Abstract
Drug delivery in the field of medicine is a system that was developed mainly for the purpose of releasing, localizing, and targeting drugs at a particular site in the body, at the right time, period, and dose. The use of natural biopolymers in drug delivery systems dates back to as far as 1970. This is due to the uniqueness of their properties, especially their ability to undergo biodegradation. The use of these biopolymers have contributed largely to the advancement of this technology by adding its own properties to the drugs. Biopolymers like polysaccharides, polyesters, and polyamides are few examples of natural biopolymers employed for this application. They are produced by microorganisms and the fact that the microorganisms can be genetically manipulated gives room for the production of a wide range of biopolymers. Their existence ranges from viscous solutions to even plastics. Also, their physical properties depend on the composition of the biopolymer as well as its molecular weight. The ability to produce natural biopolymers with personalized properties via biotechnological techniques opens them up to several medical applications such as drug delivery. The properties, structure, synthesis, and most importantly the application of some natural biopolymers in drug delivery will be discussed in this chapter.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
In medicine, the need and demand for the successful delivery of pharmacologically active materials or therapeutic compounds to cells, tissues, and organs in the system have made drug delivery techniques broadly studied. Several drug delivery methods have been developed and investigated in the past [180]. The aim is to design better approaches to treat various diseases affecting humans in the world. This has led to the development and use of different materials of natural and synthetic origin as drug delivery devices. However, certain limitations and challenges have been faced with the use of most of these materials hence the need for more suitable alternatives. Some of these limitations include material toxicity, non-biocompatibility, and non-flexibility among others. At the moment, research has brought to limelight some group of materials with unique properties that can potentially serve as drug delivery systems. They are commonly referred to as biopolymers and because they can be manipulated, they can be fabricated into nanosizes (sizes of between 1 and 100 nm); hence, they are called bionanopolymers. Bionanopolymers are generally of natural origin, they are biodegradable and biocompatible. These properties have made them widely employed in biomedical applications [178]. Bionanopolymers have gained attention in drug delivery [84, 143] and have contributed to the progress recorded in the treatment of disease conditions such as cancer [25], diabetes [13], allergy [166], infection [57], and inflammation [214]. They are known to increase the therapeutic effect of drugs at the same time minimize the side effect. Their flexibility permits the engineering of plenty of functionalities needed for delivering drugs efficiently, at the same time, maintaining biocompatibility, facile manufacturing, and formulation stability [29]. A lot of reasons account for their use in a therapeutic application; these include similarity in domain size as proteins, a large surface area which allows the display of a large number of functional groups. In addition, their high abilities of diffusion and volume change provide rapid absorption and good release behavior [138]. Another great benefit associated with the use of bionanopolymers is the ability to tailor or control their particle size and surface characteristics. For example, the surface characteristic can be modified by binding the surface to the functional molecule covalently and assembling them layer-by-layer [223]. Low drug-loading, wide size distribution, and difficulty in scaling up are some limitations of these biomaterials. These, however, have not affected the interest chemists, biologist, and pharmaceutical scientist have in them. This is because they are promising drug delivery vehicles for transporting bioactive agents to several parts of the body compared to other materials [71, 220].
This chapter thus focuses on the utilization of commonly used biopolymers such as proteins (with emphasis on collagen), polysaccharides (with emphasis on chitosan), and polyester (with emphasis on polyhydroxyalkanoates (PHAs)). Their structures, synthesis, and application as biomaterials in biomedicine for drug delivery are discussed.
2 Drug Delivery Systems
For a successful and efficient management of diseases, a valuable therapeutic approach is of great importance. Therefore, a drug delivery system must be one that permits the introduction of therapeutic agents into the body in order to improve the drug’s efficacy. Also, the safety, time, and mode of delivery of any drug delivery system should be considered at the experimental or clinical level [197, 199]. The aim of every drug delivery system is to localize administered pharmacological agent at a selectively targeted site in the body at a controlled rate with reduced side effects that may arise from systemic treatment. Research for this better alternate drug delivery system has been ongoing for over two decades and successes have been reported. The development, study, and formulation of microsphere, nanomaterials, and hydrogels among others from biopolymers are rapidly becoming promising routes for drug delivery [176]. Generally, the delivery of drugs is achieved by using the chemical formulation of the drug, medical device, or both. Currently, various systems such as particulate carriers, polymer gels, and lipids are employed as drug delivery systems [10, 90, 135, 193, 194, 212]. Some properties that make a drug delivery suitable for delivering therapeutic agents in the body include nonimmunogenic, non-toxic, biocompatible, good biodegradability, controllable drug release, and ease of consistent reproducible and clinical-grade synthesis [76]. All the various types of drug delivery system that are used to deliver bioactive agents in different fields in medicine are grouped into two. These are conventional and the novel drug delivery system.
2.1 Conventional Drug Delivery System
This type of drug delivery system is referred to as traditional drug delivery system. It involves the delivery of pharmaceutical compounds into the body by using the common and usual methods. Common examples of this type of delivery system include oral delivery, transdermal delivery, and parenteral delivery such as buccal/sublingual delivery, rectal delivery, intravenous delivery, subcutaneous delivery, and intramuscular delivery among others. These individual delivery systems have their peculiar benefits [182].
2.1.1 Oral
The oral route of drug delivery remains the most popular drug delivery system. Drugs delivered via this route are often absorbed in the small intestine which provides 100 m2 epithelia surface for drug transfer. However, if it is a drug that has poor solubility, absorption may also take place in the large intestine [70]. The advantages of this drug delivery system are (i) convenience of administration, (ii) non-invasive, (iii) accurate and measured dose, (iv) unit dosage form, (v) cheap for the patient, and (vi) reduced infection risk compared to subcutaneous injections [33, 217]. Disadvantages associated with this route of drug delivery are (i) cannot be used by unconscious patients, (ii) low solubility and permeability, (iii) degradation by gastrointestinal enzymes or flora, (iv) food interactions (v) quick clearance time of between 4 and 12 h, and (vi) irregular absorption [129, 107, 106], making this form of drug administration less effective.
2.1.2 Transdermal
Transdermal drug delivery system is one that is designed to deliver therapeutic agents in dosage form across the skin of patients [2]. This system is a discrete dosage form that is otherwise known as patches [21, 95]. The first transdermal drug delivery system (Transderm SCOP) was approved by the food and drug administration in the year 1979. This was used to prevent nausea and vomiting when traveling. The major aim of this drug delivery system is to get drugs into the systemic circulation via the skin. This is done at a rate that is predetermined with slight inter- and intra-patients variation [95]. The design of several transdermal drug delivery systems is such that the therapeutic agent is released for a period of several hours to days upon application to the surface of the skin. The advantages this drug delivery system are reduction of workload placed on digestive tracts and liver by the oral route, patients comply more to drug administration and the harmful side effect of drugs as a result of a temporary overdose is minimized [2]. In addition, it permits the continuous addition of drugs that have a short half-life and also prevents pulsed entry of drugs into the system that most times lead to harmful side effects [95]. Other benefits of this drug delivery system include limited hepatic first-pass metabolism, the enhanced therapeutic efficacy of administered drugs, and ability to maintain a steady plasma level of the drug [77]. It has been found that this drug delivery system is beneficial for prophylactic therapy in chronic disease conditions [124]. By measuring the level of the drug in the blood, detecting the excretion of the drug and its metabolic products in the urine, the percutaneous absorption of the drug is evident. The clinical response of patients that were administered drugs through this route is also a valid way to ascertain the efficacy of this drug delivery system [77].
2.1.3 Parenteral
Parenteral drug delivery is simply any non-oral means of administering bioactive agents into the body. It is generally a method that injects therapeutic compounds directly into the body, bypassing the skin and mucous membranes. The common parenteral routes include intramuscular (IM), subcutaneous (SC), and intravenous (IV). Table 1 shows some of the advantages and disadvantages of the different parenteral drug delivery routes.
First-pass metabolism is a process whereby the concentration of the drug is reduced before it eventually arrives into the systemic circulation.
2.2 Novel Drug Delivery System
These classes of drug delivery system are those designed to continuously deliver drugs at predictable and reproducible kinetics over an extended period of time in the circulation. The major advantage of this type of drug delivery system is minimized side effect of the administered drug, controlled therapeutic blood level, and improved compliance by patients. This is because the frequency and total drug dosage are greatly reduced [11, 60]. This drug delivery system consists of three individual systems, viz. targeted, controlled, and sustained/modified release drug delivery system. The ability to combine these systems further increases the therapeutic efficacy of drugs in disease management and treatment [206].
2.3 Targeted Drug Delivery System
It is a drug delivery system otherwise known as smart drug delivery system. It delivers drugs to patients in a manner that makes the drug available for a prolonged time period and in increased concentration in the targeted site compared to other body parts. As oppose conventional drug delivery systems, drugs are absorbed across a biological membrane and the therapeutic agent is released in dosage form at the site of the target. Advantages of this delivery system include a decrease in the frequency of ingesting the drug, reduced drug circulation fluctuations, reduced side effects, and required drug level in the plasma, and tissue is retainable thus prevents tissue damage. On the other hand, the disadvantages of this delivery system are limited dosage adjustment and the high cost of production. In addition, it is a system that cuts across different disciplines and requires the active collaboration of these disciplines in order to achieve the desired result. Factors to be considered before fully implementing a targeted drug delivery system are the drug properties, side effects of the drugs, the drug delivery route, the targeted site, and the disease to be treated [5, 119, 131, 210]. Due to the fact that the targeted drug delivery system seeks to improve the overall curative effect of the drug as well as reduced its toxicity, two approaches otherwise called classes of targeted drug delivery system are employed. These are
-
1.
Passive targeting: This involves drug delivery via blood circulation. Drugs embedded in drug carrier systems are gathered at the particular site of the disease in the body. The action and release of the drug are limited to particular sites in the body. For example, an anticancer agent is targeted to the tumor site and not the liver. Although this requires the specific control of the size and surface of the delivery device to avoid uptake by other organs [64, 65, 159].
-
2.
Active targeting: This takes place majorly after blood circulation and extravasations. It involves the interaction of specific type of ligand-receptor for intracellular localization. This form of targeting is further classified based on the different targeting levels.
-
First-order targeting: Here, the distribution of the drug carrier system is restricted to the capillary bed of a predetermined target site, organ, or tissue. For instance, targeting of compartments in lymphatics, peritoneal cavity, pleural cavity, cerebral ventricles and eyes and the joints [159].
-
Second-order targeting: In this case, specific site (cell type) is targeted for drug delivery. For example, tumor cell targeting rather than normal cell and selectively delivery drug to kupffer cells in the liver [4].
-
Third-order targeting: This involves the precise delivery of drugs to the intracellular site of the targeted cells. For example, receptor-based ligand-mediated entry of a drug complex into a cell by endocytosis [64, 81, 131].
-
2.4 Controlled Drug Delivery System
This is a system that involves a predesigned manner of delivery (releasing) drugs incorporated into drug delivery vehicles into the body system. Here, the concentration of the drug and the drug’s pharmacokinetic characteristics are put into consideration and the drug is administered at a predetermined rate and time. Currently, drug delivery systems that are stimuli-responsive have gained attention for controlled release of drugs because they can be controlled easily and can be triggered externally. Such external factors include pH [36, 102] temperature [34, 97], ionic strength [175], and electric field [140]. The release of the therapeutic agent may occur at a constant or cyclic rate over a long period of time. However, the ultimate goal is to obtain a better therapeutic efficiency and avoiding under/overdose. Drug carrier capable of encapsulating drugs and releasing them at controlled rates (hours, days, weeks, and even months) is widely employed in this system. Advantages of this system include tailored drug release rates, protection of fragile drugs, patient’s compliance, and comfort.
2.5 Sustained Drug Delivery System
This form of drug delivery system is a modified form of administering drugs to patients. It can serve as a more efficient substitute to the conventional drug delivery system. This is achieved by sustaining the drug release and at the same time maintaining the concentration of the drug in the plasma. Sustained drug release system prevents peak and trough in dosing and a constant concentration of the drug is found available in the therapeutic window. Benefits associated with this delivery system are uniform drug delivery, low-dose frequency, increased drug effectiveness due to the localization of the drug, evasion of multiple dosing, little or no side effect, and ability to overcome challenges faced by conventional drug delivery system [96, 98, 148]. Physiochemical factors such as dose size, ionization, partition coefficient, drug stability, half-life, therapeutic index, absorption window, plasma concentration-response relationship, drug metabolism, pKa, and aqueous solubility are linked to the manner which drugs are administered through a sustained drug delivery system [17, 78, 152].
Although sustained drug delivery system and controlled drug delivery system are different drug delivery processes, they are usually mixed up in an inconsistent and confusing way because of the similarities in their processes. The major difference between them is that controlled release is a perfect zero-order release; that is, the drug is released over time irrespective of the drug’s concentration. Whereas, the drug is slowly released over a period of time in sustained manner and it is not time dependent [20, 99]. It can either be controlled or uncontrolled [24, 105, 211].
3 Bionanopolymer
These are polymers obtained from natural origin (living organisms) during the growth cycles. They are synthesized by enzyme-catalyzed, chain growth polymerization reactions of activated monomers, which are typically formed within cells by complex metabolic processes [178]. They are non-toxic, less expensive, and easily available; therefore, they have been more greatly employed in the diverse application as delivery carriers of therapeutic drugs compared to synthetic polymers. However, for them to be used for this purpose, it is necessary for the particle size, charge, surface morphology, and drug release rate to be controlled [138]. They usually tailor-made as drug delivery devices in nano-sized forms, and this involves a various process of preparation. These macromolecular-based mucoadhesive biomaterials have been considered for several years and used as drug delivery vehicles and quite a number of successes have been reported [129]. With the use of these bionanopolymers as drug carriers, many limitations (e.g. enzymatic degradation of drug) of the different drug delivery system has been overcome. For example, increased buccal penetration has been achieved using biopolymer micro-/nano-sphere formulation [75, 169]. Hence, they have been identified as protective agents against rapid drug degradation [107, 153, 186].
3.1 Types of Bionanopolymer
Bionanopolymers are divided into various groups; some of these groups are polysaccharides, proteins, and nucleic acids. Numerous examples of each class exist but for the purpose of this chapter, a representative member of each group will be emphasized and discussed in details. The focus is on collagen, chitosan, and polyhydroxyalkanoate (PHA) which are bionanopolymers of protein, polysaccharide, and polyester origin respectively.
3.1.1 Protein
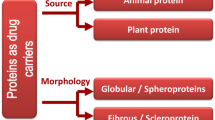
Proteins are natural, thermoplastic heteropolymers polymers. They are made up of different polar and nonpolar α-amino acids, most proteins are not soluble nor fusible [31]. They have advantages such as biodegradability, low toxicity, high stability, and excellent binding capacity of diverse drugs [42, 62, 91, 209]. They are also able to emulsify, form gels and possess water-binding capacity [49, 50, 209. In addition, they show some drug-loading mechanisms such as electrostatic attractions, hydrophobic interactions, and covalent bonding [51]. Additionally, the presence of functional groups on the surface of nanoparticles makes modification possible thus permits specific drug targeting to the site of action [138]. These properties make them different from synthetic polymers and have made protein-based nanocarriers promising candidates for drug and gene delivery. Some of these proteins of natural origin have already been studied, designed, and used as biomaterials for drug delivery. Examples are collagen [55, 145], elastin [40] and fibronectin [44]. Currently, research has led to the development of genetically engineered protein with properties that can be manipulated. Therefore, more protein-based nanocarriers with better drug delivery properties are available for drug delivery applications. Collagen, which is the protein of interest in this chapter, is a natural protein that exists in the body as a major component of the extracellular matrix of animals [113]. The total body protein consists of between 25 and 35% collagen, and they are in the form of elongated fibrils. They are abundantly found in tissues such as bone, cartilage, tendons, blood vessels, ligament, skin, cornea, intervertebral disk, and the gut [149]. They are found within and outside the cells of the body. Collagen possesses excellent tensile strength and firmness to the tissues in the body [167]. Collagen exhibits superior biocompatibility compared with other polymers of natural sources, for example, albumin and gelatin [113]. When the medical application of biomaterials began to expand in the 1970s, most research laboratories focused their studies on collagen. Since then, collagen of medical grade, improved processing technology became easier to obtain [137, 221, 222] and subsequently, collagen was employed as a drug delivery system. It has been in use for years as a biomaterial because of its biocompatibility, low antigenicity, and biodegradability [113]. At the moment, collagen is still one of the best biomaterials that have a broad range of application as a delivery vehicle for drugs, proteins, and genes. Thus far, it has been proven to function effectively in controlled release and localized drug delivery [125]. It has been used as a drug vehicle in ophthalmology, as injectable dispersions in cancer treatment, as sponges carrying antibiotics and as implantable mini-pellets loaded with protein drugs. However, the biological, physical, and chemical properties of collagen are still being studied in order to improve the overall properties, as well as overcome some of its limitations in the application as a drug carrier [83]. It is generally anticipated that collagen-based materials will become a useful matrix substance for biomedical application, especially in drug delivery.
3.1.2 Polysaccharide
Polysaccharides are a group of biopolymer of the natural source and have a number of advantages over synthetic polymers. They are non-toxic, can be produced at a relatively low cost, and are very biocompatible. Hence, they are promising ideal materials for the synthesis of drug delivery systems [45, 46]. Examples of polysaccharides include starch, cellulose, and chitosan among several others. However, the emphasis is on chitosan in this chapter. Chitosan is a linear polysaccharide that occurs naturally and one of the most abundant in the environment [12]. It is produced by chitin and found in only some species of fungi (Mucoraceae) [3]. Chitosan is a semi-crystalline polymer that is solid and exhibits a degree of polymorphism [141]. It is known to possess chemical activity thus, increasing its range of applications. Although the discovering of chitosan dates as far back as an early nineteenth century, it has only been used in biological applications and drug delivery systems (such as nanoparticle microspheres) in the last two decades [47]. It is one of the most widely studied and used marine polysaccharides for biomedical application. It has now become a great biomaterial of interest to the pharmaceutical industry in drug delivery and several publications on its use as a drug carrier has been seen [129]. Review on the biodegradation, biodistribution, and toxicity [86] as well as its formulation for DNA and siRNA [118] delivery has been considered. Its application as hydrogel for controlled, localized drug delivery [17]; nanostructures for delivery of ocular therapeutics [41] have also been reported. In addition, its use as a drug carrier for targeted delivery of low-molecular drugs have also been stated [146]. It is important to know that the mucoadhesive properties of chitosan play a key role in its usage in oral, nasal, and ocular drug delivery [68, 69, 74]. At the moment, chitosan is designed as nanoparticles, beads, and capsules for controlled drug delivery systems [3, 39, 154, 184]. The biological properties of chitosan make it an excellent candidate for applications that require contact with biological environments. Also, these properties allows the incorporation and delivery of pharmacologically active substances [73, 183]. The electrostatic interaction between a chitosan-based drug delivery system and the bioactive compound is crucial to the drug’s stability, protection and the ultimate release of the drug. In other words, a positively charged polymer like chitosan will be more suitable to deliver an anionic drug than a cationic drug [30].
3.1.3 Polyester
They are biodegradable polymers that are currently considered the most competitive for various applications in the biomedical field. They are biocompatible and their physicochemical properties make them suitable and ideal for a wide range of application in medicine [1, 43, 160]. Apart from being used as a drug delivery vehicle in controlled drug release system, medical devices are also manufactured from them [114, 134]. In drug delivery applications, they act as a biological inert support material like a mesh or drug carrier. There are different types of polyester in existence that are used in biomedicine. However, the most commonly employed are polylactic acid (PLA), poly(lactic-co-glycolic acid) (PLGA), poly(ε-caprolactone) (PCL), polyhydroxyalkanoate (PHA), e.g., poly-3-hydroxybutyrate (or poly-β-hydroxybutyric acid, PHB). Polyhydroxyalkanoates are a type of naturally occurring polyesters. Their discovery dates back as far as 1888 but was not called PHA by biochemists [161]. They have obtained from over 75 different bacteria genera both gram-positive and gram-negative bacteria. They are stored in the cytoplasm as granules and forms about 90% of the dry weight of the cell [26, 103, 112]. The molecular weight of PHA is between 50,000 and 1,000,000 Da depending on the type of bacteria it is obtained from. The monomers of PHA provide different types of materials with properties ranging from rigid and stiff to flexible and elastomeric, including polymers that degrade relatively quickly in vivo and others that are slow to degrade [72] . Typical examples of this class of polyester include Poly(3-hydroxybutyrate) P(3HB), Poly(3-hydroxyhexanoate), Poly(4-hydroxybutyrate), Poly(3-hydroxyoctanoate) P(3HO), Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate), Poly(3-hydroxyvalerate), Poly(3HHx-co-3HO) and Poly(3HB-co-3HV). Generally, the degradation of this group of polyester depends on the microbial activity of the environment, the surface they are exposed to, moisture, temperature, pH, molecular weight, polymer composition, crystallinity, and nature of the attached monomer unit of the PHA [23, 103, 189]. They are used as delivery vehicles for localized drug delivery with a controlled release of the entrapped compound over a period of time [104]. PHAs have excellent properties far beyond the synthetic polymers used in the biomedical application. Currently, these PHA are considered worthy of intense research for the possibility of modifying their properties in order for them to serve in more areas in medicine.
3.2 Structure and Synthesis
3.2.1 Collagen
The first and correct model of collagen structure was known as Madras Model. This model was proposed by Ramachandran and Kartha [170]. Collagen is made up of a triple helical structure that contains two homologous chains (α-1) and one supplementary chain with varying chemical composition (α-2) [167]. The triple helical structure of collagen is made up of three polypeptide a-chains with more than a thousand amino acids in each. The individual chains have a different turn in the reverse direction and they are linked together primarily by hydrogen bonds between nearby CO and NH groups. These various polypeptides present in collagen are made up of mainly proline, hydroxyproline, glycine, and lysine. Although, glycine makes the smallest side group, the quantity of the glycine present determines the degree of flexibility, the more the glycine content, the greater the flexibility of the collagen chain [61]. Glycine unit is repeated at every position on the sequence; thus, it permits the chains to be well closely packed, leaving very little core space for residues. Proline constitutes about 35% of the non-glycine positions in the Gly-X-Y sequence, but it is mostly found in the X-position while 4-hydroxyproline is found in the Y-position [83]. Hydroxyproline is a product of the posttranslation of proline, and it is mediated by the enzyme prolylhydroxylase [94]. This makes up about 10% of the amino acid present in collagen. Collagen also consists of unusual amino acid known as hydroxylysine, produced from lysine via enzymatic hydroxylation by lysyl hydroxylase enzyme. The formation of the unusual amino acid permits the sugar components to attach to the structure, to form the three helical collagen structures. Imino acids are also found in collagen, and they help to stabilize the triple helix structure. They also cause the A-chain and form hydrogen bonds that limit rotation [150]. There exists very little difference between collagen obtained from different species of vertebrates [196]. Generally, collagen molecule weighs 300 kda and its rope-shaped structure has a length and width of 300 and 1.5 nm, respectively [167].
The synthesis of collagen is made possible by fibroblast cells. The preliminary materials used to synthesize collagen used in biomedicine, especially drug delivery is obtained from human tissue that is rich in fibrous. For drug delivery purposes, four types of collagen are isolated and purified. These are natural salt-soluble collagen, alkali, and enzyme-treated collagen, acid-soluble collagen, and insoluble collagen. In addition, collagen of various types like procaine, bovine, and sheep can be derived from different sources such as marine sources, human placenta, and recombinant human collagen from transgenic animals [167].
3.2.2 Chitosan
Chitosan occurs naturally as linear polysaccharide produced by alkaline deacetylation and chitin [54]. It consists of amine groups that are sensitive to changes in pH. They are positively charged in an acidic medium but neutral in an alkaline medium [115]. The rigidity, compact crystallinity in structure and strong intra- and inter-molecular hydrogen bonding makes chitosan insoluble in water and alkaline media [208]. Chitosan has a very amazing structure whose fundamental skeleton does not change despite any alteration made on the structure. The original physicochemical and biochemical properties are still maintained while possessing new and improved properties at the same time. Their wide application in pharmaceutics, biomedicine, and biotechnology is as a result of the broad range of derivatives available, each with its own unique properties [130]. Chitosan can be modified for various applications by oligomerization, alkylation, acylation, hydroxy alkylation, carboxy alkylation, sulfation, phosphorylation, enzymatic modifications [47]. The ability to modify chitosan is a good approach to increase the effectiveness of drug release, protection, and stability [6]. Chitosan has been reported to have a rigid rod-type structure [35, 52, 53, 82, 128, 195] or a semi-flexible-coil [15, 27, 100, 123, 171, 204, 207]. It has also been reported that the degree of flexibility (in terms of persistence length) is reasonably influenced by DA [123, 195]. Figure 1 shows the chemical structure of chitosan. The chemical structure is shown in Fig. 1.
As earlier stated, chitosan is synthesized by the deacetylation of chitin. This leads to the production of a compound having a d-glucosamine residue and n-acetyl-d-glucosamine which represents the deacetylated and acetylated unit, respectively. These residues are randomly distributed within the compound, but glucosamine units are known to be predominant [6, 162, 208]. The degree of deacetylation is dependent on the ratio of glucosamine to acetyl glucosamine; there are between the ranges of 30 and 100% [147]. The degree of acetylation of chitosan can vary from zero (full deacetylation) to one (full acetylation). This is made possible due to the ability to replace the n-acetyl group present in chitosan by NH2 [129]. The acetylated and deacetylated monomers which are GlcNAc; A-unit and GlcN; D-unit, respectively, are either randomly distributed or distributed in a block-wise manner [201, 202]. Chitosan can be designed for drug delivery purposes using different techniques such as multiple emulsion, solvent evaporation, coating deposition, and successive bark chitosan [109].
3.2.3 PHA
PHAs are biodegradable, hydrophobic, and crystalline biopolymer that has gained increasing interest among other class of biodegradable biopolymers. PHAs are a class of polyester, accumulated in the granules of different bacterial cells as energy storage material under growth conditions that are not balanced [180]. They are often modified to take care of problems such as low cell adhesion, hydrophobicity, and inflammatory side effects associated with the use of some synthetic polymers [173, 93]. PHA consists of over 100 types of hydroxyl acid monomers, the monomers units of hydroxyalkanoate ranges from 2- to 6-hydroxy acids. These monomers can be substituted with a broad range of side groups such as alkyl, aryl, alkenyl, halogen, cyano, epoxy, ether, acyl, ester, and acid groups [190]. These monomers help provide additional structural properties to the polymer [72]. They are grouped into short chain length (SCL) and medium chain length (MCL) consisting of 3–5 carbon atoms and 6–14 carbon atoms, respectively. They consist of only chirally pure (R)-configuration monomers [180]. Even though they are of biological origin, they have few resemblances in structure to synthetic polymers used in medical applications [47]. The general structure of PHA is shown in Fig. 2.
PHAs are generally synthesized by different gram-positive and gram-negative bacteria such as Azotobacter sp., Pseudomonas sp., Bacillus sp., and Methylobacterium sp. [188]. PHA synthesis is achieved by the metabolic biosynthesis of PHAs molecules by these microorganisms when there is excess carbon but limited nutrient [87, 189]. Most PHAs are synthesized by metabolic biosynthesis, and this pathway has been studied. For example, the metabolic pathway that leads to the synthesis of PHB involves the formation of a carbon–carbon bond of two acetyl-CoA moieties by the action of β-ketothiolase. 3-hydroxybutyryl-CoA is further formed by the reduction of acetoacetyl-CoA by the enzyme NADPH-dependent acetoacetyl-CoA reductase [88]. PHB, an example of PHA is synthesized by the polymerization of (R)-3-hydroxybutyryl-CoA molecules by the PHB synthase thus, the formation of PHB granules [151]. The synthesis of PHA is dependent on the NADH to NAD+ ratio, an increased NADH/NAD+ ratio is observed when the nitrogen source is finished. This inhibits the action of the enzyme β-ketothiolase, thus preventing the entering of Acetyl-CoA into the PHA biosynthetic pathway to produce 3HB monomers [7, 139]. The most bacteria strain used for the industrial production of poly-(R)-3- hydroxybutyrate (PHB), poly((R)-3-hydroxybutyrate-co-4-hydroxybutyrate) (P3HB4HB), and poly((R)-3-hydroxybutyrate-co-(R)-3-hydroxyvalerate) (PHBV) is Cupriavidus necator. Generally, PHA that is synthesized in vivo is of high-molecular weights. Recently, synthesis of PHA from plant origin for biomedical application is increasingly considered and investigated [180].
4 Biodegradation
Biodegradable polymers generally have labile bonds in their backbones that can be hydrolytically or proteolytically degraded [187]. One property that makes bionanopolymers unique is their ability to degrade in the different environment. They degrade within the body due to natural biological processes, thus when used as a drug delivery system, it eliminates the need to remove them from the body system [58]. Most often, they degrade by a process known as hydrolysis, into smaller compounds or molecules that are biologically acceptable by the body hence their broad application in biomedicine [177]. For example, PHAs can be broken down completely into water and carbon dioxide by a lot of microorganisms present in the soil, water, and sewage environment [28]. Some bacteria and fungi are known to excrete enzymes capable of degrading solid PHAs into water-soluble monomers and oligomers which are subsequently used as nutrients within the cells [180]. Chitosan is degradable by enzymes such as chitosanase or lysozymes, hence their wide application in biomedicine [133]. The stability/degradability of chitosan is crucial to the pharmaceutical industry because it plays a vital role in the function of chitosan in different formulations [132, 185]. Factors such as temperature are also considered in the storage of chitosan as this may cause depolymerization. Depolymerization may be detrimental to the purposed application based on the significant functional changes that occur [127, 136]. The degree of deacetylation of chitosan is also a parameter that can be used to control the rate of its degradation [3, 19]. Collagen, on the other hand, degrades by denaturation in most cases; however, this can be coupled with physical and/or chemical processes. This results into the production of a polypeptide with high-molecular weight known as gelatine. This protein (gelatine) is further degraded by the enzyme proteases by the hydrolysis of the amine functional group that is present [31].
5 Formulations of Bionanopolymers for Drug Delivery
The use of bionanopolymers as drug carriers for various drug delivery systems in the treatment of several diseases is currently been studied in depth [178]. For this to be successfully achieved, these bionaopolymers are formulated to suit the particular application. These formulations can either be capsules, gels, nanoparticles, tablets, etc. The main reason behind this specific design of bionanopolymer is to adequately protect certain therapeutic agents that degrade or metabolize rapidly as soon as they are administered [58]. This technology is seen overtime to modify, control the distribution of drug in tissues, cells, and specific target sites within the body.
5.1 Nanoparticles
One of the technologies used to target drugs to site of interest is the use of nanoparticles that are polymer based. This has been ongoing since 1980s when new discoveries in polymer chemistry led to the design of biodegradable and biocompatible materials [58]. Recently, research focus has been on designing drug delivery systems with sizes between the range of nanometers which are otherwise known as nanoparticles [9, 181]. Nanoparticles are submicron colloidal system that comprises of polymers and they are usually less than 1 μm. That is, they are between 7 and 70 times smaller in size than red blood cells. Generally, nanoparticles loaded with drugs are able to interact with biomolecules within and outside the cell without leading to any irreversible damage. This is because the diameter of the cells in the human body is between 10 and 20 microns while different structural units of cells are between a few several hundred nanometers [89]. Biopolymer-based nanoparticles can be prepared either as nano-spheres or nanocapsules. In nanosphere formulation, drugs are dispersed throughout the entire particle within the polymer while nanocapsules are formed by a liquid core containing drug and surrounded by a polymeric membrane [58]. The use of proteins to prepare nanoparticles helps to obtain the precise size of the desired nanoparticles because their secondary structure determines their molecular sizes. Collagen among other proteins is good examples of proteins that have been designed and utilized as nanoparticles for therapeutic application [144, 165]. Collagen nanoparticle is prepared with additional chemical treatments in order to enhance the mechanical strength [121]. A sustained drug release was displayed by collagen nanoparticle in a recent study. This was attributed to the ease of controlling the particle size, a large surface area, high adsorption capacity, and dispersion ability in water [144]. Furthermore, properties such as large surface area small size, great absorptive capability, capacity to diffuse in water to form a colloidal solution has made collagen-based readily used in sustained and delayed release formulation for steroids and antibiotics. Some of the useful benefits generally offered by nanoparticles are:
-
Improved drug solubility and compatibility
-
Ability to target tissues and organs that are pathogenic
-
Sensitivity to stimuli from external environment (e.g., heating effect of magnetic field or the pathogen stimuli environment (e.g., changes in temperature or pH)
-
Ability to transfer a metabolic information reporter. That is, a reporter that supplies information on metabolism [181, 197,198,199].
The most widely employed methods for fabricating protein-based nanoparticles are emulsification, desolvation, coacervation, spray drying. Other methods include jet-milling technique, fluidization, and solvent precipitation method, and interfacial polymerization. Apart from proteins, polysaccharides have also been used for the manufacture of nanoparticles. They are classified by their native charges, for example, cationic (chitosan), anionic (alginate, heparin, hyaluronic acid), and nonionic (pullulan, dextran). They can also be classified based on the mechanism of their formation, viz. chemically crosslinked nanoparticles, physically crosslinked nanoparticles, polyion complex, and self-assembled nanoparticles [126]. Chitosan remains the most commonly used polysaccharide for fabricating nanoparticles [163]. Nanoparticles are expected to be excreted from the body after they have delivered the incorporated therapeutic agents. Also, they should be without the risk of uncontrolled accumulation, non-toxic and should not cause any immune response [203].
5.2 Tablets/Pellets
Over the years, polymers have been used as excipients in traditional oral drug release, and they have also been employed to protect drugs from degradation during storage [58]. Pellets are very suitable for local delivery of certain compounds. Bionanopolymers are often used in tablet/pellet formulations as diluents for highly potent low dose drugs. Collagen-based mini-pellets have been synthesized as for the delivery of various compounds. This pellet was used as a carrier for the local delivery of minocycline and lysozyme [56, 113, 122, 192]. Lucas and his coworkers also described a pellet drug delivery vehicle made of purified type I collagen in 1989. This was used as a delivery device for water-soluble osteogenic proteins [111]. Collagen pellets are commonly found in use extensively in Japan, and they are referred to as monolithic devices. They are usually tiny, cylindrical structures (rod-like in shape) with a diameter of about 1 mm and length of 15 mm. They are administered through injection with the aid of a syringe and a plugar [167]. Polysaccharides such as starch and cellulose are used in tablet formation to prevent drug disintegration. These bionanopolymers swell upon hydration, leading to a burst of the tablet. This results in the increased surface area of the drug that is exposed and improved the dissolution characteristics of the formulation [108].
5.3 Gel
Gel happens to be another form in which bionanopolymers are used as drug delivery systems. These gels which can be of protein, polysaccharide, or polyester origin possess unique properties such as the ability to soak and swell when in contact with liquid (biological fluids). They are also able to maintain their integrity after hydration. In addition, they have high bio-adhesion property, easy to apply, and they are compatible with a wide variety of drugs and therapeutic agents [167]. A very common bionanopolymer fabricated in this form is collagen via crosslinking with the chemical. However, they are still capable of forming hydrogels without the use of chemical crosslinking [178]. The non-fibrillar viscous solution in aqueous medium and fibers injectables suspensions are the most common forms by which collagen is used as an injectable hydrogel. Drugs to be administered are mixed in these suspensions and injected into the body. Initially, it remains as a liquid but after a while, it becomes gel-like and has the potential for controlled and sustained drug delivery [167]. Hydrogels produced by crosslinking chitosan have also been investigated by Wu and his colleagues. They are known to be pH sensitive and have the ability to promote a sustained release of therapeutic agents upon nasal administration [215].
5.4 Capsules
Capsules are alternatives used for tablets and materials that are poorly compressible. They mask the bitterness of some drugs and at times increase the bioavailability of drugs. Over the years, bionanopolymers have been used to “bulk out” capsule fills. Polysaccharides have been exclusively used as a shell material for hard (two‐piece) and soft (one‐piece) capsules [58]. Capsules that are chitosan-based have also been synthesized for delivery of drugs. This is observed in the novel thermoresponsive ELR/chitosan microcapsules that were developed for the delivery of active molecule [37]. Chitosan-based capsules remedy electrostatic complexation that occurs in most delivery systems. Also, they respond to other external stimuli apart from pH [38].
6 Application of Bionanopolymer in Drug Delivery
The use of bionanopolyers as drug delivery system has been ongoing ever since the discovery of their unique properties. A lot of in vitro and in vivo studies have been undertaken to understand more clearly the mechanism of delivering several therapeutic agents using bionanopolymers. Some of the results obtained have formed the basis for their clinical applications. Protein-based pellets have been proven to be effective and successful for the in vivo delivery of interleukin-2. It was discovered that the half-life of interleukin-2 was prolonged with the use of pellets (half-life 360 min) compared to subcutaneous and intravenous injections of an aqueous preparation (15 and 8 min respectively). It was also proven that a prolonged retention of interleukin-2 was experienced when these mini-pellets were injected subcutaneously [167]. Collagen-based nanoparticles have shown their potential as sustained release formulations for steroids and antimicrobial agents because of their small size, a large surface area, high adsorptive capacity, and ability to disperse in water to form a clear colloidal solution [48]. Rossler and co-workers likewise, observed an enhanced dermal delivery of retinol using collagen nanoparticles as the drug carrier. They reported that the collagen facilitated a quicker and higher transportation of retinol through the skin than the freshly precipitated drug and the drug was stable in the system [164]. There has been an extensive study of collagen-based pellet as a gene delivery vehicle. One such study is the investigation of the effect of collagen-based mini-pellet on the mRNA expression and functional status of the facial nerve. This experiment was however carried on rat model [92].
Delivery systems that are chitosan-based have been reported for oral, parenteral, ocular, nasal, and transdermal delivery; this is particularly attributed to their mucoadhesive property. Chitosan-based gene delivery system to oral and nasal route gene therapy has successfully been applied. Nanoparticles made from chitosan have also been employed as delivery carriers for growth factors (e.g., epidermal growth factor and fibroblast growth factor) which can be impregnated into a construct for application in tissue engineering [158, 168]. In another study, a chitosan-based biopolymer was used to deliver a hydrophobic drug (ketoprofen). The results from the in vitro release revealed that the bionanopolymer has a good potential for hydrophobic drugs in a pH-sensitive controlled release [155, 156]. In addition, it was observed in another study that grafting acetylated chitosan with a fatty acid such as palmitoyl led to the development of chitosan-based excipients capable of entrapping and releasing drugs that are hydrophobic [79, 101, 120]. Wijekoon and his colleagues also developed a chitosan-based hydrogel that was used to deliver oxygen for wound healing [213]. The hydrogel was synthesized by photo-crosslinking; this hydrogel allowed for the control of both the capacity and rate of the oxygen delivery. It was also able to maintain the level of oxygen for up to five days. Other studies have shown that chitosan has the ability to improve and prolong the absorption of hydrophilic drugs that are ingested orally [213] and via pulmonary administration [8]. Reduced toxicity and enhanced intestinal absorption capability was also demonstrated by chitosan-based nanocapsules for the oral delivery of peptides as reported by Prego and his fellow workers [157]. Similarly, this approach can be applied for the delivery of other drugs like insulin as shown by other studies [80, 116, 117, 224]. Bhattarai and coworkers developed a chitosan-based hydrogel for the controlled release of albumin [18]. The observation from the in vitro release studies is that there was a high release in the first 5 h and subsequently a sustained release over the next days of up to 80% cumulative release. Advantages associated with the use of bionanopolymers as drug carriers have also been reported by some scientist. Nanoparticles of bionanopolymer origin can be absorbed by the reticuloendothelial system [121]. It can also enhance the uptake of exogenous compounds (e.g., anti-HIV drugs) into a number of cells like macrophages [14]. Reports on the use of such nanoparticles as carriers for cytotoxic agents and other therapeutic materials such as camptothecin and hydrocortisone have been made by Yang and his coworkers as well as Berthold and his coworkers respectively [16, 219]. Other advantages that make bionanopolymer widely employed in drug delivery application is the ease of nasal and parenteral administration [191]. This was demonstrated by Wu and his coworkers in an experiment where hydrogels synthesized from bionanopolymers. The hydrogels were used as smart devices for the controlled release of drugs via nasal administration as drops/spray. This study carried out on rat models showed an increased absorption of the therapeutic agent in the nasal cavities, decreased level in the blood, and no sign of cytotoxicity. These results demonstrated that bionanopolymeric hydrogels have potentials as drug carriers for controlled release of therapeutic agents, especially molecules that are hydrophilic in nature [216]. Currently, several in vivo and in vivo studies are still going in order to discover other possible applications of bionanopolymers for the successful delivery of different therapeutic agents used in the treatment and management of several diseases.
PHAs like other degradable biopolymers have gained grounds in the application as a drug delivery vehicle for the administering several drugs since the early 1990s. When converted into films, matrices, microcapsules, microsphere and nanoparticles, drugs can be entrapped into this formulation for different drug delivery application. Microcapsules made of PHAs have been widely used for the delivery of drugs such as anesthetics, antibiotics, anti-inflammatory agents, anticancer agents, hormones, steroids, and vaccines [139, 142]. The potential application of some PHAs such as P(3HB) and P(3HB-co-3HV) have been investigated in a number of in vitro and in vivo studies for drug delivery purposes. Gangrade and price reported the use of PHA microsphere as vehicles for steroids [59]. In their investigation, PHB and P(3HB-3HV) were used to synthesize microspheres entrapped with progesterone and the in vitro release was carefully studied. Also, PHB, PHBV, and P(3HB-4HB) showed to be advantageous in the design of biodegradable, implantable rods for delivering antibiotics in the treatment of chronic osteomyelitis [66, 200, 218]. PHAs are known to have the ability to provide and maintain adequate concentrations of antibiotics at sites of infections [63, 67]. In addition, the in vivo and in vitro releases of anticancer agent (lomustine) from PHA and a synthetic polymer were compared, and it was discovered that the drug release kinetics from the PHA microsphere was better [22]. In another study done by Sendhil and coworkers, microspheres and microcapsules of polyhydroxybutyrate-co-hydroxyvalerates (PHBV) of various 3-hydroxyvalerate contents were loaded with tetracycline. The drug carrier (PHBV) was subsequently used for the targeted treatment of some periodontal disease, and it was observed that the release of the drug was complete before any sign of degradation of the carrier [172]. Similarly, PHB (polyhydroxybutyrate) microsphere was used to deliver an antitumor drug (rubomycin) to mice, and it was observed that the proliferation of the cancer cells was greatly inhibited compared to the free drug [179]. In the same vein, Kawaguchi and colleagues synthesized PHB-based microsphere for the delivery of 2, 3-diacyl-5-fluoro-2-deoxyuridine in mice and rats. Results showed low toxicity, good compatibility, and excellent drug efficiency [85]. In a recent study by Lu et al., a sustained release of P13K inhibitor (TGX221) was achieved using PHA nanoparticles and the proliferation of cancer cell lines was successfully hindered [110]. Furthermore, the efficacy and bioavailability of cisplatin embedded in a novel amorphous amphiphilic block copolymer P(3HV-co-4HB)-b-mPEG was studied by Shah and fellow researchers [174]. Reports from their observation showed that there was a sustained release of the drug from the drug carrier as well as tumor growth suppression by the nanoparticles. It was concluded that the PHA-based nanoparticle had an enhanced apoptotic effect on the tumor cells. These and other reports from several scientists globally have proven that bionanopolymers are potential drug delivery systems for a wide range of therapeutic agents.
7 Challenges
Despite the fact that, bionanopolymers have great properties that make them widely employed in biomedicine as drug delivery devices. They have somewhat been limited in use because they have not been able to successfully and completely deliver certain classes of drugs. Thus, there is still a big gap that needs to be bridged in order for major needs in drug delivery systems to be met. Also, at the moment, there is a need for a lot of improvements and modifications to be made on these bionanopolymers before they can be largely applied clinically. Furthermore, another challenge is the feasibility of scaling up the production of this bionanopolymer in the market. Also, the possibility of designing multifunctional drug delivery systems that can meet the different biological and therapeutic need still pose to be a challenge with the use of these bionaopolymers.
8 Conclusion
There has been increased interest in the use of bionanopolymers for medical application in response to the need to efficiently manage several diseases. This has led to considerable advances in drug delivery technology. Bionanopolymers have various advantages over most synthetic polymers in several biomedical applications. Hence, the main reason behind its application in the area of drug delivery. The use of bionanopolymer as drug carrier is majorly attributed to their biodegradability, versatility, and broad range of properties. Various successful studies using bionanopolymers have proven that they are biocompatible for drug carrier use. They can be formulated for targeted drug delivery in a controlled manner. Thus, bionanopolymer-based drug delivery vehicles hold tremendous promise in the management of several disease conditions such as cancer. With the intense research ongoing in this area, bionanopolymers will emerge as one of the most economical and environmentally friendly material for the next generation in a wide range of biomedical application. Their synergism with other bioactive compounds will greatly contribute to further development and advancement of drug delivery technology. This will also bring many bionanopolymer-based products to the market.
References
Ad EA, Sin A (2013) Handbook of biopolymers and biodegradable plastics. Elsevier/William Andrew
Aggarwal G, Dhawan S (2009) Development, fabrication and evaluation of transdermal drug delivery system—a review. Pharmainfo.net, 7
Agnihotri SA, Mallikarjuna NN, Aminabhavi TM (2004) Recent advances on chitosan-based micro-and nanoparticles in drug delivery. J Controlled Release 100:5–28
Akanksha B, Ganesh K, Preeti K (2014) Indian J Novel Drug Delivery 6:215–222
Allen TM, Cullis PR (2004) Drug delivery systems: entering the mainstream. Science 303:1818–1822
Alves N, Mano J (2008) Chitosan derivatives obtained by chemical modifications for biomedical and environmental applications. Int J Biol Macromol 43:401–414
Anderson AJ, Dawes EA (1990) Occurrence, metabolism, metabolic role, and industrial uses of bacterial polyhydroxyalkanoates. Microbiol Rev 54:450–472
Andrade F, Goycoolea F, Chiappetta DA, Das Neves J, Sosnik A, Sarmento B (2011) Chitosan-grafted copolymers and chitosan-ligand conjugates as matrices for pulmonary drug delivery. Int J Carbohyd Chem 2011
Arruebo M, Fernández-Pacheco R, Ibarra MR, Santamaría J (2007) Magnetic nanoparticles for drug delivery. Nano Today 2:22–32
Bae Y, Kataoka K (2006) Significant enhancement of antitumor activity and bioavailability of intracellular pH-sensitive polymeric micelles by folate conjugation. J Controlled Release 116:e49–e50
Banakar UV (1987) Drug delivery systems of the 90s: innovations in controlled release. Am Pharm 27:39–44
Bansal V, Sharma PK, Sharma N, Pal OP, Malviya R (2011) Applications of chitosan and chitosan derivatives in drug delivery. Adv Biol Res 5:28–37
Basarkar A, Singh J (2009) Poly (lactide-co-glycolide)-polymethacrylate nanoparticles for intramuscular delivery of plasmid encoding interleukin-10 to prevent autoimmune diabetes in mice. Pharm Res 26:72–81
Bender AR, Von Briesen H, Kreuter J, Duncan IB, Rübsamen-Waigmann H (1996) Efficiency of nanoparticles as a carrier system for antiviral agents in human immunodeficiency virus-infected human monocytes/macrophages in vitro. Antimicrob Agents Chemother 40:1467–1471
Berth G, Dautzenberg H, Peter MG (1998) Physico-chemical characterization of chitosans varying in degree of acetylation. Carbohyd Polym 36:205–216
Berthold A, Cremer K, Kreuter J (1998) Collagen microparticles: carriers for glucocorticosteroids. Eur J Pharm Biopharm 45:23–29
Bhattarai N, Gunn J, Zhang M (2010) Chitosan-based hydrogels for controlled, localized drug delivery. Adv Drug Deliv Rev 62:83–99
Bhattarai N, Ramay HR, Gunn J, Matsen FA, Zhang M (2005) PEG-grafted chitosan as an injectable thermosensitive hydrogel for sustained protein release. J Controlled Release 103:609–624
Bhise KS, Dhumal RS, Paradkar AR, Kadam SS (2008) Effect of drying methods on swelling, erosion and drug release from chitosan–naproxen sodium complexes. AAPS PharmSciTech 9:1–12
Bhowmik D, Gopinath H, Kumar BP, Duraivel S, Kumar KS (2012) Controlled release drug delivery systems. Pharma Innov 1
Bhowmik D, Kumar KS, Bhanot R (2017) Recent advances in transdermal drug delivery system. LAP LAMBERT Academic Publishing
Bissery M, Valeriote F, Thies C (1985) Therapeutic efficacy of CCNU-loaded microspheres prepared from poly (D, L) lactide (PLA) or poly-B-hydroxybutyrate (PHB) against Lewis lung (LL) carcinoma. In: Proceedings of the American Association for Cancer Research. AMER ASSOC CANCER RESEARCH PUBLIC LEDGER BLDG, SUITE 816, 150 S. INDEPENDENCE MALL W., PHILADELPHIA, PA 19106, 355
Boopathy R (2000) Factors limiting bioremediation technologies. Biores Technol 74:63–67
Brahmankar D, Jaiswal S (2009) Biopharmaceutics and Pharmacokinetics: pharmacokinetics. Vallabh Prakashan, pp 399–401
Brigger I, Dubernet C, Couvreur P (2012) Nanoparticles in cancer therapy and diagnosis. Adv Drug Deliv Rev 64:24–36
Brigham CJ, Sinskey AJ (2012) Applications of polyhydroxyalkanoates in the medical industry. International Journal of Biotechnology for Wellness Industries 1:52–60
Brugnerotto J, Desbrières J, Roberts G, Rinaudo M (2001) Characterization of chitosan by steric exclusion chromatography. Polymer 42:09921–09927
Byrom D (1987) Polymer synthesis by microorganisms: technology and economics. Trends Biotechnol 5:246–250
Cammas S, Bear M-M, Moine L, Escalup R, Ponchel G, Kataoka K, Guérin P (1999) Polymers of malic acid and 3-alkylmalic acid as synthetic PHAs in the design of biocompatible hydrolyzable devices. Int J Biol Macromol 25:273–282
Cardoso MJ, Costa RR, Mano JF (2016) Marine origin polysaccharides in drug delivery systems. Marine drugs 14:34
Chandra R, Rustgi R (1998) Biodegradable polymers. Prog Polym Sci 23:1273–1335
Chang S, Kramer W, Feldman S, Ballentine R, Frankel L (1981) Bioavailability of allopurinol oral and rectal dosage forms. Am J Health-Syst Pharm 38:365–368
Chen H, Langer R (1998) Oral particulate delivery: status and future trends. Adv Drug Deliv Rev 34:339–350
Chen S, Li Y, Guo C, Wang J, Ma J, Liang X, Yang L-R, Liu H-Z (2007) Temperature-responsive magnetite/PEO–PPO–PEO block copolymer nanoparticles for controlled drug targeting delivery. Langmuir 23:12669–12676
Cölfen H, Berth G, Dautzenberg H (2001) Hydrodynamic studies on chitosans in aqueous solution. Carbohyd Polym 45:373–383
Connal LA, Li Q, Quinn JF, Tjipto E, Caruso F, Qiao GG (2008) pH-responsive poly (acrylic acid) core cross-linked star polymers: morphology transitions in solution and multilayer thin films. Macromolecules 41:2620–2626
Costa RR, Custódio CA, Arias FJ, Rodríguez-Cabello JC, Mano JF (2013) Nanostructured and thermoresponsive recombinant biopolymer-based microcapsules for the delivery of active molecules. Nanomed Nanotechnol Biol Med 9:895–902
Costa RR, Martín L, Mano JF, Rodríguez‐Cabello JC (2012) Elastin‐like macromolecules. In: Biomimetic approaches for biomaterials development, pp 93–116
Couto DS, Hong Z, Mano JF (2009) Development of bioactive and biodegradable chitosan-based injectable systems containing bioactive glass nanoparticles. Acta Biomater 5:115–123
Daamen WF, Veerkamp J, Van Hest J, Van Kuppevelt T (2007) Elastin as a biomaterial for tissue engineering. Biomaterials 28:4378–4398
De La Fuente M, Raviña M, Paolicelli P, Sanchez A, Seijo B, Alonso MJ (2010) Chitosan-based nanostructures: a delivery platform for ocular therapeutics. Adv Drug Deliv Rev 62:100–117
Desai N, Trieu V, Yao Z, Louie L, Ci S, Yang A, Tao C, De T, Beals B, Dykes D (2006) Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel. Clin Cancer Res 12:1317–1324
Díaz A, Katsarava R, Puiggalí J (2014) Synthesis, properties and applications of biodegradable polymers derived from diols and dicarboxylic acids: From polyesters to poly (ester amide)s. Int J Mol Sci 15:7064–7123
Doillon CJ, Silver FH, Berg RA (1987) Fibroblast growth on a porous collagen sponge containing hyaluronic acid and fibronectin. Biomaterials 8:195–200
Donaldson K, Stone V, Tran C, Kreyling W, Borm PJ (2004) Nanotoxicology. BMJ Publishing Group Ltd
Doshi N, Mitragotri S (2009) Designer biomaterials for nanomedicine. Adv Func Mater 19:3843–3854
Efthimiadou EK, Metaxa A-F, Kordas G (2015) Modified polysaccharides for drug delivery. Polysaccharides: Bioactivity and Biotechnology, pp 1805–1835
El-Samaligy M, Rohdewald P (1983) Reconstituted collagen nanoparticles, a novel drug carrier delivery system. J Pharm Pharmacol 35:537–539
Elzoghby AO, El-Fotoh WSA, Elgindy NA (2011) Casein-based formulations as promising controlled release drug delivery systems. J Controlled Release 153:206–216
Elzoghby AO, Samy WM, Elgindy NA (2012) Albumin-based nanoparticles as potential controlled release drug delivery systems. J Controlled Release 157:168–182
Elzoghby AO, Samy WM, Elgindy NA (2012) Protein-based nanocarriers as promising drug and gene delivery systems. J Controlled Release 161:38–49
Errington N, Harding S, Vårum K, Illum L (1993) Hydrodynamic characterization of chitosans varying in degree of acetylation. Int J Biol Macromol 15:113–117
Fee M, Errington N, Jumel K, Illum L, Smith A, Harding SE (2003) Correlation of SEC/MALLS with ultracentrifuge and viscometric data for chitosans. Eur Biophys J 32:457–464
Felt O, Buri P, Gurny R (1998) Chitosan: a unique polysaccharide for drug delivery. Drug Dev Ind Pharm 24:979–993
Friess W (1998) Collagen–biomaterial for drug delivery1. Eur J Pharm Biopharm 45:113–136
Fujioka K, Takada Y, Sato S, Miyata T (1995) Novel delivery system for proteins using collagen as a carrier material: the minipellet. J Controlled Release 33:307–315
Furno F, Morley KS, Wong B, Sharp BL, Arnold PL, Howdle SM, Bayston R, Brown PD, Winship PD, Reid HJ (2004) Silver nanoparticles and polymeric medical devices: a new approach to prevention of infection? J Antimicrob Chemother 54:1019–1024
Gandhi KJ, Deshmane SV, Biyani KR (2012) Polymers in pharmaceutical drug delivery system: a review. International journal of pharmaceutical sciences review and research 14:10
Gangrade N, Price JC (1991) Poly (hydroxybutyrate-hydroxyvalerate) microspheres containing progesterone: preparation, morphology and release properties. J Microencapsul 8:185–202
Gates KA, Grad H, Birek P, Lee PI (1994) A new bioerodible polymer insert for the controlled release of metronidazole. Pharm Res 11:1605–1609
Gelse K, Pöschl E, Aigner T (2003) Collagens—structure, function, and biosynthesis. Adv Drug Deliv Rev 55:1531–1546
Ghuman J, Zunszain PA, Petitpas I, Bhattacharya AA, Otagiri M, Curry S (2005) Structural basis of the drug-binding specificity of human serum albumin. J Mol Biol 353:38–52
Gould PL, Holland SJ, Tighe BJ (1987) Polymers for biodegradable medical devices. IV. Hydroxybutyrate-valerate copolymers as non-disintegrating matrices for controlled-release oral dosage forms. Int J Pharm 38:231–237
Gref R, Minamitake Y, Peracchia MT, Trubetskoy V, Torchilin V, Langer R (1994) Biodegradable long-circulating polymeric nanospheres. Science 263:1600–1603
Gupta M, Sharma V (2011) Targeted drug delivery system: a Review. ResJ Chem Sci 1:134–138
Gürsel İ, Korkusuz F, Türesin F, Alaeddinoǧlu NG, Hasrc V (2000) In vivo application of biodegradable controlled antibiotic release systems for the treatment of implant-related osteomyelitis. Biomaterials 22:73–80
Gursel I, Yagmurlu F, Korkusuz F, Hasirci V (2002) In vitro antibiotic release from poly (3-hydroxybutyrate-co-3-hydroxyvalerate) rods. J Microencapsul 19:153–164
Harding SE (2006) Trends in muco-adhesive analysis. Trends Food Sci Technol 17:255–262
Harding SE, Davis SB, Deacon MP, Fiebrig I (1999) Biopolymer mucoadhesives. Biotechnol Genet Eng Rev 16:41–86
Hardy JG, Davis SS, Wilson CG (1989) Drug delivery to the gastrointestinal tract. Ellis Horwood, UK
Heiati H, Phillips NC, Tawashi R (1996) Evidence for phospholipid bilayer formation in solid lipid nanoparticles formulated with phospholipid and triglyceride. Pharm Res 13:1406–1410
Holmes P (1988) Biologically produced (R)-3-hydroxy-alkanoate polymers and copolymers. In: Developments in crystalline polymers. Springer
Ilium L (1998) Chitosan and its use as a pharmaceutical excipient. Pharm Res 15:1326–1331
Illum L (2002) Nasal drug delivery: new developments and strategies. Drug Discovery Today 7:1184–1189
Jain D, Panda AK, Majumdar DK (2005) Eudragit S100 entrapped insulin microspheres for oral delivery. AAPS PharmSciTech 6:E100–E107
Jain KK (2008) Nanomedicine: application of nanobiotechnology in medical practice. Med Principles Pract 17:89–101
Jalwal P, Jangra A, Dahiya L, Sangwan Y, Saroha R (2010) A review on transdermal patches. Pharma Res 3:139–149
Jantzen GM, Robinson JR (1996) Sustained-and controlled-release drug delivery systems. Drugs Pharm Sci 72:575–610
Jiang G-B, Quan D, Liao K, Wang H (2006) Novel polymer micelles prepared from chitosan grafted hydrophobic palmitoyl groups for drug delivery. Mol Pharm 3:152–160
Jintapattanakit A, Junyaprasert VB, Mao S, Sitterberg J, Bakowsky U, Kissel T (2007) Peroral delivery of insulin using chitosan derivatives: a comparative study of polyelectrolyte nanocomplexes and nanoparticles. Int J Pharm 342:240–249
Kannagi R, Izawa M, Koike T, Miyazaki K, Kimura N (2004) Carbohydrate-mediated cell adhesion in cancer metastasis and angiogenesis. Cancer Sci 95:377–384
Kasaai MR (2007) Calculation of Mark–Houwink–Sakurada (MHS) equation viscometric constants for chitosan in any solvent–temperature system using experimental reported viscometric constants data. Carbohyd Polym 68:477–488
Kasoju N, Ali SS, Dubey VK, Bora U (2013) Exploiting the potential of Collagen as a natural biomaterial in drug delivery. J Proteins Proteomics 1
Kataoka K, Harada A, Nagasaki Y (2001) Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv Drug Deliv Rev 47:113–131
Kawaguchi T, Tsugane A, Higashide K, Endoh H, Hasegawa T, Kanno H, Seki T, Juni K, Fukushima S, Nakano M (1992) Control of drug release with a combination of prodrug and polymer matrix: Antitumor activity and release profiles of 2′, 3′-diacyl-5-fluoro-2′-deoxyuridine from poly (3-hydroxybutyrate) microspheres. J Pharm Sci 81:508–512
Kean T, Thanou M (2010) Biodegradation, biodistribution and toxicity of chitosan. Adv Drug Deliv Rev 62:3–11
Keshavarz T, Roy I (2010) Polyhydroxyalkanoates: bioplastics with a green agenda. Curr Opin Microbiol 13:321–326
Kessler B, Witholt B (2001) Factors involved in the regulatory network of polyhydroxyalkanoate metabolism. J Biotechnol 86:97–104
Kim GJ, Nie S (2005) Targeted cancer nanotherapy. Mater Today 8:28–33
Kim J, Conway A, Chauhan A (2008) Extended delivery of ophthalmic drugs by silicone hydrogel contact lenses. Biomaterials 29:2259–2269
Koch-Weser J, Sellers EM (1976) Binding of drugs to serum albumin. N Engl J Med 294:311–316
Kohmura E, Yuguchi T, Yoshimine T, Fujinaka T, Koseki N, Sano A, Kishino A, Nakayama C, Sakaki T, Nonaka M (1999) BDNF atelocollagen mini-pellet accelerates facial nerve regeneration. Brain Res 849:235–238
Kretlow JD, Klouda L, Mikos AG (2007) Injectable matrices and scaffolds for drug delivery in tissue engineering. Adv Drug Deliv Rev 59:263–273
Kucharz EJ (1992) Degradation. In: The collagens: biochemistry and pathophysiology. Springer
Kumar JA, Pullakandam N, Prabu SL, Gopal V (2010) Transdermal drug delivery system: an overview. Int J Pharm Sci Rev Res 3:49–54
Kumar KS, Bhowmik D, Srivastava S, Paswan S, Dutta AS (2012) Sustained release drug delivery system potential. Pharma Innov 1
Kurkuri MD, Nussio MR, Deslandes A, Voelcker NH (2008) Thermosensitive copolymer coatings with enhanced wettability switching. Langmuir 24:4238–4244
Kutmalge M, Jadhav A, Ratnaparkhi M, Chaudhari S (2014) Sustained release drug delivery system. Terminology 1:2
Lachman L, Lieberman HA, Kanig JL (1986) The theory and practice of industrial pharmacy. Lea & Febiger
Lamarque G, Lucas J-M, Viton C, Domard A (2005) Physicochemical behavior of homogeneous series of acetylated chitosans in aqueous solution: role of various structural parameters. Biomacromol 6:131–142
Le Tien C, Lacroix M, Ispas-Szabo P, Mateescu M-A (2003) N-acylated chitosan: hydrophobic matrices for controlled drug release. J Controlled Release 93:1–13
Lee JS, Bae JW, Joung YK, Lee SJ, Han DK, Park KD (2008) Controlled dual release of basic fibroblast growth factor and indomethacin from heparin-conjugated polymeric micelle. Int J Pharm 346:57–63
Lee SY (1996) Biotechnol Bioeng 49:1–14
Lenz RW, Marchessault RH (2005) Bacterial polyesters: biosynthesis, biodegradable plastics and biotechnology. Biomacromol 6:1–8
Li VH, Robinson J, Lee V, Hui H (1987) Controlled drug delivery: fundamentals and applications. Marcel Dekker, Inc., New York, pp 373–432
Lin Y-H, Chen C-T, Liang H-F, Kulkarni AR, Lee P-W, Chen C-H, Sung H-W (2007) Novel nanoparticles for oral insulin delivery via the paracellular pathway. Nanotechnology 18:105102
Liu L, Fishman ML, Kost J, Hicks KB (2003) Pectin-based systems for colon-specific drug delivery via oral route. Biomaterials 24:3333–3343
Longer MA, Ch’ng HS, Robinson JR (1985) Bioadhesive polymers as platforms for oral controlled drug delivery III: oral delivery of chlorothiazide using a bioadhesive polymer. J Pharm Sci 74:406–411
Lu C, Mu B, Liu P (2011) Stimuli-responsive multilayer chitosan hollow microspheres via layer-by-layer assembly. Colloids Surf, B 83:254–259
Lu X-Y, Ciraolo E, Stefenia R, Chen G-Q, Zhang Y, Hirsch E (2011) Sustained release of PI3K inhibitor from PHA nanoparticles and in vitro growth inhibition of cancer cell lines. Appl Microbiol Biotechnol 89:1423–1433
Lucas PA, Syftestad GT, Goldberg VM, Caplan AI (1989) Ectopic induction of cartilage and bone by water-soluble proteins from bovine bone using a collagenous delivery vehicle. J Biomed Mater Res, Part A 23:23–39
Madison LL, Huisman GW (1999) Metabolic engineering of poly (3-hydroxyalkanoates): from DNA to plastic. Microbiol Mol Biol Rev 63:21–53
Maeda M, Tani S, Sano A, Fujioka K (1999) Microstructure and release characteristics of the minipellet, a collagen-based drug delivery system for controlled release of protein drugs. J Controlled Release 62:313–324
Makadia HK, Siegel SJ (2011) Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 3:1377–1397
Mano JF (2008) Stimuli-responsive polymeric systems for biomedical applications. Adv Eng Mater 10:515–527
Mao S, Germershaus O, Fischer D, Linn T, Schnepf R, Kissel T (2005) Uptake and transport of PEG-graft-trimethyl-chitosan copolymer–insulin nanocomplexes by epithelial cells. Pharm Res 22:2058–2068
Mao S, Shuai X, Unger F, Wittmar M, Xie X, Kissel T (2005) Synthesis, characterization and cytotoxicity of poly (ethylene glycol)-graft-trimethyl chitosan block copolymers. Biomaterials 26:6343–6356
Mao S, Sun W, Kissel T (2010) Chitosan-based formulations for delivery of DNA and siRNA. Adv Drug Deliv Rev 62:12–27
Mark S, Torchilin VP (2011) Drug delivery systems. Access Science. McGraw-Hill Companies
Martin L, Wilson CG, Koosha F, Uchegbu IF (2003) Sustained buccal delivery of the hydrophobic drug denbufylline using physically cross-linked palmitoyl glycol chitosan hydrogels. Eur J Pharm Biopharm 55:35–45
Marty J (1978) Nanoparticles-a new colloidal drug delivery system. Pharm Acta Helv 53:17–23
Matsuoka J, Sakagami K, Shiozaki S, Uchida S, Fujiwara T, Gohchi A, Orita K (1988) Development of an interleukin-2 slow delivery system. ASAIO Trans 34:729–731
Mazeau K, Rinaudo M (2004) The prediction of the characteristics of some polysaccharides from molecular modeling. Comparison with effective behavior. Food Hydrocolloids 18:885–898
Mehta R (2004) Topical and transdermal drug delivery: what a pharmacist needs to know. Inet Continuing education, InetCE.com, 1–10
Miyata T, Rubin AL, Stenzel KH, Dunn MW (1979) Collagen drug delivery device. Google Patents
Mizrahy S, Peer D (2012) Polysaccharides as building blocks for nanotherapeutics. Chem Soc Rev 41:2623–2640
Morris GA, Castile J, Smith A, Adams GG, Harding SE (2009) The kinetics of chitosan depolymerisation at different temperatures. Polym Degrad Stab 94:1344–1348
Morris GA, Castile J, Smith A, Adams GG, Harding SE (2009) Macromolecular conformation of chitosan in dilute solution: a new global hydrodynamic approach. Carbohyd Polym 76:616–621
Morris GA, Kök SM, Harding SE, Adams GG (2010) Polysaccharide drug delivery systems based on pectin and chitosan. Biotechnol Genet Eng Rev 27:257–284
Mourya V, Inamdar NN (2008) Chitosan-modifications and applications: opportunities galore. React Funct Polym 68:1013–1051
Muller RH, Keck CM (2004) Challenges and solutions for the delivery of biotech drugs—a review of drug nanocrystal technology and lipid nanoparticles. J Biotechnol 113:151–170
Muzzarelli R, Muzzarelli C (2009) Chitin and chitosan hydrogels. In: Handbook of hydrocolloids, 2nd edn. Elsevier
Nair LS, Laurencin CT (2007) Biodegradable polymers as biomaterials. Prog Polym Sci 32:762–798
Nazemi K, Azadpour P, Moztarzadeh F, Urbanska A, Mozafari M (2015) Tissue-engineered chitosan/bioactive glass bone scaffolds integrated with PLGA nanoparticles: a therapeutic design for on-demand drug delivery. Mater Lett 138:16–20
Nguyen DN, Raghavan SS, Tashima LM, Lin EC, Fredette SJ, Langer RS, Wang C (2008) Enhancement of poly (orthoester) microspheres for DNA vaccine delivery by blending with poly (ethylenimine). Biomaterials 29:2783–2793
Nguyen TTB, Hein S, Ng CH, Stevens WF (2008) Molecular stability of chitosan in acid solutions stored at various conditions. J Appl Polym Sci 107:2588–2593
Nimni ME, Cheung D, Strates B, Kodama M, Sheikh K (1987) Chemically modified collagen: a natural biomaterial for tissue replacement. J Biomed Mater Res, Part A 21:741–771
Nitta SK, Numata K (2013) Biopolymer-based nanoparticles for drug/gene delivery and tissue engineering. Int J Mol Sci 14:1629–1654
Nobes G, Maysinger D, Marchessault R (1998) Polyhydroxyalkanoates: materials for delivery systems. Drug Delivery 5:167–177
Nolkrantz K, Farre C, Brederlau A, Karlsson RI, Brennan C, Eriksson PS, Weber SG, Sandberg M, Orwar O (2001) Electroporation of single cells and tissues with an electrolyte-filled capillary. Anal Chem 73:4469–4477
Ogawa K, Yui T (1994) Effect of explosion on the crystalline polymorphism of chitin and chitosan. Biosci Biotechnol Biochem 58:968–969
Orts WJ, Nobes GA, Kawada J, Nguyen S, Yu G-E, Ravenelle F (2008) Poly (hydroxyalkanoates): biorefinery polymers with a whole range of applications. The work of Robert H. Marchessault. Can J Chem 86:628–640
Panyam J, Labhasetwar V (2003) Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev 55:329–347
Papi M, Palmieri V, Maulucci G, Arcovito G, Greco E, Quintiliani G, Fraziano M, De Spirito M (2011) Controlled self assembly of collagen nanoparticle. J Nanopart Res 13:6141–6147
Parenteau-Bareil R, Gauvin R, Berthod F (2010) Collagen-based biomaterials for tissue engineering applications. Materials 3:1863–1887
Park JH, Saravanakumar G, Kim K, Kwon IC (2010) Targeted delivery of low molecular drugs using chitosan and its derivatives. Adv Drug Deliv Rev 62:28–41
Park SY, Lee BI, Jung ST, Park HJ (2001) Biopolymer composite films based on κ-carrageenan and chitosan. Mater Res Bull 36:511–519
Patnaik AN, Nagarjuna T, Thulasiramaraju T (2013) Sustained release drug delivery system: a modern formulation approach. Int J Res Pharm Nano Sci 2:586–601
Piez K (1985) Collagen. Encycl Polymer Sci 3:699–727
Piez KA (1984) Molecular and aggregate structures of the collagens. Elsevier, New York
Poirier Y, Nawrath C, Somerville C (1995) Production of polyhydroxyalkanoates, a family of biodegradable plastics and elastomers, in bacteria and plants. Nat Biotechnol 13:142
Popli H, Sharma S (1989) Trends in oral sustained release formulation-I. Eastern Pharm 32:99–103
Pourjavadi A, Barzegar S (2009) Smart pectin-based superabsorbent hydrogel as a matrix for ibuprofen as an oral non-steroidal anti-inflammatory drug delivery. Starch-Stärke 61:173–187
Prabaharan M, Mano J (2004) Chitosan-based particles as controlled drug delivery systems. Drug Delivery 12:41–57
Prabaharan M, Mano JF (2005) Hydroxypropyl chitosan bearing β-cyclodextrin cavities: synthesis and slow release of its inclusion complex with a model hydrophobic drug. Macromol Biosci 5:965–973
Prabaharan M, Reis R, Mano J (2007) Carboxymethyl chitosan-graft-phosphatidylethanolamine: amphiphilic matrices for controlled drug delivery. React Funct Polym 67:43–52
Prego C, Fabre M, Torres D, Alonso M (2006) Efficacy and mechanism of action of chitosan nanocapsules for oral peptide delivery. Pharm Res 23:549–556
Rajam M, Pulavendran S, Rose C, Mandal A (2011) Chitosan nanoparticles as a dual growth factor delivery system for tissue engineering applications. Int J Pharm 410:145–152
Rani K, Paliwal S (2014) A review on targeted drug delivery: its entire focus on advanced therapeutics and diagnostics. Sch J App Med Sci 2:328–331
Ratner BD, Hoffman AS, Schoen FJ, Lemons JE (2013) Introduction-biomaterials science. In: Biomaterials science: an introduction to materials, 3rd edn. Elsevier Inc
Reddy C, Ghai R, Kalia VC (2003) Polyhydroxyalkanoates: an overview. Biores Technol 87:137–146
Rinaudo M (2006) Chitin and chitosan: properties and applications. Prog Polym Sci 31:603–632
Roldo M, Hornof M, Caliceti P, Bernkop-Schnürch A (2004) Mucoadhesive thiolated chitosans as platforms for oral controlled drug delivery: synthesis and in vitro evaluation. Eur J Pharm Biopharm 57:115–121
Rössler B, Kreuter J, Ross G (1994) Effect of collagen microparticles on the stability of retinol and its absorption into hairless mouse skin in vitro. Pharmazie 49:175–179
Rössler B, Kreuter J, Scherer D (1995) Collagen microparticles: preparation and properties. J Microencapsul 12:49–57
Roy K, Mao H-Q, Huang S-K, Leong KW (1999) Oral gene delivery with chitosan–DNA nanoparticles generates immunologic protection in a murine model of peanut allergy. Nat Med 5:387
Sahithi B, Ansari S, Hameeda S, Sahithya G, Prasad DM, Lakshmi Y (2013) A review on collagen based drug delivery systems. Indian J Res Pharm Biotechnol 1:461
Sarmento B, Ribeiro A, Veiga F, Ferreira D, Neufeld R (2007) Insulin-loaded nanoparticles are prepared by alginate ionotropic pre-gelation followed by chitosan polyelectrolyte complexation. J Nanosci Nanotechnol 7:2833–2841
Sarmento B, Ribeiro A, Veiga F, Ferreira D, Neufeld R (2007) Oral bioavailability of insulin contained in polysaccharide nanoparticles. Biomacromol 8:3054–3060
Sasisekharan V, Yathindra N (1999) The Madras Group and the structure of collagen. In: Proceedings of the Indian Academy of Sciences-Chemical Sciences. Springer, pp 5–12
Schatz C, Viton C, Delair T, Pichot C, Domard A (2003) Typical physicochemical behaviors of chitosan in aqueous solution. Biomacromol 4:641–648
Sendil D, Gürsel I, Wise DL, Hasrc V (1999) Antibiotic release from biodegradable PHBV microparticles. J Controlled Release 59:207–217
Seyednejad H, Gawlitta D, Dhert WJ, Van Nostrum CF, Vermonden T, Hennink WE (2011) Preparation and characterization of a three-dimensional printed scaffold based on a functionalized polyester for bone tissue engineering applications. Acta Biomater 7:1999–2006
Shah M, Ullah N, Choi MH, Kim MO, Yoon SC (2012) Amorphous amphiphilic P (3HV-co-4HB)-b-mPEG block copolymer synthesized from bacterial copolyester via melt transesterification: nanoparticle preparation, cisplatin-loading for cancer therapy and in vitro evaluation. Eur J Pharm Biopharm 80:518–527
Shah NM, Pool MD, Metters AT (2006) Influence of network structure on the degradation of photo-cross-linked PLA-b-PEG-b-PLA hydrogels. Biomacromol 7:3171–3177
Shantha Kumar T, Soppimath K, Nachaegari S (2006) Novel delivery technologies for protein and peptide therapeutics. Curr Pharm Biotechnol 7:261–276
Sharma K, Singh V, Arora A (2011) Natural biodegradable polymers as matrices in transdermal drug delivery. Int J Drug Dev Res 3
Sharma R (2014) An overview of future prospect of Aloe vera gel as nano drug carrier. In: Shukla JP (ed) Technologies for sustainable rural development having potential of socioeconomic upliftment. Allied Publishers, New Delhi, 173–179
Shishatskaya E, Goreva A, Voinova O, Inzhevatkin E, Khlebopros R, Volova T (2008) Evaluation of antitumor activity of rubomycin deposited in absorbable polymeric microparticles. Bull Exp Biol Med 145:358–361
Shrivastav A, Kim H-Y, Kim Y-R (2013) Advances in the applications of polyhydroxyalkanoate nanoparticles for novel drug delivery system. BioMed Res Int 2013
Singh R, Lillard JW Jr (2009) Nanoparticle-based targeted drug delivery. Exp Mol Pathol 86:215–223
Singh S, Pandey VK, Tewari RP, Agarwal V (2011) Nanoparticle based drug delivery system: advantages and applications. Indian J Sci Technol 4:177–180
Singla A, Chawla M (2001) Chitosan: some pharmaceutical and biological aspects—an update. J Pharm Pharmacol 53:1047–1067
Sinha V, Singla A, Wadhawan S, Kaushik R, Kumria R, Bansal K, Dhawan S (2004) Chitosan microspheres as a potential carrier for drugs. Int J Pharm 274:1–33
Skaugrud Ø, Hagen A, Borgersen B, Dornish M (1999) Biomedical and pharmaceutical applications of alginate and chitosan. Biotechnol Genet Eng Rev 16:23–40
Sriamornsak P (2003) Chemistry of pectin and its pharmaceutical uses: a review. Silpakorn Univ Int J 3:206–228
Srivastava A, Yadav T, Sharma S, Nayak A, Kumari AA, Mishra N (2015) Polymers in drug delivery. J Biosci Med 4:69
Steinbüchel A, Füchtenbusch B (1998) Bacterial and other biological systems for polyester production. Trends Biotechnol 16:419–427
Steinbüchel A, Schlegel H (1991) Physiology and molecular genetics of poly (β-hydroxyalkanoic acid) synthesis in Alcaligenes eutrophus. Mol Microbiol 5:535–542
Steinbüchel A, Valentin HE (1995) Diversity of bacterial polyhydroxyalkanoic acids. FEMS Microbiol Lett 128:219–228
Tahrir F, Ganji F, Ahooyi TM (2015) Injectable thermosensitive chitosan/glycerophosphate-based hydrogels for tissue engineering and drug delivery applications: a review. Recent Pat Drug Delivery Formulation 9:107–120
Takenaka H (1986) New formulation of bioactive materials. Pharm Technol Jpn 2:1083–1091
Tamilvanan S, Venkateshan N, Ludwig A (2008) The potential of lipid-and polymer-based drug delivery carriers for eradicating biofilm consortia on device-related nosocomial infections. J Controlled Release 128:2–22
Tang Y, Singh J (2008) Controlled delivery of aspirin: effect of aspirin on polymer degradation and in vitro release from PLGA based phase sensitive systems. Int J Pharm 357:119–125
Terbojevich M, Cosani A, Conio G, Marsano E, Bianchi E (1991) Chitosan: chain rigidity and mesophase formation. Carbohyd Res 209:251–260
Timpl R (1984) Immunology of the collagens. In: Extracellular matrix biochemistry, pp 159–190
Torchilin V (2009) Multifunctional and stimuli-sensitive pharmaceutical nanocarriers. Eur J Pharm Biopharm 71:431–444
Torchilin VP (2007) Targeted pharmaceutical nanocarriers for cancer therapy and imaging. AAPS J 9:E128–E147
Torchilin VP (2012) Multifunctional nanocarriers. Adv Drug Deliv Rev 64:302–315
Türesin F, Gürsel I, Hasirci V (2001) Biodegradable polyhydroxyalkanoate implants for osteomyelitis therapy: in vitro antibiotic release. J Biomater Sci Polym Ed 12:195–207
Vårum KM, Anthonsen MW, Grasdalen H, Smidsrod O (1991) 13C-Nmr studies of the acetylation sequences in partially N-deacetylated chitins (chitosans). Carbohyd Res 217:19–27
Vårum KM, Antohonsen MW, Grasdalen H, Smidsrod O (1991) Determination of the degree of N-acetylation and the distribution of N-acetyl groups in partially N-deacetylated chitins (chitosans) by high-field nmr spectroscopy. Carbohyd Res 211:17–23
Vauthier C, Bouchemal K (2009) Methods for the preparation and manufacture of polymeric nanoparticles. Pharm Res 26:1025–1058
Velásquez CL, Albornoz JS, Barrios EM (2008) Viscosimetric studies of chitosan nitrate and chitosan chlorhydrate in acid free NaCl aqueous solution. e-Polymers 8
Verma P, Thakur A, Deshmukh K, Jha A, Verma S (2010) Routes of drug administration. Int J Pharm Stud Res 1:54–59
Vikas K, Arvind S, Ashish S, Gourav J, Vipasha D (2011) Recent advances in Ndds (Nov el Drug Delivery System) for delivery of anti-hypertensive drugs. Int J Drug Dev Res 3
Vold IMN (2004) Periodate oxidised chitosans: structure and solution properties
Vroman I, Tighzert L (2009) Biodegradable polymers. Materials 2:307–344
Vuignier K, Schappler J, Veuthey J-L, Carrupt P-A, Martel S (2010) Drug–protein binding: a critical review of analytical tools. Anal Bioanal Chem 398:53–66
Vyas SP, Khar RK (2004) Targeted & controlled drug delivery: novel carrier systems. CBS Publishers & Distributors
Wani MS (2008) Controlled release system—a review. Pharm Rev 6:41–46
Watanabe M, Kawano K, Toma K, Hattori Y, Maitani Y (2008) In vivo antitumor activity of camptothecin incorporated in liposomes formulated with an artificial lipid and human serum albumin. J Controlled Release 127:231–238
Wijekoon A, Fountas-Davis N, Leipzig ND (2013) Fluorinated methacrylamide chitosan hydrogel systems as adaptable oxygen carriers for wound healing. Acta Biomater 9:5653–5664
Wilson DS, Dalmasso G, Wang L, Sitaraman SV, Merlin D, Murthy N (2010) Orally delivered thioketal nanoparticles loaded with TNF-α–siRNA target inflammation and inhibit gene expression in the intestines. Nat Mater 9:923
Wu J, Su Z-G, Ma G-H (2006) A thermo-and pH-sensitive hydrogel composed of quaternized chitosan/glycerophosphate. Int J Pharm 315:1–11
Wu J, Wei W, Wang L-Y, Su Z-G, Ma G-H (2007) A thermosensitive hydrogel based on quaternized chitosan and poly (ethylene glycol) for nasal drug delivery system. Biomaterials 28:2220–2232
Yadav N, Morris G, Harding S, Ang S, Adams G (2009) Various non-injectable delivery systems for the treatment of diabetes mellitus. Endocr Metab Immune Disord-Drug Targets (Formerly Current Drug Targets-Immune, Endocrine & Metabolic Disorders) 9:1–13
Yagmurlu MF, Korkusuz F, Gürsel I, Korkusuz P, Örs Ü, Hasirci V (1999) Sulbactam-cefoperazone polyhydroxybutyrate-co-hydroxyvalerate (PHBV) local antibiotic delivery system: In vivo effectiveness and biocompatibility in the treatment of implant-related experimental osteomyelitis. J Biomed Mater Res, Part A 46:494–503
Yang SC, Lu LF, Cai Y, Zhu JB, Liang BW, Yang CZ (1999) Body distribution in mice of intravenously injected camptothecin solid lipid nanoparticles and targeting effect on brain. J Controlled Release 59:299–307
Yang YY, Wang Y, Powell R, Chan P (2006) Polymeric core-shell nanoparticles for therapeutics. Clin Exp Pharmacol Physiol 33:557–562
Yannas I, Burke J, Gordon P, Huang C, Rubenstein R (1980) Design of an artificial skin. II. Control of chemical composition. J Biomed Mater Res, Part A 14:107–132
Yannas I, Burke JF (1980) Design of an artificial skin. I. Basic design principles. J Biomed Mater Res, Part A 14:65–81
Yu D-G, Lin W-C, Yang M-C (2007) Surface modification of poly (L-lactic acid) membrane via layer-by-layer assembly of silver nanoparticle-embedded polyelectrolyte multilayer. Bioconjug Chem 18:1521–1529
Zhang X, Zhang H, Wu Z, Wang Z, Niu H, Li C (2008) Nasal absorption enhancement of insulin using PEG-grafted chitosan nanoparticles. Eur J Pharm Biopharm 68:526–534
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Fasiku, V.O. et al. (2019). Bionanopolymers for Drug Delivery. In: Gnanasekaran, D. (eds) Green Biopolymers and their Nanocomposites. Materials Horizons: From Nature to Nanomaterials. Springer, Singapore. https://doi.org/10.1007/978-981-13-8063-1_8
Download citation
DOI: https://doi.org/10.1007/978-981-13-8063-1_8
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-8062-4
Online ISBN: 978-981-13-8063-1
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)