Abstract
In recent decades, mycotoxin contamination of agricultural food items has garnered considerable attention because to their high acute or chronic toxicity in humans and animals, resulting from consumption and exposure duration to contaminated food or feed. This is exacerbated by the impact of the Covid-19 pandemic, civil wars, and conflicts (e.g., the Russia-Ukraine conflict, Yemen, Ethiopia, Afghanistan, and others), which further strain the food security and nutritional status of the most vulnerable demographic groups, which are predicted to continue to deteriorate due to health and socioeconomic factors. The presence of these mycotoxins in food and animal feed has a negative impact on public health and the economy; consequently, it is crucial to detect and quantify these toxins in agricultural lots. Maintaining food quality and minimizing adverse effects on human and animal health are dependent on early detection. Conventional techniques for detecting mycotoxins include enzyme-linked immunoassay (ELISA), gas chromatography (GC), thin-layer chromatography (TLC), and high-performance liquid chromatography (HPLC). Nanomaterial-based sensor technologies provide diverse mitigation methods for quantifying single or multiple analytes, as mycotoxin co-occurrence in a single matrix has become more common. In this chapter, we describe recent advancements in nanodiagnostic techniques that permit multiplex detection of mycotoxins on a single platform. In addition, we discuss certain commercially available lateral flow immunoassay (LFIA) test strips that often use gold nanoparticles (AuNPs) or quantum dots (QDs) as colored labels for signal amplification, as well as some commercial goods with nanoformulations used in agriculture. For the commercialization of nano-based assays (nanosensors), nanodisks (nanoparticles-based artificial sensing), and that may be used as point-of-care testing (POCT) devices for mycotoxin detection, it will be necessary to conduct additional research and make additional investments to overcome the difficulties identified.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
15.1 Introduction
Mycotoxigenic fungi have received special attention due to their threat to food safety and toxicological profiles to human and animal health. Mycotoxins are secondary metabolic products of toxigenic fungi, secreted in food and feed (Rai et al. 2015). They have a great capacity to cause damage to cells, through the activation a cascade of variety of signaling pathways (e.g., MAPK, NRF2, Wnt, P53, and PI3K), known to have detrimental effects to health through causing oxidative stress, cytotoxicity, and genotoxicity to the liver and kidneys (Chen et al. 2022), thus making them extremely dangerous for both humans and animals, even resulting in death, depending on the amount and type of mycotoxin ingested. Fungal growth occurs naturally in food and is more common in grains (e.g., maize, peanuts, etc.), but this growth can be enhanced by humidity and temperature, as well as irregular conditions of production and storage. Fungal poisoning in food and feed through mycotoxin contaminations causes large economic losses; it is estimated that approximately 25% of food worldwide is contaminated by mycotoxins (SILVA et al. 2021).
These mycotoxigenic fungi exist in diverse environments and can contaminate a wide range of agricultural products (Shanakhat et al. 2018). The contamination of agricultural food products by mycotoxins has received a lot of attention in recent decades, due to their high acute or chronic toxicity in humans and animals and due to consumption and exposure time to food or feed contaminated with mycotoxins. This is exacerbated by the impact of the Covid-19 pandemic, civil wars, and conflicts (e.g., Russia-Ukraine conflict, Yemen, Ethiopia, Afghanistan, and other others), further straining food security and nutritional status of the most vulnerable demographic groups, which are anticipated to continue to deteriorate because of health and socioeconomic factors. According to the UN report in 2020, one in three people in the world (~2.37 billion) lack access to adequate safe and nutritional food – an increase of nearly 320 million people from 2019 (FAO, IFAD, UNICEF 2021; Vos et al. 2022). The presence of these mycotoxins in food and feed affects public health and the economy; therefore, it is of great importance to detect and quantify these toxins in agricultural lots. Early detection is essential to maintain food quality and reduce the impact on human and animal health (Li et al. 2021).
Efforts of killing the fungus does not certify nor guarantees that the mycotoxins have been eliminated, because mycotoxins are highly stable (SILVA et al. 2021). There are different types of mycotoxins, mainly categorized into the following groups with the prevalently occurring being: aflatoxins (AFs), AFB1; ochratoxins (OTs), OTA, fumonisins (FMs), FB1; deoxynivalenol (DON), patuline, and zearalenone (ZEA), respectively (Rai et al. 2015). The most common mycotoxigenic genera include Aspergillus, Alternaria, Fusarium, Penicillium, and Stachybotrys (Dobrucka and Długaszewska 2016). A single species of fungus has the capabilities of producing different types of mycotoxins, just as different fungi can produce different types of mycotoxins (SILVA et al. 2021).
It is estimated that more than 300 mycotoxins, which are of concern, have been identified; the growth and proliferation of fungi producing these toxins occur mainly in field cultivation, during the transport and storage of commodities. The main fungi responsible for mycotoxins are Aspergillus, Penicillium, and Fusarium species. The Aspergillus fungal species generally produces mycotoxins that are divided into three different groups. Aflatoxins B (AFB1 and AFB2), aflatoxins G (AFG1 and AFG2), and aflatoxins M (AFM1 and AFM2), where AFB1 is considered the most dangerous to health due to its high carcinogenic potential. Additionally, the mycotoxin ochratoxin A (OTA) is highly toxic and prevalent; it is produced by Aspergillus and Penicillium species (Nayaka et al. 2013), exhibiting nephrotoxic and nephrocarcinogenic effects (Ingle et al. 2020), and can be found in several animal products. Another mycotoxin produced by these two species is patulin, which is more common in agricultural commodities such as vegetables, fruits, and cereals. Patulin toxicity is associated with gastrointestinal disorders (Oancea and Stoia 2008).
Fumonisins are mainly produced by Fusarium proliferatum, Fusarium verticillioides, and Fusarium nygamai and are a group of non-fluorescent mycotoxins. The main contaminant is corn and its derivatives; consumption of food contaminated with fumonisins leads to leukoencephalomalacia in horses, hydrothorax, and pulmonary edema in swine (Nayaka et al. 2013). Many other mycotoxins, such as trichothecenes (T-2) and zearalenone (ZEA), are present in agricultural products. The identification of multiple toxins in large batches is of great importance to reduce the negative impact on public health. Effective, sensitive/selective, and low-cost methods are required for the qualitative and quantitative detection of mycotoxigenic fungi that can produce mycotoxin in small quantities.
The main toxicological effects caused by mycotoxins predominantly include carcinogenesis, hepatotoxicity, neurotoxicity, immunosuppression, and mutagenicity (SILVA et al. 2021). A variety of mycotoxins have been classified as carcinogenic, with Aflatoxin B1 (AFB1) being the most potent carcinogen and usually the major aflatoxin produced by toxigenic strains (Rai et al. 2015). Mycotoxin exposure is increasingly becoming a global problem, especially with the increase in a significant number of people switching to a vegan diet (Penczynski et al. 2022). Chronic exposure can lead to the development of serious pathologies already mentioned above; therefore, mitigation and elimination of these mycotoxins is essential.
15.2 Conventional Diagnostics for Mycotoxins in Agriculture
All conventional analytical procedures used for mycotoxin detection and quantification include three basic steps: (i) extraction, (ii) purification and cleaning, and (iii) identification and quantification (Shanakhat et al. 2018) as shown in Fig. 15.1 (Li et al. 2021).
-
(i)
Extraction
The detection of mycotoxins is governed by effective sample extraction, assigning the correct methods according to their specificity. In this situation, good sampling guarantees a more accurate result for the overall sample. Generally, the sample is ground, homogenized in extraction solvent and filtered for the purification step. In the extraction process, the analyte (mycotoxin) will move in the extraction solvent and thus the desired mycotoxin compound will be removed for analysis (Shanakhat et al. 2018).
-
(ii)
Purification and cleaning
Before identification and quantification of mycotoxins, sample extracts must undergo cleanup to remove co-extracted materials (such as interfering compounds such as proteins, lipids, and carbohydrates). Cleanup is done with an immunoaffinity column (IAC), which has a high selectivity which is achieved by passing the sample through prepackaged cartridges, centrifugation, and extraction techniques in solid phase (Nayaka et al. 2013).
-
(iii)
Identification and quantification
The detection of these mycotoxins in samples can be done through several conventional techniques, such as thin-layer chromatography (TLC), high-performance liquid chromatography (HPLC), gas and/or liquid chromatography often coupled with an ultraviolet detector or with mass spectrometric detectors (GC/GC-MS and LC/LC-MS, respectively), or immunochemical methods such as radioimmunoassay (RIA), enzyme-linked immunosorbent assay (ELISA), column immunity assay (ICA), and lateral flow immunochromatographic strips (ICSTs).
The TLC technique are widely used for their speed, simplicity, and low cost. This technique is very popular, because it can detect more than one mycotoxin in a sample with a detection limit of approximately 0.01 ppm (Oancea and Stoia 2008). TLC is based on the separation of substances by their migration in a specific matrix with a specific solvent (Nayaka et al. 2013). On the other hand, HPLC method has become popular and is often used for analyzing aflatoxins with UV fluorescence detection, with detection limit below ng/g of the product (Oancea and Stoia 2008). Thus, it is a technique that has high sensitivity and a high degree of precision. In agricultural samples, reversed-phase chromatography (RP) is widely used.
Gas chromatography has a limitation, as it requires volatilization. It has a detection of approximately 0.0001 ppm and can be coupled to mass spectrometry (GC-MS), where it combines superior separation in capillary columns with a variety of specific and general detectors. In immunochemical methods, the RIA has a high sensitivity that is due to radiolytic detection, and, therefore, a large amount of sample is not required. The ELISA is the most used immunoassay to identify OTA; the method has simplicity, the ability to immobilize antibodies, and efficiency in analyzing multiple mycotoxins with low molecular weight that would hardly be detected in a single sample with other available methods (Ingle et al. 2020).
15.3 Nanosurveillance to Mitigate Mycotoxins
There is a myriad of conventional techniques for detecting mycotoxins, which include enzyme-linked immunoassay (ELISA), gas chromatography (GC), thin-layer chromatography (TLC), high-performance liquid chromatography (HPLC), etc. The burden of mycotoxin contamination in the global market for testing is at compound annual growth rate (CAGR) 7.9% from 2021 to 2026, which will account for 1052.86 million USD (BCC Research Publishing 2022). Extreme rainfall and drought promote mycotoxin production, and once mycotoxins are released, they are difficult to control and nearly impossible to eradicate. Mycotoxins are the largest toxin that contaminates food and feedstuff, thus causing the largest burden in global food market (Fig. 15.2). Therefore, it is crucial to do mycotoxin testing on every crop produced. Small food processors rely on visual inspection and only test when mycotoxin contamination is identified or suspected. In addition, the risk of mycotoxin contamination in crops and stored food products is anticipated to grow, leading to an increase in the incidence of both human and animal diseases.
Global market for testing toxins. (Adapted from BCC Research Publishing 2022)
The Food and Agriculture Organization (FAO) advocated for improved surveillance and traceability and acknowledged the need to invest in radical new technologies such as nanodiagnostic tools to achieve these goals. Thus, there is a need to develop more advanced, specific, selective, sensitive, and portable methods that require minimal expertise for operation. Microfluidics, lab-on-a-chip, smart nanospectroscopy, and sensor technologies are some of the most important technical interfaces that have been refined from micro- to nano-sizes. The beneficial properties of this size transformation are the result of increased surface-to-volume ratio due to the availability of surface atoms, multifunctionality, better catalysis, and reactivity. Moreover, the antimicrobial potential of nanomaterials increases due to the high contact surface-to-volume ratio with fungal surfaces or biomolecules (Kalia et al. 2020b). By interacting with fungal cells, nanomaterials adsorb to oppositely charged functional groups and exhibit the advantage of bypassing intact cell membranes. They can form complexes with biomolecules leading to damage and inactivation of a cascade of pathways involved in maintaining fungal cell homeostasis. These interactions and transformations of biomolecules result in inhibition of fungal growth and mycotoxin production (Kalia et al. 2020a). Microfluidic/optofluidic lab-on-a-chip technologies are a common detection method for mycotoxins. The use of nanotechnology opens up the avenue for the miniaturization and development of nanobiosensors that can be used to detect mycotoxins (Eskola et al. 2020; Thipe et al. 2018).
15.4 Nanodiagnostics for Mycotoxins
15.4.1 Sensors Based on Nanomaterials for Mycotoxin Surveillance
Nanomaterial-based sensor technologies provide diversified mitigation methods for quantifying single or multiple analytes, since mycotoxin co-occurrence in a single matrix has become more prevalent. This can aid in early detection with high sensitivity/selectively of mycotoxigenic fungi and the respective mycotoxin they produce (Kalia et al. 2020a).
15.4.2 Metallic Nanoparticles
Many chemical, physical, and biological methods have been used to synthesis of metallic nanoparticles, such as gold (Thipe et al. 2015), silver (Guilger-Casagrande and Lima 2019), copper (Raafat et al. 2021), iron/iron oxide (Devi et al. 2019), zinc (Kalia et al. 2020a), and many others, including their bi- or tri-metallic complexes. Metal and metal oxide nanoparticles demonstrate excellent antimicrobial activities and have bactericidal, fungicidal, viricidal, and algaecide action (Kalia et al. 2020a). Kalia and coworkers showed that oxide nanoparticles can control the production of mycotoxins, in addition to neutralizing or adsorbing already secreted mycotoxins. Zinc-derived nanomaterials at substantially low concentrations demonstrate sporicidal activity and inhibit vegetative mycelial growth of filamentous fungal plant pathogens (Kalia et al. 2020a; Li et al. 2021).
15.5 Smart Nanosensors
15.5.1 Nanoparticles with Conductivity-Based Sensors
Biosensors are the combination of a biological component (in this case, secondary metabolites) with a physicochemical detector or transducer. The transducer transforms the signal received from the interaction between the analyte and the biological component into quantified and easily measurable signals. These signals are then displayed by the signal processor by digital output signals (Rai et al. 2015). Sheini (2020) produced a paper-based sensor array with gold and silver nanoparticles; color changes provide colorimetric signatures attributed by aggregation of nanoparticles from the interaction with mycotoxins (AFB1, AFG1, AFM1, OTA, and ZEN) with a LOD of 2.7, 7.3, 2.1, 3.3, and 7.0 ng/mL, respectively (Sheini 2020).
Liu et al. (2020) designed a smartphone-based multiplexed dual detection mode device integrated AuNPs and time-resolved fluorescence microspheres (TRFMs) LFIA for mycotoxins (AFB1, ZEN, DON, T-2, and FB1) in cereals with LODs of 2.5, 0.5, 0.5, 2.5, and 0.5 μg/kg, respectively. Nanoparticles have been incorporated into biosensors to improve analytical parameters, which include limit of detection/quantification, linear range, assay stability/selectivity/sensitivity, and cheap production cost, among other things. In the development of nanosensors, nanoparticles serve as an immobilization support, signal amplifier, mediator, and artificial enzyme label in mycotoxin analysis as shown in Fig. 15.3 (Zhao et al. 2022).
Schematic overview of multiplex detection of mycotoxins employing the enrichment for samples with various nanoparticles based on laminar flow strips and smartphone readouts. This utility functions by (i) using multimodal nanoparticles coated with mycotoxin antibodies to enhance the limit of detection (LOD) of the mycotoxin; (ii) utilizing different labels (e.g., magnetic particles, quantum dots, fluorophores on gold nanoparticles), which may be feasible choices for multiplexed detection; and (iii) developing smartphone apps that can examine the color intensities and be transformed into concentrations. (Adapted with permission from Zhao et al. 2022)
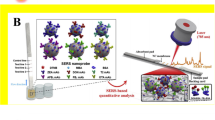
Xiulan and coworkers developed an immunochromatographic method for detecting AFB1 using a combination of an antibody and a conjugated nanogold probe (Xiulan et al. 2005). Several mycotoxigenic fungi produce diffusible exotoxins, which can be used as markers for the identification and confirmation of phytopathogenic fungi. An indium-tin oxide electrochemical impedance sensor with nano-ZnO film was developed by co-immobilizing antibodies and BSA protein to detect ochratoxin-A in agricultural and other plant-derived products (Ansari et al. 2010). Hernandez et al. (2020) developed a label-free surface-enhanced Raman spectroscopy (SERS) and localized surface plasmon resonance (LSPR) gold nanosensor constructed by immobilizing OTA-, FB1-, and AFB1-aptamers on gold nanoprisms (AuNTs) for detecting OTA, FB1, and AFB1 in cereals and grains (e.g., green coffee beans, wheat, and amaranth) in complex matrixes, demonstrated by the response of the plasmonic nanosensors with detection at 10–22 ppb (Hernandez et al. 2020). Similarly, Zhang et al. (2020) developed a multiplex SERS-based lateral flow immunosensor for the simultaneous detection of AFB1, ZEN, OTA, T-2, FB1, and DON in maize, with LOD of 0.96, 6.2, 15.7, and 8.6 pg/mL, while FB1 and DON were at 0.26 and 0.11 ng/mL, respectively (Zhang et al. 2020). The work by Zhao et al. (2021) demonstrated the use of novel α-Fe2O3 nanocubes as lateral flow immunoassays (LFIA) labels for the simultaneous detection of AFB1 and DON in corn, mung bean, and millet with the visual LOD of 0.01 and 0.18 ng/mL, respectively (Zhao et al. 2021).
Wu et al. (2020) developed a novel multicolor immunochromatographic test strip nanosensor composed of gold nanoparticles that exhibit different multicolored labels based on the SERS and LSPR of the different morphologies and size of the gold nanoparticles (gold nanospheres (AuNSs), gold nanocacti (AuNCs), gold nanoflowers (AuNFs), and hyperbranched Au plasmonic blackbodies (AuPBs)) for simultaneous and accurate detection of AFB1, FB1, OTA, and ZEN at 0.06, 3.27, 0.10, and 0.70 ng/mL, respectively, as shown in Fig. 15.4 (Wu et al. 2020).
Multicolor immunochromatographic test strip (ICTS) nanosensor: (a) characterization of four different sized or shaped AuNPs (AuNSs, AuNCs, AuNFs, and AuPBs); (b) schematic illustration of multicolor AuNP-based multiplex ICTS nanosensor, where test lines (T1, T2, T3, and T4) represent the simultaneous detection FB1, ZEN, OTA, and AFB1, and one control line indicates the validity of our method; (c) interpretation of qualitative test results; and (d) quantitative test results of multicolor AuNP-based multiplex ICTS nanosensor. (Adapted with permission from Wu et al. 2020)
This can also be achieved utilizing quantum dots (QDs), which are semiconductor nanoparticles with exceptional optical properties (e.g., size-tunable emission, wide adsorption, narrow photoluminescence spectra, strong photostability, a significant Stokes’s shift, and a long fluorescence lifespan). Duan et al. (2019) designed a tricolor QD nanobead (QB)-based multiplex immunochromatographic assay (mICA) for simultaneous qualitative detection of FB1, OTA, and ZEN in maize that serves as point-of-care testing (POCT) device. The QBs exhibited distinguishable yellow, orange, and red luminescence and conjugated with anti-FB1, anti-OTA, and anti-ZEN monoclonal antibodies for the detection of FB1, OTA, and ZEN, respectively. The QB-mICA revealed an LOD of 5, 20, and 10 ng/mL for OTA, FB1, and ZEN within 10 min, respectively, as shown in Fig. 15.5 (Duan et al. 2019).
Tricolor QD nanobead (QB)-based multiplex immunochromatographic assay (mICA) for simultaneous qualitative detection of FB1, OTA, and ZEN: (a) schematic illustration of emulsification evaporation method for the synthesis of tricolor QB; (b) schematic representation of tricolor QB-based mICA, T1 – ZEN, T2 – OTA, T3 - FB1, and C - control; (c) qualitative result visualization with naked eye; (d) evaluation and optimization of the cross-reaction for each QB-mAbs probe on three T lines, where “√” represents the optimal interpret time; (e) relationship between reaction time and the gray values on T lines and C line; and (f) sensitivity of QB-based mICA for ZEN, OTA, and FB1 detection, where “√” represents LOD for ZEN, OTA, and FB1
15.5.2 Antibody-Coupled Nanomaterials
Antibodies are widely used as molecular recognition receptors for toxin detection due to their specificity and sensitivity (Tothill 2011). Nanomaterials are not only promising absorbents but are also capable of coupling different molecules. Recently, synthetic receptors, such as aptamers, peptides, proteins, and printed polymers, have been coupled to nanomaterials for the development of nanosensors for the detection of mycotoxins (Thipe et al. 2018). Direct coupling via adsorption requires specific surface properties that allow interaction with the antibody. Preferably, the covalent binding of the antibody allows interaction with antigen-binding sites (Horky et al. 2018). Table 15.1 presents and compares studies of LFIAs utilized for the detection of mycotoxins in recent years. Additionally, activated carbon has been used as an absorbent to eliminate mycotoxins for a long time, so the use of carbon nanoforms was thought to be a promising successor to activated carbon. Carbon nanomaterials have high adsorption, stability, inertness, large surface area by weight, and colloidal stability at various pH ranges. Chemically, the carbon-carbon covalent bonds and the crystal structure provide specific properties, such as strength, elasticity, and optimal conductivity. Graphene, graphene oxide, nanodiamonds, fullerenes, fibers, and nanotubes have great potential to become new adsorbents for mycotoxins. Nanocarbon structures are amphoteric, and their surface can be protonated or deprotonated, which results in the binding capacity of polar or nonpolar compounds (Horky et al. 2018).
For the detection of mycotoxins, commercial test kits are often used as an appropriate alternative as a more inexpensive, user-friendly, and quick analysis. These commercial kits often include called the ELISA kits, fluorescence polarization immunoassays (FPIAs), membrane-based immunoassays such as LFIAs, and immunoaffinity column coupled with fluorometric assay. These kits are predominantly based on an immunoassay format that relies on the specific interaction between antigen and antibody. All the commercial kits are designed with nanoparticles to facilitate increase quantification, sensitivity, and selectivity, all attributed to the properties of nanoparticles. Additionally, colorimetric kits are preferred due to its ability to show results for the naked eye. LFIAs are strong competitors in the mycotoxin analysis market due to its acceptable sensitivity, portability, accuracy, ease of use, short detection time, and no need for specialized personnel (Majdinasab et al. 2020).
The commercially available LFIAs test strips commonly use AuNPs or QDs for signal amplification as colored labels. This method can deliver qualitative or semiquantitative results. For semiquantitative analysis, portable readers are developed for on-site detection. Commercially available test kits are developed for the determination of individual or multiple mycotoxins in single sample matrix. The latest trend in LFIA technology is the development of strips with multiple test lines for simultaneous detection of various mycotoxins. ELISA-based kits hold a major portion of the market of mycotoxin detection methods, after the LFIA test strips. Numerous companies offer ELISA kits for the detection of the most commonly occurring mycotoxins. Some of which can detect more than one type of mycotoxins in a single sample matrix. Commercial ELISA kits are selective, sensitive, and high throughput, with minimum sample separation steps. Furthermore, the detection time has been shortened, so that most of them can detect a targeted mycotoxin within 1–2 h. In some kits, cross-reactivity of antibodies can lead to overestimation of results, while matrix effect can play a key role in false-positive results. In order to avoid those effects, most kits define the limited matrices to which ELISA kits can be used (Majdinasab et al. 2020). There are many commercial detection kits available in the current market, as shown in Table 15.2.
While conventional methods are always improving, current research is looking for more innovative solutions. The utilization of nanotechnology is a promising, low-cost, and effective way to minimize the impact of mycotoxins (Horky et al. 2018). There are a number of commercial nanoformulations already in the market used as nanodiagnostic tools for mycotoxins detection (Thipe et al. 2018), as shown in Table 15.3.
15.6 Smart and Antifungal Packaging Nanosurveillance
Mycotoxin contamination predominately occurs in the field, during pre- and postharvest, transportation, processing, and improper storage of food and feedstuff. This is evident, since when food is stored properly and exposed to air and moisture, it often deteriorates allowing for the manifestation of fungi with subsequent mycotoxin production. Individual packets of food are not amenable to laboratory-based food deterioration testing. Most recently, technological advances through nanotechnology have utilized nanoparticles-based artificial sensing as nanodisks in spot indicators for sensitive mycotoxin detection on packages (Akhila et al. 2022; Kumar et al. 2017), as shown in Fig. 15.6, while Table 15.4 shows some studies that utilize nanoparticle-based active antifungal packaging.
15.7 Concluding Remarks
Due to their unique physiochemical properties, nanomaterials provide a multitude of design options for mycotoxin nanodiagnosis utilizing nanosensor fabrications. The nanomaterials enhanced sensitivity due to surface-atom availability, when functionalized, can exhibit structure-switchable conformational changes upon mycotoxin binding for multiplex detection of mycotoxin. Due to their nanoscale size and reversibility, structure-switchable nano-based assays are widely used for continuous and real-time monitoring of mycotoxins. In this approach, a great number of innovative and new nanomaterials for mycotoxin detection platforms have been investigated. The production of composite/hybrid nanomaterials, quantum structures, and nanomaterials with functionalized surfaces allows for multiplex detection of different mycotoxins within a single sample matrix. In the construction of smart nanosensors, gold and silver nanoparticles are the mostly utilized nanoparticles in the development of nanosensors, particularly due to their plasmon resonance for signal amplification. However, depending on the pH, temperature, medium, and size of the nanoparticles, their properties can change dramatically under a variety of physiological conditions. In contrast to immunoassays, immunochromatographic assay composed of a variety of nanoparticles or composite/hybrid nanoparticles for the detection of major mycotoxins (AFB1, FB1, OTA, and ZEN) are still in the development phase. Future research and investment are required to address the obstacles in the approach for the commercialization of nano-based assays that can be utilized as point-of-care testing (POCT) devices for mycotoxin detection.
Abbreviations
- AFs:
-
aflatoxins
- AuNPs:
-
gold nanoparticles
- DON:
-
deoxynivalenol
- FMs:
-
fumonisins
- FPIAs:
-
fluorescence polarization immunoassays
- LFIA:
-
lateral flow immunoassays
- LOD:
-
limit of detection
- LSPR:
-
localized surface plasmon resonance
- mICA:
-
multiplex immunochromatographic assay
- OTs:
-
ochratoxins
- POCT:
-
point-of-care testing
- QB:
-
QD nanobeads
- QDs:
-
quantum dots
- SERS:
-
surface-enhanced Raman spectroscopy
- T-2:
-
trichothecenes
- TRFMs:
-
time-resolved fluorescence microspheres
- ZEA:
-
zearalenone
References
Akhila PP, Sunooj KV, Navaf M, Aaliya B, Sudheesh C, Sasidharan A, Sabu S, Mir SA, George J, Mousavi Khaneghah A. Application of innovative packaging technologies to manage fungi and mycotoxin contamination in agricultural products: current status, challenges, and perspectives. Toxicon. 2022;214:18–29.
Anfossi L, Di Nardo F, Cavalera S, Giovannoli C, Spano G, Speranskaya ES, Goryacheva IY, Baggiani C. A lateral flow immunoassay for straightforward determination of fumonisin mycotoxins based on the quenching of the fluorescence of CdSe/ZnS quantum dots by gold and silver nanoparticles. Microchim Acta. 2018;185:94.
Ansari AA, Kaushik A, Solanki PR, Malhotra BD. Nanostructured zinc oxide platform for mycotoxin detection. Bioelectrochemistry. 2010;77:75–81.
BCC Research Publishing. (2022). Global markets and technologies for food safety testing.
Caon T, Martelli SM, Fakhouri FM. In: Grumezescu AMBT-N, editor. New trends in the food industry: application of nanosensors in food packaging, vol. 18. Academic Press; 2017. p. 773–804.
Chen J, Yang S, Li P, Wu A, Nepovimova E, Long M, Wu W, Kuca K. MicroRNA regulates the toxicological mechanism of four mycotoxins in vivo and in vitro. J Anim Sci Biotechnol. 2022;13:37.
da Silva JVB, de Oliveira CAF, Ramalho LNZ. An overview of mycotoxins, their pathogenic effects, foods where they are found and their diagnostic biomarkers. Food Sci Technol. 2021;
Devi HS, Boda MA, Shah MA, Parveen S, Wani AH. Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity. Green Process Synth. 2019;8:38–45.
Dobrucka R, Długaszewska J. Biosynthesis and antibacterial activity of ZnO nanoparticles using Trifolium pratense flower extract. Saudi J Biol Sci. 2016;23:517–23.
Duan H, Li Y, Shao Y, Huang X, Xiong Y. Multicolor quantum dot nanobeads for simultaneous multiplex immunochromatographic detection of mycotoxins in maize. Sensors Actuators B Chem. 2019;291:411–7.
Eskola M, Kos G, Elliott CT, Hajšlová J, Mayar S, Krska R. Worldwide contamination of food-crops with mycotoxins: validity of the widely cited ‘FAO estimate’ of 25%. Crit Rev Food Sci Nutr. 2020;60:2773–89.
FAO, IFAD, UNICEF, W. and W. Brief to the state of food security and nutrition in the world 2021. In: Brief to the state of food security and nutrition in the world 2021. FAO; 2021.
Fonseca C, Ochoa A, Ulloa MT, Alvarez E, Canales D, Zapata PA. Poly(lactic acid)/TiO2 nanocomposites as alternative biocidal and antifungal materials. Mater Sci Eng C. 2015;57:314–20.
Ghorbani HR, Alizadeh V, Mehr FP, Jafarpourgolroudbary H, Erfan K, Yeganeh SS. Preparation of polyurethane/CuO coating film and the study of antifungal activity. Prog Org Coatings. 2018;123:322–5.
Goryacheva OA, Guhrenz C, Schneider K, Beloglazova NV, Goryacheva IY, De Saeger S, Gaponik N. Silanized luminescent quantum dots for the simultaneous multicolor lateral flow immunoassay of two mycotoxins. ACS Appl Mater Interfaces. 2020;12:24575–84.
Guilger-Casagrande M, de Lima R. Synthesis of silver nanoparticles mediated by fungi: a review. Front Bioeng Biotechnol. 2019;7:1–16.
Hernandez Y, Lagos L, Galarreta B. Development of a label-free-SERS gold nanoaptasensor for the accessible determination of ochratoxin a. Sens Bio-Sensing Res. 2020;28:100331.
Horky P, Skalickova S, Baholet D, Skladanka J. Nanoparticles as a solution for eliminating the risk of mycotoxins. Nano. 2018;8:727.
Huang X, Huang T, Li X, Huang Z. Flower-like gold nanoparticles-based immunochromatographic test strip for rapid simultaneous detection of fumonisin B1 and deoxynivalenol in Chinese traditional medicine. J Pharm Biomed Anal. 2020a;177:112895.
Huang X, Huang X, Xie J, Li X, Huang Z. Rapid simultaneous detection of fumonisin B1 and deoxynivalenol in grain by immunochromatographic test strip. Anal Biochem. 2020b;606:113878.
Ingle, A., Gupta, I., Jogee, P., Rai, M., 2020. Role of nanotechnology in the detection of mycotoxins. pp. 11–33.
Jiang H, Zhang W, Li J, Nie L, Wu K, Duan H, Xiong Y. Inner-filter effect based fluorescence-quenching immunochromotographic assay for sensitive detection of aflatoxin B1 in soybean sauce. Food Control. 2018;94:71–6.
Jin Y, Chen Q, Luo S, He L, Fan R, Zhang S, Yang C, Chen Y. Dual near-infrared fluorescence-based lateral flow immunosensor for the detection of zearalenone and deoxynivalenol in maize. Food Chem. 2021;336:127718.
Kalia A, Abd-Elsalam KA, Kuca K. Zinc-based nanomaterials for diagnosis and management of plant diseases: ecological safety and future prospects. J Fungi. 2020a;6:1–29.
Kalia A, Kaur J, Kaur A, Singh N. Antimycotic activity of biogenically synthesised metal and metal oxide nanoparticles against plant pathogenic fungus Fusarium moniliforme (F. fujikuroi). Indian J Exp Biol. 2020b;58:263–70.
Kumar V, Guleria P, Mehta SK. Nanosensors for food quality and safety assessment. Environ Chem Lett. 2017;15:165–77.
Li S, Wang J, Sheng W, Wen W, Gu Y, Wang S. Fluorometric lateral flow immunochromatographic zearalenone assay by exploiting a quencher system composed of carbon dots and silver nanoparticles. Microchim Acta. 2018;185:388.
Li R, Meng C, Wen Y, Fu W, He P. Fluorometric lateral flow immunoassay for simultaneous determination of three mycotoxins (aflatoxin B1, zearalenone and deoxynivalenol) using quantum dot microbeads. Microchim Acta. 2019;186:748.
Li R, Bu T, Zhao Y, Sun X, Wang Q, Tian Y, Bai F, Wang L. Polydopamine coated zirconium metal-organic frameworks-based immunochromatographic assay for highly sensitive detection of deoxynivalenol. Anal Chim Acta. 2020;1131:109–17.
Li R, Wen Y, Wang F, He P. Recent advances in immunoassays and biosensors for mycotoxins detection in feedstuffs and foods. J Anim Sci Biotechnol. 2021;12:108.
Liu Z, Hua Q, Wang J, Liang Z, Li J, Wu J, Shen X, Lei H, Li X. A smartphone-based dual detection mode device integrated with two lateral flow immunoassays for multiplex mycotoxins in cereals. Biosens Bioelectron. 2020;158:112178.
Majdinasab M, Aissa SB, Marty JL. Advances in colorimetric strategies for mycotoxins detection: toward rapid industrial monitoring. Toxins 2021. 2020;13:13.
Nayaka SC, Ramana MV, Udayashankar AC, Niranjana SR, Mortensen CN, Prakash HS. Chemical and molecular methods for detection of toxigenic fungi and their mycotoxins from major food crops BT – laboratory protocols in fungal biology: current methods in fungal biology. In: Gupta VK, Tuohy MG, Ayyachamy M, Turner KM, O’Donovan A, editors. Laboratory protocol for fungi. New York: Springer; 2013. p. 73–90.
Oancea S, Stoia M. Mycotoxins: a review of toxicology, analytical methods and health risks. Acta Univ Cibiniensis Ser E Food Technol. 2008;XII:19–36.
Penczynski KJ, Cramer B, Dietrich S, Humpf H-U, Abraham K, Weikert C. Mycotoxins in serum and 24-h urine of vegans and omnivores from the risks and benefits of a vegan diet (RBVD) study. Mol Nutr Food Res. 2022;66:2100874.
Raafat M, El-Sayed ASA, El-Sayed MT. Biosynthesis and anti-mycotoxigenic activity of Zingiber officinale roscoe-derived metal nanoparticles. Molecules. 2021;26:2290.
Rai M, Jogee PS, Ingle AP. Emerging nanotechnology for detection of mycotoxins in food and feed. Int J Food Sci Nutr. 2015;66:363–70.
Shanakhat H, Sorrentino A, Raiola A, Romano A, Masi P, Cavella S. Current methods for mycotoxins analysis and innovative strategies for their reduction in cereals: an overview. J Sci Food Agric. 2018;98:4003–13.
Shao Y, Duan H, Zhou S, Ma T, Guo L, Huang X, Xiong Y. Biotin–streptavidin system-mediated ratiometric multiplex immunochromatographic assay for simultaneous and accurate quantification of three mycotoxins. J Agric Food Chem. 2019;67:9022–31.
Sheini A. Colorimetric aggregation assay based on array of gold and silver nanoparticles for simultaneous analysis of aflatoxins, ochratoxin and zearalenone by using chemometric analysis and paper based analytical devices. Microchim Acta. 2020;187:167.
Tang X, Li P, Zhang Q, Zhang Z, Zhang W, Jiang J. Time-resolved fluorescence immunochromatographic assay developed using two idiotypic nanobodies for rapid, quantitative, and simultaneous detection of aflatoxin and Zearalenone in maize and its products. Anal Chem. 2017;89:11520–8.
Thakur M, Wang B, Verma ML. Development and applications of nanobiosensors for sustainable agricultural and food industries: recent developments, challenges and perspectives. Environ Technol Innov. 2022;26:102371.
Thipe VC, Njobeh PB, Mhlanga SD. Optimization of commercial antibiotic agents using gold nanoparticles against toxigenic aspergillus spp. Mater Today Proc. 2015;2:4136–48.
Thipe V, Keyster M, Katti K. Sustainable nanotechnology: mycotoxin detection and protection; 2018. p. 323–49.
Tothill I. Biosensors and nanomaterials and their application for mycotoxin determination. World Mycotoxin J. 2011;4:361–74.
Vos R, Food I, Dc W, Programme WF, Dc W, States U, Turn E, Nations U, Change C, Security WF. War in Ukraine and the challenge to global food security. Nature. 2022;
Wu J, Sun Q, Huang H, Duan Y, Xiao G, Le T. Enhanced physico-mechanical, barrier and antifungal properties of soy protein isolate film by incorporating both plant-sourced cinnamaldehyde and facile synthesized zinc oxide nanosheets. Colloids Surfaces B Biointerfaces. 2019;180:31–8.
Wu Y, Zhou Y, Huang H, Chen X, Leng Y, Lai W, Huang X, Xiong Y. Engineered gold nanoparticles as multicolor labels for simultaneous multi-mycotoxin detection on the immunochromatographic test strip nanosensor. Sensors Actuators B Chem. 2020;316:128107.
Xiulan S, Xiaolian Z, Jian T, Zhou J, Chu FS. Preparation of gold-labeled antibody probe and its use in immunochromatography assay for detection of aflatoxin B1. Int J Food Microbiol. 2005;99:185–94.
Xu Y, Ma B, Chen E, Yu X, Ye Z, Sun C, Zhang M. Dual fluorescent immunochromatographic assay for simultaneous quantitative detection of citrinin and zearalenone in corn samples. Food Chem. 2021;336:127713.
You KH, Luo XE, Hu WJ, Xu Y, Guo JB, He QH. Environmental-friendly gold nanoparticle immunochromatographic assay for ochratoxin a based on biosynthetic mimetic mycotoxin-conjugates. World Mycotoxin J. 2020;13:267–75.
Youssef AM, Abdel-Aziz MS, El-Sayed SM. Chitosan nanocomposite films based on Ag-NP and Au-NP biosynthesis by Bacillus Subtilis as packaging materials. Int J Biol Macromol. 2014;69:185–91.
Zhang X, Xiao G, Wang Y, Zhao Y, Su H, Tan T. Preparation of chitosan-TiO2 composite film with efficient antimicrobial activities under visible light for food packaging applications. Carbohydr Polym. 2017a;169:101–7.
Zhang X, Yu X, Wen K, Li C, Mujtaba Mari G, Jiang H, Shi W, Shen J, Wang Z. Multiplex lateral flow immunoassays based on amorphous carbon nanoparticles for detecting three Fusarium mycotoxins in maize. J Agric Food Chem. 2017b;65:8063–71.
Zhang W, Tang S, Jin Y, Yang C, He L, Wang J, Chen Y. Multiplex SERS-based lateral flow immunosensor for the detection of major mycotoxins in maize utilizing dual Raman labels and triple test lines. J Hazard Mater. 2020;393:122348.
Zhao S, Bu T, He K, Bai F, Zhang M, Tian Y, Sun X, Wang X, Zhangsun H, Wang L. A novel α-Fe2O3 nanocubes-based multiplex immunochromatographic assay for simultaneous detection of deoxynivalenol and aflatoxin B1 in food samples. Food Control. 2021;123:107811.
Zhao X, Byrne HJ, O’Connor CM, Curtin J, Tian F. Limits of detection of mycotoxins by laminar flow strips: a review. Appl Nano. 2022;
Acknowledgments
The authors would like to thank the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) Grant No. 2019/15154-0 for support to Velaphi Clement Thipe.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Thipe, V.C. et al. (2023). Nanodiagnostic Tools for Mycotoxins Detection. In: Lim, KT., Abd-Elsalam, K.A. (eds) Nanorobotics and Nanodiagnostics in Integrative Biology and Biomedicine. Springer, Cham. https://doi.org/10.1007/978-3-031-16084-4_15
Download citation
DOI: https://doi.org/10.1007/978-3-031-16084-4_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-16083-7
Online ISBN: 978-3-031-16084-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)