrying capacity of this oil is low or it wouldn’t precipitate wax (Becker 2000). Formation damage due to different reasons can also happen during hot oiling treatments (Barker 1989).
Hot watering and hot watering surfactant were introduced as an alternative to hot oiling, however, plain hot water treatments cannot provide the solvency that hot oiling offers. Hot watering in the presence of surfactants usually produce large water wet particles and as a result more demulsifiers are required to address water wet waxes. Aromatic and aliphatic solvents can also be used as a method to address the wax problem; however, this method is used without the benefit of using heat. Molecular level separation can be achieved through using the solvents with high wax carrying capacity (Becker 2000).
Mechanical means are the oldest techniques to remove wax, which are economical. The main disadvantages with these methods are perforation plugging and wireline scrapers being stuck in wells during post-cleaning stages (White et al. 2017).
Chemical methods can be very effective since they work at molecular level. Wax inhibitors (WI) can be added to oils to diminish oil transportation issues, and they categorize into wax crystal modifiers, dispersants, and pour point depressants (PPD) (White et al. 2017). The crystal growth of waxes can be reduced through the use of WI and as a result the smaller crystals allow larger free space of the liquid fraction of crude oil to flow freely (Halim et al. 2011). One of the disadvantages of PPDs and polymeric WIs is that their performance is poor at temperatures below WAT under higher shear rates. The latter can be due to the breakage of crystal-crystal bond under high shear rate and lack of its reforming (Daraboina et al. 2016). Another problem is with the degradation of large molecules of PPDs so smaller molecules are favorable. On the other hand, non-ionic surfactants are becoming more important since they don’t pose any incompatibility issues with other additives and surfactants and they are soluble in water and many organic solvents. Non-ionic surfactants are better choices than ionic surfactants due to some advantages such as more degradability and low toxicity. The most typical and used non-ionic surfactants are ethoxylated compounds due to availability of many hydrophobes and lower cost (El-Shamy et al. 2011). Therefore, the objectives of the present study were to: (1) Investigate the effect of non-ionic surfactants as rheology modifiers (2) Study the impact of hydrophobe structure, number of EO and PO in non-ionic surfactants as wax inhibitor/dispersant, (3) Assess the performance of these non-ionic surfactants through cold finger experiments.
To investigate the deliverability of these objectives, laboratory tests were conducted to study: steady shear viscosity measurements as crude was cooled down below its WAT, microscopic studies of untreated and treated crude, and wax deposition rate of treated and untreated crude through cold finger.
2 Materials and Experimental Studies
2.1 Crude Oil Samples
Oil samples were received from Lagoa do Paulo field, and they were heated and homogenized. Saturates, Aromatic, Resin, Asphaltene (SARA) analysis and HTGC were conducted to determine crude oil properties and carbon distribution based on ASTM D2007-11 and ASTM D5442-93, respectively. Tables 1 and 2 show the crude oil characteristics. Wax appearance temperature of the crude oil was determined based on the viscosity deflection method (Fig. 1). High shear forces are required for better flow of crude oil through pipelines based on the surface tension of 29 m N m−1. It can be seen that this crude is high in saturates (Fig. 2).
2.2 Non-ionic Surfactants
Different non-ionic surfactants were used in the present study with varying hydrophobe chain length, structure, and EO/PO (Table 3). The physical properties of selected surfactants are shown in Table 4. The fact that some of these surfactants have a very low pour point is very favorable since it eases field application under cold temperature conditions.
2.3 Viscosity Measurements
A coaxial cylinder, rotational viscometer with R1/B1 combination and ±0.5% of torque span or better was used to assess the viscosity profile of untreated and treated crude oil. Refrigerated and Heating Circulator for heating and cooling purposes was used in rheology measurements with a temperature stability of ±0.01 °C. The oil sample was heated to 50 °C and then cooled down to −10 °C at a cooling rate of 1.2 °C/min. The viscosity measurements for each sample were conducted at shear rates of 5 and 300 s−1. The viscosity profile of treated crude oil was compared against the viscosity of the control sample (untreated crude).
2.4 Optical Microscopy
A small aliquot of sample was placed onto a microscope slide then a cover slip was placed on top. Images were collected utilizing a 10×, 20×, and 50× objective lenses. The data were collected on an Olympus BX51 research grade optical compound microscope with a ColorView IIIu or CoolSNAP digital color charged-coupled device (CCD) camera. All the images were collected in the bright field reflectance mode with polarized light.
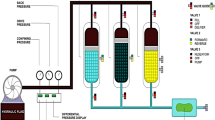
2.5 Cold Finger
The cold finger consists of a closed stainless steel tube (straight, diameter 12 mm, length 72 mm), inside which flows a cooling fluid with temperature range accuracy of ±0.2 °C. The cold finger is submerged inside the oil sample that is heated and homogenized with the aid of a stirrer. The stirring rate was set at 500 RPM throughout the test. When the cold finger is cooled below the WAT of the crude, wax starts to deposit on the cold finger surface and as a result the cold finger mimics the oil pipeline’s wall. The instrument is a multi-cold finger and six cold finger tests can be run simultaneously and each one is temperature controlled through valves allowing the specific amount of cooling fluid to flow through each cold finger.
3 Results and Discussion
3.1 Effect of Non-ionic Surfactants Structures on Their Performance as Wax Inhibitors/Dispersants
Nine different surfactants were tested in this study, and their characteristics are summarized in Table 3. The crude oil was treated with 500 ppm of surfactant above the WAT of the crude oil and then the viscosity was measured as the sample was cooled down to temperatures well below the WAT. It can be seen from Figs. 3 and 4 that TERRAVIS M4 outperformed TERRAVIS M7 and M9, however, TERRAVIS S4, S7, and S9 almost performed the same. Figure 5 shows the effect of each surfactant on the viscosity of crude oil as it was cooled down to 10 °C. All of the surfactants were effective to decrease the viscosity of crude oil at the applied shear rate of 5 s−1 and temperature ramp of 40 to 10 °C. Moreover, based on the comparison between the surfactants with medium chain length hydrophobe (TERRAVIS M4 and TERRAVIS S4) TERRAVIS M4 performed better and this can be due to the degree of branching of hydrophobe. They both have the same chain length of hydrophobe and the same number of EO; however, TERRAVIS M4 is highly branched, whereas TERRAVIS S4 is semi-branched.
Any fluid in the oil and gas industry is exposed to different shear rates during different stages of any treatment. For example high shear rates are applied to waxy crudes during long distance transportations whereas low shear rates inside the oil pipelines is existed during long-term shutdowns. Therefore, it is crucial to assess the performance of any treatment fluid under both high and low shear rates. Based on the results of the present study, the effective concentration of surfactant to reduce the crude oil viscosity may change under high shear environment. The viscosity of the crude oil after treating it with 500 ppm of each surfactant was measured at 300 s−1 (Fig. 6). TERRAVIS M4 and TERRAVIS S4 were not able to inhibit/disperse wax crystals in the crude oil at 300 s−1. TERRAVIS N outperformed all the other surfactants and TERRAVIS N’ also was able to reduce the viscosity of the crude compared to untreated crude oil. A better performance of TERRAVIS N compared to TERRAVIS N’ due to the fact that TERRAVIS N has less linear hydrophobe since all the other factors (hydrophobe chain length, number of PO and EO) are the same for both surfactants. A possible explanation is that the side chain or branching can cause more hindrance effect so the crystal growth would be retarded more effectively (Daraboina et al. 2016).
Poor performance of TERRAVIS M4 and S4 (at 500 ppm dosage) at high shear rate can be attributed to higher heat transfer rate and increased rate of molecular collisions. Moreover, wax crystals can break under shear and these fragments can serve as a new nucleation sites for future crystal growth (Blake and Marangoni 2015). Higher shear rate and faster cooling rate also can decrease the interaction time between the treatment chemical and wax crystals. Moreover, for an increased porosity lattice, such as the one TERRAVIS N would form, the entrapped liquid oil would still be able to flow through the pores, especially at higher shear, which supports again the observed behavior of TERRAVIS N and TERRAVIS M4 at higher shear rates.
To investigate the effect of concentration of TERRAVIS M4 on its performance at shear rate of 300 s−1, concentrations of 1000 and 1500 ppm were examined. Furthermore, it was found that 1500 ppm of TERRAVIS M4 is able to reduce the viscosity of crude oil compared to untreated crude when the temperature is decreased from 50 to 10 °C at a fixed shear rate of 300 s−1. However, 500 ppm of TERRAVIS N still outperformed 1500 ppm of TERRAVIS M4 (Fig. 7).
3.2 Microscopic and Particle Size Studies
The optical polarized microscope can recognize the crystalline areas inside the sample due to the fact that they have different refractive indexes compared to the surrounding hydrocarbon environment. The wax crystals can be seen in Fig. 8a–c. It is obvious that the wax crystals in untreated crude are denser (Fig. 8a), which triggers agglomeration and as a result, higher viscosity of crude as it cools down. Treated crude using TERRAVIS N leads to less nuclei number density and different wax crystals sizes (Fig. 8b) that can be the reason behind its better performance compared to other surfactants regarding the ability to reduce the viscosity of the crude oil. In the case of TERRAVIS M4, the wax crystals are significantly smaller (Fig. 8c) than the wax crystals in the presence of TERRAVIS N, but higher density of nuclei is observed.
Wax crystals shape and distribution at 22 °C and a magnification of 20×, a blank crude oil, b crude oil + 500 ppm of TERRAVIS N, c crude oil + 500 ppm of TERRAVIS M4. The wax crystals are bigger but less and more disperse (in the presence of TERRAVIS N) and smaller and denser wax crystals can be seen (in the presence of TERRAVIS M4). Both surfactants were successful to disperse/inhibit wax crystals from further growth
The results of this section are in agreement with the findings from the rheology section, which showed that TERRAVIS N outperformed TERRAVIS M4 in reducing the crude oil viscosity as it was exposed to temperatures well below WAT.
3.3 Cold Finger Studies
The wax inhibition studies were conducted to assess the performance of each surfactant. The samples were heated to 55 °C and then the fingers were cooled down to 25 °C. Figure 9 shows the wax deposition at the end of the tests. TERRAVIS M4 could reduce the wax deposition by 30%, whereas TERRAVIS N did not appear to decrease the wax deposition although its performance was better than the other surfactants in regards to reducing the viscosity of crude and producing less wax crystals based on microscopic studies. Second round of cold finger experiments was run where the samples were cooled down from 55 to 18 °C. The wax deposit on the cold finger wax and crude oil entrained within the deposit matrix (Jennings and Weispfennig 2006).
It should be noted that the reason why TERRAVIS M4 a thinner deposition compared to TERRAVIS N can be due to the wax porosity. The free space between the wax crystals, which is filled by the confined liquid oil, defines wax porosity and a high wax porosity normally results in a thicker but softer wax deposit (Labes-Carrier et al. 2002). In addition, the small crystals favored by TERRAVIS M4 are more likely to fill the pore spaces inside the deposit, leading to a higher degree of compaction and a thinner deposit. However, larger crystals can buildup on the deposit surface and create a thicker deposit (Daraboina et al. 2016).
The wax deposit on the fingers was analyzed using optical microscope (Fig. 10). Figure 10a shows a continuous network of wax crystals. In the presence of 500 ppm of TERRAVIS N and TERRAVIS M4 (Fig. 10 a, b), the wax crystals became dispersed and the continuous network is not existed anymore.
Wax crystals shape and distribution at 22 °C and a magnification of 20×, a blank crude oil, b crude oil + 500 ppm of TERRAVIS N, c crude oil + 500 ppm of TERRAVIS M4. The wax crystals are more disperse (in the presence of TERRAVIS N) and smaller and denser wax crystals can be seen (in the presence of TERRAVIS M4) compared to blank crude. Both surfactants were successful to disperse/inhibit wax crystals from further growth
4 Conclusions
Different non-ionic surfactants were used as wax inhibitors/dispersants for high paraffinic crude. Several techniques and experiments were run to assess the performance of each surfactant. The conclusions below are based on the results and findings of the present study:
-
1.
Medium and long chain non-ionic surfactants in this study are capable of reducing the precipitation and deposition of wax.
-
2.
Two surfactants outperformed the other ones, one with low HLB and the other one with high HLB. The latter is advantageous since some surfactants are only effective in the presence of water.
-
3.
The hydrophobe with higher degree of branching and more EO (smaller and more polar ones) can be considered more as a wax inhibitor rather than dispersant due to the hindrance effect and polar-non-polar repulsive interactions. Longer chain hydrophobes are effective more as dispersant.
This study shows that despite the complex nature of the possible interactions occurring between rheology modifiers or pour point depressants and different types of crudes with a wide SARA distribution, a good understanding of a few basic facts about the latter ones allows for a good choice of surfactants and thus for the operational success sought for by the service companies or operators dealing with paraffin/asphaltene deposits of heavy and/or high paraffinic oils.
References
Barker, K.M.: Formation damage related to hot oiling. SPE Prod. Eng. 4(4): 371–375 (1989). SPE-16230-PA. https://doi.org/10.2118/16230-PA
Becker, J.R.: Oilfield paraffin treatments: hot oil and hot water compared to crystal modifiers. In: Presented at the SPE Annual Technical Conference and Exhibition, 1–4 October, Dallas, Texas. SPE-63123-MS. https://doi.org/10.2118/63123-MS (2000)
Blake, A.I., Marangoni, A.G.: The effect of shear on the microstructure and oil binding capacity of wax crystal networks. Food Biophys. 10(04), 403–415 (2015). https://doi.org/10.1007/s11483-015-9398-z
Daraboina, N., Soedarmo, A., Sarica, C.: Microscopic study of wax inhibition mechanism. In: Presented at the Offshore Technology Conference, 2–5 May, Houston, Texas. SPE-26973-MS. https://doi.org/10.4043/26973-MS (2016)
El-Shamy, O.A.A., Khid, T.T., Doheim, M.M.: Effect of ethoxlate chain length on the pour point depressant of middle distillate fuel oil. J. Disp. Sci. Technol. 32(05), 654–658 (2011). https://doi.org/10.1080/01932691003800023
Halim, N., Ali, S., Nadeem, M., et al.: Synthesis of wax inhibitor and assessment of squeeze technique application for malaysian waxy crude. In: Presented at the SPE Asia Pacific Oil and Gas Conference and Exhibition, 20–22 September, Jakarta, Indonesia. SPE-142288-MS. https://doi.org/10.2118/142288-MS (2011)
Harun, A., Ab Lah, N.K.I.N., Husin, H., et al.: An overview of wax crystallization, deposition mechanism and effect of temperature and shear. In: Presented at the 2016 International Conference on Industrial Engineering, Management Science and Application (ICIMSA) (2016)
Jennings, D.W., Weispfennig, K.: Effect of shear on the performance of paraffin inhibitors: coldfinger investigation with Gulf of Mexico crude oils. Energy Fuels 20(06), 2457–2464 (2006)
Kumar, R., Banerjee, S., Mandal, A., et al.: Improvement in transportability of indian heavy crude oil using novel surfactant. Indian J. Chem. Technol. 23(04), 262–270 (2016)
Labes-Carrier, C., Ronningsen, H.P., Kolnes, J., et al.: Wax deposition in north sea gas condensate and oil systems: comparison between operational experience and model prediction. In: Presented at the SPE Annual Technical Conference and Exhibition, 29 Sept 2 October, San Antonio, Texas. SPE-77573-MS. https://doi.org/10.2118/77573-MS (2002)
Struchkov, I.A., Rogachev, M.K.: Risk of wax precipitation in oil well. Nat. Resour. Res. 26(1), 67–73 (2017)
Turbakov, M.S., Riabokon, E.P.: Improving of cleaning efficiency of oil pipeline from paraffin. In: Presented at the SPE Russian Oil and Gas Exploration and Production Technical Conference and Exhibition, 14–16 Oct, Moscow, Russia. SPE-171295-MS. https://doi.org/10.2118/171295-MS (2014)
White, M., Pierce, K., Acharya, T.: A review of wax-formation/mitigation technologies in the petroleum industry. SPE Prod. Oper. 33(03): 476–485. SPE-189447-PA. https://doi.org/10.2118/189447-PA (2017)
Acknowledgements
The authors thank Sasol Performance Chemicals for permission to present and publish this paper. Riaan Bekker and Rebecca Sanders are acknowledged for their analytical support.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Sokhanvarian, K., Diarra, A., Fernandez, J.M., Stanciu, C. (2020). Wax Control in Paraffinic Crudes: Investigating the Effectiveness of Novel Non-ionic Surfactants as Wax Inhibitor/Dispersant. In: Lin, J. (eds) Proceedings of the International Petroleum and Petrochemical Technology Conference 2019. IPPTC 2019. Springer, Singapore. https://doi.org/10.1007/978-981-15-0860-8_21
Download citation
DOI: https://doi.org/10.1007/978-981-15-0860-8_21
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-0859-2
Online ISBN: 978-981-15-0860-8
eBook Packages: EnergyEnergy (R0)