Abstract
Mercury exists in the earth’s crust mostly as cinnabar (HgS) and hence is present as an impurity in most of the fuel and minerals. Coal is the primary fuel source for 40.7% of the world according to 2014 data. In recent years, there is a decline in coal usage in countries like Europe, USA, and China. However, India’s energy consumption has doubled since year 2000 with coal being the major fuel source. With the rising coal demand, India is predicted to be the largest coal importer in the world before 2020. Mercury content in coal varies from 0.05 to 0.2 g/MT according to the United Nations Environmental Program’s mercury estimation toolkit. Mercury pollution monitoring and its intercontinental transport modeling done for Arctic region concludes that 32% of this pollution originated from the Asian countries. Various world mercury emission estimates done in the past researches consider India to be the second largest mercury emitter after China. Last mercury emission estimate for India was done in the year 2004. This paper compares the mercury emissions from coal combustion in India to that of the world and studies the effectiveness of various air pollution control devices in the removal of mercury from coal combustion emissions.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
8.1 Introduction
Mercury exists in the environment in three forms elementary mercury vapors, ionic mercury, and organic methyl mercury [1]. Most of the mercury in nature is elemental Hg vapor which circulates in the atmosphere for up to one year and hence can be widely dispersed around the globe. Inorganic mercury which is emitted into the atmosphere associated with airborne particles or in gaseous form is removed by dry or wet deposition over short distances (few tens to few hundreds of kilometer) [2]. Inorganic mercury deposited changes its form to organic methylmercury by various naturally occurring biological processes [3]. This methyl mercury can bio-concentrate more than a million-fold in the aquatic food chain, and hence, mercury emissions become a major health concern in the environment [2]. The transport and deposition of mercury from the source depend on the chemical form of mercury emitted, the stack height, the area surrounding the site, topography, and meteorology [2, 4]. Minamata Convention, which was established in October 2013, has an objective to protect human health and environment from anthropogenic emissions and releases of mercury and mercury compounds by a set of control and phase-out regulations [5]. India joined the convention on September 30, 2014. However, India lacks a reliable data source for mercury used in industrial processes and products. Elemental mercury vapors enter the bloodstream on inhalation while inorganic mercury enters the bloodstream through the gastrointestinal system and dermal contact [1]. Elemental mercury is highly lipophilic which allows it to cross blood–brain and placental barriers [1]. Inorganic mercury exposure damages kidney [4]. Organic methylmercury exposure to humans occurs through ingestion of polluted food items, especially fish. Critical target for methylmercury toxicity is the nervous system. A fetus, newborn, and young children are at a higher risk of developing neurological diseases due to methylmercury poisoning [4]. Individuals with past history of diseases related to liver, kidneys, and lungs are also at higher risk [4].
8.2 Intercontinental Mercury Transportation
Intercontinental transport of mercury is estimated using modeling studies. These models require various measurements of atmospheric mercury concentrations, airborne particulate mercury concentration, and concentrations of mercury deposited through wet deposition [6]. The modeling also requires data like wind direction, mixing height, various mercury transformations in the atmosphere, dry deposition velocity of mercury forms, and mercury emission estimate data [7]. Global anthropogenic atmospheric mercury modeling studies conducted in the year 2014 using GEOS-Chem global chemical transport model estimated East Asia to be the primary source of mercury pollution for all the continents. This study also evaluated Indian subcontinent to be the second largest contributor to particulate mercury around the globe [8]. Similar study conducted using emission data of 1996 concluded that 67% of total depositions to North America originated from outside the continent including 24% from Asin sources. About half the mercury deposition in Arctic regions originated from other continents majorly Asia and Europe [9]. Anthropogenic mercury emissions to atmosphere and releases to environment can be associated with various mercury sources. Various mercury sources are divided into broad categories which includes extraction and combustion of fuels, primary metal production, production of other materials with mercury impurities (cement, pulp and paper, and lightweight aggregates), intentional use of mercury in industrial processes (chlor-alkali, vinyl chloride monomer), intentional use in consumer products (batteries, thermometers, paints, and biocides), and other intentional uses like dental amalgam fillings and production of recycled metals [10]. Various studies in the past have estimated global mercury emissions from various sources over the years. In 1995, a worldwide mercury emissions estimate concluded that 3/4th of the total emissions (56%) were caused due to fossil fuel combustion in China, India, and Korea [11]. Estimates of year 2005 states that out of a total 1930 tonnes of mercury released from anthropogenic sources, 400 tonnes comes from fossil fuel combustion in China and 180 tonnes come from fossil fuel combustion in India [12]. Such studies in the past have recognized coal combustion to be a major source of both elemental and oxidized mercury in the atmosphere. Mercury emissions estimation in India for the period 2000–2004 quantifies mercury release from coal-fired power plants and residential and commercial boilers to be 104.09 and 124.55 tonnes, respectively [13].
8.3 Mercury Emissions from Coal in India and World
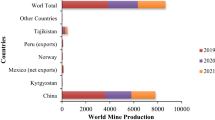
Coal occurring in India is of Gondwana origin, low in hydrogen, and high in nitrogen content. Most of them are characterized by high ash content, low Sulfur content, and low chloride content. Most of the coal reserves are confined to eastern and south-central parts of the country in states of Jharkhand, Orissa, Chhattisgarh, West Bengal, Andhra Pradesh, Maharashtra, and Madhya Pradesh [14]. India’s coal consumption in the period 2005–2012 increased from 6% of world production to 7.6% of world production in 2012 (Table 8.1) [15]. Within India, consumption of coal is distributed in sectors like steel and washery, power generation, cement, textile, fertilizer and chemicals, paper, brick, colliery consumption, and others. Among the various sectors, power generation consumes the largest fraction of coal available for consumption in the country. In 2005, 71.56% of the available raw coal was consumed in power generation which increased to 78.91% in 2007 after which it decreased to 71.17% in 2008 and reached 63.4% in the year 2011. After 2012, coal consumption in power generation increased again to 69.43%. Lignite consumption by the power generation industry also averaged at around 65% of total lignite available [16–23]. Mercury emissions from coal combustion estimation require data regarding activity rate of coal, emission rate, and output factor or distribution factor [10]. Activity rate for coal combustion is derived from the national provisional coal statistics which is published every year. Input factor in this study is decided as 0.005–0.5 g/MT (0.376 g/MT) which has been decided referring the UNEP toolkit for identification and quantification of mercury releases as well as on Mukherjee report on mercury emissions from industrial sources in India and its effects on the environment. Selection of an output factor is done based on the abatement measures for various pollutants included in the coal combustion facilities. Almost all thermal power plants in India are equipped with electrostatic precipitators with more than 99% efficiency [24]. Output factor for this study has been taken from UNEP toolkit for estimation of mercury emissions from anthropogenic sources. Output factor for mercury emissions to air from coal combustion is taken as 0.75 in case of coal used in power plant (PP) combustion and 0.9 for rest of the activities.
Mercury emission graph shows an increasing trend of mercury emissions from India as compared to a decreasing trend in world mercury emissions (Table 8.2). Considering median values for both global and Indian emission data, India contributed to 15% of world mercury emissions in 2005 which increased to 16.02% in 2006 and 37.25% in 2010. This trend can be associated with increasing coal consumption in India (Table 8.2). India’s coal consumption rate is estimated to increase further in coming years which implies the need to include effective mercury emissions control devices in our power plants (PP) and industrial boilers (Tables 8.1 and 8.2).

8.4 Mercury Emissions Control Measures
Statistics regarding mercury emissions in the above data show that majority of the mercury in the atmosphere is released due to coal combustion in power plants (PP). A study on mercury emissions from three power plants in India, two public sectors, and one private sector was carried out each having boiler with unit generation capacities of 210, 250, and 500 MW. These plants are equipped with electrostatic precipitator for particulate capture with outlet of electrostatic precipitator (ESP) having a flue gas temperature of 150, 130, and 127 °C. Results for these plants suggested that 60–70% of mercury emissions in flue gases are in the form of gaseous elemental mercury. This is due to low chloride content in the feed coal which does not oxidize mercury vapors [24]. Mercury emissions’ reduction from coal combustion in power plants depends on the mercury content of the coal feed, species of mercury emitted, efficiency of the boiler, and type of pollution control measures employed in the plant. Various control devices that are usually provided in the power plants include fabric filters (FF) and electrostatic precipitators (ESP) for fly ash removal and flue gas desulfurization (FGD) and selective catalytic reactors (SCR) for SO2 and NOx removal, respectively. The efficiency of these devices for mercury removal depends on the speciation of mercury in the flue gas. Elemental mercury (Hg0) is rather inert to most of these pollution control devices and requires to be converted to its oxidized form for its removal [25]. All the particulate control devices can easily remove mercury associated with fly ash and unburnt carbon while FGD and SCR as well as FF can remove or aid removal of oxidized mercury (Hg2+) from flue gases. Coal blending and coal additives can be used to oxidize the flue gas mercury hence aiding mercury removal by these control devices. Special mercury control techniques such as activated carbon injection (ACI) uses activated carbon or activated carbon impregnated with chloride ions to aid oxidation of elemental mercury vapors on the AC surface. Effectiveness of any co-benefit or dedicated mercury removal technology will depend on the extent of oxidation of elemental mercury in the control devices. Latest regulations on pollution from thermal power plants have limited mercury emissions to 0.03 mg/Nm3 for all facilities. However, limits for SO2 and NOx are limited to 100 mg/Nm3 each for plants installed after 2017 and 60 mg/Nm3 (< 500 MW capacity units)/200 mg/Nm3 (>500 MW) for SO2 along with 300 mg/Nm3 for NOx in case of plants established between 2003 and 2016, respectively [26]. Guidelines for mercury recently introduced in India is similar to the regulations in place in China; however, it is very high as compared to the USA where mercury limit for bituminous coal combustion facility is 0.0017 mg/Nm3 and in case of lignite combustion is 0.0153 mg/Nm3 [27]. Since Indian coal is characterized by low sulfur content and high ash content, particulate matter control devices commonly ESP is installed almost in all thermal power plants. Mercury removal options which co-benefit PM control is a good option for mercury emissions control from thermal power plants in India.
8.4.1 CO-benefit Removal Techniques
Electrostatic precipitators (ESP) which use electrostatic charges to separate dust particles from flue gas stream are useful for removing particulate mercury from flue gases. However, no oxidation of mercury occurs in ESP which results in passage of elemental mercury vapor from this device. A study conducted using coal from Talcher, Orissa, evaluated mercury removal performance of conventional and low-temperature ESP. The coal used had a mercury concentration of 220 g/kg and chlorine content of 100 mg/kg. Mercury removal rate in high-performance ESP with an inlet temperature of 90 °C showed a 68% mercury removal percent while conventional one only 19%. This temperature drop (up to 90 °C as compared to 170 °C in conventional ones) was brought about by gas–gas heat exchanger (GGH) [28]. Another study with SCR, coal side ESP, and wet FGD suggested a positive correlation between mercury oxidation in SCRs and removal in CS-ESPs [29]. This suggests that decreased temperatures in CS-ESP increase the efficiency of capture of particulate as well as oxidized mercury while it requires some other technologies to aid oxidation of mercury vapors. ESP alone showed a mercury removal efficiency of 28% for a study conducted in China [14]. Fabric filters which are another particulate control device consisting of cloth or fiber bags that retain fly ash and unburnt carbon on its pores are another device which showed a 67% mercury removal efficiency.
Selective catalytic reactor (SCR) is one of the few devices used for NOx removal and the only one of them which facilitates mercury oxidation. It uses NH3 as the reducing agent to reduce NOx to N2 using oxides of vanadium, molybdenum, and tungsten as the catalyst. This device in association with ESP and wet fluidized gas desulfurization (WFGD) can give a mercury removal efficiency of 69% and with the addition of fabric filters (FF), it increases to 90–95% [14]. A study done for mercury removal efficiency of SCR from flue gas from lignite burning suggests the important role played by the presence of halogen in coal. Oxidation in SCR occurs by two methods, homogenous oxidation and heterogeneous oxidation. Homogeneous oxidation occurs in the gaseous phase and heterogeneous oxidation occurs at the interface of solids. Since chloride content in lignite is less, the addition of various halogen gases and its effect on the oxidation of mercury in SCR is studied. For all the halogen gases (HCl, HF, HI, and HBr), mercury oxidation percentage increased with increasing the gas concentration up to a certain point after which it remained unchanged. Addition of HBr and HI showed stronger impact on Hg(0) oxidation than Hcl and HF. One more factor affecting the oxidation in SCR is the presence of NH3. The reactions, NOx reduction by NH3 and Hg(0) oxidation by halogen species, compete with each other except when HBr and HI is used [30]. Gas flow rate, temperature of the flue gas, and presence of other gases are some of the parameters that affect Hg(0) oxidation in SCR. Positive correlation between Hg oxidation in SCRs and CS-ESPs as well as between SCR and wet FGDs indicates the importance of SCR in mercury removal from coal combustion flues gases [29]. Fluidized gas desulfurization (FGD) is used to scrub SO2 from the flue gases. Two types of FGD are available, one dry FGD and other wet FGD. Water-soluble nature of oxidized Hg2+ makes wet FGD a good device for oxidized mercury removal. However, it is necessary to have an increased Hg2+ concentration in flue gases prior to wet FGD. Oxidized mercury ions in wet FGD are prone to re-emission which was seen to be suppressed by addition of chemicals like Na2S4, NaHS, and HI. Also, increase in scrubber temperature and decreased pH of slurry due to increase in O2 concentration further enhanced re-emission in scrubber [29]. Wet FGD with ESP present upstream shows a 64% mercury removal while FF installed upstream as particulate removal device increases the removal efficiency to 86%. Including a SCR upstream of particulate removal device further increases the removal efficiency to 69 and 90%, respectively.
8.4.2 Dedicated Removal Techniques
Activated carbon injection uses activated carbon as an adsorbent for mercury removal. Some of the parameters important while commercially tapping ACI are even distribution of sorbent in flue gases, sufficient contact time between sorbent and flue gases and maintaining system operating temperature within range. Standard PAC injection was effective for mercury capture in low sulfur bituminous coal but not in case of low rank fuel fitted with ESPs and those burning low rank fuel with FF as control equipment. This is so because such fuel shower lower chlorine levels. Also plants using high sulfur fuel showed no considerable effect of PAC injection [31]. ACI upstream of a particulate control device like ESP or FF alone gives a 90% removal efficiency [14]. TOXECON is a combination of ESP, ACI downstream of ESP, and pulse jet FF to capture the activated carbon. This system is considered to be more effective and more expensive than ESP/FF + ACI system [32]. This increased cost is to separate toxic fly ash and activated carbon from usual fly ash and unburnt carbon in primary PM control equipment usually ESP. TOXECON 2 addresses this problem without the additional cost of pulse jet FF by injecting PAC into the initial collecting field of ESP thus keeping the fly ash collected in the downstream collecting fields relatively free from toxic mercury [33]. Regeneration of used activated carbon using thermogravimetric analyzer and high-temperature air slide apparatus at a temperature around 900 F has been studied. A 46% Hg removal at 800 F and 100% at 900 F was observed. Mercury adsorption capacity of ACI increases with catalyst, 1% CuO and 4% Fe2O3 and 5% CuCl, and decrease in flue gas temperature. In case of flue gas with low halogen content, halogen-treated activated carbon is effective at capturing mercury. Presence of high sulfur levels in coal or increased SO3 concentrations in flue gases hamper the removal efficiency of activated carbon. Separation of AC ash from fly ash becomes a major concern while using ACI technique, especially, when the ash is utilized in other boilers [32]. The study done on Talcher coal suggested that no effective mercury removal took place on activated carbon surface without the aid of denitrification catalysts [28]. In another study, ACI was used in conjunction with SCR, FF, and wet FGD and also with ESP in addition to achieve a removal efficiency of 97 and 99%, respectively. Activated carbon along with some advanced PM control technologies is believed to achieve removal efficiency as high as 90% [14]. Another study points out some of the limitations that come in the use of AC. The effectiveness of AC reduces in the presence of SO2 and at high temperatures. Also, halogenation of AC is required prior to its use as an adsorbent. A high operating temperature has an adverse effect when plants with hot-side ESP are used. Few scenarios which limit the use of PAC for mercury removal are plants where complete removal of Hg was obtained at 700 F [34]. Choice of any pollution control measure depends not only on the removal efficiency but also on the cost incurred in the lifetime of the device. Presently, thermal power plants in India is equipped with only ESPs, a few of them do have FGD unit (Mumbai) [24]. Hence, a mercury removal system in Indian power plants should revolve around particulate matter control device. Among all the control devices that could act as co-benefit for mercury removal in power plants, wet FGD has highest capital cost and maintenance cost followed by SCR and FF which have around same capital cost though SCR has higher maintenance cost. ACI when introduced in a system consisting of SCR, FF and wet FGD would require an additional capital cost of around 10 CNY/kW and O&M cost of 11 CNY/kW/year [14]. While another study gives the capital cost of APC as $5/kW in the USA which is fairly low compared to other devices. Brominated carbon though higher in capital cost requires much lesser injection rate.
8.4.3 Coal Pre-treatment Techniques
Another option to control mercury in flue gases is coal washing and other coal pre-treatment, which reduces fly ash, sulfur, and mercury content of coal hence reducing the mercury emissions as well as increasing the efficiency of plant. Physical coal cleaning consists of reducing size of coal and screening, gravity separation of coal from sulfur-bearing mineral impurities, dewatering, and lastly drying. 10–50% Hg removal can be achieved by physical coal washing. The variation depends on the type of process used for cleaning and the nature of mercury in coal [33]. A conventional coal washing removes mercury associated with incombustible minerals and not the ones associated with organic carbon in the coal matrix [35]. Another study conducted to know mercury removal efficiency of coal washing and coal pre-treatment processes suggested 8–96% removal by coal cleaning and deshaling process while a 45–70% removal when treated at 300 °C. A combination of coal cleaning and thermal pre-treatment is ideal to achieve good mercury removal [36]. Coal beneficiation and coal blending are two additional treatments that can be provided to coal for enhanced mercury removal. K-Fuel which is widely used for sub-bituminous and lignite coal is a method of coal beneficiation which includes coal washing. It removes particulate matter, increases the heating value, and reduces emissions from the coal combustion [33]. Coal blending is commonly used to reduce SO2 emissions from coal combustion. However, coal blending also provides an additional advantage of mercury removal. Coal blending increases chlorine in flue gases thus resulting in increased Hg removal. A mix of 60% sub-bituminous and 40% bituminous coal increased mercury oxidation to 63% in the absence of SCR and 97% with SCR. Some of the salts that are added to coal are hydrochloric acid (HCl) or ammonium chloride (NH4Cl). These can be either sprayed on coal or injected in the boiler. It can also be added in the form of solid upstream of coal pulverizer [33, 35].
8.5 Summary
A total 1332.959 MT of total mercury has been emitted by coal combustion sector in India over the period of eight years starting 2005. The rate of emission is likely to increase further in the coming years with increase in coal consumption. Major coal consumption sector in India is thermal power plants consuming 71.56% of total coal available in India. Currently, the mercury emissions from thermal power plant are estimated between 19 and 130 g/Nm3 [37]. Bituminous coal is the prevalent coal type available in thermal power plants in India. Thermal power plants being a major coal consumer in India require strict regulations, effective control devices, and continuous monitoring system to mitigate mercury emissions. Coal burned in India has high fly ash content and low sulfur and chloride content. Hence, approximately 60–70% of the mercury emitted in the flue gases is in elemental state and hence is emitted into the atmosphere. Almost all the power plants in India have ESP installed for PM control. Mercury removal efficiency of conventional ESP varies from 19 to 28%. However, the performance of cold-side ESP is seen to be better than the conventional one. ESP majorly removes particulate mercury from the flue gas which constitutes around 20–40% of the total mercury in the flue gases from thermal power plant. Removal of mercury in elemental vapor state and also oxidized mercury in flue gases require other treatment options which are currently not used in thermal power plants in India. Wet fluidized gas desulfurization (WFGD) removes oxidized mercury from flue gases as they are water soluble. However, WFGD has the highest capital and O&M cost among all the co-benefit control devices. Studies have shown a 64% mercury removal efficiency when a combination of ESP and WFGD is used. Fabric filters (FFs), another PM control device with a higher PM removal efficiency than ESP and also has a better mercury removal efficiency when compared to ESP. FF along with WFGD gives a mercury removal efficiency of 86%. Fabric filters though involving a higher capital investment has a lower O&M investment and quite high removal efficiency than ESP. Hence, replacement of ESP with FF can be considered with more stringent PM regulations introduced recently. Since only 30% of the mercury in flue gases exists in the form of oxidized mercury (Hg2+) and rest 60–70% is in elemental form, it makes sense to supplement WFGD with control devices or techniques that would oxidize elemental mercury in flue gases hence improving the removal efficiency of WFGD. Selective catalytic reactor (SCR) is one such device placed upstream of PM control device, which reduces NOx concentration in the flue gases also aiding the oxidation of elemental mercury. One study gives the removal efficiency of SCR, cold-side ESP, and WFGD combination as 69% which is just 5% increase from when ESP + WFGD was considered. SCR has an initial capital cost comparable with FF but a higher O&M cost. In India’s context, use of SCR should not be considered unless NOx removal from thermal power plants is desired. Activated carbon injection (ACI) is a dedicated mercury removal technique which uses powered activated carbon or sometimes a halogenated activated carbon for removal of oxidized as well as elemental mercury from flue gases. Spent AC is then removed using a PM control device downstream. ACI provided upstream of an ESP or FF showed a mercury removal efficiency of 90%. ACI requires less capital and O&P cost according to the study conducted in USA and China; however, costs involved in India still requires to be studied. TOXECON is an advancement in ACI mercury removal, which provides an ESP upstream and a pulse jet FF downstream of ACI. This separates usual fly ash from toxic AC and high-mercury fly ash. Taking into consideration the efficiency and costs involved, a TOXECON or halogenated ACI + PM control seems to be a good option for mercury removal in Indian thermal power plants. Coal cleaning and blending with additives are also cheap and effective techniques to reduce mercury as well as sulfur impurities from coal. Study of efficiency of coal washing for mercury removal in case of Indian coal varied from around 15 to 30%. Implementation of coal washing should be considered based on the nature of coal mined from different regions. Also, costs involved in handling of effluents released from washing should also be considered when using this technique. One of the most important steps to ensure mercury emissions reduction from coal combustion is to put a transparent emission monitoring system in place.
References
Risher, J.F., World Health Organization.: Elemental mercury and inorganic mercury compounds: human health aspects
Schroeder, W.H., Munthe. J.: Atmospheric mercury-an overview. Atmos. Environ. 32(5) 809–822 (1998). Elsevier, Great Britain
Pirrone, N.: Emissions, Hemispheric Transport of Air Pollution 2010, pp 75–96. United Nations Economic Commission for Europe
UNEP., WHO.: Guidance for identifying population at risk from mercury exposure (2008)
UNEP.: Minamata Convention on Mercury (2013)
Chen.Y., Wang, R., Shen, H., Li, W., Chen, H., Huang, Y., Zhang, Y.: Global mercury emissions from combustion in light of international fuel trading. Environ. Sci. Technol. 48, 1727–1735 (2014)
Berg, T., Bartnicki, J., Munthe, J., Lattila, H., Hrehoruk, J., Mazur, A.: Atmospheric mercury species in the Europian Arctic: measurements and modelling. Atmos. Environ. 35, 2569–2582 (2001). Elsevier
Chen, L., Wang, H.H., Liu, J.F., Tong, Y.D., Ou, L.B., Zhang, W., Hu, D., Chen, C., Wang, X.J.: Intercontinental transport and deposition patterns of atmospheric mercury from anthropogenic emissions. Atmos. Chem. Phys. 14, 10163–10176 (2014)
Travnikov, O.: Contribution of the intercontinental atmospheric transport to mercury pollution in the Northern Hemisphere. Atmos. Environ. 39, 7541–7548 (2004). Elsevier
United Nations Environment Programme.: Toolkit for Identification and Quantification of Mercury Releases Version 1.4 (2017)
Pacyna, E.G., Pacyna, J.M.: Global Emission of Mercury from Anthropogenic Sources in 1995 (2001)
Pacyna, E.G., Pacyna, J.M., Sundseth, K., Munthe, J., Kindbom, K., Wilson, S., Steenhuisen, F., Maxson, P.: Global emission of mercury to the atmosphere from anthropogenic sources in 2005 and projections to 2020. Atmos. Environ. 44, 2487–2499 (2010)
Mukherjee, A.B., Bhattacharya, P., Sarkar, A., Zevenhoven, R.: Mercury emissions from industrial sources in India and its effects in the environment. In: Mason, R., Pirrone, N. (eds.) Mercury Fate and Transport in the Global Atmosphere. Springer, Boston, MA (2009)
Ancora, M.P., et al.: Economic analysis of atmospheric mercury emission control for coal-fired power plants in China, pp 1-11. JES, Elsevier B.V (2015)
International Energy Agency.: https://www.iea.org/
Coal Controller’s Organisation, GOI, Ministry of Coal, Provisional Coal Statistics, 2005–06
Coal Controller’s Organisation, GOI, Ministry of Coal, Provisional Coal Statistics 2006–07
Coal Controller’s Organisation, GOI, Ministry of Coal, Provisional Coal Statistics 2007–08
Coal Controller’s Organisation, GOI, Ministry of Coal, Provisional Coal Statistics 2008–09
Coal Controller’s Organisation, GOI, Ministry of Coal, Provisional Coal Statistics 2009–10
Coal Controller’s Organisation, GOI, Ministry of Coal, Provisional Coal Statistics 2010–11
Coal Controller’s Organisation, GOI, Ministry of Coal, Provisional Coal Statistics 2011–12
Coal Controller’s Organisation, GOI, Ministry of Coal, Provisional Coal Statistics 2012–13
Prepared, R.: Assessment of the mercury content in coal fed to power plants and study of mercury emissions from the sector in India (2014)
UNEP.: Guidance on best available techniques and best environmental practices to control (2015)
The Gazette of India.: Ministry of Environment, Forest and Climate Change Notification. Mercury Emissions from Coal-fired Power Plants and Coal-fired Industrial Boilers’, pp. 1–45 (2016)
NRDC.: Health facts summary of recent mercury emission limits for power plants in the united (2012)
Japan Coal Energy Centre.: Environmental Study through combustion test of Indian coal (2017)
Pudasainee, D., et al.: Oxidation, reemission and mass distribution of mercury in bituminous coal-fired power plants with SCR, CS-ESP and wet FGD. Fuel 93(2012), 312–318 (2012). Elsevier Ltd
Cao, Y., et al.: Impacts of halogen additions on mercury oxidation, in a slipstream Selective Catalyst Reduction (SCR), reactor when burning sub-bituminous coal. Environ. Sci. Technol. 42(1), 256–261 (2008)
Srivastava, R.K., et al.: Control of mercury emissions from coal-fired electric utility boilers. Environ. Sci. Technol. 40(5), 1385–1393 (2006). https://doi.org/10.1021/es062639u
Sjostrom, S., et al.: Activated carbon injection for mercury control: overview. Fuel 89(6), 1320–1322 (2010). Elsevier Ltd
Pacyna, J.M., et al.: An assessment of costs and benefits associated with mercury emission reductions from major anthropogenic sources. J. Air Waste Manag. Assoc. 60(3), 302–315 (2010)
Okwadha, G.D.O., et al.: Thermal removal of mercury in spent powdered activated carbon from TOXECON Process, pp 1032–1040 (2009)
UNEP Chemicals Branch., Geneva., S.: Process optimization guidance for reducing mercury emissions from coal combustion in power plants, p. 82 (2010)
Dziok, T., Strugała, A.: Method selection for mercury removal from hard coal 2007 (2017)
Centre for Science and Environment.: Clearing the air: pollution-control technology for coal-based power plants (2016)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Bhave, P.P., Shrestha, R. (2020). Mercury Air Pollution from Coal Combustion in India and Its Control Measures. In: Sivasubramanian, V., Subramanian, S. (eds) Global Challenges in Energy and Environment. Lecture Notes on Multidisciplinary Industrial Engineering. Springer, Singapore. https://doi.org/10.1007/978-981-13-9213-9_8
Download citation
DOI: https://doi.org/10.1007/978-981-13-9213-9_8
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-9212-2
Online ISBN: 978-981-13-9213-9
eBook Packages: EnergyEnergy (R0)