Abstract
Polycyclic aromatic hydrocarbons (PAHs) are active members of the group of multi-aromatic organic compounds, considered to be the most ubiquitous environmental pollutants, mainly engendered from partial combustion of wood, coal, oil or other organic materials. Currently, more than 500 PAHs are prevalent in the atmosphere; reactions between PAHs and various chemicals such as ozone, sulfur dioxide and nitrogen oxides lead to the formation of more toxic chemicals such as diones, nitro- and dinitro-PAHs and sulfonic acids. Due to high global concern, studies are being carried out by researchers to remove PAHs in an eco-friendly and cost-effective manner. Biodegradation of PAHs is a widely used strategy in which diverse types of bacterial, fungal, algal, earthworms, protozoans, plant species and their derived compounds such as biocatalysts, and biosurfactants are being used. Though the microbial degradation of PAHs has been extensively explored, it is a quite progressive area with many research findings being added to the literature. This chapter focuses on a critical overview of current knowledge around the biodegradation of PAHs. It also discusses the recent advancement including ‘omics’ approaches in bioremediation techniques to illuminate fundamental challenges and future prospects.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
9.1 Introduction
Soil contamination through industrial and agricultural activities is the main cause of environmental and health-related issues. Prevalent soil or water contaminants include petroleum hydrocarbons (PHCs), polycyclic aromatic hydrocarbons (PAHs), halogenated hydrocarbons, pesticides, chlorophenols, heavy metals, metals and salt. An increase of various pollutants in the soil or water, contributing to detrimental effect on human and environmental health, is well documented. PAHs are aromatic organic compounds containing two or more fused aromatic rings, specifically benzene rings, in linear, angular or cluster arrangements (Latimer and Zheng 2003; Baklanov et al. 2006). These compounds are ubiquitously distributed in the environment, persistent with various structures; they bear toxic, carcinogenic and mutagenic properties (Arey and Atkinson 2003; Di Toro et al. 2000; Gupta et al. 2015).
9.1.1 Characteristics of PAHs
Polycyclic (or polynuclear) aromatic hydrocarbons (PAHs) fall under the category of persistent organic hydrocarbons that result when two or more aromatic rings fuse together. The benzene ring usually contains only carbon (C) and hydrogen (H) atoms, the latter of which may readily substitute nitrogen (N), sulfur (S) and oxygen (O) atoms to form heterocyclic aromatic compounds. They are mostly colourless to white or pale-yellow solids. The water solubility of PAHs decreases with each additional aromatic ring. PAHs are high melting and boiling solids with low vapour pressure (Arey and Atkinson 2003; Di Toro et al. 2000; Phillips 1999). These properties decrease with increasing molecular weight; as a result, the resistance property towards oxidation and reduction also increases. PAHs are hydrophobic in nature, making them very-low solubility in aqueous medium and high persistence in soil.
9.1.2 Environmental Distribution and Toxicity of PAHs
PAHs are one of a series of the most hazardous pollutants released into the environment primarily during the incomplete combustion and pyrolysis of organic materials throughout natural (biogenic and geochemical) and anthropogenic activities. Natural sources of PAHs generation are forest and rangeland fires, oil seeps, volcanic eruptions and exudates from trees. Most remarkable anthropogenic sources of PAHs are the ones that include burning of fossil fuels, coal tar, wood, garbage, used lubricating oil and oil filters, electric power generation, home heating, municipal solid waste incineration and petroleum spill and discharge (Di Toro et al. 2000; Haritash and Kaushik 2009). Other anthropogenic activities that release PAHs include internal combustion engines (driving), agricultural burning, roofing or working with coal tar products, sound- and waterproofing, coating pipes, steelmaking and paving with asphalt.
Wide distribution of PAHs from anthropogenic and environmental sources results in its bioaccumulation in food chains at various trophic levels. However, their accumulation in foods is substantial, depending on the mode of handling, and is detected in a wide range of vegetables, fruits, meats and fishes (Phillips 1999). PAHs can be easily detected in their forms and their metabolites in blood and urine, mutagenicity in urine and faeces, chromosome aberrations in peripheral blood lymphocytes and DNA, and protein adduct formation in the latter and in other tissues (Baird et al. 2005). Severe hematological disorders have been noticed in animals when they are being exposed to elevated concentration of PAHs. Aplastic anaemia, pancytopenia, decrease in peripheral blood leukocytes and bone marrow depression with almost complete destruction of pluripotent haematopoietic stem cells were observed in non-responsive mice after oral administration of benzo[a]pyrene (BaP), while responsive mice were shown resistance to bone marrow toxicity (Novosad et al. 2002; Page et al. 2004).
PAHs are in a priority pollutant list of the U.S. Environmental Protection Agency (USEPA) and the European Community (EC) (Anyakora et al. 2005). On the basis of abundance and toxicity, USEPA has listed 16 PAH compounds as priority environmental pollutants in water, soil and sediments (Liu et al. 2001).
Several physical, chemical and biological methods are currently available for the remediation of PAHs in water/soils (Abdel-Shafy and Mansour 2016; Kuppusamy et al. 2017). Biological approaches are considered the most efficient and low-cost technique for total removal of PAHs. In biological processes, known collectively as bioremediation, microbes and their combinations are being used for the degradation of hazardous chemicals present in soil, water and/or other contaminated sites (Wang et al. 2014). PAHs-contaminated sites have been remediated in several countries for many years using biological methods. Over the past decade, several reviews and book chapters (Abdel-Shafy and Mansour 2016; Aitken and Long 2004; Haritash and Kaushik 2009; Kuppusamy et al. 2017; Morelli et al. 2013) have focused on potential biological remediation strategies and their distinct metabolic pathways for the degradation of PAHs. This chapter focuses on current knowledge around various technologies such as microbial remediation (bacteria and fungi), phyco (algae) remediation and phyto (plant)-remediation including recent advancements of PAHs bioremediation on bench-, pilot- and field-scale. Further, it also discusses genetic, transcriptomic, proteomic and metabolomic approaches in the field of PAHs bioremediation.
9.2 Biological Remediation Technologies
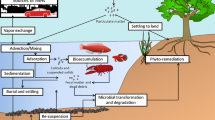
Bioremediation is the emerging application process of biological means (including bacteria, fungi, algae, plants, etc.) for cleaning up contaminated soil, groundwater and wastewater that contains a mixture of contaminant types including salts, organic compounds, radionuclides and heavy metals at widely varying concentrations. It is a cost-effective, alternative pollutant removal method for the degradation or/and transformation (mineralisation) of contaminants without deleterious effects on the environment. Bioremediation technologies hasten biodegradation processes originating principally from biodegradation—a natural process that takes place without human intervention (Congress 1991). In the process of biodegradation, complex organic contaminants may have completely degraded through biotransformation into less complex metabolites and through mineralisation into inorganic materials, water, carbon dioxide (aerobic) or methane (anaerobic) (Das and Chandran 2011; Dean 1999) by the metabolic activity of microbes. Nevertheless, the microbial activity can be affected by many factors such as temperature, oxygen, pH, water, bioavailability of PAHs, salinity, toxicity of endproducts, microbial community and nutrients; these are believed to play a significant role in the performance process (Abdel-Shafy and Mansour 2016; Kuppusamy et al. 2017). There are several biological remediation techniques (bioremediation; bacteria and fungi, phycoremediation; algae, phytoremediation; plants and rhizoremediation; plant and microbe) for the treatment of PAHs-contaminated soil (Fig. 9.1). Based on the selection of the proper remediation approach, these remediation techniques are carried out by two basic types: (i) in-situ (land farming, biostimulation, bioaugmentation, composting and phytoremediation) and (ii) ex-situ (bioreactors) (Kuppusamy et al. 2017).
9.2.1 In-Situ Remediation
This method involves an on-site remediation process where the pollutant is spread vertically and horizontally. In such a method, excavation and transport of the contaminated soil is not required to go to off-site treatment facilities and is therefore considered less expensive. However, the status of weather, soil permeability, contamination depth and potential deep leaching of chemicals, moisture content, nutrient availability, pH and temperature are amongst the critical environmental conditions that must be carefully considered for success with in-situ bioremediation (Gupta et al. 2016). In-situ remediation methods involve cleaning the PAHs contamination site mainly in four ways: (i) land farming (ii) biostimulation, (iii) bioaugmentation and (iv) bioventing. Land farming is a process in which contaminated soils are mixed with fertilizer, then tilled and irrigated to provide aeration, moisture and promote soil homogeneity for biodegradation; and to stimulate indigenous microbes (to enhance natural attenuation process) (Hansen et al. 2004; Juhasz and Naidu 2000; Wang et al. 2016). Biostimulation involves the application of nutrients or substrates to stimulate the growth and metabolic rate of indigenous microorganisms to substantially improve the degradation process (Sayara et al. 2011). Bioaugmentation is the addition of laboratory cultured indigenous or exogenous biodegraders (bacteria, fungi or algae) into contaminated soils to stimulate the degradation of a contaminant in the soil (Sayara et al. 2011; Vidali 2001). Bioventing is a combined process of bioaugmentation and biostimulation. This technique was successfully used to treat organic pollutants such as PAHs in soils and aquifers (Kuppusamy et al. 2016).
9.2.2 Ex-Situ Remediation
These methods involve a transfer of pollutants from contaminated sites to an off-site for the treatment purpose. Although these processes incur extra costs for soil excavation, transport, treatment, disposal and site refilling, these treatments can be precise and controlled, and as a result attaining good outcomes in significantly less time. Bioreactors are some of the examples of ex situ treatment methods (Mohan et al. 2006) used for PAHs remediation.
9.3 Bacteria Degradation of PAHs
Bacteria are known to be involved in the degradation of PAHs in contaminated sites. Many studies on successful applications of different bacterial strains, individually or as consortia, isolated from soil or sediments have been done in the treatment of PAHs-contaminated soils. A group of microbes (consortia) have diverse enzymatic activities, which can degrade more complex organic pollutants than their individual applications. The principal metabolic pathways, those of degradation-related enzymes and catabolic genes of bacteria for the degradation and metabolism of PAH, have been widely studied (Haritash and Kaushik 2009; Nzila 2018; Peng Jing-Jingwang Ningli 2011). In nature, bacteria degrade PAH contaminants by aerobic and anaerobic processes (Qin et al. 2018). Aerobic degradation of PAHs have been extensively studied in various environments (soil, sea, sediments and bioreactor), but the ability of bacteria to degrade PAHs under strictly anaerobic conditions is limited to a few strains (Nzila 2018). Many polluted environments such as aquifers, sediments and submerged soils are often of lead anoxic condition, where anaerobic bacteria can play a key role in PAHs biodegradation (Callaghan 2013; Lu et al. 2011; Nzila 2018).
Several studies have reported using bacteria as pure culture or consortium for the degradation of PAHs. The most commonly reported PAHs-degraded bacterial genera are Arthrobacter, Bacillus, Stenotrophomaonas, Vibrio, Corynebacterium, Flavobacterium, Marinobacter, Micrococcus, Nocardia, Ochrobactrum, Sphingomonas, Burkholderia, Pseudomonas, Mycobacterium and Sphingomonas (Kumari et al. 2018; Singh and Tiwary 2017; Varjani and Upasani 2016).
Tarafdar et al. (2017) reported the degradation of anthracene by Bacillus thuringiensis strain isolated from the fly ash deposition. Eskandari et al. (2017) identified two bacterial species B. licheniformis and B. mojavensis among studied oil-contaminated soil microorganisms that are able to remove a mixture of PAHs. The strain B. licheniformis was capable of destroying cenaphtylene, acenaphtene and indeno pyrene in 72, 96 and 96 h, respectively while the species B. mojavensis was able to destroy naphthalene in 72 h and acenaphtene, acenaphtylene, benzo(ghi)prylene, dibenzo(ah)anthracene and indeno pyrene in 96 h.
Jauhari et al. (2018) studied three bacterial strains, Pseudomonas aeruginosa, Cronobacter sp. and Rhodococcus sp., isolated from petroleum-contaminated soil with very high efficiency (94, 84 and 78%, respectively) for degradation of anthracene (1000 mg/L) over 10 day growth periods. It was confirmed that anthracene gets mineralised by these strains through the O-phthalate pathway with specific activities of catabolic enzymes like C120, C230, 3,4-PCD and 4,5-PCD. However, aromatic rings of anthracene cleaved by Rhodococcus sp. mainly through meta-cleavage pathway were also confirmed. Recently, a novel species of the genus Gordonia, isolated from oilfield-produced water, utilized naphthalene and pyrene as its sole carbon source and was degraded with mixed naphthalene, phenanthrene, anthracene and pyrene (each at a concentration of 500 mg/L) in ratios of 100, 95.4, 73.8% and 53.4%, respectively, over 7 days (Qi et al. 2017).
Kamil and Talib (2016) reported potential of a Corynebacterium urealyticum in degrading artificially contaminated phenanthrene soil at different concentrations. A bacterial community from Actinobacteria, Firmicutes, a- and g-Proteobacteria, and Bacteroidetes isolated from the tidal flats near Sinduri Beach in Taean, Korea, able to degrade PAHs has been demonstrated by Lee et al. (2018). A microbial consortium can improve PAH degradation due to their synergetic coordinated metabolic activities (Tauler et al. 2016). Pugazhendi et al. (2017) studied the application of bacterial consortium for degradation of high molecular weight PAH compounds present in crude oil. Kumari et al. (2018) investigated the ability of a consortium, Stenotrophomonas maltophilia, Ochrobactrum anthropi, Pseudomonas mendocina, Microbacterium esteraromaticum and Pseudomonas aeruginosa for biodegradation of multiple PAHs. It was found that a consortium of these bacteria showed enhanced biodegradation of naphthalene (89.1%), fluorine (63.8%), phenanthrene (81%) and benzo(b)fluoranthene (72.8%) compared to their individual bacterial activity.
Many natural habitats such as aquifers, sediments and submerged soils contaminated with large amounts of PAHs are often lead anoxic where PAHs can degrade by anaerobic bacteria via anaerobic catabolism (Callaghan 2013; Lu et al. 2011). Researchers from a different group have reported the degradation of PAHs without oxygen by addition of alternative final electron acceptors such as nitrate, sulfate or ferric ions (Ambrosoli et al. 2005; Chang et al. 2008; Coates et al. 1996; Liang et al. 2014). Qin et al. (2018) isolated a novel strain Cellulosimicrobium cellulans CWS2 from PAHs-contaminated soil, capable of utilizing BaP (2.5–50 mg/L) as the sole carbon and energy source under nitrate-reducing conditions (1 g/L of NaNO3). The capacity of CWS2 on the removal of BaP was 78.8% in 13 days when the initial BaP concentration was 10 mg/L.
Li et al. (2010) demonstrated the use of NaHCO3 (20 mM) for the anaerobic biodegradation of four mixed PAHs (10 mg/kg of sediment; fluorene, phenanthrene, fluoranthene and pyrene) in mangrove sediment with enriched PAHs-degrading bacterial consortium. PAH degradation was increased by consortium compared to without consortium in medium. However, no effect of NaHCO3 was observed in the biodegradation of PAHs, leading to the conclusion that the presence of other terminal electron acceptors such as nitrate and sulfate or CO2 produced by anaerobes might be utilised by consortium. Marozava et al. (2018) initiated an anaerobic degradation of 1-methylnaphthalene by a member of the Thermoanaerobacteraceae in an iron-reducing enrichment culture. Zafra et al. (2016) investigated the two microbial (bacteria and fungi) mixed consortia with the ability to degrade the phenanthrene (92%), pyrene (64%) and BaP (65%) in the PAHs-polluted soils. Some cyanobacteria can also break down PAHs in water environments. Ibraheem (2010) reported five cyanobacterial species Phormidium, Anabaena, Nostoc, Aphanothece conferta, and Synechocystis aquatilis could enable degradation of phenanthrene by 12% (on day 60), 51% (on day 40), 43% (on day 60), 40% (on day 40) and 46% (on day 40) (Ibraheem 2010).
9.4 Fungal Degradation of PAHs
Fungi are potential candidates for degradation of PAHs due to their certain advantage over bacteria with respect to resistance to different environmental conditions, ability to grow on a variety of media and to produce enzymes for the degrading of organic pollutants (Harms et al. 2011; Messias et al. 2009; Venkatesagowda et al. 2012). Unlike bacteria, few fungi can only utilize PAHs as a sole source of carbon and energy, and co-metabolise them into a wide variety of detoxified-oxidised metabolites (Bamforth and Singleton 2005; Hadibarata et al. 2012; Hadibarata and Kristanti 2012; Wu et al. 2010). Several groups of fungi, namely, Phanerochaete, Pleurotus, Trametes, Bjerkandera, Chrysosporium, Cunninghamella, Coriolopsis, Aspergillus, Penicillium, Trichoderma, Mucor and Cladosporium, were found to be potential degraders of PAHs (Aydin et al. 2017; Gupta et al. 2017; Kadri et al. 2017; Marco-Urrea et al. 2015). However, many fungal species with undefined potential for remediation of PAHs remain in nature, still to be identified. Fungal metabolism of PAHs is usually mediated by ligninolytic and non-ligninolytic enzymes (Gupta et al. 2016; Marco-Urrea et al. 2015). Nevertheless, many fungi can produce both types of enzymes, but one cannot rule out what levels of each enzyme secreted and contributed to the breakdown the PAH (Chigu et al. 2010; Marco-Urrea et al. 2015; Ning et al. 2010; Wu et al. 2010).
9.4.1 Ligninolytic Fungi
Most white-rot fungi are ligninolytic, producing extracellular ligninolytic enzymes such as lignin peroxidase, manganese-dependent peroxidase, phenol oxidase (laccases and tyrosinases) and H2O2-producing enzymes to oxidise lignin present in wood and other organic matter (Mester and Tien 2000). These ligninolytic enzymes have been proven to degrade PAHs (Kadri et al. 2017; Lee et al. 2014). PAH biodegradation by ligninolytic white-rot basidiomycete fungi has been studied mostly in Phanerochaete chrysosporium, Pleurotus ostreatus, T. versicolor and Bjerkandera adusta P. chrysosporium has been shown to degrade the pyrene, anthracene, phenanthrene, benz[a]anthracene and BaP in various levels in soils and solutions (Bishnoi et al. 2008; Haemmerli et al. 1986; Kadri et al. 2017; Wang et al. 2014; Wang et al. 2009). Andersson et al. (2003) assessed the efficacy of Pleurotus ostreatus and Antrodia vaillantii to degrade several PAHs in the artificially contaminated soil. P. ostreatus strains were employed for biodegradation of different PAHs in lab experiments (Dubrovskaya et al. 2017; Pozdnyakova et al. 2018), soil (Márquez-Rocha et al. 2000) and cultivation substrates in a pilot plant (Di Gregorio et al. 2016). Phenanthrene-degrading fungus T. versicolor, isolated in Korea, was able to remove 76% of phenanthrene from the fungal culture (Han et al. 2004). Moreira et al. (2000) reported the ability of three fungi, Bjerkandera adusta, Irpex lacteus and Lentinus tigrinus, to degrade phenanthrene, fluoranthene, pyrene and chrysene in forest and salt marsh soils. In a study by Valentín et al. (2006), biodegradation of four different PAHs by the white-rot fungus Bjerkandera adusta in spiked marsh soil in a bioreactor was around 30 mg PAH/kg soil. Juhasz and Naidu (2000) reported the capability of Bjerkandera sp. in degradation of BaP and benzo(a)anthracene.
Hidayat and Yanto (2018) isolated a new tropical fungus, Trametes hirsuta, shown to degrade 0.8, 0.17 and 0.46% of phenanthrene, chrysene and BaP per mg dry weight of biomass, respectively. Agrawal and Shahi (2017) isolated a white-rot fungus Coriolopsis byrsina from the Surguja district of Chhattisgarh, India, and were able to degrade 96.1% of pyrene in mineral salt broth and 51.85% in soil. Vieira et al. (2018) observed an efficient degradation of pyrene (100%) by marine-derived basidiomycete fungus Marasmiellus sp. under saline conditions. It was reported that Ganoderma lucidum was capable of degrading 99.65% of phenanthrene and 99.58% of pyrene in mineral salt broth (Agrawal et al. 2018). Pozdnyakova et al. (2018) studied the ability of Pleurotus ostreatus and Agaricus bisporus for mineralisation of phenanthrene and anthracene in the presence of laccase and versatile peroxidase enzymes.
9.4.2 Non-ligninolytic Fungi
Non-ligninolytic fungi metabolize PAHs mostly mediated by Phase I and Phase II detoxification metabolism by cytochrome P450 monooxygenase and epoxide hydrolase-catalyzed reactions (Aydin et al. 2017; Kadri et al. 2017; Marco-Urrea et al. 2015). However, extracellular enzymes such as laccase were also produced by some of these fungi (Aydin et al. 2017; Kadri et al. 2017; Marco-Urrea et al. 2015). Non-ligninolytic fungi for PAH degradation include strain(s) of Aspergillus, Cladosporium, Cunninghamella, Fusarium, Mucor, Penicillium, and Trichoderma (Bamforth and Singleton 2005; Marco-Urrea et al. 2015). Biotransformation of fluoranthene by Cunninghamella elegans in a bioreactor under biofilm-based and niche-mimicking conditions was demonstrated (Mitra et al. 2013).
Saraswathy and Hallberg (2005) reported a Penicillium ochrochloron, which was found effective in degrading pyrene. The strain was able to degrade a maximum of 75% of 50 mg/L pyrene over 28 days of incubation. Moreover, Penicillium sp. isolated from Antarctic soil was able to degrade acenaphthene (10.0%) and BaP (2.0%) under low-temperature conditions (at 20 °C) (Govarthanan et al. 2017). Liu et al. (2010) showed that Penicillium sp., Aspergillus niger and white-rot fungus could remove 48, 58 and 16% of BaP, respectively, in a sterile, artificially polluted soil after 35 days. Potin et al. (2004) performed degradation studies in a PAHs-contaminated soil with two inoculation treatments by spore or mycelial inoculum of 21 native filamentous fungi. However, the extent of total PAH degradation occurred with Coniothyrium sp. and Fusarium sp. mycelial inoculum.
Hesham et al. (2017) demonstrated that Fusarium Solani could degrade naphthalene (84.82%), phenanthrene (40.09%), chrysene (57.84%) and BaP (71.06%) at the end of 10 days. Zafra et al. (2015) found that Trichoderma asperellum was able to degrade phenanthrene (74%), pyrene (63%), and BaP (81%) at a concentration of 1000 mg/kg in 14 days. Moreover, Trichoderma genus was found to utilize pyrene, resulting in its degradation, and it was further enhanced using an alternative carbon source such as yeast extract, sucrose or lactose (Mineki et al. 2015). Mao and Guan (2016) reported a fungus Scopulariopsis brevicaulis isolated from an aged PAHs-contaminated soil, having the ability to degrade phenanthrene (60%), fluoranthene (62%), pyrene (64%) and BaP (82%) over 30 days incubation with PZ-4 strain. In microcosm studies for 28 days, 77% of total PAHs was removed from the soil, and the highest removal was observed for phenanthrene (89%) and BaP (75%).
Birolli et al. (2018) demonstrated the degradation of anthracene (14 days, 50 mg/mL initial concentration in malt 2% medium) by five marine-derived fungi, Trichoderma harzianum CBMAI 1677, Cladosporium sp. CBMAI 1237, A. sydowii CBMAI 935, Penicillium citrinum CBMAI 1186 or Mucor racemosus CBMAI 847). Among them, Cladosporium sp. CBMAI 1237 showed the highest degradation of anthracene and 16% more in the presence than in the absence of artificial seawater. Further experiments with different PAHs (50 mg/L) in malt for 21 days resulted in Cladosporium sp. CBMAI 1237 biodegrading anthracene 71%, anthrone 100%, anthraquinone 32%, acenaphthene 78%, fluorene 70%, phenanthrene 47%, fluoranthene 52%, pyrene 62% and nitropyrene 64%. In another study, M. racemosus and T. harzianum strains were reported for having potential biodegradation activity of anthracene (Jové et al. 2016). de Lima Souza et al. (2017) isolated Hypoxylon sp. from sediments contaminated with various levels of PAHs and showed the lowest growth of inhibition rates over the other six species tested for tolerance to phenanthrene and pyrene.
Fungi have been shown to efficiently degrade PAHs by producing ligninolytic enzymes under anaerobic conditions (Aydin et al. 2017). Silva et al. (2009) studied the soil fungi which could remediate PAHs and produce ligninolytic enzymes under microaerobic and very low-oxygen (<1%) conditions. In this study, naphthalene (46.5%), phenanthrene (25.1%), perylene (37%) and decacyclene (37.7%) by Aspergillus sp., chrysene (40.9%) by Trichocladium canadense and naphthol[2,3-a]pyrene (40.8%) by Verticillium sp. were degraded under microaerobic conditions. Under very low oxygen, T. canadense removed 22.1% of naphthalene, 9.8% of chrysene, 18.8% of decacyclene; F. oxysporum removed 13.2% of phenanthrene; Achremonium sp. removed 20.5% of perylene; and Basidiomycete strain H2 and Verticillium sp. removed 12.5% of naphthol[2,3-a]-pyrene. Cobas et al. (2013) studied phenanthrene remediation both in an aqueous medium (90% after 14 d) and in soil (70% after 28 d) using Trichoderma longibrachiatum-mediated permeable reactive biobarriers (PRBBs).
9.5 Microalgae-Mediated Degradation of PAHs
PAHs are subject to removal or degradation by a range of naturally occurring microorganisms, but research has focused on the removal of these contaminants using bacteria and fungi, and microalgae have been explored to a limited extent. However, particular attention should be paid to microalgae as they play an important role in wastewater treatment processes and biodegradation of toxic organic pollutants. Multiple microalgae help in the degradation of PAHs. Hong et al. (2008) assessed the accumulation and biodegradation of phenanthrene and fluoranthene, by the diatoms Skeletonema costatum (Greville) Cleve and Nitzschia sp., enriched from a mangrove aquatic ecosystem. In a another study, the removal and transformation of seven high-molecular weight PAHs, namely, benz[a]anthracene, benzo[b]fluoranthene, benzo[k]fluoranthene, BaP, dibenzo[a,h]anthracene, indeno[1,2,3-c,d]pyrene and benzo[g,h,i]perylene, by a freshwater microalga Selenastrum capricornutum under gold and white light irradiation was studied. A PAH compound-dependent removal efficiency was observed (Luo et al. 2014).
Warshawsky et al. (2007) studied the combination of Mycobacterium sp. and Sphingomonas yanoikuyae and a green alga Selanastrum capricornutum on the degradation of BaP to various intermediates. The rate of degradation was greater in bacterial cultures grown with the algal/BaP extract than those grown with BaP alone. Takáčová et al. (2014) reported the biodegradation efficiency for BaP by Chlorella kessleri. In a separate study, sorption and degradation of BaP was determined by two microalgal species, Selenastrum capricornutum and Scenedesmus acutus (García de Llasera et al. 2016). It was found that that the removal rate of BaP was 99% by S. capricornutum after 15 h exposure, whereas it was 95% by S. acutus after 72 h exposure. Biodegradation efficiency for the anthracene and pyrene was determined by Anabaena fertilissima (Patel et al. 2015). In another study, a microalga Chlorella sp. was capable of degrading 70% of 50 μM pyrene in 7 days incubation (Subashchandrabose et al. 2017). Further, the rate of degradation was nearly 20% increased with use of Tween-80 when compared with Triton X-100.
9.6 Phytoremediation of PAHs
Phytoremediation is the use of a variety of plants to degrade, extract and immobilize contaminants considered capable of eliminating PAHs from the soil. Based on processes and applicability, there are various categories like phytoextraction, phytovolatilisation, phytofiltration, phytostabilisation, phytotransformation and rhizoremediation. Phytotransformation, or phytodegradation, and rhizoremediation are two primary mechanisms involved in the degradation of the organic compounds into simple molecules in the soil or plant tissue. However, some studies reported that the accumulation of PAHs by plants is associated with a phytoextraction process (Alagić et al. 2015; Košnář et al. 2018; Sivaram et al. 2018) . In phytodegradation process, plant roots exude various enzymes such as dehalogenase and oxygenase, which can directly degrade organic contaminants (Dubrovskaya et al. 2017).
Various plant species such as fescue grass (Festuca arundinacea), switch grass (Panicum virgatum), maize (Zea mays L.), soybean (Glycine max L.), Sorghum (Sorghum bicolor L. Moench) and alfalfa (Medicago sativa L.) have the ability to phytodegrade the PAHs. Guo et al. (2017) examined the influence of maize root and soybean root exudates on pyrene degradation. D’Orazio et al. (2013) reported a pot culture study on three plant species for pyrene degradation. The results showed that the amount of pyrene in soils decreased by 32, 30 and 28%, with Medicago sativa, Brassica napus, and Lolium perenne , respectively, and 18% in the control soil without plants. Košnář et al. (2018) showed the removal of 16 individual PAHs using maize from ash-treated soil ranged between 4.8 and 87.8% in a 120-day pot experiment. The phytoremediation potential of Fire Phoenix on the degradation of 8 PAHs was investigated (Liu et al. 2014b). This study demonstrated that plants have a prominent role in enhancing the degradation of 8 PAHs with an increase in planting time. After a 150-day culture growth, the concentrations of the 8 PAHs by Fire Phoenix were decreased to 96.18%. Sivaram et al. (2018) studied bioaccumulation and biodegradation ability of 14 plant species grown in soils spiked with BaP and pyrene in a glasshouse. The rate of PAHs removal efficacy of BaP and pyrene were varied among the plant species over a period of 50 days for BaP (6–26%) and pyrene (14–40%) and the maximum removal of both PAHs was observed in Sudan grass (C4), vetiver (C4), maize (C4) and sunflower (C3).
9.7 Rhizoremediation of PAHs
Rhizoremediation is a combined process of micro- and phytoremediation, considered as a promising technology because it offers ecological and natural aesthetic benefits at low cost. Rhizodegradation can also be referred as plant-based bioremediation technology, rhizosphere bioremediation or microbe-assisted phytoremediation technology (MAPT). Microbe-assisted phytoremediation has been used for removal of PAHs contaminants synergistically. Rhizodegradation of polluted soils mainly relies on stimulating the population of degrading microorganisms through plant rhizospheric effects (Merkl et al. 2004; Newman and Reynolds 2004; Truu et al. 2015). Many studies have attempted rhizoremediation of PAHs-polluted soils by the combination of plants with bacteria, or/and fungi-assisted bioremediation; this combination has shown to enhance the rate of rhizosphere degradation of PAHs. Guo et al. (2017) investigated rhizoremediation of 12 PAHs in rhizosphere and non-rhizosphere soils using Mycobacterium sp. and ryegrass (Lolium multiflorum L.) under glasshouse conditions. After 60 days, the removal percentages of PAHs in the bacteria added rhizospheric soil were higher (5-ring PAHs, 25.6%; 6-ring PAHs, 27.6%; and 3- and 4-ring PAHs only 2.2–9.7%) compared to the non-inoculated soil.
Liu et al. (2014a) investigated a phenanthrene-degrading endophytic bacterium, Massilia sp., for their ability to promote plant growth and phenanthrene uptake as well as community structure of root endophytic bacteria in wheat. They indicated that Massilia sp. is able to reduce the phenanthrene levels in wheat, enhance biomass and change the bacterial community structure in phenanthrene-contaminated wheat in a contamination level-dependent manner. A study performed in the combination of Sorghum plant and Pseudomonas aeruginosa to improve phytoremediation of soil contaminated with pyrene showed that inoculated bacterium play a key role in pyrene removal (66–82% for pyrene concentrations of 150 and 300 mg/kg, respectively) from contaminated soil. The results of the current study also showed that P. aeruginosa inoculation increased pyrene removal rate and number of bacteria in the soil (Rostami et al. 2017).
Biotransformation and conjugation of phenanthrene in pak choi at the subcellular level by endophytic bacteria Pseudomonas sp. was investigated (Sun et al. 2018). Inoculation of Pseudomonas sp. reduced the subcellular distribution of phenanthrene to plant subcellular fractions (i.e., cell wall, cell membrane, cell solution and cell organelles). Eskandary et al. (2017) demonstrated the effective use of B. licheniformis and B. mojavensis isolated from oil-contaminated soils to ameliorate 10 PAHs in oil-contaminated soil grown with Festuca arundinacea under controlled greenhouse conditions. They stated that the concentration of some PAHs in rhizosphere soil samples inoculated with both bacteria was decreased, and even some PAHs (e.g., naphthalene, phenanthrene, benzo[a]anthracene and dibenzo[a,h]anthracene) reached below the detection limit of the method.
Košnář et al. (2018) observed a synergistic association of maize and ligninolytic fungus P. ostreatus in soil contaminated with 16 PAHs and wood chip of waste apple tree trunks as a lignocellulosic substrate for fungus. It was found that fungus in the treated soil improved fungal biomass, enzyme and microbial activities, and it removed total PAHs by 36.2% after the 120-day period.
Mycorrhizal fungi have also been known to promote plant growth and PAHs biodegradation and rhizoremediation (Gao et al. 2017; Ren et al. 2017). Ren et al. (2017) conducted a greenhouse experiment to evaluate the potential interaction between legume (Sesbania cannabina), rhizobia (Ensifer sp.) and AM fungus (Glomus mosseae) on PAHs dissipation in spiked soil. The triple symbiosis showed more than 97 and 85% degradation of phenanthrene and pyrene, respectively, in soil, whereas it showed 81 and 72% degradation in only plant-treated soil. In treatments where Glomus etunicatum or Glomus lamellosum inoculation were combined with alfalfa (Medicago sativa L.), the loss of pyrene was increased with 38.3–68.9% and 39.4–71.3% in soils, respectively (Gao et al. 2017).
9.8 Recent Advancements in PAHs Degradation
Recently, novel approaches such as combined organic composting, enzyme-mediated bioremediation, biosurfactant-enhanced bioremediation, and microbial fuel cells for the process of PAHs biodegradation have received particular attention.
9.8.1 Vermi- and Protozoan Degradation of PAHs
Many bioremediation studies have reported on bacteria, cyanobacteria, fungi and algae. However, only scant information is available on earthworm and protozoan, which are part of the aquatic and terrestrial community involved in PAHs degradation. Earthworms play a significant role in removal of PAHs from soil. They absorb PAHs from soil to biotransform or biodegrade them, rendering them harmless. Earthworms can accumulate PAHs, mainly the 3- or 4-ringed PAH compounds (Achten and Andersson 2015). In another study, Sinha et al. (2008) confirmed PAH removal from soil with 11,820 mg/kg PAH concentration by a consortium of earthworms (E. fetida, E. euginae and Perionyx excavates). Schmidt et al. (2017) demonstrated that E. fetida was able to transform pyrene and phenanthrene to conjugated phase II metabolites in hydroponic culture. The results of Deng and Zeng (2017) demonstrated that the inoculation of alfalfa with earthworms and/or white-rot fungus prompted the growth of alfalfa and removed the phenanthrene in the soil, and the removal rate in soil was highest (93%) under alfalfa + earthworms + white-rot fungus treatment.
According to Kachieng’a and Momba (2018), protozoa are considered more active agents in PAHs degradation and could be potential candidates for remediation of PAHs-contaminated soil and water. They reported a consortium of symbiotic protozoan isolates (Paramecium sp., Vorticella sp., Epistylis sp. and Opercularia sp.) able to degrade approximately 70%, while individual isolates could degrade 65% at the end of the study.
9.8.2 Enzymes in PAHs Degradation
Enzymes are biocatalytic macromolecules that facilitate the conversion of substrates into products by lowering the activation energy of molecules. In the last few years, degradation of contaminants with enzymes separated from their cells has been used for PAHs bioremediation. At present, fungal enzymes are studied most for remediation of organic-contaminated soils. Wu et al. (2008) studied the direct application of free laccase enzyme for the transformation of 15 priority PAHs in soil. Similarly, Zhang et al. (2016) studied degradation of PAHs using purified manganese peroxidase form Trametes sp. and found it very efficient at degrading both individual PAHs (fluorene, fluoranthene, pyrene, phenanthrene and anthracene) and PAHs in mixtures. Although enzymatic remediation is an alternative to conventional bioremediation, the cost of purification and enzymes is the major constraints of this method. Immobilization of enzymes with various carrier materials allows an alternative technology that enables an increase in stability and repeat utilization and it reduces degradation. Wang et al. (2018) demonstrated that immobilised laccase could increase degradation of pyrene (Pyr) and BaP around 10–30% compared with free laccase. In the past few years, researchers have paid more attention to molecular docking in environmental remediation to find the suitable orientation of molecules in the enzyme active sites to predict binding affinity.
9.8.3 Combined Organic Addition for PAHs Degradation
Traditional bioremediation techniques combined with organic additives such as biochar, compost, sludge residue and poultry manure incorporation positively enhance overall PAHs remediation. Lignocellulosic materials such as wheat straw, corncobs and straw pellets as carriers have been shown to improve the growth capacity and soil PAH degradation performance of fungi (Covino et al. 2010). Fernández-Luqueño et al. (2016) observed a rapid biodegradation rate of phenanthrene and anthracene in the soil amended with wastewater sludge. Kong et al. (2018) observed a significant reduction of PAH content when wheat straw biochars was applied to petroleum-polluted soil. Additionally, adding biochar to soils increased specific taxa, including PAH degraders. Han et al. (2017) reported that biomass wastes from wheat stalk, mushroom compost and cow manure accelerated the degradation of aged PAHs and significantly increased abundances of the bacterial community. Košnář et al. 2018 studied the combination of biostimulation and bioaugmentation showed superior removal efficiencies of phenanthrene, fluoranthene and pyrene. Phytoremediation of PAHs derived from biomass fly ash-soil using maize (Zea mays L.) amended with compost or vermicompost was studied. The results of this study showed that compost and vermicompost applied in planted ash-soil efficiently removed PAHs in a range between 62.9 and 64.9%, respectively.
9.8.4 Biosurfactant-Enhanced Degradation of PAHs
PAHs bioavailability in soil is usually low and can be overcome with the application of biosurfactants to enhance PAHs bioavailability for microbial degradation in soil/aqueous phase. Biosurfactants are amphiphilic secondary compounds (both hydrophobic and hydrophilic) derived from microorganisms and plants. Compared to chemical surfactants, biosurfactants are readily biodegradable and perform with excellence in the remediation process (Lamichhane et al. 2017; Liang et al. 2017). In general, two different types of biosurfactants (microbial-based and plant-based) have been widely used in surfactant-enhanced bioremediation (SEBR) and surfactant-enhanced phytoremediation (SEPR). A number of researchers have studied the remediation of PAHs-contaminated soil by various biosurfactants (Lamichhane et al. 2017; Liang et al. 2017).
The use of rhamnolipid biosurfactant at low concentration (25 mg/L) increased (96.2%) the degradation of fluorene (280 mg/L) by Paenibacillus sp., but increased it only 90.6% with Tween-40 (3% v/v) and 96.5% with Tween-60 (3.5% v/v) ((Reddy et al. 2018). (Bezza and Chirwa 2017), recognised the degradation enhancement potential of pyrene by lipopeptide biosurfactant produced by Paenibacillus dendritiformis and found that lipopeptide at 600 and 300 mg/L enhanced pyrene degradation to 67.5 and 51%, respectively, compared to its absence.
PAHs biodegradation was 86.5% in the presence of biosurfactant, which was dramatically higher.
Moreover, the addition of 0.2 and 0.6% biosurfactant enhanced the removal of 13 PAHs by 34.2 and 63%, respectively, from only 6% without surfactant (Bezza and Chirwa 2017). Liao et al. (2015) investigated the biosurfactant-amended phytoremediation of phenanthrene and pyrene by maize plant. This study suggest that the use of rhamnolipid and saponin could increase desorption of phenanthrene (10 and 29%) and pyrene (9 and 28%, respectively) in soil.
9.8.5 Microbial Fuel Cells (MFC) in PAHs Degradation
A microbial fuel cell is a bio-electrochemical device that generates power through the oxidation of organic and inorganic matter by microbes. This technique is increasingly considered for the remediation of (in)organic-contaminated soils including PAHs. However, very few studies have reported the ability of MFCs in remediation of PAHs (Gambino et al. 2017; Hamdan et al. 2017). Sherafatmand and Ng (2015) demonstrated a sediment microbial fuel cell (SMFC) for the degradation of PAHs under aerobic or anaerobic environment. A significant removal of naphthalene (41.7 and 76.9%), acenaphthene (31.4 and 52.5%) and phenanthrene (36.2 and 36.8%) in the aerobic and anaerobic environment, respectively, was observed. Gambino et al. (2017) investigated consortia of Bacillaceae, Enterobacteriaceae, Staphilococcaceae, Xanthomonadaceae and Pseudomoniadaceae to study the degradation ability of PAHs in MFCs. The results showed that anode enrichment with microelectrogenic bacteria decreased overall PAH concentration to 90%. However, this technique may not be suited for the large scale and the suitability of this technique for the degradation of PAHs will need to be investigated further.
9.8.6 Omics Approaches in Degradation of PAHs
Omics (metagenomics, transcriptomics, proteomic and metabolomic) approaches to understanding the role of rhizosphere microbiome and plants for the development of genetically modified microorganisms (GMOs) and plants play a significant role in the enhancement of bioremediation/transformation of PAHs (Aydin et al. 2017; Kotoky et al. 2018). These techniques are used to investigate total genome content (also known as metagenomics), catabolic gene expression (mRNA collection; transcriptomics), protein profile (proteomics) and key metabolites (metabolomics) from the microbiome including uncultured microbes during the bioremediation practice. The potential of “omics” approaches suggests the use of engineered rhizospheric microbiome and plants for decreasing the toxicity of PAHs (El Amrani et al. 2015). However, an insufficient amount of studies have focused on omics approaches for enhancement of bioremediation/transformation of PAHs, and so more practice performance is still needed.
Metagenomic approaches provide total genetic content of a microbial community for identification of different groups of microbes and their functional genes involved in a particular habitat. Metagenomics analysis of soils contaminated with anthracene increased the percentages of sequences belonging to the Actinobacteria and reduced the percentage of Proteobacteria while increasing percentages of sequences belonging to Proteobacteria in unamended soil after 14 days of study (Castro-Silva et al. 2013). Zafra et al. (2016) reported metagenomics sequence analysis of a fungal-bacterial consortium on degradation of PAH in soil. The results from this analysis demonstrated that the inoculated consortia could change the native microbial diversity of soil and enhance the degradation rate of PAHs in soil presumably due to co-metabolic degradation. Thus, metagenomics analysis enabled researchers to exploit knowledge about uncultivable microorganisms and their various probable metabolic pathways for the degradation of PAHs in polluted soils.
Transcriptomics or metatranscriptomics provides examination of mRNA expression of a single microbe or microbiome with a change in environmental situations (Hautefort and Hinton 2000). de Menezes et al. (2012) studied the microbial expression analysis of dioxygenase genes in soil stressed with phenanthrene and reported that a higher abundance of transcripts in the soil was due to stress response and detoxification activity of soil microbial communities. Similarly, rhizosphere soil of willows significantly enriched in transcripts was related to PAH degradation (Yergeau et al. 2018). Pagé et al. (2015) revealed that Salix purpurea growing in PAHs-contaminated soil stimulated the expression of 4 oxygenase genes out of the 10 studied within the bacterial orders Actinomycetales, Rhodospirillales, Burkholderiales, Alteromonadales, Solirubrobacterales, Caulobacterales and Rhizobiales. Herbst et al. (2013) confirmed that Burkholderiales, Actinomycetales and Rhizobiales were the most abundant microbes in the communities of groundwater amended with either C13-naphthalene or C13-fluorene by metaproteomic analysis and protein-stable-isotope probing (SIP).
Proteomics analysis deals with information about proteins and their functions in microbial communities involved in the bioremediation of pollutants in a contaminated environment. The proteogenomics approach was applied to Mycobacterium vanbaalenii for investigation of aromatic hydrocarbon catabolic pathways in presence of high molecular-PAHs (Kim et al. 2009). The various expressed proteins were identified as catalase-peroxidase, putative monooxygenases, dioxygenases, naphthalene-inducible dioxygenases and aldehyde dehydrogenase. Verdin et al. (2005) reported the overexpression of cytochrome P450 monooxygenase enzyme in Fusarium solani fungus under the presence of benzopyrene.
Metabolomics provides information of degradation products and primary metabolites in response to pollutant exposures. Transcriptomic and proteomic studies can be helpful in unweaving important information about the different metabolic pathways. Keum et al. (2008) studied metabolic intermediates during degradation of phenanthrene by Sinorhizobium sp. Metabolic pathways such as catechol, gentisic acid and protocatechuic acid pathway of phenanthrene degradation and expression of catabolic genes involved in these pathways by halophilic consortium were studied under different salinities (Wang et al. 2018).
GMOs are any organisms whose genetic material has been altered to enhance the catabolic efficiency associated with pollutant-degrading pathways. Such strategies are now common to accelerate the rate of biological degradation of PAHs using genetically modified bacteria and plants. Cytochrome P450 monooxygenases (CYPs) for the degradation of naphthalene, fluorine, acenaphthalene and acenaphthylene were altered at the different active sites within the enzyme which enhanced oxidation potential of the enzyme in Pseudomonas putida and B. megaterium (Carmichael and Wong 2001; Harford-Cross et al. 2000). Peng et al. (2014) generated transgenic plants with enhanced tolerance to and uptake of phenanthrene by transferring the complex dioxygenase system of Pseudomonas into Arabidopsis and rice.
9.9 Summary and Future Outlook
Natural and anthropogenic activities contribute to generate PAHs, which are becoming a great concern due to their persistence in living beings and the environment. Several strategies have been employed for effective bioremediation of PAHs over the past century. Bioremediation technologies discussed in this chapter have been recognised as suitable technologies that contribute to PAH remediation. However, current PAHs biodegradation rates are low due to several environmental, biological and physico-chemical factors (Kuppusamy et al. 2017). Despite having multiple bioremediation technologies for PAHs removal, no single remediation technology can be the ultimate solution for different types of PAHs (Mohan et al. 2006). Thus, depending on the severity of contaminant and remedial objectives, a proper remediation approach has to be carefully considered. In the past few decades, a plethora of microbes have been screened and characterised with PAHs-degrading capabilities. However, microbial interactions, measurement and control of biochemical pathways within different PAHs-degrading microbial consortium (bacteria, bacteria and fungi, bacteria and algae or cyanobacteria and algae) have yet to be explored. This is the case because different types of consortium are shown to be much more beneficial in the remediation of PAHs than their single or individual consortium (García de Llasera et al. 2016).
It has been observed that in contaminated soils and sediments, bioavailability of PAHs is one of the most limiting factors, strongly affecting the feasibility of remediation; but this can be overcome moderately by the use of biosurfactants. Some biosurfactant producer microbes can also be used to enhance PAHs bioavailability for microbial degradation; however, application for field-level remediation is quite expensive. Thus, cost-effective production of biosurfactant is required for its broader application in the field (Bezza and Chirwa 2017). In addition, many unique enzymes associated with PAHs-degradation have been isolated and purified, and their role in PAHs-degradation has been characterised. Nevertheless, larger production and wide application in enzymatic bioremediation is a time-consuming process and has financial constraints; this problem can be overcome by the use of omics approaches. The use of organic additives is promising for an effective PAHs-degradation strategy. However, their characterisation, quantification and interactions must be considered because complex composition of natural amendments may affect the metabolism of soil microbiota as well as of the plants involved in phytoremediation.
An effective bioremediation strategy requires selection of efficient microbes, algae, plants or combinations thereof. So far, undefined pathways of many potential microbial species are related to PAHs-remediation, so there is a need to use an omics approach to study the complex behaviour of novel species and their degradation pathways. Advances in omics technologies have provided an opportunity to develop genetically modified organisms and plants to boost bioremediation of PAHs. Researchers, to some extent, are convinced of the safe use of GMOs as a potential alternative for biodegradation of PAHs at reasonably low cost. Some non-technical factors such as environmental laws and mandates, aside from technical constraints, limit the use of GMOs. Therefore, it can be concluded from the present review that the application of coupled green degradation methods such as various types of microbial consortium, rhizoremediation, combined organic addition, and microbes with biosurfactants may be enough to manage the cleanup of the PAHs-contaminated sites.
References
Abdel-Shafy, H. I., & Mansour, M. S. M. (2016). A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egyptian Journal of Petroleum, 25, 107–123.
Achten, C., & Andersson, J. T. (2015). Overview of Polycyclic Aromatic Compounds (PAC). Polycyclic Aromatic Compounds, 35, 177–186.
Agrawal, N., & Shahi, S. K. (2017). Degradation of polycyclic aromatic hydrocarbon (pyrene) using novel fungal strain Coriolopsis byrsina strain APC5. International Biodeterioration and Biodegradation, 122, 69–81.
Agrawal, N., Verma, P., & Shahi, S. K. (2018). Degradation of polycyclic aromatic hydrocarbons (phenanthrene and pyrene) by the ligninolytic fungi Ganoderma lucidum isolated from the hardwood stump. Bioresources and Bioprocessing, 5, 11.
Aitken, M. D., & Long, T. C. (2004). Biotransformation, biodegradation, and bioremediation of polycyclic aromatic hydrocarbons. In A. Singh & O. P. Ward (Eds.), Biodegradation and bioremediation (pp. 83–124). Berlin/Heidelberg: Springer.
Alagić, S. Č., Maluckov, B. S., & Radojičić, V. B. (2015). How can plants manage polycyclic aromatic hydrocarbons? May these effects represent a useful tool for an effective soil remediation? A review. Clean Technologies and Environmental Policy, 17, 597–614.
Ambrosoli, R., Petruzzelli, L., Luis Minati, J., & Ajmone Marsan, F. (2005). Anaerobic PAH degradation in soil by a mixed bacterial consortium under denitrifying conditions. Chemosphere, 60, 1231–1236.
Andersson, B. E., Lundstedt, S., Tornberg, K., Schnürer, Y., Oberg, L. G., & Mattiasson, B. (2003). Incomplete degradation of polycyclic aromatic hydrocarbons in soil inoculated with wood-rotting fungi and their effect on the indigenous soil bacteria. Environmental Toxicology and Chemistry, 22, 1238–1243.
Anyakora, C., Ogbeche, A., Palmer, P., Coker, H., Ukpo, G., & Ogah, C. (2005). GC/MS analysis of polynuclear aromatic hydrocarbons in sediment samples from the Niger Delta region. Chemosphere, 60, 990–997.
Arey, J., & Atkinson, R. (2003). Photochemical reactions of PAHs in the atmosphere. In P. E. T. Douben (Ed.), PAHs: An ecotoxicological perspective (pp. 47–63). Chichester: Wiley.
Aydin, S., Karaçay, H. A., Shahi, A., Gökçe, S., Ince, B., & Ince, O. (2017). Aerobic and anaerobic fungal metabolism and Omics insights for increasing polycyclic aromatic hydrocarbons biodegradation. Fungal Biology Reviews, 31, 61–72.
Baird, W. M., Hooven, L. A., & Mahadevan, B. (2005). Carcinogenic polycyclic aromatic hydrocarbon-DNA adducts and mechanism of action. Environmental and Molecular Mutagenesis, 45, 106–114.
Baklanov, A., Hänninen, O., Slørdal, L. H., Kukkonen, J., Bjergene, N., Fay, B., Finardi, S., Hoe, S. C., Jantunen, M., Karppinen, A., Rasmussen, A., Skouloudis, A., Sokhi, R. S., & Sørensen, J. H. (2006). Integrated systems for forecasting urban meteorology, air pollution and population exposure. Atmospheric Chemistry and Physics Discussions, 6, 1867–1913.
Bamforth, S. M., & Singleton, I. (2005). Bioremediation of polycyclic aromatic hydrocarbons: Current knowledge and future directions. Journal of Chemical Technology and Biotechnology, 80, 723–736.
Bezza, F. A., & Chirwa, E. M. N. (2017). Pyrene biodegradation enhancement potential of lipopeptide biosurfactant produced by Paenibacillus dendritiformis CN5 strain. Journal of Hazardous Materials, 321, 218–227.
Birolli, W. G., de A Santos, D., Alvarenga, N., Garcia, A. C. F. S., Romão, L. P. C., & Porto, A. L. M. (2018). Biodegradation of anthracene and several PAHs by the marine-derived fungus Cladosporium sp. CBMAI 1237. Marine Pollution Bulletin, 129, 525–533.
Bishnoi, K., Kumar, R., & Bishnoi, N. R. (2008). Biodegradation of polycyclic aromatic hydrocarbons by white rot fungi Phanerochaete chrysosporium in sterile and unsterile soil.
Callaghan, A. V. (2013). Metabolomic investigations of anaerobic hydrocarbon-impacted environments. Current Opinion in Biotechnology, 24, 506–515.
Carmichael, A. B., & Wong, L. L. (2001). Protein engineering of Bacillus megaterium CYP102. The oxidation of polycyclic aromatic hydrocarbons. European Journal of Biochemistry, 268, 3117–3125.
Castro-Silva, C., Ruíz-Valdiviezo, V. M., Valenzuela-Encinas, C., Alcántara-Hernández, R., Navarro-Noya, Y., Vázquez-Núñez, E., Luna-Guido, M., Marsch, R., & Dendooven, L. (2013). The bacterial community structure in an alkaline saline soil spiked with anthracene. Electronic Journal of Biotechnology, 16. https://doi.org/10.2225/vol16-issue5-fulltext-14.
Chang, B.-V., Chang, I. T., & Yuan, S. Y. (2008). Anaerobic degradation of phenanthrene and pyrene in mangrove sediment. Bulletin of Environmental Contamination and Toxicology, 80, 145–149.
Chigu, N. L., Hirosue, S., Nakamura, C., Teramoto, H., Ichinose, H., & Wariishi, H. (2010). Cytochrome P450 monooxygenases involved in anthracene metabolism by the white-rot basidiomycete Phanerochaete chrysosporium. Applied Microbiology and Biotechnology, 87, 1907–1916.
Coates, J. D., Anderson, R. T., Woodward, J. C., Phillips, E. J. P., & Lovley, D. R. (1996). Anaerobic hydrocarbon degradation in petroleum-contaminated harbor sediments under sulfate-reducing and artificially imposed iron-reducing conditions. Environmental Science & Technology, 30, 2784–2789.
Cobas, M., Ferreira, L., Tavares, T., Sanromán, M. A., & Pazos, M. (2013). Development of permeable reactive biobarrier for the removal of PAHs by Trichoderma longibrachiatum. Chemosphere, 91, 711–716.
Congress, U. S. (1991). Office of technology assessment, bioremediation for marine oil spills-background paper. Washington, DC: Government Printing Office OTA-BP-O-70.
Covino, S., Svobodová, K., Cvancarová, M., D’Annibale, A., Petruccioli, M., Federici, F., Kresinová, Z., Galli, E., & Cajthaml, T. (2010). Inoculum carrier and contaminant bioavailability affect fungal degradation performances of PAH-contaminated solid matrices from a wood preservation plant. Chemosphere, 79, 855–864.
D’Orazio, V., Ghanem, A., & Senesi, N. (2013). Phytoremediation of pyrene contaminated soils by different plant species. Clean Soil Air Water, 41, 377–382.
Das, N., & Chandran, P. (2011). Microbial degradation of petroleum hydrocarbon contaminants: An overview. Biotechnology Research International, 2011, 941810.
de Lima Souza, H. M., Barreto, L. R., da Mota, A. J., de Oliveira, L. A., dos Santos Barroso, H., & Zanotto, S. P. (2017). Tolerance to polycyclic aromatic hydrocarbons (PAHs) by filamentous fungi isolated from contaminated sediment in the Amazon region. Acta Scientiarum Biological Sciences, 39, 481–488.
de Menezes, A., Clipson, N., & Doyle, E. (2012). Comparative metatranscriptomics reveals widespread community responses during phenanthrene degradation in soil. Environmental Microbiology, 14, 2577–2588.
Dean, R. B. (1999). Book review: Biodegradation and Bioremediation (2nd ed., Martin Alexander, 470 pp. $59.95). San Diego: Academic Press. Waste Management & Research 17, 390–391.
Deng, S., & Zeng, D. (2017). Removal of phenanthrene in contaminated soil by combination of alfalfa, white-rot fungus, and earthworms. Environmental Science and Pollution Research International, 24, 7565–7571.
Di Gregorio, S., Becarelli, S., Siracusa, G., Ruffini Castiglione, M., Petroni, G., Masini, G., Gentini, A., de Lima e Silva, M. R., & Lorenzi, R. (2016). Pleurotus ostreatus spent mushroom substrate for the degradation of polycyclic aromatic hydrocarbons: The case study of a pilot dynamic biopile for the decontamination of a historically contaminated soil. Journal of Chemical Technology and Biotechnology, 91, 1654–1664.
Di Toro, D. M., McGrath, J. A., & Hansen, D. J. (2000). Technical basis for narcotic chemicals and polycyclic aromatic hydrocarbon criteria. I. Water and tissue. Environmental Toxicology and Chemistry, 19, 1951.
Dubrovskaya, E., Pozdnyakova, N., Golubev, S., Muratova, A., Grinev, V., Bondarenkova, A., & Turkovskaya, O. (2017). Peroxidases from root exudates of Medicago sativa and Sorghum bicolor: Catalytic properties and involvement in PAH degradation. Chemosphere, 169, 224–232.
El Amrani, A., Dumas, A.-S., Wick, L. Y., Yergeau, E., & Berthomé, R. (2015). “Omics” insights into PAH degradation toward improved green remediation biotechnologies. Environmental Science & Technology, 49, 11281–11291.
Eskandari, S., Hoodaji, M., Tahmourespour, A., Abdollahi, A., Baghi, T. M., Eslamian, S., & Ostad-Ali-Askari, K. (2017). Bioremediation of polycyclic aromatic hydrocarbons by Bacillus Licheniformis ATHE9 and Bacillus Mojavensis ATHE13 as newly strains isolated from oil-contaminated soil. Journal of Geography Environment and Earth Science International, 11, 1–11.
Eskandary, S., Tahmourespour, A., Hoodaji, M., & Abdollahi, A. (2017). The synergistic use of plant and isolated bacteria to clean up polycyclic aromatic hydrocarbons from contaminated soil. Journal of Environmental Health Science and Engineering, 15, 12.
Fernández-Luqueño, F., López-Valdez, F., Dendooven, L., Luna-Suárez, S., & Ceballos-Ramírez, J. M. (2016). Why wastewater sludge stimulates and accelerates removal of PAHs in polluted soils? Applied Soil Ecology, 101, 1–4.
Gambino, E., Toscanesi, M., Del Prete, F., Flagiello, F., Falcucci, G., Minutillo, M., Trifuoggi, M., Guida, M., Nastro, R. A., & Jannelli, E. (2017). Polycyclic Aromatic Hydrocarbons (PAHs) degradation and detoxification of water environment in single-chamber air-cathode Microbial Fuel Cells (MFCs). Fuel Cells, 17, 618–626.
Gao, Y., Zong, J., Que, H., Zhou, Z., Xiao, M., & Chen, S. (2017). Inoculation with arbuscular mycorrhizal fungi increases glomalin-related soil protein content and PAH removal in soils planted with Medicago sativa L. Soil Biology and Biochemistry, 115, 148–151.
García de Llasera, M. P., Olmos-Espejel, J. d. J., Díaz-Flores, G., & Montaño-Montiel, A. (2016). Biodegradation of benzo(a)pyrene by two freshwater microalgae Selenastrum capricornutum and Scenedesmus acutus: A comparative study useful for bioremediation. Environmental Science and Pollution Research International, 23, 3365–3375.
Govarthanan, M., Fuzisawa, S., Hosogai, T., & Chang, Y.-C. (2017). Biodegradation of aliphatic and aromatic hydrocarbons using the filamentous fungus Penicillium sp. CHY-2 and characterization of its manganese peroxidase activity. RSC Advances, 7, 20716–20723.
Guo, M., Gong, Z., Miao, R., Su, D., Li, X., Jia, C., & Zhuang, J. (2017). The influence of root exudates of maize and soybean on polycyclic aromatic hydrocarbons degradation and soil bacterial community structure. Ecological Engineering, 99, 22–30.
Gupta, S., Pathak, B., & Fulekar, M. H. (2015). Molecular approaches for biodegradation of polycyclic aromatic hydrocarbon compounds: A review. Reviews in Environmental Science and Technology, 14, 241–269.
Gupta, G., Kumar, V., & Pal, A. K. (2016). Biodegradation of polycyclic aromatic hydrocarbons by microbial consortium: A distinctive approach for decontamination of soil. Soil and Sediment Contamination: An International Journal, 25, 597–623.
Gupta, G., Kumar, V., & Pal, A. K. (2017). Microbial degradation of high molecular weight polycyclic aromatic hydrocarbons with emphasis on pyrene. Polycyclic Aromatic Compounds, 1–13.
Hadibarata, T., & Kristanti, R. A. (2012). Fate and cometabolic degradation of benzo[a]pyrene by white-rot fungus Armillaria sp. F022. Bioresource Technology, 107, 314–318.
Hadibarata, T., Khudhair, A. B., & Salim, M. R. (2012). Breakdown products in the metabolic pathway of anthracene degradation by a Ligninolytic fungus Polyporus sp. S133. Water, Air, and Soil Pollution Focus, 223, 2201–2208.
Haemmerli, S. D., Leisola, M. S., Sanglard, D., & Fiechter, A. (1986). Oxidation of benzo(a)pyrene by extracellular ligninases of Phanerochaete chrysosporium. Veratryl alcohol and stability of ligninase. The Journal of Biological Chemistry, 261, 6900–6903.
Hamdan, H. Z., Salam, D. A., Hari, A. R., Semerjian, L., & Saikaly, P. (2017). Assessment of the performance of SMFCs in the bioremediation of PAHs in contaminated marine sediments under different redox conditions and analysis of the associated microbial communities. Science of the Total Environment, 575, 1453–1461.
Han, M.-J., Choi, H.-T., & Song, H.-G. (2004). Degradation of phenanthrene by Trametes versicolor and its laccase. Journal of Microbiology, 42, 94–98.
Han, X., Hu, H., Shi, X., Zhang, L., & He, J. (2017). Effects of different agricultural wastes on the dissipation of PAHs and the PAH-degrading genes in a PAH-contaminated soil. Chemosphere, 172, 286–293.
Hansen, L. D., Nestler, C., Ringelberg, D., & Bajpai, R. (2004). Extended bioremediation of PAH/PCP contaminated soils from the POPILE wood treatment facility. Chemosphere, 54, 1481–1493.
Harford-Cross, C. F., Carmichael, A. B., Allan, F. K., England, P. A., Rouch, D. A., & Wong, L.-L. (2000). Protein engineering of cytochrome P450cam (CYP101) for the oxidation of polycyclic aromatic hydrocarbons. Protein Engineering, Design & Selection, 13, 121–128.
Haritash, A. K., & Kaushik, C. P. (2009). Biodegradation aspects of polycyclic aromatic hydrocarbons (PAHs): A review. Journal of Hazardous Materials, 169, 1–15.
Harms, H., Schlosser, D., & Wick, L. Y. (2011). Untapped potential: Exploiting fungi in bioremediation of hazardous chemicals. Nature Reviews Microbiology, 9, 177–192.
Hautefort, I., & Hinton, J. C. (2000). Measurement of bacterial gene expression in vivo. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 355, 601–611.
Herbst, F.-A., Bahr, A., Duarte, M., Pieper, D. H., Richnow, H. -H., von Bergen, M., Seifert, J., & Bombach, P. (2013). Elucidation of in situ polycyclic aromatic hydrocarbon degradation by functional metaproteomics (protein-SIP). Proteomics, 10. https://doi.org/10.1002/pmic.201200569.
Hesham, A. E.-L., Mohamed, E. A., Mawad, A. M. M., Elfarash, A., Abd El-Fattah, B. S., & El-Rawy, M. A. (2017). Molecular characterization of degrades a mixture of low and high molecular weight polycyclic aromatic hydrocarbons. Open Biotechnology Journal, 11, 27–35.
Hidayat, A., & Yanto, D. H. Y. (2018). Biodegradation and metabolic pathway of phenanthrene by a new tropical fungus, Trametes hirsuta D7. Journal of Environmental Chemical Engineering, 6, 2454–2460.
Hong, Y.-W., Yuan, D.-X., Lin, Q.-M., & Yang, T.-L. (2008). Accumulation and biodegradation of phenanthrene and fluoranthene by the algae enriched from a mangrove aquatic ecosystem. Marine Pollution Bulletin, 56, 1400–1405.
Ibraheem, I. B. M. (2010). Biodegradability of hydrocarbons by Cyanobacteria1. Journal of Phycology, 46, 818–824.
Jauhari, N., Mishra, S., Kumari, B., Singh, S. N., Chauhan, P. S., & Upreti, D. K. (2018). Bacteria induced degradation of anthracene mediated by catabolic enzymes. Polycyclic Aromatic Compounds, 1–13.
Jové, P., Olivella, M. À., Camarero, S., Caixach, J., Planas, C., Cano, L., & De Las Heras, F. X. (2016). Fungal biodegradation of anthracene-polluted cork: A comparative study. Journal of Environmental Science and Health. Part A, Toxic/Hazardous Substances & Environmental Engineering, 51, 70–77.
Juhasz, A. L., & Naidu, R. (2000). Bioremediation of high molecular weight polycyclic aromatic hydrocarbons: A review of the microbial degradation of benzo[a]pyrene. International Biodeterioration and Biodegradation, 45, 57–88.
Kachieng’a, L., & Momba, M. N. B. (2018). The synergistic effect of a consortium of protozoan isolates (Paramecium sp., Vorticella sp., Epistylis sp. and Opercularia sp.) on the biodegradation of petroleum hydrocarbons in wastewater. Journal of Environmental Chemical Engineering, 6, 4820–4827.
Kadri, T., Rouissi, T., Kaur Brar, S., Cledon, M., Sarma, S., & Verma, M. (2017). Biodegradation of polycyclic aromatic hydrocarbons (PAHs) by fungal enzymes: A review. Journal of Environmental Sciences, 51, 52–74.
Kamil, N. A. F., & Talib, S. A. (2016). Biodegradation of PAHs in soil: Influence of initial PAHs concentration. IOP Conference Series: Materuals Science Engineering, 136, 012052.
Keum, Y. S., Seo, J. S., Li, Q. X., & Kim, J. H. (2008). Comparative metabolomic analysis of Sinorhizobium sp. C4 during the degradation of phenanthrene. Applied Microbiology and Biotechnology, 80, 863–872.
Kim, S.-J., Kweon, O., & Cerniglia, C. E. (2009). Proteomic applications to elucidate bacterial aromatic hydrocarbon metabolic pathways. Current Opinion in Microbiology, 12, 301–309.
Kong, L., Gao, Y., Zhou, Q., Zhao, X., & Sun, Z. (2018). Biochar accelerates PAHs biodegradation in petroleum-polluted soil by biostimulation strategy. Journal of Hazardous Materials, 343, 276–284.
Košnář, Z., Mercl, F., & Tlustoš, P. (2018). Ability of natural attenuation and phytoremediation using maize (Zea mays L.) to decrease soil contents of polycyclic aromatic hydrocarbons (PAHs) derived from biomass fly ash in comparison with PAHs-spiked soil. Ecotoxicology and Environmental Safety, 153, 16–22.
Kotoky, R., Rajkumari, J., & Pandey, P. (2018). The rhizosphere microbiome: Significance in rhizoremediation of polyaromatic hydrocarbon contaminated soil. Journal of Environmental Management, 217, 858–870.
Kumari, S., Regar, R. K., & Manickam, N. (2018). Improved polycyclic aromatic hydrocarbon degradation in a crude oil by individual and a consortium of bacteria. Bioresource Technology, 254, 174–179.
Kuppusamy, S., Palanisami, T., Megharaj, M., Venkateswarlu, K., & Naidu, R. (2016). In-situ remediation approaches for the management of contaminated sites: A comprehensive overview. In P. de Voogt (Ed.), Reviews of environmental contamination and toxicology (Vol. 236, pp. 1–115). Cham: Springer.
Kuppusamy, S., Thavamani, P., Venkateswarlu, K., Lee, Y. B., Naidu, R., & Megharaj, M. (2017). Remediation approaches for polycyclic aromatic hydrocarbons (PAHs) contaminated soils: Technological constraints, emerging trends and future directions. Chemosphere, 168, 944–968.
Lamichhane, S., Bal Krishna, K. C., & Sarukkalige, R. (2017). Surfactant-enhanced remediation of polycyclic aromatic hydrocarbons: A review. Journal of Environmental Management, 199, 46–61.
Latimer, J. S., & Zheng, J. (2003). The sources, transport, and fate of PAHs in the marine environment. In P. E. T. Douben (Ed.), PAHs: An ecotoxicological perspective (pp. 7–33). Chichester: Wiley.
Lee, H., Jang, Y., Choi, Y.-S., Kim, M.-J., Lee, J., Lee, H., Hong, J.-H., Lee, Y. M., Kim, G.-H., & Kim, J.-J. (2014). Biotechnological procedures to select white rot fungi for the degradation of PAHs. Journal of Microbiological Methods, 97, 56–62.
Lee, D. W., Lee, H., Lee, A. H., Kwon, B.-O., Khim, J. S., Yim, U. H., Kim, B. S., & Kim, J.-J. (2018). Microbial community composition and PAHs removal potential of indigenous bacteria in oil contaminated sediment of Taean coast, Korea. Environmental Pollution, 234, 503–512.
Li, C.-H., Wong, Y.-S., & Tam, N. F.-Y. (2010). Anaerobic biodegradation of polycyclic aromatic hydrocarbons with amendment of iron(III) in mangrove sediment slurry. Bioresource Technology, 101, 8083–8092.
Liang, L., Song, X., Kong, J., Shen, C., Huang, T., & Hu, Z. (2014). Anaerobic biodegradation of high-molecular-weight polycyclic aromatic hydrocarbons by a facultative anaerobe Pseudomonas sp. JP1. Biodegradation, 25, 825–833.
Liang, X., Guo, C., Liao, C., Liu, S., Wick, L. Y., Peng, D., Yi, X., Lu, G., Yin, H., Lin, Z., & Dang, Z. (2017). Drivers and applications of integrated clean-up technologies for surfactant-enhanced remediation of environments contaminated with polycyclic aromatic hydrocarbons (PAHs). Environmental Pollution, 225, 129–140.
Liao, C., Liang, X., Lu, G., Thai, T., Xu, W., & Dang, Z. (2015). Effect of surfactant amendment to PAHs-contaminated soil for phytoremediation by maize (Zea mays L.). Ecotoxicology and Environmental Safety, 112, 1–6.
Liu, K., Han, W., Pan, W. P., & Riley, J. T. (2001). Polycyclic aromatic hydrocarbon (PAH) emissions from a coal-fired pilot FBC system. Journal of Hazardous Materials, 84, 175–188.
Liu, S.-L., Luo, Y.-M., Wu, L.-H., & Cao, Z.-H. (2010). Effects of fungi on co-metabolic degradation of benzo [a] pyrene in droughty red soil. Huan Jing Ke Xue, 31, 1944–1950.
Liu, J., Liu, S., Sun, K., Sheng, Y., Gu, Y., & Gao, Y. (2014a). Colonization on root surface by a phenanthrene-degrading endophytic bacterium and its application for reducing plant phenanthrene contamination. PLoS One, 9, e108249.
Liu, R., Xiao, N., Wei, S., Zhao, L., & An, J. (2014b). Rhizosphere effects of PAH-contaminated soil phytoremediation using a special plant named fire Phoenix. Science of the Total Environment, 473–474, 350–358.
Lu, X., Zhang, T., Han-Ping Fang, H., Leung, K. M. Y., & Zhang, G. (2011). Biodegradation of naphthalene by enriched marine denitrifying bacteria. International Biodeterioration and Biodegradation, 65, 204–211.
Luo, L., Wang, P., Lin, L., Luan, T., Ke, L., & Tam, N. F. Y. (2014). Removal and transformation of high molecular weight polycyclic aromatic hydrocarbons in water by live and dead microalgae. Process Biochemistry, 49, 1723–1732.
Mao, J., & Guan, W. (2016). Fungal degradation of polycyclic aromatic hydrocarbons (PAHs) by Scopulariopsis brevicaulis and its application in bioremediation of PAH-contaminated soil. Acta Agriculturae Scandinavica Section B Soil and Plant Science, 66, 399–405.
Marco-Urrea, E., García-Romera, I., & Aranda, E. (2015). Potential of non-ligninolytic fungi in bioremediation of chlorinated and polycyclic aromatic hydrocarbons. New Biotechnology, 32, 620–628.
Marozava, S., Mouttaki, H., Müller, H., Laban, N. A., Probst, A. J., & Meckenstock, R. U. (2018). Anaerobic degradation of 1-methylnaphthalene by a member of the Thermoanaerobacteraceae contained in an iron-reducing enrichment culture. Biodegradation, 29, 23–39.
Márquez-Rocha, F. J., Hernández-Rodríguez, V. Z., & Vázquez-Duhalt, R. (2000). Biodegradation of soil-adsorbed polycyclic aromatic hydrocarbons by the white rot fungus Pleurotus ostreatus. Biotechnology Letters, 22, 469–472.
Merkl, N., Schultze-Kraft, R., & Infante, C. (2004). Phytoremediation in the tropics—The effect of crude oil on the growth of tropical plants. Bioremediation Journal, 8, 177–184.
Messias, J. M., da Costa, B. Z., de Lima, V. M. G., Dekker, R. F. H., Rezende, M. I., Krieger, N., & Barbosa, A. M. (2009). Screening Botryosphaeria species for lipases: Production of lipase by Botryosphaeria ribis EC-01 grown on soybean oil and other carbon sources. Enzyme and Microbial Technology, 45, 426–431.
Mester, T., & Tien, M. (2000). Oxidation mechanism of ligninolytic enzymes involved in the degradation of environmental pollutants. International Biodeterioration and Biodegradation, 46, 51–59.
Mineki, S., Suzuki, K., Iwata, K., Nakajima, D., & Goto, S. (2015). Degradation of Polyaromatic hydrocarbons by Fungi isolated from soil in Japan. Polycyclic Aromatic Compounds, 35, 120–128.
Mitra, S., Pramanik, A., Banerjee, S., Haldar, S., Gachhui, R., & Mukherjee, J. (2013). Enhanced biotransformation of fluoranthene by intertidally derived Cunninghamella elegans under biofilm-based and niche-mimicking conditions. Applied and Environmental Microbiology, 79, 7922–7930.
Mohan, S. V., Kisa, T., Ohkuma, T., Kanaly, R. A., & Shimizu, Y. (2006). Bioremediation technologies for treatment of PAH-contaminated soil and strategies to enhance process efficiency. Reviews in Environmental Science and Technology, 5, 347–374.
Moreira, M. T., Feijoo, G., & Lema, J. M. (2000). Manganese peroxidase production by Bjerkandera sp. BOS55. Bioprocess Engineering, 23, 657–661.
Morelli, I. S., Saparrat, M. C. N., Panno, M. T. D., Coppotelli, B. M., & Arrambari, A. (2013). Bioremediation of PAH-contaminated soil by fungi. In E. M. Goltapeh, Y. R. Danesh, & A. Varma (Eds.), Fungi as bioremediators (pp. 159–179). Berlin/Heidelberg: Springer.
Newman, L. A., & Reynolds, C. M. (2004). Phytodegradation of organic compounds. Current Opinion in Biotechnology, 15, 225–230.
Ning, D., Wang, H., Ding, C., & Lu, H. (2010). Novel evidence of cytochrome P450-catalyzed oxidation of phenanthrene in Phanerochaete chrysosporium under ligninolytic conditions. Biodegradation, 21, 889–901.
Novosad, J., Fiala, Z., Borská, L., & Krejsek, J. (2002). Immunosuppressive effect of polycyclic aromatic hydrocarbons by induction of apoptosis of pre-B lymphocytes of bone marrow. Acta Medica, 45, 123–128.
Nzila, A. (2018). Biodegradation of high-molecular-weight polycyclic aromatic hydrocarbons under anaerobic conditions: Overview of studies, proposed pathways and future perspectives. Environmental Pollution, 239, 788–802.
Page, T. J., MacWilliams, P. S., Suresh, M., Jefcoate, C. R., & Czuprynski, C. J. (2004). 7-12 Dimethylbenz[a]anthracene-induced bone marrow hypocellularity is dependent on signaling through both the TNFR and PKR. Toxicology and Applied Pharmacology, 198, 21–28.
Pagé, A. P., Yergeau, É., & Greer, C. W. (2015). Salix purpurea stimulates the expression of specific bacterial xenobiotic degradation genes in a soil contaminated with hydrocarbons. PLoS One, 10, e0132062.
Patel, J. G., Nirmal Kumar, J. I., Kumar, R. N., & Khan, S. R. (2015). Enhancement of pyrene degradation efficacy of Synechocystis sp., by construction of an artificial microalgal-bacterial consortium. Cogent Chemistry, 1, 221.
Peng Jing-Jingwang Ningli. (2011). Microbial degradation mechanisms of soil high molecular weight PAHs and affecting factors: A review. Chinese Journal of Ecology.
Peng, R.-H., Fu, X.-Y., Zhao, W., Tian, Y.-S., Zhu, B., Han, H.-J., Xu, J., & Yao, Q.-H. (2014). Phytoremediation of Phenanthrene by transgenic plants transformed with a naphthalene dioxygenase system from Pseudomonas. Environmental Science & Technology, 48, 12824–12832.
Phillips, D. H. (1999). Polycyclic aromatic hydrocarbons in the diet. Mutation Research, 443, 139–147.
Potin, O., Rafin, C., & Veignie, E. (2004). Bioremediation of an aged polycyclic aromatic hydrocarbons (PAHs)-contaminated soil by filamentous fungi isolated from the soil. International Biodeterioration and Biodegradation, 54, 45–52.
Pozdnyakova, N., Dubrovskaya, E., Chernyshova, M., Makarov, O., Golubev, S., Balandina, S., & Turkovskaya, O. (2018). The degradation of three-ringed polycyclic aromatic hydrocarbons by wood-inhabiting fungus Pleurotus ostreatus and soil-inhabiting fungus Agaricus bisporus. Fungal Biology, 122, 363–372.
Pugazhendi, A., Qari, H., Al-Badry Basahi, J. M., Godon, J. J., & Dhavamani, J. (2017). Role of a halothermophilic bacterial consortium for the biodegradation of PAHs and the treatment of petroleum wastewater at extreme conditions. International Biodeterioration and Biodegradation, 121, 44–54.
Qi, Y.-B., Wang, C.-Y., Lv, C.-Y., Lun, Z.-M., & Zheng, C.-G. (2017). Removal capacities of polycyclic aromatic hydrocarbons (PAHs) by a newly isolated strain from oilfield produced water. International Journal of Environmental Research and Public Health, 14. https://doi.org/10.3390/ijerph14020215.
Qin, W., Fan, F., Zhu, Y., Huang, X., Ding, A., Liu, X., & Dou, J. (2018). Anaerobic biodegradation of benzo(a)pyrene by a novel Cellulosimicrobium cellulans CWS2 isolated from polycyclic aromatic hydrocarbon-contaminated soil. Brazilian Journal of Microbiology, 49, 258–268.
Reddy, P. V., Karegoudar, T. B., & Nayak, A. S. (2018). Enhanced utilization of fluorene by Paenibacillus sp. PRNK-6: Effect of rhamnolipid biosurfactant and synthetic surfactants. Ecotoxicology and Environmental Safety, 151, 206–211.
Ren, C.-G., Kong, C.-C., Bian, B., Liu, W., Li, Y., Luo, Y.-M., & Xie, Z.-H. (2017). Enhanced phytoremediation of soils contaminated with PAHs by arbuscular mycorrhiza and rhizobium. International Journal of Phytoremediation, 19, 789–797.
Rostami, S., Azhdarpoor, A., & Samaei, M. R. (2017). Removal of pyrene from soil using phytobioremediation (Sorghum Bicolor-Pseudomonas). Health Scope, 6.
Saraswathy, A., & Hallberg, R. (2005). Mycelial pellet formation by Penicillium ochrochloron species due to exposure to pyrene. Microbiological Research, 160, 375–383.
Sayara, T., Borràs, E., Caminal, G., Sarrà, M., & Sánchez, A. (2011). Bioremediation of PAHs-contaminated soil through composting: Influence of bioaugmentation and biostimulation on contaminant biodegradation. International Biodeterioration and Biodegradation, 65, 859–865.
Schmidt, N., Boll, E. S., Malmquist, L. M. V., & Christensen, J. H. (2017). PAH metabolism in the earthworm Eisenia fetida – Identification of phase II metabolites of phenanthrene and pyrene. International Journal of Environmental Analytical Chemistry, 97, 1151–1162.
Sherafatmand, M., & Ng, H. Y. (2015). Using sediment microbial fuel cells (SMFCs) for bioremediation of polycyclic aromatic hydrocarbons (PAHs). Bioresource Technology, 195, 122–130.
Silva, I. S., Grossman, M., & Durrant, L. R. (2009). Degradation of polycyclic aromatic hydrocarbons (2–7 rings) under microaerobic and very-low-oxygen conditions by soil fungi. International Biodeterioration and Biodegradation, 63, 224–229.
Singh, P., & Tiwary, B. N. (2017). Optimization of conditions for polycyclic aromatic hydrocarbons (PAHs) degradation by Pseudomonas stutzeri P2 isolated from Chirimiri coal mines. Biocatalysis and Agricultural Biotechnology, 10, 20–29.
Sinha, R. K., Bharambe, G., & Ryan, D. (2008). Converting wasteland into wonderland by earthworms—A low-cost nature’s technology for soil remediation: A case study of vermiremediation of PAHs contaminated soil. Environmentalist, 28, 466–475.
Sivaram, A. K., Logeshwaran, P., Lockington, R., Naidu, R., & Megharaj, M. (2018). Impact of plant photosystems in the remediation of benzo[a]pyrene and pyrene spiked soils. Chemosphere, 193, 625–634.
Subashchandrabose, S. R., Logeshwaran, P., Venkateswarlu, K., Naidu, R., & Megharaj, M. (2017). Pyrene degradation by Chlorella sp. MM3 in liquid medium and soil slurry: Possible role of dihydrolipoamide acetyltransferase in pyrene biodegradation. Algal Research, 23, 223–232.
Sun, K., Habteselassie, M. Y., Liu, J., Li, S., & Gao, Y. (2018). Subcellular distribution and biotransformation of phenanthrene in pakchoi after inoculation with endophytic Pseudomonas sp. as probed using HRMS coupled with isotope-labeling. Environmental Pollution, 237, 858–867.
Takáčová, A., Smolinská, M., Ryba, J., Mackuľak, T., Jokrllová, J., Hronec, P., & Čík, G. (2014). Biodegradation of benzo[a]pyrene through the use of algae. Central European Journal of Chemistry, 12, 1133–1143.
Tarafdar, A., Sinha, A., & Masto, R. E. (2017). Biodegradation of anthracene by a newly isolated bacterial strain, Bacillus thuringiensis AT.ISM.1, isolated from a fly ash deposition site. Letters in Applied Microbiology, 65, 327–334.
Tauler, M., Vila, J., Nieto, J. M., & Grifoll, M. (2016). Key high molecular weight PAH-degrading bacteria in a soil consortium enriched using a sand-in-liquid microcosm system. Applied Microbiology and Biotechnology, 100, 3321–3336.
Truu, J., Truu, M., Espenberg, M., Nõlvak, H., & Juhanson, J. (2015). Phytoremediation and plant-assisted bioremediation in soil and treatment wetlands: A review. The Open Biotechnology Journal, 9, 85–92.
Valentín, L., Feijoo, G., Moreira, M. T., & Lema, J. M. (2006). Biodegradation of polycyclic aromatic hydrocarbons in forest and salt marsh soils by white-rot fungi. International Biodeterioration and Biodegradation, 58, 15–21.
Varjani, S. J., & Upasani, V. N. (2016). Biodegradation of petroleum hydrocarbons by oleophilic strain of Pseudomonas aeruginosa NCIM 5514. Bioresource Technology, 222, 195–201.
Venkatesagowda, B., Ponugupaty, E., Barbosa, A. M., & Dekker, R. F. H. (2012). Diversity of plant oil seed-associated fungi isolated from seven oil-bearing seeds and their potential for the production of lipolytic enzymes. World Journal of Microbiology and Biotechnology, 28, 71–80.
Verdin, A., Lounès-Hadj Sahraoui, A., Newsam, R., Robinson, G., & Durand, R. (2005). Polycyclic aromatic hydrocarbons storage by Fusarium solani in intracellular lipid vesicles. Environmental Pollution, 133, 283–291.
Vidali, M. (2001). Bioremediation. An overview. Journal of Macromolecular Science, Part A Pure and Applied Chemistry, 73, 1163–1172.
Vieira, G. A. L., Magrini, M. J., Bonugli-Santos, R. C., Rodrigues, M. V. N., & Sette, L. D. (2018). Polycyclic aromatic hydrocarbons degradation by marine-derived basidiomycetes: Optimization of the degradation process. Brazilian Journal of Microbiology. https://doi.org/10.1016/j.bjm.2018.04.007.
Wang, C., Sun, H., Li, J., Li, Y., & Zhang, Q. (2009). Enzyme activities during degradation of polycyclic aromatic hydrocarbons by white rot fungus Phanerochaete chrysosporium in soils. Chemosphere, 77, 733–738.
Wang, C., Sun, H., Liu, H., & Wang, B. (2014). Biodegradation of pyrene by Phanerochaete chrysosporium and enzyme activities in soils: Effect of SOM, sterilization and aging. Journal of Environmental Sciences, 26, 1135–1144.
Wang, J., Hang Ho, S. S., Huang, R., Gao, M., Liu, S., Zhao, S., Cao, J., Wang, G., Shen, Z., & Han, Y. (2016). Characterization of parent and oxygenated-polycyclic aromatic hydrocarbons (PAHs) in Xi’an, China during heating period: An investigation of spatial distribution and transformation. Chemosphere, 159, 367–377.
Wang, C., Huang, Y., Zhang, Z., & Wang, H. (2018). Salinity effect on the metabolic pathway and microbial function in phenanthrene degradation by a halophilic consortium. AMB Express, 8, 67.
Warshawsky, D., Ladow, K., & Schneider, J. (2007). Enhanced degradation of benzo[a]pyrene by Mycobacterium sp. in conjunction with green alga. Chemosphere, 69, 500–506.
Wu, Y., Teng, Y., Li, Z., Liao, X., & Luo, Y. (2008). Potential role of polycyclic aromatic hydrocarbons (PAHs) oxidation by fungal laccase in the remediation of an aged contaminated soil. Soil Biology and Biochemistry, 40, 789–796.
Wu, Y.-R., Luo, Z.-H., & Vrijmoed, L. L. P. (2010). Biodegradation of anthracene and benz[a]anthracene by two Fusarium solani strains isolated from mangrove sediments. Bioresource Technology, 101, 9666–9672.
Yergeau, E., Tremblay, J., Joly, S., Labrecque, M., Maynard, C., Pitre, F. E., St-Arnaud, M., & Greer, C. W. (2018). Soil contamination alters the willow root and rhizosphere metatranscriptome and the root-rhizosphere interactome. The ISME Journal, 12, 869–884.
Zafra, G., Moreno-Montaño, A., Absalón, Á. E., & Cortés-Espinosa, D. V. (2015). Degradation of polycyclic aromatic hydrocarbons in soil by a tolerant strain of Trichoderma asperellum. Environmental Science and Pollution Research International, 22, 1034–1042.
Zafra, G., Taylor, T. D., Absalón, A. E., & Cortés-Espinosa, D. V. (2016). Comparative metagenomic analysis of PAH degradation in soil by a mixed microbial consortium. Journal of Hazardous Materials, 318, 702–710.
Zhang, H., Zhang, S., He, F., Qin, X., Zhang, X., & Yang, Y. (2016). Characterization of a manganese peroxidase from white-rot fungus Trametes sp.48424 with strong ability of degrading different types of dyes and polycyclic aromatic hydrocarbons. Journal of Hazardous Materials, 320, 265–277.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Babu, A.G., Reja, S.I., Akhtar, N., Sultana, M., Deore, P.S., Ali, F.I. (2019). Bioremediation of Polycyclic Aromatic Hydrocarbons (PAHs): Current Practices and Outlook. In: Arora, P. (eds) Microbial Metabolism of Xenobiotic Compounds. Microorganisms for Sustainability, vol 10. Springer, Singapore. https://doi.org/10.1007/978-981-13-7462-3_9
Download citation
DOI: https://doi.org/10.1007/978-981-13-7462-3_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-7461-6
Online ISBN: 978-981-13-7462-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)