Abstract
Enthalpy recovery wheel is a high-efficiency heat recovery device with fresh air and return air, which can recycle both the obvious and latent heat from return air at the same time, and reduced energy consumption for handling fresh air. In this paper, a mathematical model of enthalpy recovery wheel was established, taking into account the air side and adsorbent side. The heat and mass transfer equation of the enthalpy recovery wheel through the coupled heat and mass transfer equations on the air side and the adsorbent side was established. The COMSOL Multiphysics which multiphysics coupling software is used to simulate the effect of fresh air inlet temperature, air inlet humidity, and face velocity on the enthalpy recovery wheel under summer conditions, revealing the heat and mass exchange performance of the enthalpy recovery wheel. This provides a reference for the optimal operation of the enthalpy recovery wheel.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
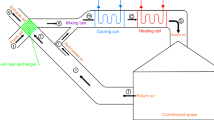
Desiccant wheels have two major applications: air dehumidification [1, 2] and enthalpy recovery [3, 4]. For dehumidification wheels, process air is dried after it flows through the wheel, which rotates constantly between the process air and a hot regenerative air stream. However, enthalpy recovery wheels are used to recover energy by transferring sensible and latent heat between supply air and exhaust air. Due to different operating conditions, heat and moisture transfer behaves quite differently in the wheels [5]. The enthalpy recovery wheel handles a large volume of air, flexible in layout, high heat recovery efficiency, and easy to clean. It is widely used as a heat recovery component in large air handling units. Enthalpy recovery wheel uses the wheel as the only heat exchange core to maintain 10–25 r/min speed rotation, and its performance determines the efficiency of the entire heat recovery system [6].
Pan and Gang [7] analyzed the working characteristics and energy-saving effect of the enthalpy recovery wheel. Nóbrega and Brum [8] established a mathematical model for the enthalpy wheel, an effectiveness number of thermal units (NTU) analysis is carried out. La [9] compared the effects of the changes of wheel thickness, rotation speed, and face velocity on the enthalpy recovery wheel efficiency when silica gel and lithium chloride were used as moisture absorbents. Horton [10] established a one-dimensional transient heat and mass transfer model and analyzed the performance of enthalpy recovery wheels both with and without purge air.
In this paper, the physical model of the enthalpy recovery wheel was established, taking into account the air side and adsorbent side. A coupled heat and mass transfer equation for the air and adsorbent side was established. The porous media saturated heat and mass transfer model is applied to the adsorbent side. Numerical simulation was carried out by using COMSOL Multiphysics, investigated the effect of the temperature of process air, humidity of process air, and face velocity on the performance of enthalpy recovery wheel under typical summer conditions.
2 Model of Enthalpy Recovery Wheel
Simulation study on the enthalpy recovery wheel with silica gel as adsorption material and rotational speed as 10 r/min, and the shape of the honeycomb channel is sinusoidal. The enthalpy recovery wheel is composed of the air and the adsorbent side, in which the adsorbent side is a porous medium composed of solid skeleton and pores, and the pores contain liquid water and gaseous steam. Silica gel remains solid during adsorption and desorption, and the adsorption process is generally physical adsorption [11].
2.1 Teat and Mass Transfer Model
For the air side and the adsorbent side of the enthalpy recovery wheel, establish the two-dimensional geometric model shown in Fig. 1 and assume that:
-
(1)
The diffusivity of steam and air are assumed to be constant.
-
(2)
The inlet air conditions are uniform in space.
-
(3)
The adsorbent material is isotropic.
-
(4)
All honeycombed channels in the wheel are identical and evenly distributed over the entire wheel.
-
(5)
The analytical heat of silica gel is approximately equal to the adsorption heat.
-
(6)
The adsorption potential energy of the pore surface of the adsorption material is negligible.
-
(7)
The physical parameters of steam and liquid water are constant.
-
(8)
The heat dissipation of the wheel shell is negligible.
-
(9)
The effect of centrifugal force is negligible
Based on the above assumptions, the mathematical model of enthalpy recovery wheel is established:
-
(1)
Energy balance differential equation on the air side:
$$\frac{{\partial \left( {\rho_{a} C_{pa} T_{a} } \right)}}{\partial t} - \frac{\partial }{\partial x}\left( {\lambda_{a} \frac{{\partial T_{a} }}{\partial x}} \right) - \frac{\partial }{\partial y}\left( {\lambda_{a} \frac{{\partial T_{a} }}{\partial y}} \right) = \frac{1}{{d_{e} }}h\left( {T_{d} - T_{a} } \right)$$(1) -
(2)
Mass balance differential equation on the air side:
$$\frac{{\partial c_{a} }}{\partial t} - \frac{\partial }{\partial x}\left( {K_{a} \frac{{\partial c_{a} }}{\partial x}} \right) - \frac{\partial }{\partial y}\left( {K_{a} \frac{{\partial c_{a} }}{\partial y}} \right) = K\left( {c_{v} - c_{a} } \right)$$(2) -
(3)
Energy balance differential equation on the adsorbent side:
$$\frac{{\partial \left( {\rho_{cp} C_{cp} T_{d} } \right)}}{\partial t} - \frac{\partial }{\partial x}\left( {\lambda_{cp} \frac{{\partial T_{d} }}{\partial x}} \right) - \frac{\partial }{\partial y}\left( {\lambda_{cp} \frac{{\partial T_{d} }}{\partial y}} \right) = \frac{1}{{d_{z} }}h\left( {T_{a} - T_{d} } \right) + Mq_{st}$$(3) -
(4)
Gas phase mass balance differential equation on the adsorbent side:
$$\frac{{\partial c_{v} }}{\partial t} - \frac{\partial }{\partial x}\left( {K_{dv} \frac{{\partial c_{v} }}{\partial x}} \right) - \frac{\partial }{\partial y}\left( {K_{dv} \frac{{\partial c_{v} }}{\partial y}} \right) = K\left( {c_{v} - c_{a} } \right) + M$$(4) -
(5)
Liquid phase mass balance differential equation on the adsorbent side:
$$\frac{{\partial c_{l} }}{\partial t} - \frac{\partial }{\partial x}\left( {K_{dl} \frac{{\partial c_{l} }}{\partial x}} \right) - \frac{\partial }{\partial y}\left( {K_{dl} \frac{{\partial c_{l} }}{\partial y}} \right) = M$$(5)
The initial conditions for the adsorbent and air are:
The temperature and humidity boundary conditions for the air are:
The above governing Eqs. (1)–(5), initial conditions (6)–(7) and boundary conditions (8)–(9), constitute a complete mathematical model of the enthalpy recovery wheel.
2.2 Performance Indexes
-
(1)
Apparent heat exchange:
$$Q_{t} = G_{p} c_{p} \left( {T_{1} - T_{2} } \right)$$(10) -
(2)
Latent heat exchange:
$$Q_{d} = G_{p} \gamma \left( {Y_{1} - Y_{2} } \right)$$(11)
3 Model Parameters
The predefined interface [12, 13] of COMSOL Multiphysics is used to simulate. The standard inlet parameters and parameter changes during simulation are shown in Table 1, and the defined parameters in the model are shown in Table 2.
4 Results and Discussion
When the return air parameters are constant, the face velocity, inlet temperature, and relative humidity of fresh air are, respectively, changed to simulate the change of the performance of the wheel in one turn (6 s).
-
(1)
Face velocity
When other conditions are under standard conditions, the face velocity is changed from 1 to 3 m/s, simulating the effect of face velocity on the performance of the enthalpy recovery wheel.
Figure 2a, b, c shows the single-channel temperature distribution of fresh air when the face velocity increases from 1 to 3 m/s, and it can be observed that the temperature range near the wall surface of the micro-channel is large. With the increase of face velocity, only the temperature near the wall of the micro-channel changes and the amplitude is small. The analysis shows that the increase of the face velocity shortens the time that the fresh air stays in the micro-channel and shortens the heat transfer time between fresh air and adsorbent.
As shown in Figs. 3 and 4, \(Q_{t}\) and \(Q_{d}\) decrease with an increasing face velocity. The analysis shows that the increase of face velocity makes the time of air stay in the wheel shorter, and the heat and moisture exchange is not sufficient. In addition, the hygroscopic ability of adsorbent decreases with the increase in face velocity, which deteriorates the performance of the adsorbent and weakens the hygroscopic ability to fresh air. Therefore, \(Q_{t}\) and \(Q_{d}\) are gradually reduced. In practical application, under the condition of ensuring airflow, a larger wheel should be chosen to increase the airflow channel area and reduce the face velocity.
-
(2)
Fresh air temperature
Temperature of fresh air increases from 28 to 37 °C when other operating conditions are in standard conditions, simulating the influence of fresh air temperature on the performance of the enthalpy recovery wheel.
As shown in Fig. 5, \(Q_{t}\) increases with temperature of fresh air, and the rise of fresh air temperature increases the temperature difference between fresh air and adsorbent and strengthens the heat transfer of the fresh air through the wheel, and so, \(Q_{t}\) increases. As shown in Fig. 6, the increase of air temperature causes a slight reduction of \(Q_{d}\), The surface temperature of absorbent rises with the fresh air temperature, which causes the water vapor partial pressure difference between the fresh air and the adsorbent to decrease, and the mass transfer driving potential difference is reduced, which weakens the moisture transfer of the fresh air through the wheel, so \(Q_{d}\) decreases.
-
(3)
Fresh air relative humidity
Relative humidity is increased by 65% from 50% (the corresponding moisture content increased from 17.7 to 23.21 g/kg) when other operating conditions are in standard conditions, simulating the influence of fresh air relative humidity on the performance of the enthalpy recovery wheel.
As shown in Fig. 7, \(Q_{t}\) remains basically unchanged with the increase of the relative humidity, and the fresh air humidity has no influence on the sensible heat exchange. As shown in Fig. 8, \(Q_{d}\) increase with inlet humidity, When fresh air is more humid, a higher difference of vapor partial pressure between fresh air and the surface of the adsorbent side, which enhances the moisture transfer, so \(Q_{d}\) increases.
5 Conclusion
In this paper, the heat and mass transfer equations are established for the air side and the adsorbent side of the single channel of the enthalpy recovery wheel, and the numerical simulation is solved using COMSOL Multiphysics. The temperature distribution characteristics of the enthalpy recovery wheel are studied, and the influence of face velocity, fresh air temperature, and fresh air humidity on the performance parameters of the enthalpy recovery wheel is analyzed. By simulation results, it obtains the following conclusions: (1) The temperature distribution on the fresh air side of the enthalpy recovery wheel indicates that the temperature range near the wall is large, and the heat exchange mainly occurs near the wall surface. (2) \(Q_{t}\) and \(Q_{d}\) gradually decrease with an increasing face velocity. Therefore, in practical applications, the face velocity should not be too large in the case of ensuring airflow. (3) \(Q_{t}\) gradually increases and \(Q_{d}\) decreases slightly with the increase of fresh air temperature. (4) remains basically unchanged and the \(Q_{d}\) increases with the increase of relative humidity of fresh air.
Abbreviations
- c :
-
Water vapor concentration, g/m3
- \(c_{p}\) :
-
Constant pressure specific heat capacity, J/(kg k)
- \(c_{\text{l}}\) :
-
Liquid water concentration on the side of the adsorbent, g/m3
- \(C_{pa}\) :
-
Specific heat of air, J/(kg K)
- \(C_{cp}\) :
-
Adsorption, liquid phase and gas combined with specific heat, J/(kg K)
- \(d_{z}\) :
-
Adsorbent layer thickness, m
- \(d_{e}\) :
-
Hydraulic diameter, m
- \(G_{p}\) :
-
Exhaust volume, kg/s
- h :
-
Heat transfer coefficient, W/(m2 K)
- K :
-
Mass transfer rate, 1/s
- \(K_{a}\) :
-
Air–steam diffusion coefficient, m2/s
- \(K_{dv}\) :
-
Air–Steam effective diffusion coefficient of gas phase, m2/s
- \(K_{dl}\) :
-
Liquid water diffusion coefficient on the side of the adsorbent, m2/s
- M :
-
Dehumidification (parsing) volume, g/(m3 s)
- \(q_{st}\) :
-
Heat of sorption, J/kg
- \(Q_{t}\) :
-
On apparent heat exchange, kw
- \(Q_{d}\) :
-
Latent heat exchange, kw
- W :
-
Extent of adsorption kg adsorbate/kg adsorbent
- \(\lambda_{cp}\) :
-
Adsorbent, liquid phase and gas phase combined with thermal
- t :
-
Time, s
- T :
-
Temperature, K
- u :
-
Face velocity, m/s
- x :
-
Axial coordinate, m
- y :
-
Axial coordinate, m
- Y :
-
Moisture content, g/kg
- T :
-
Temperature, K
- γ :
-
Latent heat of vaporization, kJ/kg
- \(\lambda\) :
-
Thermal Conductivity, W/(m K)
- \(\rho_{da}\) :
-
Gas phase density on the adsorbent side, kg/m3
- \(\rho\) :
-
Density, kg/m3
- \(\rho_{cp}\) :
-
Adsorbent, liquid phase and gas phase bonding density, kg/m3
- a :
-
Air
- d :
-
Adsorbent
- f :
-
Fresh air
- r :
-
Return air
- 0:
-
Initial state
- 1:
-
Import
- 2:
-
Export
References
Kodama, et al.: Experimental study of optimal operation for a honeycomb adsorber operated with thermal swing. J. Chem. Eng. Jpn. 26(5), 530–535 (1993)
Tauscher, R., et al.: Transport processes in narrow channels with application to rotary exchangers’. Heat Mass Transf. 35(2), 123 (1999)
Besant, R.W., Simonson, C.J.: Heat and moisture transfer in energy wheels during sorption, condensation, and frosting conditions, J. Heat Transf.: Trans. ASME 120(3), 699–708 (1998). Contribution, F.: Title. In: 9th International Proceedings on Proceedings, pp. 1–2. Publisher, Location (2010)
Kassai, M.: Experimental investigation on the effectiveness of sorption energy recovery wheel in ventilation system. Exp. Heat Transf. 31(2), 106–120 (2018)
Zhang, L.Z., Niu, J.L.: Performance comparisons of desiccant wheels for air dehumidification and enthalpy recover. Appl. Therm. Eng. 22(12) (2002)
Fang, J.H., et al.: Mathematical model performance analysis with variable condition of enthalpy recovery wheel. J. Shanghai Jiaotong Univ. 48(6), 809–815 (2014)
Pan S.H, Gang, Z.: Character analysis on the running wheels of air ventilator with total thermal recovery. Refrig. Air Cond. Electr. Power Mach. 28(5), 76–78 (2009)
Nóbrega, C.E.L., Brum, N.C.L.: Modeling and simulation of heat and enthalpy recovery wheels. Energy 34(12), 2063–2068 (2009)
La, D., et al.: Numerical simulation of characteristics of enthalpy recovery wheel with different desiccants. J. Chem. Ind. Eng. 59(S2), 64–69 (2008)
Horton, W.T., et al.: Modeling analysis of an enthalpy recovery wheel with purge air. Int. J. Heat Mass Transf. 55(17–18), 4665—4672 (2012)
Rouquerol, etc., Adsorption by powders and porous solids: principles, methodology and applications, Academic Press (2013)
Datta, A.K.: Porous media approaches to studying simultaneous heat and mass transfer in food processes. II: property data and representative results. J. Food Eng. 80 (2013)
Datta, A.K.: Porous media approaches to studying simultaneous heat and mass transfer in food processes. I: problem formulations. J. Food Eng. 80 (2007)
Acknowledgements
This work is supported by the Natural Science Foundation of China (NSFC) (Number51176104).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Fan, H., Chen, L. (2020). Analysis of Heat and Mass Exchange Performance of Enthalpy Recovery Wheel. In: Wang, Z., Zhu, Y., Wang, F., Wang, P., Shen, C., Liu, J. (eds) Proceedings of the 11th International Symposium on Heating, Ventilation and Air Conditioning (ISHVAC 2019). ISHVAC 2019. Environmental Science and Engineering(). Springer, Singapore. https://doi.org/10.1007/978-981-13-9524-6_52
Download citation
DOI: https://doi.org/10.1007/978-981-13-9524-6_52
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-9523-9
Online ISBN: 978-981-13-9524-6
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)