Abstract
Mycotoxins such as aflatoxin and ochratoxin A are secondary metabolites secreted by Aspergillus and Penicillium species. These fungal species flourish in foodstuff and feeds under appropriate temperature and humidity conditions to produce mycotoxins. Aflatoxins are known carcinogens and ochratoxin A causes nephrotoxicity. The contamination of mycotoxins in food and feed, persistence during food processing, and toxicity make them a primary health hazard. Therefore, determination of aflatoxin and ochratoxin A contamination bears a critical importance. Classical methods like chromatographic separation including thin-layer chromatography, high-performance liquid chromatography, and mass spectroscopy are described. Detection of the causal organism by molecular approaches employing PCR and real-time PCR may contribute in early detection. Recently, immunochemical-based methods like enzyme-linked immunosorbent assay and electrical, optical, and piezoelectric immunosensors are being used for the screening purposes. Such detection platforms are portable, reducing the dependence on costly instrumentation. Current strategies to improve the mycotoxin detection involve nanotechnology-enabled sensors. One of the main challenges for the detection of mycotoxin contamination is the co-occurrence of two or more toxins in food and feed samples. The incorporation of novel recognition elements such as antibodies, peptides, or aptamers with nanoparticles for LFA and immunosensors has immense potential for simultaneously sensitive, specific, and cost-effective multitoxin analysis. Such devices will contribute to improved detection of toxic secondary fungal metabolites critical in food safety, human health, and food trade.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Aflatoxin
- Ochratoxin A
- Chromatographic detection
- Immunochemical methods
- PCR
- Nanotechnology
- Lateral flow assay
- Multitoxin detection
1 Introduction
Mycotoxins are secondary metabolites produced by fungi that contaminate food and feed leading to mycotoxicosis. FAO has estimated that 25% of food is contaminated with mycotoxins causing annual losses of around 1 billion metric tons of foods and food products globally (Smith et al. 2016). Mycotoxins are hepatotoxic and nephrotoxic causing diarrhea, vomiting, hemorrhage, and immune suppression, thus resulting in increased susceptibility to disease and possible death among animals and humans (Binder 2007; Bryden 2012). Mycotoxins are thermostable and generally resistant to sterilization. A recent study among patients suffering from kidney disease in Sri Lanka reported 93.5% of patients with presence of OTA in urine (Desalegn et al. 2011). The apathy toward the problem of mycotoxins is mainly due to low awareness about mycotoxins, their adverse health effects, and the approaches needed to control mycotoxin contamination at pre- and post-harvest stages. Mycotoxin detection and monitoring will contribute to better grain quality, health, and trade when carried out as a preliminary and routine activity in food testing. Remediation approaches incorporate strategies for prevention of mycotoxin contamination at pre- and post-harvest stages, detoxification of mycotoxins in food and feed, and inhibition of mycotoxin absorption in the gastrointestinal tract.
Major mycotoxins found in cereal grains such as wheat, maize, barley, oats, and rye and cereal-based products are aflatoxins (AF), ochratoxin A (OTA), deoxynivalenol, and zearalenone produced by the fungal genera of Aspergillus, Penicillium, and Fusarium (Smith et al. 2016). Mycotoxins are produced by growth of the fungus in adverse conditions of temperature, water activity, and oxygen content (Magan et al. 2002). Recent climate change with untimely rain and floods increases risk of mycotoxin contamination. According to hazard associated critical control point (HACCP) concept, mycotoxin contamination should be detected at every stage of production, harvesting, processing, and distribution; that warrants the requirement of toxin detection systems (FAO/WHO/UNEP 2001). Worldwide, HACCP is implemented for aflatoxin, while other mycotoxins are largely ignored. Worldwide, one or more mycotoxins may be found to contaminate cereal grains (Alshannaq and Yu 2017). In Europe, multi-mycotoxin studies reported that 75%–100% of animal feed samples contained more than one mycotoxin which could impact animal health and in turn the human health (Streit et al. 2013). In India 78% of feed is contaminated with more than one mycotoxin (https://en.engormix.com 2010). Aflatoxins and ochratoxins are secondary metabolites produced by the polyketide synthase pathway of the fungus. Aflatoxins produced by A. flavus and A. parasiticus in food and feedstuff are implicated in hepatocarcinogenesis. Aflatoxins have a difuranocoumarin structure, aflatoxins B1 and B2 exhibit blue fluorescence, and G1 and G2 display green fluorescence. Aflatoxins M1 and M2, found in milk and milk products, are the hydroxylated products of AFB1 and AFB2. The carcinogenicity of AFB1 is due to its interaction with DNA and alternation of the base sequence by transversion (Wacoo et al. 2014). Another mycotoxin, ochratoxin A, displaying a dihydroisocoumarin structure coupled to a phenylalanine amino acid by peptide linkage, is produced mainly by fungal strains belonging to Aspergillus ochraceus, Penicillium verrucosum , and P. carbonarius. OTA exposure leads to kidney toxicity due to the competitive binding of OTA with phenylalanine t-RNA synthase and interference in protein synthesis (Ha 2015).
Mycotoxins in human and animal food supply have been recognized as safety issue for many years and regulatory levels are defined by WHO and other national agencies. Wheat is a staple cereal consumed worldwide. Wheat and its by products are commonly contaminated by microbial organisms and their metabolites, contamination may survive and carry through cereal grain and their processed food. The European Commission has established regulatory limit of 5 μg/kg for OTA in processed cereal and baby foods (EC 2006). In India, a limit of 20 μg/kg of OTA and 15 μg/kg AFT has been defined by the Food Safety and Standards Authority of India in wheat and wheat-based products (Table 15.1).
Analytical methods for rapid, sensitive, and accurate determination of these mycotoxins in unprocessed food products are generally applied for the assessment of toxicological risk in tune with the regulatory levels. These methods generally involve toxin extraction from the matrix with an adequate extraction solvent, cleanup step designed to eliminate interference from the extract, and detection/determination by suitable analytical instruments/technologies. Often, commercial immunochromatographic assays, such as enzyme-linked immunosorbent assays (ELISA) are frequently used for screening purposes (Schneider et al. 2004). In addition, chromatographic methods such as high-performance liquid chromatography (HPLC) coupled with ultraviolet (UV); diode array (DAD), fluorescence, (FD) or mass spectrometry (MS) detectors; and gas chromatography (GC) coupled with electron capture (ECD), flame ionization (FID), or MS detectors are used (Shephard 2008). These methods though available are expensive and require skilled personnel. A variety of rapid methods are proposed for mycotoxin analyses including infrared spectroscopy, immunochromatographic detection, electrochemical sensors, and biosensors (Wacoo et al. 2014).
Identification and characterization of fungal spoilage organisms by molecular methods such as polymerase chain reaction (PCR) or quantitative PCR (qPCR) can contribute to their early detection. The main efforts taken into ochratoxin and aflatoxin detection are directed toward causal organisms, i.e., Aspergillus and Penicillium spp. Molecular methods are used for validation with ITS regions of 18S RNA gene, conserved genes, and specific mycotoxigenic genes (Schmidt-Heydt et al. 2011; Sadhasivam et al. 2017).
Recently, several nanotechnology-enabled detection sensors are being developed for mycotoxin detection. Nanotechnology can contribute in rapid, sensitive detection of mycotoxins and their producers with nanoparticles as labels for signal detection. Nanoparticles can be used in monitoring food quality by detection of aflatoxins, OTA, as well as mycotoxin producers to provide safe and healthy food and improve food security regulations. Nanoparticles conjugated with antibodies, peptides, enzymes, or oligonucleotides are devised into sensitive detection sensors or lateral flow formats in diagnostic approaches (Baptista et al. 2005; Gao et al. 2009; Piro et al. 2016). Improved detection of toxic secondary fungal metabolites in food safety is of critical importance for human health and food trade.
2 Ochratoxin and Aflatoxin Detection
2.1 Spectroscopic Methods
Fluorescence spectrophotometry is used for recording the fluorescence emitted by ochratoxin and aflatoxins. The presence of aflatoxins and ochratoxin in grains and raw peanut was determined with this method. However, since crude samples show interference in fluorescence, may require derivatization, and often have a high LOD, these methods are limited to the initial screening for presence of toxins in food and feed samples (Wacoo et al. 2014). Fourier transform spectroscopy identifies organic compounds on basis of their specific infrared spectrum. FTIR analyses was used to identify the presence of aflatoxins in peanuts, peanut cake, and single corn kernels. Mirghani et al. (2001) used transmittance and reflectance spectroscopy to detect aflatoxin in maize kernels that were grouped with high (>100 ppb) or low (<10 ppb) aflatoxin concentrations.
2.1.1 Thin-Layer Chromatography
Thin-layer chromatography (TLC) is simple, robust, and qualitative method used for mycotoxin detection and can be optimized for their rapid screening. The samples are loaded on TLC plates, separated chromatographically by solvent mobile phase and analyzed by UV exposure by comparison to the standard. The presence of ochratoxin A (OTA) was analyzed in different cereals and feeds using TLC. Boudra et al. (1995) extracted OTA from Aspergillus ochraceus contaminated wheat using acidified chloroform and analyzed it by bidirectional TLC. Samples were loaded on silica gel aluminum sheet activated for 1 h at 100°C and developed first with mobile phase of anhydrous diethyl ether and later with toluene/ethyl acetate/formic acid (6:3:1) and exposed to UV for OTA detection (Boudra et al. 1995). Production of OTA by Aspergillus ochraceus on feed-grade wheat was confirmed by chloroform extraction followed by TLC detection using ethyl acetate and acetic acid for separation (Xiao et al. 1996). Pittet and Royer (2002) reported a rapid, low-cost method for detection of OTA in green coffee using bidirectional TLC with the detection limit of 10 μg/kg. Two varieties of green coffee obtained from Thailand, India, Kenya, Uganda, Salvador, and Guatemala were extracted using dichloromethane containing 0.1 M phosphoric acid and qualitatively screened using bidirectional TLC method that gave 98% OTA recovery.
A reliable, rapid, and inexpensive method to screen cereals for total and individual B1, B2, G1, G2 aflatoxins (AFs), as well as OTA, was developed by Braicu et al. (2008). After chloroform extraction, 43 cereal samples (wheat, maize, rye, and triticale) were separated by TLC with toluene/ethyl acetate/acetic acid and quantified using densitometry. Among them, 25 samples (58.14%) were contaminated with different mycotoxins: total aflatoxin, 11.2–10.8 mg/kg; individual AB1, AB2, AG1, and AG2, ranged from 0.89 to 5.7 mg/g; and ochratoxin A, 4.3–30 mg/kg. Wheat samples (62.5%) showed the highest contamination. Kushiro et al. (2017) carried out TLC-based rapid analysis of AFs, in presence of dichlorvos, an inhibitor of aflatoxin biosynthesis, using toluene/ethyl acetate/acetic acid (60:30:4) as a mobile phase. Inhibition of AF production was observed in aflatoxigenic A. oryzae, A. parasiticus, and A. flavus strains.
TLC determination of aflatoxin B1, B2, G1, and G2 was carried out for a total of 2668 brown rice, white rice, broken rice, Sella rice, and parboiled rice samples from Pakistan during 2006–2010 (Nisa et al. 2014). Anhydrous ether, chloroform, and acetone were used as the mobile phase. AFB1 contamination in these samples ranged from 22% to 39%, and AFG1 was low (3.6%), while AFG2 was absent in all samples. Aflatoxin B2 was found in 33% white rice, 23% brown rice, and 3.03% broken rice samples. In Ghana, maize kernels and groundnut seeds were studied for aflatoxin contamination, and the distribution of aflatoxin-producing potential of Aspergillus species were associated with both crops (Agbetiameh et al. 2018). Out of 326 maize and 183 groundnut samples, aflatoxin level exceeded the threshold limits of 15 and 20 ppb, respectively, set by the Ghana Standards Authority for 15% of maize and 11% of groundnut samples.
2.1.2 High-Performance Thin-Layer Chromatography
High-performance thin-layer chromatography (HPTLC) is an automated and sophisticated form of TLC. The efficiency of HPTLC is much enhanced due to the automated sampler, thinly layered sorbent HPLTC plates, and short migration distance in a controlled development chamber. The qualitative and quantitative scanning is recorded by an advanced densitometer. The semi-qualitative method is easy to use, robust and utilized for preliminary screening of food and feed for mycotoxin contamination.
Skarkova and Ostry (2000) employed HPTLC for the confirmation of aflatoxin M1 level in human urine samples using HPTLC after cleanup on immunoaffinity columns, with the mobile phase comprising of chloroform/acetone/2-propanol (85:10:5). The limit of quantification (LOQ) of aflatoxin M1 in urine was 5 ng/L. Twofold enhancement of the sensitivity of the HPTLC method was achieved by immersion of the chromatographic plate in a solution of paraffin oil in n-hexane.
OTA in wines was analyzed with HPTLC and HPLC after a dispersive liquid–liquid microextraction (Antep and Merdivan 2012). A linearity of 0.03–1.00 μg/L was established for HPTLC with a high correlation with the HPLC method. Additionally, the HPTLC method achieved the simultaneous analysis of different wine samples and standard of OTA on the same plate.
Welke et al. (2010) optimized the HPLTC method for wine that showed a mean recovery of 90.4% with a limit of quantification (LOQ) and limit of detection (LOD) of 0.1 μg/L and 0.016 μg/L, respectively. Quantification of OTA in 34 Brazilian red wine samples demonstrated that one sample exceeded (4.5 μg/L) the 2 μg/L limit of the Scientific Commission of the European Communities.
Kupski and Badiale-Furlong (2015) standardized the HPTLC method using hexane, ethyl acetate, and acetic acid (18:4:1.5) as mobile phase. The typical fluorescent spots of mycotoxin were observed under UV light plate after the development of plate with aluminum chloride in 15% methanol. The addition of dried magnesium sulfate and sodium chloride as salts to the extraction solvent acetonitrile/water (2:1) resulted in improved recovery of the toxin. The developed method could detect contamination of OTA from 0.22 to 0.85 μg/kg in 20 wheat flour samples.
Aflatoxin M1 (AFM1) levels in dairy products of South Korean origin were determined with HPTLC after immunoaffinity column purification (Yoon et al. 2016). The LOQ for milk, yoghurt, and cheese was 0.003, 0.07, and 0.05 μg/kg, respectively. Among 224 samples, the AFM1 contamination ranged from 0.001 to 0.1 μg/kg for milk and 0.015 to 0.136 μg/kg for yoghurt and cheese. The dairy products were safe for consumption according to the maximum limit of 0.5 μg/kg set by Korea Food and Drug Administration.
Contamination of livestock feed causes detrimental effects in both animal and human beings (Kotinagu et al. 2015). Analysis of 97 livestock feed and feed ingredient samples for aflatoxin B1 contamination showed that 33% of livestock feed and 24.5% of feed ingredients were positive.
2.1.3 High-Performance Liquid Chromatography
High-performance liquid chromatography (HPLC) allows detection and identification of aflatoxin and ochratoxin from food samples by recording the retention time through sensitive fluorescent, ultraviolet, or diode array detectors and mass spectroscopy (MS). In practice, the HPLC technique employs a chromatography column such as C-18 as the stationary phase, a mobile phase that moves through the column and a detector that displays the retention times of the separating molecules. Often, HPLC analysis for mycotoxin requires immunoaffinity column purification to eliminate other contaminants.
Commonly, the reversed phase HPLC method is used for separation and determination of aflatoxins. HPLC detection of aflatoxins with a sensitivity of 0.1 ng/kg using FLD (fluorescent detector) has been reported (Herzallah 2009). Occasionally, chemical derivatization using acid and halogens was employed to enhance the sensitivity of aflatoxins B1 and G1 detection when their natural fluorescence was not sufficient to reach the required detection limit (Kok et al. 1986). Recently, HPLC coupled to mass spectroscopy was able to overcome the challenges associated with derivatization processes in aflatoxins analysis. The HPLC–MS/MS uses low sample volumes to generate structural information (Rahmani et al. 2009). However, HPLC–MS/MS is a bulky, expensive equipment and requires skilled personnel for operation.
Aboul Enein et al. (2002) reported the use of HPLC-FLD for the determination of OTA from wheat, corn, red pepper, cheese, and wine. The retention time was 11.7 min using a mobile phase consisting of acetonitrile/water/acetic acid (99:99:2, v/v/v). The LOD and LOQ was 0.1 ng/ml and 3.3 ng/ml, respectively. Durguti et al. (2014) analyzed 54 wine samples by HPLC-FLD after sample cleanup using immunoaffinity columns. The OTA levels were below the EU limit of 2 ng/ml, and the LOD and LOQ were 0.05 and 0.1 ng/ml, respectively.
Infant formulas are an important food source for infants during the early stages. Ochratoxin A levels were analyzed from 150 samples of infant foods (50 infant formulas, 50 follow-on formulas, and 50 cereal-based supplementary foods for infants and children) from various supermarkets and pharmacies from Istanbul (Hampikyan et al. 2015). Among these, 52 (34.7%) analyzed samples were contaminated with OTA, though none exceeded the Turkish Food Codex maximum limit of OTA (0.5 μg/kg) in baby, infant, and young children foods.
Meuccia et al. (2010) surveyed the presence aflatoxin M1 (AFM1) and OTA in 14 leading brands of infant formulas marketed in Italy. Mycotoxin levels were determined by immunoaffinity column cleanup and HPLC-FLD. Among 185 samples, OTA was detected in 133 (72%) samples (range 35.1–689.5 ng/L). Ready-to-use preparations and powdered samples displayed 80% and 63% OTA contamination, respectively. The aflatoxin AFM1 was detected in two samples at levels below the European legislation limit of 25 ng/L.
Aflatoxin-contaminated livestock feeds are a potential health hazard as they can move up the food chain (Eun-mee et al. 2006). Screening of 249 feed samples collected in Korea by ELISA showed that ~10% contained AFB1. Among these, HPLC-FLD and LC/MS analysis confirmed that only one sample contained 11 ppb AFB1, while other samples did not contain AFB1. It was suggested that screening by ELISA should be followed by HPLC-FLD analysis for rapid and accurate detection of AFB1.
Yazdanpanah et al. (2013) analyzed AFB1 in rice, bread, puffed corn snack, wheat flour, and peanut samples with a HPLC method and obtained an average recovery of 94.4–100%. The LOD was 0.01 ng/g. Among 90 samples collected from Tehran retail market in June 2005, the bread and wheat flour samples were not contaminated, while rice, puffed corn snack, and peanut samples showed AFB1 level below 5 ng/g, the maximum tolerated level (MTL) in Iran. One rice sample and two peanuts samples tested positive for AFB1 contamination. In a study from Karaman, Turkey, aflatoxin analysis for 45 dried apricots, raisins, dried figs, nuts, peanuts, almonds, corn, red pepper, black pepper, bread, and moldy cheese samples was carried out by HPLC-FLD method after post-column derivatization (Kilicel et al. 2017). The total AF concentration in eight red pepper samples exceeded the MTL of 10 μg/kg that also exceeded the European MTL for AFB1 of 8 μg/kg. The amount of aflatoxin in other samples was negligible. AFs were present in 100% of raisins, dried figs, black pepper, red pepper, and corn, 75% of dried apricots, nuts, bread, and moldy cheese, and 50% of peanuts and almonds.
Kim et al. (2017) detected aflatoxins (B1, B2, G1, and G2) and OTA in animal feeds by a sensitive and robust HPLC-FLD method with a photochemical reaction device after immunoaffinity column purification. Aflatoxin and OTA were found in 44 of 496 samples with a concentration range of 1.76–162.69 μg/kg for AFB1 and 3.38–45.42 μg/kg for OTA for 2 years. The developed method was suitable for the routine analysis of aflatoxin in animal feed.
2.1.4 Liquid Chromatography Coupled Mass Spectroscopy
The technique of liquid chromatography coupled with tandem mass spectroscopy utilizing database searching has a high degree of sensitivity and accuracy that can be used in specific mycotoxin analysis. The accurate masses of protonated fungal metabolite molecule ions are obtained by optimizing the electrospray conditions, retention times, and UV spectra. Sulyok et al. (2006) used a single extraction step, without any cleanup step, followed by liquid chromatography with electrospray ionization triple quadrupole mass spectrometry (LC/ESI-MS/MS). They developed a validated method for the determination of 39 different mycotoxins in wheat and maize that included trichothecenes, zearalenone, fumonisins, ergot alkaloids, ochratoxins, aflatoxins, moniliformin, etc. The multianalyte analyses was carried out with positive as well as the negative ion ESI mode in two consecutive runs to accommodate the diverse mycotoxins. The limits of detection ranged from 0.03 to 220 mg/kg.
Jung et al. (2012) carried out the quantitative determination of aflatoxins (B1, B2, G, G2), OTA, deoxynivalenol, fumonisins, zearalenone, etc. in roasted and ground grains using LC–MS. The co-extraction of 11 mycotoxins was carried out by a double extraction using a phosphate buffer solution and methanol followed by cleanup with a multitoxin immunoaffinity column. The LODs of mycotoxins were 0.1–6.1 μg/kg, and LOQs were 0.3–18.4 μg/kg. Among 47 samples collected from Seoul, Korea, OTA was detected in 17% of samples, while aflatoxins were not detected in all samples.
LC–MS-based determination was carried out in a study on occurrence and levels of OTA in 98 infant formula (milk- and soy-based samples) and 155 infant cereal (barley-, rice-, oat-, wheat-, and mixed grain-based) products available in the US market. None of the infant formula samples tested positive, while 30% of infant cereals were contaminated with OTA in the range of 0.6–22.1 ng/g. Regulatory limits for OTA are lacking in the USA; however all positive samples were above the European Commission limits of 0.5 ng/g for OTA in baby foods. Oat-based infant cereals (59%) and mixed grain cereals (34%) showed the highest incidence and concentrations of ochratoxin contamination. Surveillance for OTA levels in grains used in infant foods can reduce exposure of infants and young children to OTA from cereal products.
The presence of aflatoxins B1, B2, G1, and G2 in four categories (oils, proteins, polysaccharides, and fatty oils) of traditional Chinese medicines was analyzed using ultra-performance liquid chromatography tandem mass spectrometry (Zhao et al. 2016). The aflatoxin concentrations ranged from 0.2 to 7.5 μg/Kg in 14 out of 22 samples. Fatty oils were most prone to contamination as compared to polysaccharides, proteins, and volatile oils.
2.1.5 Gas Chromatography
As other chromatographic methods, gas chromatography (GC) is based primarily on differential partitioning of analytes between the stationary phase consisting of inert particles coated with a layer of liquid in a column by the carrier gas as the mobile phase. The sample is vaporized into gaseous phase, and detection of the volatile products is carried out using either a flame ionization detector or an electron capture detector and mass spectrometer (MS). In case of aflatoxins, there is a need for derivatization in order to be detected, due to their nonvolatile nature (Scott 1995). Detection by GC-MS and an electronic nose did not find a correlation between odor and ochratoxin level, as samples with OTA levels both below and above 5 μg/Kg displayed pronounced or strong off-odors (Olsson et al. 2002).
However, due to the availability of other cheaper chromatographic methods, gas chromatography is less common in commercial analysis of mycotoxins (Wacoo et al. 2014). Besides, gas chromatography also requires a preliminary cleanup and derivatization before analysis, and it is therefore limited to analysis of a few mycotoxins, such as type A and B trichothecenes. Even in such analyses, the GC displays disadvantages of non-linearity of calibration curves, drifting responses, memory effects from previous samples, and high variation in reproducibility and repeatability.
2.1.6 PCR and Quantitative Real-Time PCR Detection
Mycotoxins are metabolites produced by the interaction of several biochemical pathways during later stages in fungal growth, while genes are involved in mycotoxin pathway expressed much earlier. Therefore, detection of the mycotoxin gene expression may contribute to early detection of mycotoxins. Conventionally, polymerase chain reaction (PCR)-based molecular methods are used for detection of mycotoxins producing fungi such as Aspergillus and Penicillium from food (Edwards et al. 2001). Gene-based methods reported in the literature are mainly based on unique ITS (internal transcribed spacer) regions of fungal species which are genus- and species-specific for identification purpose but do not confirm the production of toxins by the isolates. Genes of important enzymes in the mycotoxin biosynthetic pathway can be good targets for diagnostic tests as they can add to the specificity for detection of mycotoxin production and sensitivity of the test (Schmidt-Heydt et al. 2011).
Shapira et al. (1996) employed the genes coding for enzymes involved in Aspergillus parasiticus polyketide synthase pathway, namely, apa-2, ver-1, and omt-1, which are responsible for regulation, oxidation, and methylation, respectively, during aflatoxin synthesis. The presence of the fungus was detected by PCR method when 102 spores/g were inoculated in corn flour.
In a Korean study, multiplex PCR and high-performance liquid chromatography (HPLC) analyses were used to assess the ability of mycotoxin production in 32 A. niger isolates (Kim et al. 2014). Though multiplex PCR and HPLC analyses of A. niger isolates showed that OTA-producing strains exhibited positive PCR patterns for ochratoxin biosynthetic genes, it did not explain the presence of positive PCR products in the non-mycotoxin-producing strains.
A PCR method for differentiation and detection of OTA-producing Penicillium species was developed by utilizing polyketide synthase and non-ribosomal peptide synthetase genes of the ochratoxin A biosynthetic pathway (Bogs et al. 2006). An analysis of 62 strains showed that 11 PCR-positive (18%) strains produced ochratoxin A. However, some OTA-negative strains also tested PCR-positive, that was attributed to the presence of non transcribed biosynthetic gene.
Currently, ochratoxin A contamination in coffee beans was found to be associated with Aspergillus carbonarius, A. niger, and A. ochraceus (Sartori et al. 2006). A multiplex PCR method that detected PCR amplicons of 809, 372, and 260 bps for detection of A. carbonarius, A. niger, and A. ochraceus species, respectively, in coffee beans was developed.
A highly specific multiplex PCR method was developed using species-specific and mycotoxin metabolic pathway gene primers for detection of Fusarium and Aspergillus species (Sadhasivam et al. 2017). Stored wheat grain samples (34) were analyzed for the presence of mycotoxin-producing fungi by PCR and mycotoxin production by LC/MS/MS. The analyses had a strong correlation, and contamination of six samples with at least one mycotoxin, above EU regulatory limits was confirmed by both the methods.
As many of the genes involved in toxin biosynthetic pathways also contribute to other secondary metabolites, there is a lack of specificity for the PCR detection of toxin production. Detection of aflatoxin production by Aspergillus versicolor and Aspergillus nidulans is difficult as the genes involved in aflatoxin production are also involved in sterigmatocystin biosynthesis (Levin 2012). Aflatoxin and ochratoxin gene pathway provide an advantage in early monitoring of the mycotoxins producing Aspergillus and Penicillium spp. (Baptista et al. 2005). Dao et al. (2005) optimized the PCR amplification using primers toward the acyl transferase domain of a polyketide synthase gene involved in the OTA biosynthesis in OTA-producing fungi such as A. carbonarius, A. melleus, A. sulfurous, A. ochraceus, and P. verrucosum. Other studies indicate the role of several enzymes, such as polyketide synthase, non-ribosomal peptide synthase, halogenase, and P450 oxidase in OTA biosynthesis. Recently a multiplex PCR, based on genes of aflatoxin and sterigmatocystin biosynthesis metabolic pathways, was reported for the identification of AFT producers among Aspergillus spp. (Criseo et al. 2001). Such studies promote our understanding of the mechanisms of OTA production and regulation.
Quantitative real-time PCR systems may be useful for determining associations between detection of a gene at critical control points in food production and quantification of the mycotoxin contamination in the final product (Rodríguez et al. 2012). Though quantitative real-time PCR has been applied to monitor expression of mycotoxin biosynthetic genes, it is expensive.
The rapid and accurate differentiation of toxigenic and atoxigenic isolates was evaluated with PCR and real-time PCR in 22 A. flavus isolates from peanut kernels (Mahmoud 2015). The PCR amplification of genes did not correlate with aflatoxin production capability. Among the four aflatoxin biosynthetic pathway genes (aflD, aflM, aflP, and aflQ), the expression of aflD and aflQ was a good marker for differentiating the toxigenic from atoxigenic isolates. The real-time PCR and agar culture method for detection of A. flavus presence had 95% agreement. Aflatoxin production by these strains was confirmed by HPLC with 72% of isolates producing the toxin.
Rodríguez et al. (2012) proposed real-time PCR for the early detection and quantification of aflatoxin in peanut, spices, and sausages. The sensitivity and specificity of real-time quantitative PCR (qPCR)-based on SYBR Green and TaqMan were evaluated using the o-methyltransferase gene (omt-1) aflatoxin biosynthetic gene. Both qPCR methods gave a good linear correlation from 4 to 1 log cfu/g per reaction and a detection limit of 1–2 log cfu/g in the different food matrices tested.
The ochratoxin-producing fungus Aspergillus carbonarius is often associated with grapes and wine contamination (Atoui et al. 2007). Specific primers to the acyltransferase (AT) domain of polyketide synthase sequence of A. carbonarius amplified a 141 bp PCR product. These primers were also used for qPCR in 72 grape samples for direct quantification of the fungus. The expression of acyltransferase gene showed a positive correlation with the OTA concentration. Therefore, rapid detection of A. carbonarius by qPCR in grapes may offer an alternative to the traditional methods of OTA detection and culture identification.
2.2 Immunochemical Methods
Immunochemical techniques are based on the high affinity and specificity of antibody–antigen (Ab-Ag) binding. The binding event is recognized by signal amplification using labels such as enzymes, fluorophores, etc. Immunochemical methods such as immunosensors, immunoaffinity column assay (ICA), and enzyme-linked immunosorbent assay (ELISA) are sensitive, specific, and less labor-intensive, require less time, and allow simultaneous processing of several samples. Though radioimmunoassay technique was used for determination of aflatoxin B1 and aflatoxin M1 levels, the disadvantages such as requirement of antigen in a pure state and potential health hazards due to use of radioactive isotope have discouraged the method (Rauch et al. 1987). However, other immunochemical methods such as ELISA and immunosensors are in wide usage.
2.2.1 Enzyme-Linked Immunosorbent Assay (ELISA)
ELISA methods for mycotoxin detection rely on the recognition of specific mycotoxin by specific antibodies coated in microtiter plate wells. Mycotoxin capture is subsequently detected by antibodies, often labeled with horseradish peroxidase or alkaline phosphatase enzymes. The assay is rapid, simple, specific, and sensitive for the detection of mycotoxins in foods and feeds. This technology is available for more than two decades, and ELISA test kits require low sample volumes with less sample cleanup procedure as compared to conventional TLC and HPLC methods. This method has application for high throughput assays and simultaneous detection in several samples.
The ELISA technique is presently applied for detection of aflatoxins in food and feedstuffs, and a number of commercially available ELISA kits are widely used (Devi et al. 1999, 2002; Prestani et al. 2011). A commercial ELISA system and HPLC method were used to analyze the total aflatoxins in 178 foodstuff samples. The two methodologies had a high correlation for nuts, nut products, peanuts, and peanut butter samples (Azer and Cooper 1991).The presence of four types of aflatoxin (B1, B2, G1, and G2) in dairy cattle feeds and aflatoxin M1 in the milk samples was measured by HPLC and ELISA methods (Prestani et al. 2011). There was no significant difference by the two procedures, though the sensitivity and specificity of HPLC method were higher. Li et al. (2016) generated specific monoclonal antibodies that recognized G class aflatoxins, G1 and G2. A competitive indirect ELISA (CI-ELISA) was developed with a LOD of 0.06 ng/ml and low cross-reactivity toward aflatoxin B1. The CI-ELISA and HPLC methods were comparable for analysis of uncontaminated peanut samples spiked with different concentrations of aflatoxins G1 and G2.
Though antibodies are highly specific and sensitive toward the target mycotoxin, many a time, compounds with similar chemical groups also interact with the antibodies. Additionally, ELISA methods are restricted to certain food matrices for which they were validated. Sun et al. (2015) conducted an extensive study to evaluate the quality of five commercial ELISA kits from different suppliers for detecting aflatoxin B1 in 30 HPLC-verified feed samples such as corn, distillers dried grains, wheat, soybean meal, and poultry feed. The accuracy and precision of these kits were tested with positive controls. The authors reported that the qualities of five tested ELISA kits were significantly different and two kits had high false positive rates. Therefore, the complete validation for an ELISA method is critical for its application to a wide range of commodities.
ELISA technique for OTA was used for analyses of dry fruit samples from Central Iran that exhibited average concentrations of 6.7 ± 3.9 ng/g in 21% of samples (Rahimi and Shakerian 2013). Concentrations of OTA higher than the European Union maximum tolerance limit of 10 ng/g were detected in 7.9% of dried raisin samples and 2.1% of dried fig samples.
An anti-OTA monoclonal antibody-based indirect competitive ELISA was developed for OTA detection in coffee and coffee products with a detection limit of 3.73 ng/g (Fuji et al. 2007). The comparison between ELISA and HPLC methods resulted in high correlation for the green, roasted, and instant coffee samples.
A sandwich dot-ELISA method was developed for the simple, fast, and sensitive detection of OTA from contaminated food grain samples with a limit of detection of 5.0 ng/ml (Venkataramana et al. 2015). The ELISA method detected contaminations in 72 (19 maize, 38 wheat, 15 rice) of 195 samples, which was in agreement with the HPLC method. The mAb-based method was specific and did not show any cross-reactivity toward deoxynivalenol, fumonisin B1 fumonisin, or AFB1.
Similarly, Ekhtelat et al. (2018) established a good correlation between the ELISA and HPLC methods for determination of OTA levels in the medicinal herbs of Zataria multiflora and Foeniculum vulgare from Ahvaz, Iran.
A competitive direct ELISA format was developed and showed IC50 value of 0.07 ng/ml. A simple, rapid, and efficient method for extraction with 50% methanol and the ELISA method resulted in 74–110% recoveries of spiked samples. Dilution with PBS was applied for food samples such as barley, wheat, oat, corn, rice, and raisins, grape juice, and beer samples. The developed ELISA method and the HPLC method showed a good correlation.
2.2.2 Immunosensors
Immunosensors are based on a signal transducer which detects the binding of the antibody and antigen. The electrochemical, optical, and piezoelectric immunosensors detect changes in electrical current, mass, or optical signals (color or fluorescence), respectively, during Ab–Ag binding.
2.2.2.1 Electrochemical Immunosensors
An electrochemical immunosensor records the electroactive signals generated by the Ab–Ag interactions by differential pulse voltammetry, cyclic voltammetry, chronoamperometry, electrochemical impedance spectroscopy, or linear sweep voltammetry transducers (amplifiers). A potential difference is generated by the reaction conditions which is measured to establish the relationship between the potential difference and antigen concentration. Electrochemical immunosensors developed for aflatoxin detection mostly employ enzymes as the active biological component to generate signals.
Badea et al. (2016) developed an impedimetric immunosensor by immobilizing anti-OTA antibody on bovine serum albumin-modified gold electrodes for ochratoxin A (OTA) detection.
Modification of the impedance due to the specific antibody–antigen reaction at immunosensor surface was used in order to detect OTA. Linear proportionality of the charge transfer resistance to the OTA concentration allowed OTA detection in the range of 2.5–100 ng/ml.
Li et al. (2016) developed a portable immunosensor comprising of an impedance detector and a 3D-printed USB-compatible sensor chip for rapid and in situ detection of AFB1 in rice. The detection time was below an hour, and the limit of detection was 5 ng/ml. Such portable devices for detection offer the possibility of analyses of clinical and environmental samples.
A fast, sensitive, and efficient electrochemical immunoassay was developed by forming self-assembled monolayers by 2-aminoethanethiol on a gold electrode for AFB1 capture (Kong et al. 2018). The noncompetitive immunoassays resulted in a significant current change at a minimum concentration of 0.01 ng/ml of AFB1. The immunosensor displayed excellent stability and sensitivity after storage for 7 days.
A simple, cost-effective, and portable antibody-modified screen-printed carbon working electrode with carbon counter was developed for direct aflatoxin M1 analysis with milk (Parker and Tothill 2009). An electrochemical detection scheme was constructed on the electrode surface in a competitive ELISA format with horseradish peroxidase (HRP) as the enzyme label. Milk samples were pre-treated with 18 mM calcium chloride to stabilize the whey proteins and eliminate the interfering signal. The sensor achieved a detection range up to 1000 ng/L and a LOD of 39 ng/L. The sensitivity of the immunosensor was comparable to the commercial ELISA kit and an a HPLC method.
Muchindu et al. (2011) produced an impedimetric immunosensor composed of a platinum disk electrode coated with polyaniline–polyvinyl sulfonate and anti-OTA antibody and calibrated it for OTA detection. When applied to the certified reference materials of corn, wheat, and roasted coffee, the sensor detected OTA concentrations of 21.1, 8.6, and 2.5 mg/kg, respectively. The immunosensor had a LOD of 10 pg/kg, and impedimetric estimation for corn was in agreement with the ELISA measurements.
Radi et al. (2009) modified an electrochemical immunosensor comprising of a screen-printed gold electrode with glutaraldehyde and tagged it with the anti-ochratoxin antibody. The competitive immunoassay between a horseradish peroxidase-labeled ochratoxin A (HRP-toxin) and ochratoxin A for antibody demonstrated a dynamic range up to 60 ng/ml and detection limit of 12 ng/ml. The binding of the antibody and HRP-toxin was measured by chronoamperometry in the presence of the substrate tetramethylbenzidine. The developed immunosensor displayed precision, accuracy, and stability.
Monoclonal antibodies were immobilized on disposable screen-printed electrodes to develop an electrochemical competitive enzyme-linked immunosorbent assay for quantitative determination of OTA (Alarcon et al. 2006). The assay had a working range from 0.05 to 2.5 and 0.1 to 7.5 g/L and detection limit of 60 and 100 g/L, respectively, in the direct and indirect assay formats. The immunosensor in the direct format was selected for the determination of OTA in wheat. Analysis of spiked and naturally contaminated wheat samples with the electrochemical assay and HPLC showed good correlation.
2.2.2.2 Optical Immunosensors
Optical immunosensors record the signals by surface plasmon resonance, optical waveguide light-mode spectroscopy, photoluminescence, etc. Surface plasmon resonance (SPR) measures the refractive index changes as a shift in the resonance angle during the Ab–Ag interactions. AFB1 detection and quantification was attempted with monoclonal as well as polyclonal antibodies using the SPR technology (Daly et al. 2000). Monoclonal antibodies encountered regeneration problems due to their high-affinity binding to the sensor surface. Immobilization of polyclonal anti-AFB1 antibodies on the sensor surface achieved a linear detection range of 3.0–98.0 ng/ml with good reproducibility. Regeneration was achieved using alkaline solution of methanolamine and acetonitrile.
Van der Gaag et al. (2003) utilized the SPR immunosensor for multiple detection of mycotoxins. Therefore, SPR immunosensors should offer label-free detection of aflatoxins if the current regeneration problems are overcome. Optical waveguide light-mode spectroscopy (OWLS) measures the resonance angle of polarized grating diffracted light, coupled into a thin waveguide. The Ab–Ag interaction on the surface of sensor chips results in multiple internal reflections within the waveguide layer which is detected by photodiodes. Aflatoxin and ochratoxin detection was carried out using OWLS in both competitive and direct immunoassays (Adanyi et al. 2007). A detection range of 0.5–10 ng/ml for aflatoxins was achieved when barley and wheat flour samples were analyzed.
Myndrul et al. (2018) fabricated a porous silicon (PSi) based photoluminescence immunosensor metal-assisted chemical etching procedure and modified by coating with protein A and binding with anti-OTA antibodies. The PL spectroscopy of PSi at room temperature resulted in an emission band at 680 ± 20 nm. The binding of OTA resulted in the photoluminescence quenching of the anti-OTA/protein A/PSi surface as compared to bare PSi. The response time of the immunosensor was in the range of 500–700 s.
Another immunosensor was developed by layering photoluminescent ZnO nanorods (ZnO-NRs) on glass substrate followed by coating for binding with protein A and binding anti-ochratoxin antibodies (glass/ZnO-NRs/protein A/anti-OTA) (Vitera et al. 2018). The immunosensor was integrated with portable fiber optic detection system and tested in a wide range of OTA concentrations, and the sensitivity and limit of detection were 0.1–1 ng/ml and 0.01 ng/ml, respectively. Response time of the immunosensor toward OTA was in the range of 500–800 s.
2.2.2.3 Piezoelectric Quartz Crystal Microbalances (QCMs)
This label-free direct detection method relies on changes in mass when the antibody immobilized on the quartz crystal surface interacts with the antigen (Janshoff et al. 2000). Spinella et al. (2013) immobilized anti-aflatoxin B1 antibody on gold-coated quartz crystals and achieved aflatoxin B1 detection in the range of 0.5–10 ppb. Antibody binding to the gold-coated surface was facilitated by 3,3’dithiodipropionic-acid-di-N hydroxysuccinimide ester. Jin et al. (2009) also detected 0.01–10.0 ng/ml aflatoxin B1 in spiked milk samples with the QCM-based sensor.
A label-free quartz crystal microbalance (QCM)-based immunosensor was developed by immobilization of OTA–bovine serum albumin conjugate on gold-coated quartz crystals (Vidal et al. 2009). An indirect competitive format was used to detect the binding of the excess of anti-OTA antibodies to the immobilized OTA that led to linear decrease in the resonant frequency at OTA concentration from 10 to 128 ng/ml, with a detection limit of 8 ng/ml. The quartz electrode was reusable after regeneration with a pepsin solution (pH 2.1).
Pirincci et al. (2018) developed OTA detection by direct immobilization of OTA to amine-bearing sensor surfaces using 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC)/N-hydroxysuccinimide (NHS) chemistry. The QCM immunosensor was developed with a detection range between 17.2 and 200 ng/ml with the possible use for on-site detection of contamination in feedstuffs. The sensor was reusable up to 13 times without loss of performance after regeneration with 50 mM NaOH and 1% SDS.
3 Nanotechnology for Mycotoxin Detection
Nanotechnology can contribute to the detection of contaminated food and feed. Nanoparticles can be used in detection of mycotoxins and mycotoxin producers, monitoring food quality, and help in food security regulations to provide safe and healthy food. Nanoparticles, due to their size- and shape-dependent physical and chemical properties, have potential for development in various colorimetric, fluorometric enzymatic, and electrochemical diagnostic assays (Vo-Dinh 2007, Selvan et al. 2009, Gao et al. 2009, Kim and Park 2005). Semiconductors, noble metals, and metal oxide nanoparticles are used in various imaging and sensing applications (Fig. 15.1). Nanoparticles as labels can be conjugated with molecular recognition elements such as DNA, RNA, antibodies, or enzymes to offer a detection method by signal amplification (Fig. 15.1). Gold nanoparticles are often used in the lateral flow immunochromatographic devices that operate on a simple chromatographic flow separation to give a colorimetric visual signal based on the affinity of the gold nanoparticle- labeled atibodies to the antigen in either a direct or indirect format.
The early detection of mRNA expression was demonstrated in a cultured melanoma cells using nanoparticles carrying an imaging probe. The probe incorporated citrate-capped gold nanoparticles covalently bound to thiol-terminated hairpin oligonucleotides. The hairpin DNA-coated gold nanoparticles positively identified tyrosinase mRNA in melanoma cells (Harry et al. 2010). Gold nanoparticles functionalized with oligonucleotides which are complementary to unique sequences, present on the heat shock protein 70, were used for detection of the human parasite Cryptosporidium parvum (Javier et al. 2009). The use of oligonucleotide gold NPs for the molecular diagnosis of mycotoxins offers new opportunities for the further development of point of care diagnostic assays with low-cost, robust reagents and simple colorimetric detection (Baptista et al. 2005). Nanoparticle aided mRNA detection to identify the spoilage organism leading to early intervention by regulatory agencies. Therefore, the development of a diagnostic assay for rapid, sensitive detection of mycotoxin producers is the need for proper food safety in a country like India.
3.1 Nanoparticle-Assisted Electrochemical Immunoassay
Liu et al. (2013) fabricated an electrochemical sensor in the indirect competitive immunoassay format for detection of OTA by modifying a glassy carbon electrode with a composite film of polythionine and self-assembled gold nanoparticle monolayer. The sample OTA competed with the ochratoxin antibody–ovalbumin conjugates immobilized on the film for binding with the anti-ochratoxin monoclonal antibodies. The alkaline phosphatase-labeled secondary antibodies bound to the monoclonal antibody to give the electrochemical signal by oxidation of 1-naphthyl phosphate substrate. The electrochemical response was inversely proportional to the OTA concentration from 1 to 1000 ng/ml with a low detection limit of 0.2 ng/ml.
Rivas et al. (2015) developed an electrochemical impedance-based sensor by electropolymerizing a polythionine film on a screen-printed carbon electrode followed by the assembly of iridium oxide nanoparticles (IrO2 NPs). The aminated aptamer selective to OTA was bound by electrostatic interactions to the IrO2 NPs. Aptamers are single or double-stranded synthetic oligonucleotides that bind specifically to the target and are more advantageous as compared to antibodies due to their thermal and chemical stability and low-cost production. The sensor detected 14 pM OTA in white wine samples and exhibited high reproducibility.
Sharma et al. (2017) devised an electrochemical immunosensor by deposition of poly(3,4-ethylenedioxythiophene) functionalized gold nanoparticles and immobilization of monoclonal anti-aflatoxin antibodies. The immunosensor displayed a LOD of 0.0045 ng/ml and LOQ of 0.0156 ng/ml by amperometric measurement. Spiked maize samples were detected a high reproducibility.
A sandwich-type nonenzymatic electrochemical immunosensor was reported by Masoomi et al. (2013) by modification of glassy carbon electrodes with chitosan, gold nanoparticle, anti-aflatoxin B1, and iron III oxide (Fe3O4) magnetic core with a gold shell functionalized with 3-(2-mercaptoethylimino) methyl) benzene-1,2-diol. Aflatoxin B1 detection was achieved in the range of 0.6–110 ng/ml with a detection limit of 0.2 ng/ml.
Linting et al. (2012) developed an immunosensor by electrodepositing graphene oxide and gold nanoparticles on the surface of gold electrode. A conducting polymer film, ionic liquid, and chitosan solution was dropped onto this electrode for AFB1 antibody immobilization. The detection range was 3.2–0.32 picomoles, and the detection limit was 1 femtomole with excellent long-term stability.
3.2 Nanoparticle-Assisted Lateral Flow Assay
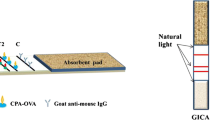
Recently, lateral flow immunochromatographic assays (LFAs), which are sensitive, simple, easy, fast, and ready-to-use devices, are used to detect the presence of a target analyte in sample without the need for specialized and costly equipment. The method uses a low-cost test device consisting of conjugation pad, membrane, sample pad, and absorbent pad. The antibody labeled with gold nanoparticles is pre-adsorbed on the conjugate pad, and the capturing reagents are immobilized in spatially confined zones on the nitrocellulose membrane (Quesada-Gonzalez and Merkoçi 2015). The detection is based upon either the competitive or direct formats (Moon et al. 2013). The high sensitivity and specificity of antibody–antigen reactions and the colorimetric visibility of the gold nanoparticles as labels are the basis for the rapid detection of analytes, as a red band on the membrane (Fig. 15.2).
Multitoxin detection by lateral flow assay. Aflatoxin and ochratoxin A can be simultaneously detected in the LFA visually and quantitatively. In the competitive format, the presence of red color bands due to gold nanoparticle on the control line indicates the presence of toxins. The presence of red bands on the test line indicates the absence of toxins. The color intensity can be compared to the standards for quantification
An immunochromatographic strip test was developed and optimized for the rapid detection of aflatoxin B1 (AFB1) by nanocolloidal gold coupled with monoclonal antibody for AFB1 (Won-bo et al. 2007). The antibody showed cross-reactivity toward aflatoxin B2, G1, and G2. The test took 15 mins and the visual detection limit 0.5 ng/ml. Analyses of 172 grain samples by the immunochromatographic strip test was in agreement with the HPLC estimation. Gold nanoparticle coated with monoclonal antibodies was used as a detection agent for aflatoxins in soybean, a major Brazilian agricultural commodity (Santos et al. 2017). The monoclonal antibody displayed specificity toward aflatoxins B1, M1, G1, G2, and B2, and the strip test detected up to 0.5 μg/kg of aflatoxins in 10 min. Similarly Delmulle et al. (2005) developed a rapid lateral flow device for detecting aflatoxin B1 in pig feed rapidly within 10 min. Though most LFA platforms employ gold nanoparticles, recently Ren et al. (2014) employed quantum dots or semiconductor nanoparticles that show robust fluorescence as detection labels. Fluorescent CdSe/ZnS quantum dot beads (QBs) were synthesized for ultrasensitive detection of AFB1 in maize by polymer encapsulation of quantum dots (QDs) (Ren et al. 2014). The surface blocking method was used to prevent non-specific binding to the lateral flow membrane. The developed sensor had an LOD of 0.42 pg/ml in maize extract and twofold higher sensitivity than a gold nanoparticle-based immunoassay. This method compared favorably with the commercial ELISA and liquid chromatography tandem mass spectrometry measurements.
Anfossi et al. (2012) detected OTA semiquantitatively in wines and grape musts by a one-step lateral flow immunoassay. Matrix-matched calibration curves carried out in blank wines showed a detection limit of 1 μg/L and IC50 of 3.2 μg/L. The developed assay could detect OTA accurately and sensitively in 5 min. The assay was in agreement with the reference method of HPLC with 38 wines and 16 musts. A rapid immunochromatographic assay was combined with a simple, sample treatment of cereals for the quantitative determination of OTA with LOD as low as 1.5 μg/kg. Ochratoxin A was extracted from the cereal samples in presence of polyethylene glycol that reduced the matrix effects caused by different cereals (maize, wheat, and durum wheat) (Anfossi et al. 2011). The recoveries ranged from 87% to 119%. Fifteen maize, four wheat, and six durum wheat samples were analyzed by the developed assay that showed a good correlation when compared with a reference method.
A monoclonal antibody against OTA was raised to develop a rapid immunochromatographic assay for efficient OTA detection (Cho et al. 2005). Up to 500 ng/ml of OTA was detected in 10 min. Wang et al. (2007) developed a colloidal gold immunoassay in a flow-through format for the rapid detection of OTA in various food matrices. OTA–BSA conjugates were used to produce polyclonal antibodies in rabbits. The assay was completed within 10 min, and the visual detection limit of the developed assay was 1.0 ng/ml.
In most cases routine LFA can only detect one target molecule at a time. By comparison, the multiplexing format of LFA can simultaneously detect several target chemicals, thus it can further reduce operating cost and improve detection efficiency (Fig. 15.2). Multiplexed antibody-based immunochromatographic LFAs are reported for detection of AFs, OTA, deoxynivalenol, and zearalenone in various studies (Kolosova et al. 2008; Song et al. 2014; Chen et al. 2016). A multiplex lateral flow immunoassay (LFA) was reported for the simultaneous, on-site determination of AFB1, OTA, and zearalenone in corn, rice, and peanut (Chen et al. 2016). The size of gold nanoparticles, conjugation of antibody-gold nanoparticle, and location of capture antigen on the assay strip were optimized for the assay. The developed LFA visually detected 10, 15, and 50 μg/kg of AFB1, OTA, and zearalenone with a LOD of 0.10–0.13, 0.19–0.24, and 0.42–0.46 μg/kg, respectively. The recovery of mycotoxins were > 86% in the spiked samples. Song et al. (2014) reported a multiplex lateral flow immunoassay for qualitative and/or semiquantitative determination of AFB1, zearalenone, and deoxynivalenol in maize samples. The monoclonal antibodies used were specific toward each mycotoxin, and cross-reactivity was not reported. The LFA strip had a visual LOD of 0.03, 1.6, and 10 μg/kg and calculated LOD of 0.05, 1, and 3 μg/kg for AFB1, zearalenone, and deoxynivalenol, respectively. The mycotoxin recoveries ranged from 80% to 122%. The multiplex LFA and LC − MS/MS analyses of naturally contaminated maize samples were in a good agreement.
3.3 Aptamer-/Peptide-Coupled Nanoparticles for Mycotoxin Detection
Aptamers and peptides can enhance the sensitivity of the sensors (Piro et al. 2016). Aptamers are interesting short strand single or double oligonucleotides that are being incorporated into various assay formats to assist the mycotoxin detection. The aptamer-target recognition and the aptamer-controlled growth of gold nanoparticles (Au NPs) were used to develop a versatile, sensitive and visual colorimetric assay for rapid detection of OTA. The aptamer coverage on the Au NPs determined the development of the varied nanostructure morphologies that resulted in formation of different colored solutions. A red-colored solution was associated with spherical Au NPs with low aptamer coverage, whereas blue colored solutions produced branched Au NPs with high aptamer coverage. Thus, Soh et al. (2015) achieved visible colorimetric response for OTA detection at nanomolar levels (1 nM) for red wine samples, as well as cocaine. The sensitive and specific visual detection of contaminants without the need for sophisticated equipment makes such assays relevant for diagnostics and food sampling.
Zhang et al. (2018) described a competitive lateral flow strip format containing a fluorescent aptamer for the one-step determination of OTA in corn samples. In short, biotin-cDNA was immobilized on the test line on the nitrocellulose membrane. In the absence of OTA, the Cy5-labeled aptamer combined with the cDNA to form a stable double helix. The presence of OTA led to the formation of Cy5-aptamer/OTA complexes, therefore reducing the capture of free aptamer in the test zone and subsequently decreasing the fluorescent signals on the test line. A linear relationship from 1 to 1000 ng/ml with the LOD of 0.40 ng/ml and recoveries from 96.4% to 104.67% were reported for spiked corn samples.
Velu and DeRosa (2018) explored two different formats using 5′-biotin-modified OTA aptamer probes in combination with silver or gold nanoparticles in lateral flow colorimetric assays for the detection of OTA. First, in the “adsorption–desorption” approach, aptamers were adsorbed onto the metal nanoparticle surface and, in addition of OTA, lead to aptamer–ochratoxin binding, thereby releasing the NPs. A detection limit of 6.3 nM was achieved for both metal nanoparticles with this approach. The second approach involved a linkage inversion assembled nano-aptasensors (LIANAs) using a DNA linker containing a 5′-5′ linkage inversion (5′-5′ linker) to assemble biotinylated aptamer-functionalized metal nanoparticles. Briefly, OTA bound specifically with its aptamer triggered the release of the linker and disassembly of LIANA aggregates into dispersed nanoparticles. A LOD of 0.63 nM was achieved in the LFA format. A comparison of the LIANA-based LFA strips and the “adsorption–desorption” LFAs showed that the former were more sensitive.
On site rapid detection of OTA contamination in Astragalus membranaceus, used in Chinese medicine, was developed with a lateral flow strip in the competitive format using aptamers against OTA (Zhou et al. 2016). The sample extraction was optimized with 2.5 ml of methanol/water (80:20, v/v) for 1 g, followed by fourfold dilution with running buffer to eliminate the matrix and methanol interferences. A visual LOD of 1 ng/ml with no significant cross-reactivity with other similar toxins was achieved within 15 min. Analyses of A. membranaceus samples showed one OTA-positive sample among nine that were in agreement with the LC–MS/MS analysis.
In place of antibodies, which are expensive and unstable, peptides can also be used for recognizing ochratoxin in the LFA format. Ochratoxin-binding peptides were identified (Bazin et al. 2013; Giraudi et al. 2007). Using such peptides in lateral flow assay technology could provide a promising approach for semiquantitative, rapid, easy, and cost-effective mycotoxin detection. In an approach to develop a user-friendly lateral flow strip and avoid the direct use of ochratoxin in the assay due to its hazardous nature, a mimotope peptide to mimic OTA was screened from a random seven-peptide M13 phage-display library (Lai et al. 2009). The user-friendly lateral flow strip assay was developed by coating the mimotope peptide on the test line, replacing the ochratoxin–BSA conjugates, for capture of the gold nanoparticle-labeled anti-ochratoxin antibodies. The rapid, inexpensive, on-site ochratoxin testing LFA could detect up to 10 ppb ochratoxin in 10 min.
3.4 Nanoparticle-Aided Molecular Detection
The use of oligonucleotide gold nanoparticles for the molecular diagnosis of mycotoxins offers new opportunities for the further development of point of care diagnostic assays with low-cost robust reagents and simple colorimetric detection (Rosi and Mirkin 2005, Harry et al. 2010). The early detection of mycotoxins was correlated with mRNA expression of toxin gene; the detection of these mRNA by nanoparticles carrying oligonucleotide probe could contribute to rapid detection for mycotoxin (Baptista et al. 2005; Schmidt-Heydt et al. 2011). Gold nanoparticles can be conjugated to the biomolecules such as DNA and RNA to develop rapid diagnostic methods.
A qPCR aptasensor for sensitive detection of AFT (M1) was used through a strong interaction with biotin–streptavidin as a molecular recognition element (Guo and Wei 2005). The aptamer was used as a molecular recognition element for the complementary ssDNA. Real-time amplification showed a linear relationship from 0.0001 to 1 μg/L with an LOD of 0.03 ng/L.
4 Conclusions
Among the mycotoxin detection methods, traditional methods like HPLC–FLD and LC–MS are the gold standards. However, these methods lack field-level operational portability and ability for screening of large number of samples simultaneously, which is a challenge for mycotoxin detection in food and feed samples. The HACCP concept requires detection at each level to ensure safe and healthy food and feed. There is still a lack of worldwide awareness toward the health problems caused by mycotoxin consumption.
European Countries have better awareness with several food alert notifications on OTA occurrence being issued over the time. In 2008, 20 alerts concerning OTA in various cereal products were issued by the Rapid Alert System of Food and Feed of the European Union. In October 2014, notification of high OTA levels (14 μg/Kg) was issued in whole emmer wheat pasta from Italy that was distributed to other European countries. The Panel on Contaminants in the Food Chain (CONTAM) of the European Food Safety Authority (EFSA) has specified that consumers like infants and children are vulnerable and have specified a tolerable weekly intake (TWI) of 120 ng/Kg body weight for OTA.
It would be highly desirable if the same kind of alertness existed worldwide; however the sheer logistics is a great challenge considering the quantum of food and feed sources. In order to meet these challenges, portable screening methods like electrochemical sensors and LFA can be utilized. Newer strategies, involving nanotechnology, harness nanomaterials to increase the sensitivity and decrease the limit of detection. The use of simple, stable nanoparticle labels to enhance signal amplification will contribute in fabrication of sensitive biosensors. One of the main challenges for detection of mycotoxin contamination is the co-occurrence of two or more toxins in food and feed samples. The incorporation of novel recognition elements such as antibodies, peptides, or aptamers with nanoparticles for LFA and immunosensors has immense potential for simultaneous multitoxin analysis.
References
Aboul Enein HY, Kutluk OB, Altiokka G, Tuncel M (2002) A modified HPLC method for the determination of ochratoxin A by fluorescence detection. Biomed Chromatogr 16:470–474
Adanyi N, Levkovets IA, Rodriguez-Gil S, Ronald A, Varadi M, Szendro I (2007) Development of immunosensor based on OWLS technique for determining Aflatoxin B1 and Ochratoxin A. Biosens Bioelectron 22:797–802
Agbetiameh D, Ortega-Beltran A, Awuah RT, Atehnkeng J, Cotty PJ, Bandyopadhyay R (2018) Prevalence of aflatoxin contamination in maize and groundnut in ghana: population structure, distribution, and toxigenicity of the causal agents. Plant Dis 102:764–772
Alarcon SG, Palleschi G, Compagnonec D, Pascale M, Visconti A, Barna-Vetron I (2006) Monoclonal antibody based electrochemical immunosensor for the determination of ochratoxin A in wheat. Talanta 69:1031–1037
Alshannaq A, Yu JH (2017) Occurrence, toxicity and analysis of major mycotoxins in food. Int J Environ Res Public Health 14:632
Anfossi L, D’Arco G, Baggiani C, Giovannoli C, Giraudi G (2011) A lateral flow immunoassay for measuring ochratoxin A: Development of a single system for maize, wheat and durum wheat. Food Cont 22:1965–1970
Anfossi L, Giovannoli C, Giraudi G, Biagioli F, Passini C, Baggiani C (2012) A lateral flow immunoassay for the rapid detection of ochratoxin A in wine and grape must. J Agric Food Chem 60:11491−11497
Antep HM, Merdivan M (2012) Determination of ochratoxin A in grape wines after dispersive liquid–liquid microextraction using high performance thin layer and liquid chromatography–fluorescence detection. J Biol Chem 40:155–163
Atoui A, Mathieu F, Lebrihi A (2007) Targeting a polyketide synthase gene for Aspergillus carbonarius quantification and ochratoxin A assessment in grapes using real-time PCR. Int J Food Microbiol 115:313–318
Azer M, Cooper C (1991) Determination of aflatoxins in foods using HPLC and a commercial ELISA system. J Food Protect 54:291–294
Badea M, Floroian L, Restani P, Codruta S, Cobzac A, Moga M (2016) Ochratoxin A detection on antibody immobilized on bsa-functionalized gold electrodes. PLoS One 11:e0160021
Baptista P, Doria GA, Henriques D, Pereira E, Franco R (2005) Colorimetric detection of eukaryotic gene expression with DNA-derivatized gold nanoparticles. J Biotechnol 119:111–117
Bazin, I , Andreotti N, IbnHadjHassine A, DeWaard M, Sabatier JM, Gonzalez C (2013) Peptide binding to ochratoxin A mycotoxin: a new approach in conception of biosensors Biosens Bioelectron 40:240–246
Binder EM (2007) Managing the risk of mycotoxins in modern feed. Anim Feed Sci Tech 133:149–166
Bogs C, Battilani P, Geisen R (2006) Development of a molecular detection and differentiation system for ochratoxin A producing Penicillium species and its application to analyse the occurrence of Penicillium nordicum in cured meats. Int J Food Microbiol 107:39–47
Boudra H, Le Bars P, Le Bars J (1995) Thermostability of ochratoxin a in wheat under two moisture conditions. Appl Environ Microbiol 61:1156–1158
Braicu C, Puia E, Bodoki E, Socaciu C (2008) Screening and quantification of aflatoxins and ochratoxin a in different cereals cultivated in Romania using thin-layer chromatography-densitometry. J Food Qual 31:108–120
Bryden WL (2012) Mycotoxin contamination of the feed supply chain: implications for productivity and feed security. Anim Feed Sci Tech 173:134–158
Chen Y, Chen Q, Han M, Zhou J, Gong L, Niu Y, Zhang Y, He L, Zhang L (2016) Development and optimization of a multiplex lateral flow immunoassay for the simultaneous determination of three mycotoxins in corn, rice and peanut. Food Chem 213: 478–484
Cho YJ, Lee DH, Kim DO, Min WK, Bong KT, Lee GG, Seo JH (2005) Production of a monoclonal antibody against ochratoxin a and its application to immunochromatographic Assay. J Agric Food Chem 53:8447−8451
Criseo G, Bagnara A, Bisignano G (2001) Differentiation of aflatoxin-producing and non-producing strains of Aspergillus flavus group. Letts Appl Microbiol 33:291–295
Daly SG, Keating GJ, Dillon PP et al (2000) Development of surface plasmon resonance-based immunoassay for aflatoxin B1. J Agri Food Chem 48:5097–5104
Dao HP, Mathieu F, Lebrihi A (2005) Two primer pairs to detect OTA producers by PCR method. Int J Food Microbiol 104:61–67
Delmulle BS, de Saeger SMDG, Sibanda L, Barna-Vetro I, van Peteghem CH (2005) Development of an immunoassaybased lateral flow dipstick for the rapid detection of aflatoxin B1 in pig feed. J Agric Food Chem 53:3364–3368
Desalegn B, Nanayakkara S, Harada KH, Hitomi T, Chandrajith R, Karunaratne U, Abeysekera T, Koizumi A (2011) Mycotoxin detection in urine samples of patients with chronic kidney disease of uncertain etiology from Sri Lanka. Bull Environ Contam Toxicol 87:6
Devi KT, Mayo M, Reddy KLN, Delfosse P, Reddy G, Reddy SV, DVR R (1999) Production and characterization of monoclonal antibodies for aflatoxin B1. Lett Appl Microbiol 29:284–288
Devi KT, Mayo M, Hall AJ, Craufurd PQ, Wheeler TR, Waliyar F, Subrahmanyam A, Reddy K (2002) Development and application of an indirect competitive enzymelinked immunoassay for aflatoxin M1 in milk and milk-based confectionery. J Agri Food Chem 50:933–937
Durguti V, Georgieva A, Angelov A, Bajrami Z (2014) Quantitative determination of ochratoxin A in wine after the clarification and filtration. Croat J Food Sci Technol 6:79–83
Edwards SG, O’Callaghan J, Dobson ADW (2001) PCR-based detection and quantification of mycotoxigenic fungi. Mycol Res 106:1005–1025
Ekhtelat M, Badpa F, Khorasgani ZN, Azemi E (2018) High-performance Liquid Chromatography analysis of Ochratoxin A in Zataria multiflora and Foeniculum vulgare in Ahvaz (Iran). Asian J Pharmaceut 12:S523
Eun-mee H, Park HR, Hu SJ, Kwon KS, Lee H, Ha M, Kim K, Ko E, Ha S, Chun H, Chung D, Bae D (2006) Monitoring of Aflatoxin B1 in Livestock Feeds Using ELISA and HPLC. J Microbiol Biotechnol 16:643–646
Fuji S, Ono EYS, Ribeiro RMR, Assunção FGA, Takabayashi CR, Moreira de Oliveir TCR, Itano EN, Ueno Y, Kawamura O, Hirooka EY (2007) A Comparison between enzyme immunoassay and hplc for ochratoxin a detection in green, roasted and instant coffee. Braz Arch Biol Technol 50:349–359
Gao J, Gu H, Xu B (2009) Multifunctional magnetic nanoparticles: design, synthesis and biomedical applications. Accounts Chem Res 42:1097–1107
Giraudi G, Anfossi L, Baggiani C, Giovannoli C, Tozzi C (2007) Solid-phase extraction of ochratoxin A from wine based on a binding hexapeptide prepared by combinatorial synthesis. J Chromatogr A 1175:174–180
Guo P, Wei C (2005) Quantum dots for robust and simple assays using single particles in nanodevices. Nanomed Nanotechnol Biol Med 1:122–124
Ha TH (2015) Recent advances for the detection of ochratoxin A. Toxins 7:5276–5300
Hampikyan H, Bingol EB, Colak H, Cetin O, Bingol B (2015) Determination of ochratoxin a in baby foods by ELISA And HPLC. Acta Aliment 44:578–584
Harry SR, Hicks DJ, Amiri KI, Wright DW (2010) Hairpin DNA coated gold nanoparticles as intracellular mRNA probes for the detection of tyrosinase gene expression in melanoma cells. Chem Commun 46:5557–5559
Herzallah SM (2009) Determination of aflatoxins in eggs, milk, meat and meat products using HPLC fluorescent and UV detectors. Food Chem 114:1141–1146
Janshoff A, Galla HJ, Steinem C (2000) Piezoelectric mass-sensing devices as biosensors - an alternative to optical biosensors?Angew Chem. Int Ed 39:4004–4032
Javier DJ, Castellanos-Gonzalez A, Weigum SE, White AC, Richards-Kortum R (2009) Oligonucleotide-gold nanoparticle networks for detection of Cryptosporidium parvum heat shock protein 70 mRNA. J Clin Microbiol 47:4060–4066
Jin X, Liu X, Chen L,Jiang J, Shen G, Yu R (2009) Biocatalyzed deposition amplification for detection of aflatoxin B1 based on quartz crystal microbalance Anal Chim Acta 645:92–97
Jung S, Choe B, Shin G, Kim J, Chae Y (2012) Analysis of roasted and ground grains on the seoul (korea) market for their contaminants of aflatoxins, ochratoxin A and Fusarium toxins by LC-MS/MS. World Academy Sci Engineer Technol 6:12–23
Kilicel F, Karapinar HS, Cimen A (2017) Quantitation of aflatoxins in food materials using HPLC-FLD method. Sci J Analy Chem 5:90–97
Kim KS, Park JK (2005) Magnetic force-based multiplexed immunoassay using superparamagnetic nanoparticles in microfluidic channel. Lab Chip 5:657–664
Kim NY, Lee I, Ji GE (2014) Reliable and simple detection of ochratoxin and fumonisin production in black Aspergillus. J Food Protect 77:653–658
Kim HJ, Lee MJ, Kim HJ, Cho SK, Park HJ, Jeong MH (2017) Analytical method development and monitoring of aflatoxin B1, B2, G1, G2 and ochratoxin A in animal feed using HPLC with Fluorescence detector and photochemical reaction device. Cogent Food Agric 3:1419788
Kok WT, van Neer TCH, Traag WA, Tuinstra LGT (1986) Determination of aflatoxins in cattle feed by liquid chromatography and post-column derivatization with electrochemically generated bromine. J Chromat A 367:231–236
Kolosova AY, Sibanda L, Dumoulin F, Lewis J, Duveiller E, Van Peteghem C, De Saeger S (2008) Lateral-flow colloidal gold-based immunoassay for the rapid detection of deoxynivalenol with two indicator ranges. Anal Chim Acta 616:235–244
Kong Z, Wang H, Zou L, Ji Z (2018) Enhancement of aflatoxin B1 detection using electrochemical immunoassay method and 2-aminoethanethiol. Mater Res Express 5:066414
Kotinagu K, Mohanamba T, Rathna Kumari N (2015) Assessment of aflatoxin B1 in livestock feed and feed ingredients by high-performance thin layer chromatography. Veterinary World. EISSN: 2231-0916
Kupski L, Badiale-Furlong E (2015) Principal components analysis: an innovative approach to establish interferences in ochratoxin a detection. Food Chem 177:354–360
Kushiro M, Hatabayashi H, Nakagawa H, Yabe K (2017) Improvement of mobile phase in thin-layer chromatography for aflatoxins and analysis of the effect of dichlorvos in aflatoxigenic fungi. JSM Mycotoxins 67:5–6
Lai W, Fung DYC, Xu Y, Liu R, Xiong Y (2009) Development of a colloidal gold strip for rapid detection of ochratoxin A with mimotope peptide. Food Control 20:791–795
Levin RE (2012) PCR detection of aflatoxin producing fungi and its limitations. Int J Food Microbiol 156:1–6
Li P, Zhou Q, Wang T, Zhou H, Zhang W, Ding X, Zhang Z, Chang PK, Zhang Q (2016). Development of an enzyme-linked immunosorbent assay method specific for the detection of g-group aflatoxins. Toxins 8:5. https://doi.org/10.3390/toxins8010005
Linting Z, Ruiyi L, Zaijun L, Qianfang X, Yinjun F, Junkang L (2012) An immunosensor for ultrasensitive detection of aflatoxin B1 with an enhanced electrochemical performance based on graphene/conducting polymer/gold nanoparticles/the ionic liquid composite film on modified gold electrode with electrodeposition. Sens Actuators B Chem 174:359–365
Liu X, Yang Z, Zhang Y, Yu R (2013) A novel electrochemical immunosensor for ochratoxin A with hapten immobilization on thionine/gold nanoparticle modified glassy carbon electrode. Anal Methods 5:1481–1486
Magan N, Hope R, Colleate A, Baxter ES (2002) Relationship between growth and mycotoxin production by Fusarium species, biocides and environment. Eur J Pl Pathol 108:685–690
Mahmoud MA (2015) Detection of Aspergillus flavus in stored peanuts using real-time pcr and the expression of aflatoxin genes in toxigenic and atoxigenic A. flavus isolates. Foodborne Pathog Dis 12:289–296
Maragos CM, Busman M (2010) Rapid and advanced tools for mycotoxin analysis: a review. Food Addit Contam 27:688–700
Masoomi L, Sadeghi O, Banitaba MH, Shahrjerdi A, Davarani SSH (2013) A non-enzymatic nanomagnetic electroimmunosensor for determination of Aflatoxin B1 as a model antigen. Sens Actuators B Chem 177:1122–1127
Meuccia V, Razzuolia E, Soldania G, Massart F (2010) Mycotoxin detection in infant formula milks in Italy. Food Addit Contam 27:64–71
Mirghani MES, Man YBC, Jinap S, Baharin BS, Bakar J (2001) A new method for determining aflatoxins in groundnut and groundnut cake using Fourier transform infrared spectroscopy with attenuated total reflectance. J Am Oil Chem Soc 78:985–992
Moon J, Kim G, Lee S (2013) Development of nanogold-based lateral flow immunoassay for the detection of ochratoxin a in buffer systems. J Nanosci Nanotechnol 13: 7245–7249
Muchindu M, Iwuoha E, Pool E, West N, Jahed N, Baker P, Waryo T, Williams A (2011) Electrochemical Ochratoxin A immunosensor system developed on sulfonated polyaniline. Electroanalysis 23:122–128
Myndrul V, Viter R, Savchuk M, Shpyrka N, Erts D, Jevdokimovs D, Silamiķelis V, Smyntyna V, Ramanavicius A, Iatsunskyi I (2018) Porous silicon based photoluminescence immunosensor for rapid and highly-sensitive detection of Ochratoxin A. Biosens Bioelectron 102:661–667
Nisa A, Zahra N, Hina S (2014) Detection of aflatoxins in rice samples. Bangladesh J Sci Ind Res 49: 189–194
Olsson J, Börjesson T, Lundstedt T, Schnürer J (2002) Detection and quantification of ochratoxin A and deoxynivalenol in barley grains by GC-MS and electronic nose. Int J Food Microbiol 72:203–214
Parker CO, Tothill IE (2009) Development of an electrochemical immunosensor for aflatoxin M1 in milk with focus on matrix interference. Biosens Bioelectron 24:2452–2457
Pirinçci SS, Ertekin O, Laguna DE,Özen FS, Öztürk ZZ , Öztürk S (2018) Label-free QCM immunosensor for the detection of ochratoxin A. Sensors 18:1161. https://doi.org/10.3390/s18041161
Piro B, Shi A, Reisberg S, Noël V, Anquetin G (2016) Comparison of electrochemical immunosensors and aptasensors for detection of small organic molecules in environment, food safety, clinical and public security. Biosensors 6:7
Pittet A, Royer D (2002) Rapid, low cost thin-layer chromatographic screening method for the detection of ochratoxin A in green coffee at a control level of 10íg/Kg. J Agric Food Chem 50:243–247
Prestani A, Tabatabaei SN, Fazeli MH, Antikchi M, Baabaei M (2011) Comparison of HPLC and Elisa for determination of aflatoxin concentration in the milk and feeds of dairy cattle. J Res Agri Sci 7:71–78
Quesada-González D, Merkoçi A (2015) Nanoparticle-based lateral flow biosensors Biosens Bioelectron. 73:47–63
Radi E, Muñoz-Berbel X, Cortina-Puig M, Marty JL (2009) An electrochemical immunosensor for ochratoxin A based on immobilization of antibodies on diazonium-functionalized gold electrode. Electrochim Acta 54:2180–2184
Rahimi E, Shakerian A (2013) Ochratoxin A in dried figs, raisings, apricots, dates on Iranian retail market. Health 5:2077–2080
Rahmani A, Jinap S, Soleimany F (2009) Qualitative and quantitative analysis of mycotoxins. Comprehen Rev Food Sci Food Saf 8:202–251
Rauch P, Fukal L, Prosek J, Brezina P, Kas J (1987) Radioimmunoassay of aflatoxin M1. J Radioanaly Nucl Chem 117:163–169
Ren M, Xu H, Huang X, Kuang M, Xiong Y, Xu H, Xu Y, Chen H, Wang A (2014) Immunochromatographic assay for ultrasensitive detection of aflatoxin b1 in maize by highly luminescent quantum dot beads. ACS Appl Mater Interfaces 6:14215−14222
Rivas L, Mayorga-Martinez CC, Quesada-Gonzalez D, Zamora-Galvez A, Escosura-Muñiz A, Merkociṃ A (2015) Label-free impedimetric aptasensor for ochratoxin-A detection using iridium oxide nanoparticles. Anal Chem 87:5167–5172
Rodriguez A, Rodriguez M, Luque MI, Martin A, Cordoba JJ (2012) Real-time PCR assays for detection and quantification of aflatoxin-producing molds in foods. Food Microbiol 31:89e99
Rosi NL, Mirkin CA (2005) Nanostructures in biodiagnostics. Chem Rev 105:1547–1562
Sadhasivam S, Britzi M, Zakin V, Kostyukovsky M, Trostanetsky A, Quinn E, Ionov E (2017) Rapid detection and identification of mycotoxigenic fungi and mycotoxins in stored wheat grain. Toxins 9:302
Santos VO, Pelegrini PB, Mulinari F, Lacerda AF, Moura, RS, Cardoso LPV, Bührer-Sékula S, Miller RNG, Grossi-de-Sa MF (2017) Development and validation of a novel lateral flow immunoassay device for detection of aflatoxins in soy-based foods. Anal Methods 9:2715–2722
Sartori D, Furlaneto MC, Martins MK, de Paula MRF, Pizzirani-Kleiner AA, Taniwaki MH, Fungaro MHP (2006) PCR method for the detection of potential ochratoxin-producing Aspergillus species in coffee beans. Res Microbiol 157:350–354
Schmidt-Heydt M, Parra R, Geisen R, Magan N (2011) Modelling the relationship between environmental factors, transcriptional genes and deoxynivalenol mycotoxin production by strains of two Fusarium species. J. R. Soc Interf 8:117–126
Schneider E, Curtui V, Seidler C, Dietrich R, Usleber E, Märtlbauer E (2004) Rapid methods for deoxynivalenol and other tricothecenes. Toxicol Lett 153:113–121
Scott P (1995) Mycotoxin methodology. Food Addit Contam 12:395–403
Selvan ST, Tan TY, Yi DK, Jana NR (2009) Functional and multifunctional nanoparticles for bioimaging and biosensing. Langmuir 26:11631–11641
Shapira R, Paster N, Eyal O, Menasherov M, Mett A, Salomon R (1996) Detection of aflatoxinogenic molds in grains by PCR. Appl Environ Microbiol 62:3270–3273. PMid:8795215
Sharma A, Kumar A, Khan R (2017) Electrochemical immunosensor based on poly (3,4-ethylenedioxythiophene) modified with gold nanoparticle to detect aflatoxin B1. Mater Sci Engineer C, Mater Biol Appl 76:802–809
Shephard GS (2008) Determination of mycotoxins in human food. Chem Soc Rev 37:2468–2477
Skarkova J, Ostry V (2000) An HPTLC method for confirmation of the presence of ultra, trace amounts of aflatoxin M1 in human urine. J Planar Chromat 13:42–49
Smith MC, Madec S, Coton E, Hymery N (2016) Natural co-occurrence of mycotoxins in foods and feeds and their in vitro combined toxicological effects. Toxins 8:94. https://doi.org/10.3390/toxins8040094
Soh JH, Lin Y, Rana S, Ying JY, Stevens MM (2015) Colorimetric detection of small molecules in complex matrixes via target-mediated growth of aptamer-functionalized gold nanoparticles. Anal Chem 87:7644–7652
Song S, Liu N, Zhao Z, Ediage EN, Wu S, Sun C, Saeger SD, Wu A (2014) Multiplex lateral flow immunoassay for mycotoxin determination. Anal Chem 86:4995−5001
Spinella K, Mosiello L, Palleschi G, Vitali F (2013) Development of a qcm (quartz crystal microbalance) biosensor to the detection of Aflatoxin B1. Open J Appl Biosensor 2:112–119
Streit E, Schwab C, Sulyok M, Naehrer K, Krska R, Schatzmayr G (2013) Multi-mycotoxin screening reveals the occurrence of 139 different secondary metabolites in feed and feed ingredients. Toxins 5:504–523
Sulyok M, Berthiller F, Krska R, Schuhmacher R (2006) Development and validation of a liquid chromatography/ tandem mass spectrometric method for the determination of 39 mycotoxins in wheat and maize. Rapid Commun Mass Spectrom 20:2649–2659
Sun D, Gu X, Li JG, Yao T, Dong YC (2015) Quality evaluation of five commercial enzyme linked immunosorbent assay kits for detecting Aflatoxin B1 in Feedstuffs. Asian Australas J Anim Sci 28:691–696
van der Gaag B, Spath S, Dietrich H et al (2003) Biosensors and multiple mycotoxin analysis. Food Cont 14:251–254
Velu R, DeRosa MC (2018) Lateral flow assays for Ochratoxin A using metal nanoparticles: comparison of “adsorption–desorption” approach to linkage inversion assembled nano-aptasensors LIANA. Analyst 143:4566–4574
Venkataramana M, Rashmi R, Uppalapati SR, Chandranayaka S, Balakrishna K, Radhika M, Gupta VK, Batra HV (2015) Development of sandwich dot-ELISA for specific detection of OchratoxinA and its application on to contaminated cerealgrains originating from India. Front Microbiol 6:article 511
Vidal JC, DuatoP BL, Castillo JR (2009) Use of polyclonal antibodies to ochratoxin a with a quartz – crystal microbalance for developing real-time mycotoxin piezoelectric immunosensors. Anal Bioanal Chem 394:575–582
Vitera R, Savchuk M, Iatsunskyic I, Pietralik Z, Starodub N, Shpyrka N, Ramanaviciene A, Ramanavicius A (2018) Analytical, thermodynamical and kinetic characteristics of photoluminescence immunosensor for the determination of Ochratoxin A. Biosens Bioelectron 99:237–243
Vo-Dinh T (2007). Nanotechnology for application in biology and medicine. CRC Press, Boca Raton
Wang XH, Liu T, Xu N, Zhang Y, Wang S (2007) Enzyme-linked immunosorbent assay and colloidal gold immunoassay for ochratoxin a: investigation of analytical conditions and sample matrix on assay performance. Anal Bioanal Chem 389:903–911
Won-bo S, Yang Z, Kim J, Kim JY, Kang SJ, Woo GJ, Chung YC, Eremin S, Chung DH (2007) Development of immunochromatography strip-test using nanocolloidal gold-antibody probe for the rapid detection of aflatoxin b1 in grain and feed samples. J Microbiol Biotechnol 17: 1629–1637
Wacoo AP, Wendiro D, Vuzi PC, Hawumba JF (2014) Methods for detection of aflatoxins in agricultural food crops. J Appl Chem 2014:Article ID 706291
Welke JE, Hoeltz M, Dottori HA, Noll IB (2010) Determination of ochratoxin A in wine by high-performance thin-layer chromatography using charged coupled device. J Braz Chem Soc 21:441–446
Xiao H, Marquardt RR, Abramson D, Frohlich AA (1996) Metabolites of Ochratoxins in rat urine and in a culture of Aspergillus ochraceus. Appl Environ Microbiol 62:648–655
Yazdanpanah H, Zarghi A, Shafaati AR, Foroutan SM, Aboul-Fathi F, Khoddam A, Nazari F, Shaki F (2013) Analysis of aflatoxin B1 in Iranian foods using hplc and a monolithic column and estimation of its dietary intake. Iranian J Pharmaceut Res 12:83–89
Yoon BR, Hong SY, Cho SM, Lee KR, Kim M, Chung SH (2016) Aflatoxin M1 levels in dairy products from South Korea determined by high performance liquid chromatography with fluorescence detection. J Food Nutrit Res 55:171–180
Zhang G, Zhu C, Huang Y, Yan J, Chen A (2018) A lateral flow strip based aptasensor for detection of ochratoxin A in corn samples. Molecules 23:291. https://doi.org/10.3390/molecules23020291
Zhao SP, Zhang D, Tan LH, Yu B, Cao WG (2016) Analysis of aflatoxins in traditional Chinese medicines: classification of analytical method on the basis of matrix variations. Sci Reports 6:30822
Zhou W, Kong W, Dou X, Zhao M, Ouyang Z, Yang M (2016) An aptamer based lateral flow strip for on-site rapid detection of ochratoxin A in Astragalus membranaceus. J Chromatogr B 1022:102–108
Acknowledgments
VG and PC thank the Department of Biotechnology and Department of Science and Technology, Government of India for the funding (BT/PR10455/PFN/20/869/2013 & DST/INT/MECICO/P-06/2016). SR thanks the Department of Science and Technology for Junior Research Fellowship under INSPIRE program.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Rahi, S., Choudhari, P., Ghormade, V. (2019). Aflatoxin and Ochratoxin A Detection: Traditional and Current Methods. In: Satyanarayana, T., Deshmukh, S., Deshpande, M. (eds) Advancing Frontiers in Mycology & Mycotechnology. Springer, Singapore. https://doi.org/10.1007/978-981-13-9349-5_15
Download citation
DOI: https://doi.org/10.1007/978-981-13-9349-5_15
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-9348-8
Online ISBN: 978-981-13-9349-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)