Abstract
Nanoparticles are promising materials in research and industrial fields because of their unique characteristics, their safety and toxicity are still being investigated. Although the safety and toxicity of nanomaterials are predicted by animal experiments, obtained results may be inconsistent with human outcomes due to the species difference. Recently, there has been an increasing interest in in vitro lung models, which allow control of experimental parameters and quantitative analyses, for the prediction of lung injuries and translocation into secondary organs of nanoparticles. In this section, we focus on developing in vitro alveolar models consisting of not only human-derived cell lines but also primary rat cells as complementary methods for intratracheal instillation in rats. We also coculture with macrophages to approach physiologically relevant alveolar environment. In addition, cytotoxicity and permeability tests of nanoparticles are presented to evaluate the in vitro alveolar coculture modles developed here. To further improve the physiological relevance of in vitro alveolar models, we discuss future issues.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- In vitro lung model
- Nanoparticles
- Pulmonary cytotoxicity
- Pulmonary permeability
- Alveolar type I and II cells
- Alveolar macrophage
1 Introduction
Suspended particulate matters, which are one of the major sources of the air pollution, have been recognized to cause health injuries, such as direct damage to alveoli and secondary diseases of other organs through their pulmonary absorption. In the meantime, the development and application of nanomaterials, which possess novel properties and functions, have recently attracted great attention in the variety of fields, while their safety and toxicity to human bodies have also been evaluated considerably [1]. For instance, in the field of medicine, nano-sized drug carriers [2, 3] for pulmonary administration have been developed and investigated to enhance their permeability into the bodies, compared to the route of drug administration through the small intestine. The safety/toxicity and drug effects of substances including nanomaterials and nanocarriers are basically predicted by their intratracheal instillation into rodents, such as rats. However, the obtained results don’t often reflect human outcomes properly due to the species difference. In addition, detailed kinetic analyses are complicated due to the inherent complexity of the living system. Hence, alveolar epithelial cell-based in vitro model systems are required as complementary and alternative methods in animal experiments.
Alveoli, in which gas exchange takes place, are located at the most distal part of the respiratory tract and surrounded by a network of blood capillaries. The alveolar epithelial cell layer consists of alveolar type I and II cells. The type I cells, which are about 0.05–0.20 μm thick flat cells, provide approximately 95% of the alveolar wall and involved in the process of gas exchange between alveoli and blood. Meanwhile, the cuboidal type II cells are known as the progenitor of type I cells and occupy a small fraction of the alveolar wall. The type II cells also secrete pulmonary surfactants, which play a significant role in physiological homeostasis in the alveoli, such as the decrease in their surface tension of an air–liquid interface and prevention of alveolar collapse and pulmonary edema. Besides the alveolar type I and II cells, alveolar macrophages as one of the immune cells are seen in the alveolar lumen and also involved in physiological homeostasis, such as clearance of foreign substances including pathogens and repairing tissues after inflammation. In order to assess alveolar injury and transport into the human body against foreign substances including nanoparticles and nanofibers in vitro, at least coculture models consisting of alveolar epithelial cells and macrophages are needed.
Previously, cell lines have frequently been used for developing in vitro alveolar coculture models [4,5,6]. However, biologically relevant functions observed in the cell lines are decreased or lost partially. Therefore, primary cells are one of the potential cell sources to achieve in vitro alveolar coculture models, which mimic the in vivo situation. In the present section, we summarized procedures for developing in vitro alveolar epithelial models toward the prediction of pulmonary toxicity and permeability of nanoparticles using not only cell lines but also primary cells. It is known that a colloidal suspension of nanoparticles is frequently administered into lungs in rodents for evaluating their lung injury and translocation to extrapulmonary organs in inhalation toxicity studies. Based on this, we therefore used primary cells isolated from rats to develop in vitro alveolar coculture models for the complementary method in animal experiments.
2 Nanoparticles
Today a numerous amount of nanomaterials, which possess novel physical, thermal, optical, and biological features, have been manufactured. Those features may depend on the chemical composition, crystal structure, size, and shape of nanomaterials. Nanoparticles as one subset of nanomaterials are contained in a variety of consumer products, such as drugs, dietary supplements, cosmetics, sunscreen, toiletry products, food packaging, clothing, sporting equipment, electronics, and batteries. To give an example, a lot of sunscreen include titanium dioxide (TiO2) or zinc oxide (ZnO) nanoparticles to block ultraviolet rays. The permeated amount of TiO2 and ZnO nanoparticles into normal skin is extremely low in general. However, TiO2 nanoparticles are known to cause DNA and chromosomal damages and inflammation in mice fed TiO2 nanoparticles for 5 days [7]. In addition, TiO2, ZnO, and silica (SiO2) nanoparticles have been reported to be toxic to human neural and lung cells in in vitro studies [8,9,10,11]. Therefore, SiO2 and TiO2 nanoparticles are helpful as a positive control for cell viability measurements (Table 11.1). Additionally, silica nanoparticles labeled with FITC or rhodamine (FITC-SiO2NPs and Rho-SiO2NPs) are useful for permeability measurements.
Cytotoxicity and pulmonary translocation of nanoparticles have been known to strongly depend on their surface characteristics, particle size, and chemical composition [1]. Among them, the size of nanoparticles is one of the key parameters. Nanoparticles often form aggregates in self-assembled manner with proteins in fetal bovine serum (FBS) and human serum via electrostatic and hydrophilic/hydrophobic interactions [12]. Not surprisingly, SiO2 and TiO2 nanoparticles used here formed their aggregates in a Dulbecco’s modified Eagle medium (DMEM) containing 10% FBS. However, SiO2 and TiO2 nanoparticles maintained their size without any aggregation in DMEM containing no FBS even after 24 h (Fig. 11.1). The culture medium without FBS might therefore be employed in cytotoxicity tests and translocation studies.
3 Properties of In Vitro Alveolar Epithelial Models
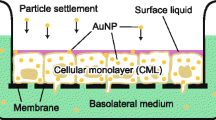
To develop in vitro lung models, transwell inserts consisting of a semipermeable membrane are commonly utilized. The semipermeable membrane allows to separate across to apical and basolateral compartments (Fig. 11.2). As one of indexes of the alveolar functions, the integrity of the blood–air barrier for alveolar epithelial cell layers cultured on the semipermeable membrane is determined from transepithelial electrical resistance (TEER), which is accepted as measuring their tight junction integrity and paracellular permeability. Here, we described the preparation and property of in vitro alveolar epithelial models using human alveolar epithelial cell lines and primary alveolar epithelial cells isolated from rats.
3.1 Human Alveolar Epithelial Cell Lines
Normal human alveolar epithelial cells are the optimal cell source for the in vitro lung model. Although primary human alveolar epithelial cells consisting of type I and II cells are commercially available, their extremely low proliferation rate causes the difficulty in obtaining a large amount of their cells in vitro inexpensively. The commercially available primary human alveolar epithelial cells are therefore unreasonable in the safety/toxicity testing and high-throughput screening. In the case of cell lines, a type I cell line has not yet been established in the present. In contrast, a human alveolar epithelial type II cell line A549 are already established [13] and serves as a potential model in the alveolar region [14], although its differentiation ability into the type I cell is lost. In order to mimic the situation in the human alveoli, we have used A549 cells and developed an in vitro human alveolar epithelial model cocultured with the human monocytic cell line THP-1, which is known to differentiate into a macrophage-like cell after stimulation of chemicals, such as phorbol 12-myristate 13-acetate (PMA) [15, 16].
A549 and THP-1 cells are basically cultured in DMEM and Roswell Park Memorial Institute medium (RPMI) 1640 containing 10% FBS and 1% antibiotics–antimycotic mixed solution (AA), respectively, in a 5% CO2 incubator at 37 °C. A549 cells were seeded onto the semipermeable membrane at a cell density of 1.0 × 105 cells cm−2 and inoculated for at least 5 days to be formed monolayer. However, A549 cells are basically unable to form the strong blood–air barrier due to the type II-derived cells. Obviously, the TEER value was over one order of magnitude smaller than those of a cell layer consisting of Calu-3 or 16HBE14o-cells (~2000 Ω cm2), which are known to possess high barrier properties because of airway and bronchial-derived epithelial cell lines, respectively [17] (Fig. 11.3). The macrophage-like cells (1.0 × 104 cells cm−2), which are differentiated from THP-1 cells, were also added onto the apical side of the A549 monoculture system and further cultured for 24 h. However, the considerable improvement of the TEER value was not also obtained.
To improve the barrier properties, A549 cells were cocultured with human umbilical vein endothelial cells HUVECs, which were adhered on the lower surface of the transwell insert (A549/HUVEC system). Unfortunately, we didn’t observe a nonlinear increase in the TEER value associated with the effect of coculture with HUVECs (Fig. 11.3). However, interestingly, the cuboidal shape of A549 cells was maintained and a rough A549 cell layer was formed on the semipermeable membrane at the monoculture system without HUVECs, whereas a flat and smooth layer of A549 cells was formed at the A549/HUVEC system (Fig. 11.4a, b). This might indicate that extracellular matrixes secreted and accumulated from HUVECs contribute to increased stability of A549 cells.
3.2 Primary Rat Alveolar Epithelial Cells
Primary alveolar epithelial cells isolated from animals are also considered as a cell source for in vitro lung models. As described above, animal experiments have been performed in the safety and toxicity testing to chemicals including nanomaterials. Therefore, developing in vitro models of animal organs is significant as with those of human organs to verify correlation between in vitro results and in vivo outcomes obtained in animal testing.
An isolation method for alveolar epithelial type I cells from animals, such as rats, has been proposed previously, but the method has been much complicated. In contrast, high purified primary alveolar epithelial type II cells are easily collected on the basis of metrizamide density centrifugation [18]. Since the type II cells are also known to differentiate into type I cells [19], the isolation method for alveolar epithelial type II cells has been accepted. The isolation method for alveolar macrophages has also been reported. Briefly, the lung was washed with saline five times and then the bronchoalveolar lavage fluid (BALF) was recovered. After centrifugation, alveolar macrophages are collected from the recovered BALF. Note that in our investigation, the amount of alveolar epithelial type II cells and alveolar macrophages isolated from one rat was about 0.8–1.4 × 107 and 1.0–1.7 × 106 cells rat−1, respectively. The isolated alveolar epithelial cells and alveolar macrophages were cultured in DMEM and RPMI 1640 containing 10% FBS and 1% antibiotics–antimycotic mixed solution (AA), respectively, in a 5% CO2 incubator at 37 °C for 24 h after cell seeding. After 24 h or later, the amount of FBS in both DMEM and RPMI 1640-based culture medium was changed from 10% to 2% for cell culturing.
First, we show differentiation behavior of isolated type II cells. As shown in Fig. 11.5, cuboidal shape of the isolated type II cells was gradually changed to thin flat cells from 48 h after cell seeding. After 5 days of culture, a complete monolayer was obtained (Fig. 11.4c). The thickness of the obtained cell layer was ~3.0 μm and about a one-third or smaller of that of the A549 cell layer (~10 μm). During the formation of the monolayer, the TEER values significantly increased from day 4 and then leveled off after day 7 (Fig. 11.6). Subsequently, the TEER value was maintained at more than 300 Ω cm2 for at least 5 days. This value was one order of magnitude larger than that for the A549 and A549/HUVEC systems due to the differentiation from type II cells into type I cells. The final cell density of differentiated type I cells was estimated to be 3.0–4.0 × 104 cells cm−2.
Next, we describe the preparation of in vitro primary rat alveolar epithelial coculture models with primary rat alveolar macrophages (macrophage/type I system). The isolated primary rat alveolar macrophages were seeded onto the differentiated type I cell monolayer on day 6 and further cultured for 24 h. The final cell density of the primary rat alveolar macrophages was estimated to be 4.8 × 103 cells cm−2. The obtained value was nearly equal to that in alveoli in vivo (3.0–6.0 × 103 cells cm−2) [20].
4 Toxicity Tests of Nanoparticles
The cell viability is known to be influenced by SiO2 and TiO2 nanoparticles, as described above. Figure 11.7a–d shows the cell viability of A549 and primary rat alveolar epithelial type I cells after 24, 48, and 72 h of exposure to P25, MP100, and MinU Silica (MUS). Here, low- and high-density culture systems were used. In the low-density culture system, the cell density was ~70% confluent. This value has generally been accepted in the cytotoxicity tests. In the high-density culture system, A549 and primary rat type I cells formed the complete monolayer. As the amount of the three nanoparticles increased, the cell viability at the low-density system for A549 cells clearly decreased (Fig. 11.7a), whereas that for primary rat type I cells slightly decreased (Fig. 11.7b). On the other hand, the cell viability for both A549 and primary rat type I cells at the high-density systems were not changed, as the amount of P25 and MP100 increased (Fig. 11.7c, d). Even after exposure to MUS, which is silica nanoparticles and known to strongly cause damage to cells, the cell viability for the both cells only slightly decreased. Notice that the cell viability for primary rat type I cells to silica nanoparticles at the low-density system was nearly equal to that at the high-density system, but that for A549 cells at the low-density system was much lower than that at the high-density system. From uptake of nanoparticles for the both cells by means of time course microscopic observation, apparently, primary rat type I cells firmly adhered on the culture dish and took up nanoparticles attached to only their cell surfaces through the endocytosis pathway. However, surprisingly, A549 cells not only took up nanoparticles attached onto their cell surfaces through the endocytosis pathway but also actively migrated and phagocytosed nanoparticles attached on the substrate surface, even though A549 cells are epithelial type II cell lines. The cytotoxicity of nanomaterials might be overestimated, when A549 cells at the low cell density are used for the toxicity tests. However, the low-density system for A549 cells would be useful as a high-sensitive detecting method of nanoparticles despite the lack of their physiologically relevant behaviors.
Similarly, Fig. 11.7e, f shows the cell viability of macrophage-like cells differentiated from THP-1 cells and isolated primary rat alveolar macrophages to the nanoparticles. As the amount of P25 and MUS increased, the cell viability of the rat macrophage significantly decreased, compared to that of the macrophage-like cells, probably due to the differences in functionality between primary cells and cell lines. As shown in Fig. 11.8, the rat macrophages were spherical shape and obviously different shapes from the macrophage-like cells. In addition, primary cells are widely believed to possess more biologically relevant functions than cell lines. A phagocytic capacity of the rat macrophages is likely higher than that of the macrophage-like cells. Indeed, the rat macrophages actively migrated and phagocytosed TiO2 nanoparticles in comparison with the macrophage-like cells (Fig. 11.8). Thus, macrophages would be the essential element for predicting not only cytotoxicity tests but also translocation studies to nanoparticles properly.
5 Permeability Tests of Nanoparticles
Inhaled nanoparticles, the size of which is 10–100 nm in diameter, tend to be deposited in the alveoli region. Here, FITC-SiO2NPs and Rho-SiO2NPs 30 nm in diameter were used in the translocation study for THP-1/A549/HUVEC and macrophage/type I systems, respectively. If cells are damaged by nanoparticles and detached from the insert surface, translocation ratio would not be determined appropriately. Based on the cell viability tests, the appropriate amount of nanoparticles, which is no influence to the cell viability, was determined to be about ≤0.02 mg mL−1 for FITC-SiO2NPs and ≤0.2 mg mL−1 for Rho-SiO2NPs. This difference might depend on the cell type and properties of fluorescent dyes on the surface of SiO2NPs. The both nanoparticles used here were also still dispersed in DMEM without FBS even after 24 h. DMEM containing FITC-SiO2NPs or Rho-SiO2NPs was added into the apical side of THP-1/A549/HUVEC and macrophage/type I systems, respectively, and further incubated for 24 h. The amount of nanoparticles translocated from the apical to the basolateral side was apparently suppressed in comparison with the A549/HVEC and type I culture systems without macrophages (Fig. 11.9). Nanoparticles were also accumulated in the cell layers and/or adsorbed at the surface of the semipermeable membrane. The increase in the amount of nanoparticles at the cell/membrane compartment is based on the fact that macrophages including differentiated macrophage-like cells phagocytosed the nanoparticles.
Compared with the THP-1/A549/HUVEC system, the macrophage/type I system may provide a physiologically relevant response of alveoli in vivo because of consisting of primary cells. The dependence of nanoparticle size on translocation characteristics was further examined at the macrophage/type I system. DMEM containing Rho-SiO2NPs 10, 30, or 100 nm in diameters was added into the apical side of the macrophage/type I system and further incubated for 24 h. Transport of the nanoparticles 10 and 30 nm in diameter from the apical to basolateral side was clearly suppressed in comparison with the case of only type I cell culture system without the rat macrophages (~2.6%, Fig. 11.10). In contrast, the penetration of nanoparticles 100 nm in diameter was apparently disrupted by even only type I cell layer without macrophages. The alveolar epithelial tight junction might prevent the paracellular pathway of nanoparticles 100 nm in diameter or larger due to its narrow gap. In any case, the obtained value is still larger than that reported in animal experiments (<1.0%). This is likely due to the quite different environment between in vitro and in vivo. In addition, the present in vitro alveolar epithelial model is still far from completely mimicking the in vivo alveolar tissue. The improvement of in vitro alveolar epithelial models is absolutely necessary, as described later.
6 Future Remarks
In this work, we have been developing physiologically relevant in vitro alveolar epithelial models for cytotoxicity tests and translocation studies of nanoparticles. However, the present in vitro alveolar epithelial models are still quite different from the in vivo situation, as mentioned above. In order to overcome such problem, the in vitro alveolar epithelial models further have to fulfill the following at least four conditions, such as (1) applying an air-liquid interface culture (ALIC) method, (2) using human induced pluripotent stem cell (hiPSC)-derived type I and II cells, (3) reconstitution of the alveolar epithelial cell layer consisting of type I and type II cells, and (4) applying mechanical strain to semipermeable membranes. The details are as follows.
6.1 Applying the ALIC Method
The ALIC system has been widely used in the field of respiratory research [21,22,23]. The ALIC method enables cells to directly contact with air and gases at their apical side, while a culture medium is supplied to the cells from the basolateral side through the semipermeable membrane. The ALIC method has also been known to improve barrier function of in vitro pulmonary models, such as alveolar and bronchial epithelial cells [24]. Note that preliminary investigation of dispersibility and stability of nanoparticles in the atmosphere is imperative, when the ALIC technique is used for the prediction of transport from the apical to basolateral side and cytotoxicity of nanoparticles.
6.2 Using hiPC-Derived Type I and II Cells
Primary human cells would provide the best prospects for obtaining more meaningful information to human bodies in the cell-based assays and high-throughput screening. However, the use of the primary human cells has generally been limited due to the donor shortage, limited source materials, heterogeneity in cell type, and donor variability. To overcome this problem, hiPSCs are promising cell sources for the in vitro assays. However, it is required that a large amount of target cells differentiated from standardized hiPSCs is produced at low cost.
6.3 Reconstitution of the Alveolar Epithelial Cell Layer Consisting of Type I and Type II Cells
In our previous investigation, it was not easy to obtain the alveolar epithelial cell layer consisting of type I and II cells, the surface area ratio of which was 95:5, respectively, just by seeding and culturing primary rat alveolar epithelial type II cells on the semipermeable membrane. The type II cells are known to usually reside at the corners of alveoli [25]. In addition, the differentiation from type II cells to type I cells is suppressed by culturing at the air-exposed apical surface [26]. Therefore, the surface morphology control and the ALIC method would be critical factors in controlling the surface area ratio of type I and II cells for the alveolar epithelial cell layer.
6.4 Applying Mechanical Strain to Semipermeable Membranes
The lungs expand and contract during normal breathing, so that alveolar epithelial cells are exposed to cyclic stretching. Mechanically applying the cyclic stretching to the alveolar epithelial cells in vitro is expected to improve their stability and physiological functions. In fact, Ingber and his coworkers have developed a lung on-a-chip with two parallel microchannels separated by a stretchable and semipermeable membrane, at the top and lower surfaces of which A549 and endothelial cells were cultured, respectively. They successfully reproduced the in vivo situation of the lung microenvironment by cyclic stretching of the membrane in addition to flowing air and culture medium at the apical and basolateral sides, respectively [27]. During cyclic stretching of the membrane, ideally, the alveolar epithelial cell layer should be covered with pulmonary surfactant secreted from type II cells.
7 Conclusion
We described the preparation and properties of in vitro alveolar epithelial models consisting of the human alveolar epithelial cell line A549 or primary rat alveolar epithelial cells. Coculture with macrophages improved the physiological performance of the in vitro alveolar epithelial models in cytotoxicity tests and translocation studies of nanoparticles. The present system would play a complementary role in the animal experiments, since the colloidal suspension of nanoparticles is frequently used to evaluate lung injury and translocation to extrapulmonary organs in rodents after its intratracheal instillation. However, the in vitro alveolar epithelial models developed here are not enough to achieve optimal in vivo performance yet. To improve the performance, the in vitro lung model consisting of human alveolar epithelial type I and II cells would be required for culturing under the ALIC with cyclic stretching condition. The improved in vitro alveolar epithelial models would be applied to accurately and kinetically understand and predict the potential toxic and alveolar permeability effects of various nanoparticles.
References
Nel A, Xia T, Mädler L, Li N. Toxic potential of materials at the nano level. Science. 2006;311:622–7.
De Jong WH, Borm PJA. Drug delivery and nanoparticles: applications and hazards. Int J Med. 2008;3:133–49.
Sung JC, Pulliam BL, Edwards DA. Nanoparticles for drug delivery to the lungs. Trends Biotechnol. 2007;25:563–70.
Klein CL, Wiench K, Wiemann M, Ma-Hock L, van Ravenzwaay B, Landsiedel R. Hazard identification of inhaled nanomaterials: making use of short-term inhalation studies. Arch Toxicol. 2012;86:1137–51.
Sayes CM, Reed KL, Warheit DB. Assessing toxicity of fine and nanoparticles: comparing in vitro measurements to in vivo pulmonary toxicity profiles. Toxicol Sci. 2007;97:163–80.
Yacobi NR, Phuleria HC, Demaio L, Liang CH, Peng CA, Sioutas C, Borok Z, Kim KJ, Crandall ED. Nanoparticle effects on rat alveolar epithelial cell monolayer barrier properties. Toxicol In Vitro. 2007;21:1373–81.
Trouiller B, Reliene R, Westbrook A, Solaimani P, Schiestl RH. Titanium dioxide nanoparticles induce DNA damage and genetic instability in vivo in mice. Cancer Res. 2009;69:8784–9.
Gurr JR, Wang ASS, Chen CH, Jan KY. Ultrafine titanium dioxide particles in the absence of photoactivation can induce oxidative damage to human bronchial epithelial cells. Toxicology. 2005;213:66–73.
Kim IS, Baek M, Choi SJ. Comparative cytotoxicity of Al2O3, CeO2, TiO2 and ZnO nanoparticles to human lung cells. J Nanosci Nanotechnol. 2010;10:3453–8.
Lai JCK, Lai MB, Jandhyam S, Dukhande VV, Bhushan A, Daniels CK, Leung SW. Exposure to titanium dioxide and other metallic oxide nanoparticles induces cytotoxicity on human neural cells and fibroblasts. Int J Nanomedicine. 2008;3:533–45.
Lin W, Huang YW, Zhou XD, Ma Y. In vitro toxicity of silica nanoparticles in human lung cancer cells. Toxicol Appl Pharmacol. 2006;217:252–9.
Mahmoudi M, Lynch I, Ejtehadi MR, Monopoli MP, Bombelli FB, Laurent S. Protein-nanoparticle interactions: opportunities and challenges. Chem Rev. 2011;111:5610–37.
Lieber M, Smith B, Szakal A, Nelson-Rees W, Todaro G. A continuous tumor-cell line from a human lung carcinoma with properties of type II alveolar epithelial cells. Int J Cancer. 1976;17:62–70.
Foster KA, Oster CG, Mayer MM, Avery ML, Audus KL. Characterization of the A549 cell line as a type II pulmonary epithelial cell model for drug metabolism. Exp Cell Res. 1998;243:359–66.
Daigneault M, Preston JA, Marriott HM, Whyte KB, Dockrell DH. The identification of markers of macrophage differentiation in PMA-stimulated THP-1 cells and monocyte-derived macrophages. PLoS One. 2010;5:e8668.
Fleit HB, Kobasiuk CD. The human monocyte-like cell line THP-1 expresses FcγRI and FcγRII. J Leukoc Biol. 1991;49:556–65.
Braakhuis HM, Kloet SK, Kezic S, Kuper F, Park MVDZ, Bellmann S, van der Zande M, Gac SL, Krystek P, Peters RB, Rietjents IMCM, Bouwmeester H. Progress and future of in vitro models to study translocation of nanoparticles. Arch Toxicol. 2015;89:1469–95.
Dobbs LG, Mason RJ. Pulmonary alveolar type II cells isolated from rats. J Clin Invest. 1979;63:378–87.
Sakagami M. In vivo, in vitro and ex vivo models to assess pulmonary absorption and disposition of inhaled therapeutics for systemic delivery. Adv Drug Deliv Rev. 2006;58:1030–60.
Wallace WAH, Gillooly M, Lamb D. Intra-alveolar macrophage numbers in current smokers and non-smokers: a morphometric study of tissue sections. Thorax. 1992;47:437–40.
Iwasawa K, Tanaka G, Aoyama T, Chowdhury MM, Komori K, Tanaka-Kagawa T, Jinno H, Sakai Y. Prediction of phthalate permeation through pulmonary alveoli using a cultured A549 cell-based in vitro alveolus model and a numerical simulation. AATEX. 2013;18:19–31.
Komori K, Murai K, Miyajima S, Fujii T, Mohri S, Ono Y, Sakai Y. Deveopment of an in vitro batch-type closed gas exposure device with an alveolar epithelial cell line, A549, for toxicity evaluations of gaseous compounds. Anal Sci. 2008;24:957–62.
Whitcutt MJ, Adler KB, Wu R. A biphasic chamber system for maintaining polarity of differentiation of cultured respiratory tract epithelial cells. In Vitro Cell Dev Biol. 1988;24:420–8.
Sakai Y, Tomita K, Suzuki M, Ono Y, Sakoda A. Development of a toxicity evaluation system for gaseous compounds using air-liquid interface culture of a human bronchial epithelial cell line. Calu-3 AATEX. 2005;11:59–67.
Weibel ER. Morphometry of the human lung: the state of the art after two decades. Bull Eur Physiopathol Respir. 1979;15:999–1013.
Dobbs LG, Pian MS, Magrio M, Dumars S, Allen L. Maintenance of the differentiated type II cell phenotype by culture with an apical air surface. Am J Physiol. 1997;273:L347–54.
Huh D, Matthews BD, Mammoto A, Montoya-Zavala H, Hsin Y, Ingber DE. Reconstituting organ-level lung functions on a chip. Science. 2010;328:1662–8.
Acknowledgements
The works shown here are collaborative work with Mr. Takuya Aoyama, Mr. Kodai Harano, Ms. Xinying Xu, and Ms. Ayaka Uemura. This work is part of the research program “Development of innovative methodology for safety assessment of industrial nanomaterials” supported by the Ministry of Economy, Trade and Industry (METI) of Japan.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Komori, K., Iwasawa, K., Ogasawara, R., Suwabe, A., Sakai, Y. (2019). In Vitro Alveolar Epithelial Models Toward the Prediction of Toxicity and Translocation of Nanoparticles: A Complementary Method for Mechanism Analyses in Humans. In: Takebayashi, T., Landsiedel, R., Gamo, M. (eds) In Vivo Inhalation Toxicity Screening Methods for Manufactured Nanomaterials. Current Topics in Environmental Health and Preventive Medicine. Springer, Singapore. https://doi.org/10.1007/978-981-13-8433-2_11
Download citation
DOI: https://doi.org/10.1007/978-981-13-8433-2_11
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-8432-5
Online ISBN: 978-981-13-8433-2
eBook Packages: MedicineMedicine (R0)