Abstract
Bioremediation is an advantageous and sustainable technology to remediate contaminated environments since it is cost-effective and environmentally safe. However, some pollutants such as most organochlorine pesticides and hydrocarbons are poorly soluble in water and thus tend to adhere tightly to soil particles. Therefore, the degradation of hydrophobic compounds is usually slow and frequently unsatisfactory due to the difficulties related to their transfer from soil particles to the aqueous phase, where these compounds are more available for degradative microorganisms. In this relation, a fundamental issue for the bioremediation processes is to overcome the limited accessibility of these hydrophobic pollutants for the microorganisms. As an alternative to synthetic surfactants, which are usually introduced into bioremediation processes with the aim of enhancing the bioavailability of hydrophobic pollutants, microemulsions have attained increasing significance both in basic research and environmental applications. Microemulsions consist of a combination of surfactants, co-surfactants, and oil phase and have demonstrated to be promising candidates due to its much higher solubilization capacity than surfactant micelles. This chapter compiles updated data related to general characteristics of microemulsions, with a special emphasis on the application of these systems as biotechnological tools for enhancing the solubilization and biodegradation of hydrophobic compounds, such as organochlorine pesticides, especially lindane.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
15.1 Introduction
Environmental pollution has been on the rise in the past decades due to increased human activities on energy reservoirs, unsafe agricultural practices, and rapid industrialization. Remediation technologies involve any operation that alters the characteristics of hazardous or polluting wastes in order to reduce their toxicity, volume, or mobility through the application of physical, chemical, and/or biological processes (Betancur-Corredor et al. 2013). Some of the physical and chemical technologies that have been used are oxidation for the treatment of a great variety of pollutants such as fuels, solvents, and pesticides (Huling and Pivetz 2006), reduction of heavy metals such as Cr (VI) using zerovalent Fe nanoparticles (US EPA 2011), stabilization or solidification based on the addition of binders in order to generate a solid material in which the contaminants are immobilized (no leaching occurs) (Al-Tabbaa and Stegemann 2005), incineration at high temperature, UV oxidation, decomposition catalyzed by acids or bases, soil washing, photocatalysis, adsorption, filtration, and precipitation, among others (Byrne et al. 2017; Carolin et al. 2017). All these technologies can be very effective in reducing the levels of a large number of pollutants, but they also have several drawbacks, such as high specificity, complexity, high costs of implementation, and lack of acceptance by the population (Niti et al. 2013). In contrast, biological treatments have received considerable attention in recent years as an effective biotechnological tool to restore contaminated environments. These eco-friendly remediation technologies are called bioremediation and include processes based on the use of biological mechanisms to reduce (degrade, detoxify, mineralize, or transform) the concentration of pollutants to an innocuous state (Azubuike et al. 2016). For this purpose, diverse biological systems may be used, including bacteria, filamentous fungi, yeasts, algae, and plants and also their derivatives (Robles-González et al. 2008; Wood 2008). Bioremediation is considered relatively cost-effective and environmentally friendly compared to other physicochemical methods such as chemical decomposition, incineration, and photodegradation (Niti et al. 2013). In addition, diverse strategies were developed to enhance the biological mechanisms and improve the bioremediation performance with the ultimate goal to effectively restore polluted environments in an eco-friendly approach, at a very low cost. In this connection, due to the unique properties of microemulsions, varied formulation possibilities, and numerous applications, these systems have attracted attention in numerous important fields, including soil washing and bioremediation (Karunaratne et al. 2017; Salam and Das 2013). On this basis, the present chapter consists of a short compilation regarding the generalities of microemulsions, such as its classification, components, and applications, with a special emphasis on the study of these systems as biotechnological tools for bioremediation purposes.
15.2 Microemulsions: Definition and General Characteristics
Microemulsions are defined as homogeneous systems formed by two immiscible fluids, hydrocarbon and water, stabilized by a surfactant or a mixture of surfactants, frequently in combination with a co-surfactant (Bera and Mandal 2015; Fanun 2012). Exhibiting a pseudo-biphasic behavior, these systems allow the solubilization of highly hydrophilic substances in oil-based systems and highly hydrophobic substances in water-based systems (Karunaratne et al. 2017).
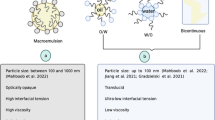
Unlike conventional emulsions, whose microstructure is static, microemulsions are dynamic systems, with a constantly fluctuating interface. In addition, microemulsions are thermodynamically stable and spontaneously formed under a specified set of experimental conditions; i.e., a change in these conditions may lead to phase separation, and, once the original conditions are restored, they reform spontaneously. Their properties are time-independent and not influenced by the production process, such as the order of mixing or mechanical energy, making them easier to prepare. In contrast, emulsions are thermodynamically unstable and require a high input of mechanical energy for their formation; therefore the cost of preparation is higher (Bragato et al. 2002; Hloucha 2014; Talegaonkar et al. 2008). Another main difference between emulsions and microemulsions is the size of the droplets of the dispersed phase; in microemulsions, it ranges between 5 and 100 nm, while in emulsions it is generally higher (>100 nm) so that they often take on a milky appearance (Karunaratne et al. 2017). On the opposite, microemulsions usually appear transparent and translucent, although they contain high amounts of water and oil (Hloucha 2014). Other significant differences between emulsions and microemulsions are described in Table 15.1.
The viscosity of microemulsions is generally low, although it is a function of its composition. For instance, it has been demonstrated that the addition of a salt such as NaCl can increase the viscosity of a microemulsion system (Moulik and Rakshit 2006). The rheology of microemulsions varies depending on the phase point. However, it is often crucial in their application because it will affect the processability, kinetics, and stability under various conditions (Karunaratne et al. 2017).
Several experimental methods are used for the characterization of the structure of microemulsions, which are often very complex systems. Some of the techniques used for this purpose include freeze-fracture electron microscopy, light scattering, and nuclear magnetic resonance spectroscopy, among others. However, it is often an advantage to use a combination of methods to obtain a complete characterization (Hloucha 2014).
15.3 Classification of Microemulsions
According to the nature of the components used in the preparation of the microemulsions and their proportions, different types of microemulsions may be formed, which, depending on their structure, can be classified in three basic types:
-
Oil in water (o/w) or direct microemulsions: consist of oil droplets dispersed in the aqueous phase. The surfactant molecules are organized so that their nonpolar tails associate with each other resulting in a globular structure with a hydrophobic core (McClements 2012), also being called oil-swollen micelles (Zheng et al. 2012a). In general, they are formed when the oil concentration is low (<30%) (Flanagan and Singh 2006) (Fig. 15.1a).
-
Water in oil (w/o) or inverse microemulsions: consist of drops of water dispersed in the oil phase. The ordering of polar and nonpolar zones of the surfactant molecules is inverse to that mentioned above so that the polar portion of the surfactant is oriented toward the inside of the micelle, while the hydrophobic chains are oriented to the outside (Muñoz Hernández et al. 2005). They are generally formed when the aqueous concentration is low (Flanagan and Singh 2006) (Fig. 15.1b).
-
Bicontinuous or middle phase microemulsions: are formed when the amounts of water and oil are similar (Talegaonkar et al. 2008) (Fig. 15.1c).
Schematic diagram of the types of microemulsions. (a) Oil in water (o/w) or direct microemulsion, (b) water in oil (w/o) or reverse microemulsion, and (c) bicontinuous microemulsion. (Adapted from Mehta and Kaur 2011)
Regarding the rheological properties of microemulsions, direct and inverse microemulsions show Newtonian behavior over a wide range of shears, while bicontinuous may undergo breakage upon medium shear forces, leading to thinning (Karunaratne et al. 2017).
Salinity can also reverse the structure of microemulsions. At high salinity levels, direct microemulsions change to reverse microemulsions, whereas at low salinity the system remains in water external phase (Bera and Mandal 2015).
15.4 Components of Microemulsions
The physicochemical properties of microemulsions depend on the nature of its components (Bera and Mandal 2015). A large number of oils, surfactants, and co-surfactants can be used for the preparation of microemulsions; however, special emphasis is put on the use of substances generally recognized as safe or GRAS. Besides, all the components should be biocompatible, nontoxic, clinically acceptable, and used in an appropriate concentration, so that they will not be aggressive (Talegaonkar et al. 2008).
15.4.1 Surfactant
Surfactants are considered to be the principal constituents of microemulsions. They consist of amphiphilic molecules, i.e., they contain a hydrophilic portion and a hydrophobic portion, and have the ability to reduce the interfacial tension between the medium in which they are dissolved and any other fluid in contact, which facilitates the dispersion during the preparation of the microemulsion (Muñoz Hernández et al. 2005; Talegaonkar et al. 2008). Surfactants can be classified based on the charge of the head group into ionic and nonionic. Ionic surfactants can also be divided into cationic, anionic, and zwitterionic. The choice of surfactants will depend on the future application of the microemulsions (Bera and Mandal 2015).
The hydrophilic-lipophilic balance (HLB) is the most characteristic parameter of a surfactant, and it relates molecular structure to interfacial packing and film curvature. The HLB of a surfactant is a measure of the degree of its hydrophilicity or lipophilicity on a scale of 0–20, where an HLB of 0 corresponds to a completely lipophilic/hydrophobic molecule and a value of 20 corresponds to a fully hydrophilic/lipophobic molecule. In general, surfactants with HLB < 12 favor the formation of inverse microemulsions, whereas surfactants with high HLB (> 12) are preferred for the formation of direct microemulsions. For bicontinuous structures, i.e., zero curvature, it was shown that HLB is around 10 (Talegaonkar et al. 2008).
Furthermore, regarding the length and volume of their hydrophobic tail, surfactants with linear aliphatic hydrocarbon chains of moderate length preferably form direct microemulsions, surfactants with voluminous hydrophobic tails form bicontinuous microemulsions, and surfactants with branched hydrophobic tails form inverse microemulsions. Often combinations of two or more surfactants are used for the formulation of microemulsion systems, and the resulting geometry of the system will depend on the geometry of the species involved (Muñoz Hernández et al. 2005).
15.4.2 Co-surfactant
Although co-surfactant is not considered as a main component of microemulsions, it is generally added to the surfactant to prepare microemulsions because it presents several well-documented roles. For instance, the co-surfactant prevents the formation of rigid structures such as gels, liquid crystals, and precipitates; alters the viscosity of the system; reduces the interfacial tension; and increases the fluidity of the interface and the mobility of the hydrocarbon tail, thus allowing greater penetration of the oil into this region, among others (Bera and Mandal 2015).
The co-surfactant inserts between the surfactant molecule constituent of the interfacial film. Short-, intermediate-, or long-chain alcohols can be used as co-surfactants. Usually, short- to medium-chain alcohols (C3-C8) such as propanol, butanol, isoamyl alcohol, pentanol, etc. are used as co-surfactants for the preparation of microemulsions, as they are able to further reduce interfacial tension and increase the interfacial fluidity. The solubility of alcohols in water depends on the aliphatic chain length. Short-chain alcohols (ethanol, propanol, and isopropanol) are more hydrophilic and slightly increase the affinity of the surfactant for the aqueous phase, whereas the longer-chain alcohols (pentanol, hexanol) show very low solubility in water; hence they are localized mainly toward the oil. Intermediate alcohols (butanol, isobutanol) have almost the same affinities for oil and water and do not significantly modify HLB (Bera and Mandal 2015; Talegaonkar et al. 2008).
Besides the components forming the microemulsion, the ratio among them is also a very important factor influencing the microemulsion existence domain. In fact, Zheng et al. (2011) postulated that the co-surfactant to surfactant ratio (C/S) is the major factor influencing the microemulsion formation and seriously influences the microemulsion area. Their results demonstrated that higher oil content was incorporated in all microemulsion systems tested when the C/S ratio increased.
15.4.3 Oil
The oil has the ability to penetrate the microemulsions and thus increase the region of the surfactant monolayer. Short-chain oils penetrate the tail group region to a greater extent than long-chain oils and thus increase this region to a greater extent, resulting in the effective reduction of HLB. Saturated fatty acids (e.g., lauric and capric acid) and unsaturated fatty acids (such as oleic and linoleic acid) have been used as the oil phase for microemulsion preparation. Fatty acid esters, such as ethyl or methyl esters of oleic acid, can also be employed for microemulsion formulation (Talegaonkar et al. 2008).
As mentioned before, the ratio of the components of the microemulsion system also plays a key role in determining its properties. In this sense, increasing the oil content of microemulsion may enhance its solubilizing capacity for a hydrophobic solute by increasing the oil volume fraction (Zheng et al. 2011).
15.5 Applications of Microemulsions
The applications of microemulsions are plenteous as they have attracted attention in various fields, including drug delivery, cosmetics, dry cleaning, food, fuels, lubricants and coatings, detergents, agrochemicals, analytical chemistry, nanoparticle synthesis, and biotechnology (Karunaratne et al. 2017). They have also been used as reaction media, as a stationary phase for capillary chromatography, and as biological membrane models, and new applications are constantly being reported (Flanagan and Singh 2006).
Microemulsions present many attractive properties. For instance, one of the interesting advantages of microemulsion-based fuels is that they contribute to the reduction of the emission rate of nitrogen oxides and carbon monoxide with an improvement in fuel economy (Worakitkanchanakul et al. 2008). Microemulsions also show extraordinary water solubilization capacity which makes them capable of injecting fluids in chemical oil recovery (Bera and Mandal 2015).
In the pharmaceutical industry, microemulsions can serve as delivery systems for both hydrophobic and hydrophilic drugs and also allow the sustained or controlled release of drugs such as chemotherapeutic agents or insulin, among others (Fanun 2012; Talegaonkar et al. 2008).
The use of microemulsions in the food industry has been extensively studied. For instance, microemulsions can be used to solubilize essential oils but are only able to deliver certain of them, and the composition of microemulsions must be carefully chosen in order not to affect their antimicrobial activities. Other practical applications of the microemulsions may be to coat semisolid foods such as cantaloupes whose surface is not consumed (Ma et al. 2016).
15.6 Microemulsions as Bioremediation Tools
Recent advances in the field of environmental restoration techniques have led to the application of microemulsions in remediation and bioremediation processes of organic and inorganic compounds (Bragato and El Seoud 2003; Castro Dantas et al. 2009; Melo et al. 2015; Vargas-Ruiz et al. 2016).
When hydrophobic pollutants such as polycyclic aromatic hydrocarbons, polychlorinated biphenyls, and organochlorine pesticides reach the soil, they are difficult to remove due to their strong adsorption onto soil particles through adsorption, electrostatic interaction, and/or covalent bonding. Therefore, there is an intense interest in soil decontamination by microemulsions (Bragato et al. 2002).This is an attractive technique due to the following reasons: high efficiency since the microemulsion decreases the interfacial tension between the contaminant and the soil and the desorbed contaminants dissolve more easily in microemulsions than in conventional surfactants emulsions; convenience based on the much smaller volumes handled in soil washing with microemulsions compared to those produced using the same technology with water or aqueous micellar solutions; and recycling possibility because the separation of microemulsions can be achieved in an aqueous surfactant-rich phase (for recycling) and an organic phase containing pollutants (for their elimination), simply by changing the temperature (Bragato and El Seoud 2003). For their application in soil remediation, nonionic surfactants are more suitable for the preparation of microemulsions than anionic or cationic surfactants; anionic surfactants can precipitate with divalent cations present in the soil, while cationic surfactants can adsorb onto the soil, both resulting in significant surfactant loss (Wang and Mulligan 2009). Also, for this type of processes, vegetable oils are more appropriate than mineral oil because of their biodegradability (Bragato et al. 2002).
Oil in water microemulsions composed of a nonionic surfactant (Tween 80 or Triton X-100), vegetable oil (linseed oil or soybean oil), and 1-pentanol as the co-surfactant has shown to effectively enhance the solubility of hydrophobic organic contaminants such as DDT and lindane compared to the solubilizing capacity of their respective surfactant solutions alone (Zheng et al. 2011). Besides pesticides, the removal of polycyclic aromatic hydrocarbons (PAHs) using microemulsions has been also studied. In this context, Zhao et al. (2005) reported a high and fast desorption of phenanthrene from contaminated soil by using a direct microemulsion prepared with sodium castor oil sulfate, due to its high solubilization capacity compared with the conventional surfactant solutions. The authors propose it as a potential agent for ex situ washing for PAH-contaminated soils since the castor oil is not toxic and the commercial sodium castor oil sulfate costs around the half of the cost of other commercial surfactants, such as Tween 80, for instance.
Microemulsions have been also applied to remove heavy metals from aqueous solutions and sludges (Castro Dantas et al. 2003; Dantas Neto et al. 2004). In fact, the extraction of heavy metals by microemulsions results advantageous, compared to conventional treatment techniques, mainly in relation to contamination with solvents and energy consumption. Castro Dantas et al. (2009) reported a very high efficiency in chromium extraction from a metal-rich solution obtained by acid digestion of a leather tannery sludge, reaching up to 93.4% of chromium removal with only one extraction stage, thus showing the high potential of microemulsions to treat tannery sediments.
On the other hand, it is known that pollutants are degraded mainly in solution since they are more available for microbial action. However, in the case of hydrophobic pollutants, they are poorly soluble in water and, therefore, tend to adhere strongly to soil particles by adsorption, electrostatic interaction, and covalent binding (Zheng and Wong 2010). Hence, degradation of this kind of pollutants in soils is usually slow and often unsatisfactory. Surfactants have been used to improve the solubility and bioavailability of hydrophobic organic compounds in soils, thus facilitating their degradation by microorganisms (Mulligan 2005; Quintero et al. 2005). In this regard, Salam and Das (2013) studied the biodegradation of lindane by the yeast Pseudozyma VITJzN01 using microemulsions prepared from a biosurfactant (BS) produced by this yeast and olive oil as the oil phase. In minimal medium, lindane degradation rate was much higher in the presence of the bio-microemulsions, reaching the complete elimination of the pesticide on the sixth day of incubation, whereas with the biosurfactant alone, the 100% removal was achieved after 10 days of incubation. The addition of the BS as well as the bio-microemulsion increased the solubility and, thus, the substrate availability for the yeast. In slurry systems, the degradation of lindane was less and slower. After 30 days of incubation, the yeast could only degrade 40% of lindane when no emulsifying agent was added to the system. The lower degradation rate in the absence of solubilizing agent clearly indicated that lindane may be adsorbed on the soil particles and therefore was not available for Pseudozyma VITJzN01. When BS was used as a solubilizing agent together with the yeast inoculum, 50% of lindane degradation was observed, whereas when the bio-microemulsion was used as a solubilizing agent, the pesticide removal reached 80% after 30 days of incubation. Consequently, the authors confirmed that the bio-microemulsion prepared with a biosurfactant of glycolipid nature, olive oil, and water, without the addition of any co-surfactant, improved the lindane degradation capacity of the yeast Pseudozyma VITJzN01 in both liquid culture and soil slurry, which can be interpreted as an eco-friendly approach.
Furthermore, the biodegradation of lindane by Sphingobium indicum B90A was also evaluated with the addition of surfactants and microemulsions. The pesticide degradation was accelerated by the addition of both the surfactant Tween 80 and microemulsions formed with Tween 80, being the microemulsions much more effective than the surfactant alone, while microemulsions formed with Triton X-100 totally inhibited the biodegradation of lindane by S. indicum B90A due to the toxicity of Triton X-100 for the bacteria (Zheng 2011). Later, Zheng et al. (2012b) also demonstrated that the use of microemulsions formed with Tween 80 favored the degradation of DDT by the fungus Phanerochaete chrysosporium around two times compared to the obtained with the Tween 80 solution. This could have occurred possibly through transporting DDT from crystalline phase to mycelium as well as the possible use of the components of the microemulsion as additional carbon source to the fungus, thus providing a positive effect on the fungal growth.
Recently, Saez et al. (2017) demonstrated for the first time the enhancement of lindane removal by an actinobacterium through the use of microemulsions as bioremediation tools. First, they obtained stable direct microemulsions using Tween 80, 1-pentanol, and three different vegetable oils, while Triton X-100 and Brij L-23 did not form stable microemulsions. Then, the ratio between the components in the microemulsions was evaluated in order to improve lindane solubilization. Thus, the authors found that an increase in the C/S ratio favored the pesticide solubilization, while an increase in the oil phase with respect to the surfactant (O/S) negatively affected the stability of the microemulsions. The microemulsion prepared with soybean oil allowed the solubilization of 66% of lindane in the aqueous medium, i.e. 4.5 times higher than the obtained by the surfactant solution at the same concentration, and the desorption of 85% of lindane in soil systems, representing around 3 and 3.5 times the obtained by the surfactant solution or water, respectively; hence this microemulsion was selected for the bioremediation studies. This microemulsion system enhanced lindane removal by Streptomyces sp. M7 in the liquid medium almost twice the achieved with the surfactant alone. This may be not only by increasing the bioavailability of the pesticide in the aqueous medium but also because the components forming the microemulsion could have exerted a stimulation effect on the microbial growth of the actinobacterium. In soil system, the addition of the microemulsion allowed an 87% of lindane removal by Streptomyces sp. M7, increasing almost 50% of the removal with respect to the obtained without the addition of surfactant agents, although it did not present significant difference with respect to the obtained with the surfactant solution. Therefore, this microemulsion could be used as potential tools in soil washing technologies or ex situ bioremediation processes of wastewaters containing not only lindane but also other hydrophobic organic compounds.
15.7 Concluding Remarks
Microemulsions are promising alternatives to synthetic surfactants in enhancing the solubilization, and hence increasing the bioremediation efficiency, of hydrophobic pollutants from wastewaters, soils, and sediments. This innovative technique combining microemulsions and bioremediation expands the scope of bioremediation and provides an efficient and safe way for the remediation of different matrices contaminated by heavy metals and hydrophobic organic compounds, such as organochlorine pesticides. However, the information available is only related to laboratory scale. Thus, deeper research is still needed in order to expand the usage of microemulsions for bioremediation purposes in field scale, assessing its effect on indigenous microorganisms, among other factors to evaluate.
References
Al-Tabbaa, A., & Stegemann, J. A. (Eds.). (2005). Stabilisation/solidification treatment and remediation. In A. A. Balkema (Ed.), Proceedings of the International Conference on Stabilisation/Solidification Treatment and Remediation, University of Cambridge, United Kingdom, CRC Press.
Azubuike, C. C., Chikere, C. B., & Okpokwasili, G. C. (2016). Bioremediation techniques–classification based on site of application: Principles, advantages, limitations and prospects. World Journal of Microbiology and Biotechnology, 32, 180. https://doi.org/10.1007/s11274-016-2137-x.
Bera, A., & Mandal, A. (2015). Microemulsions: A novel approach to enhanced oil recovery: a review. Journal of Petroleum Exploration and Production Technologies, 5, 255–268. https://doi.org/10.1007/s13202-014-0139-5.
Betancur-Corredor, B., Pino, N., Peñuela, G. A., & Cardona-Gallo, S. (2013). Bioremediation of a pesticide polluted soil: case DDT. Gestión y Ambient, 16, 119–135.
Bragato, M., & El Seoud, O. A. (2003). Formation, properties, and “ex situ” soil decontamination by vegetable oil-based microemulsions. Journal of Surfactants and Detergents, 6, 143–150.
Bragato, M., Subklew, G., Schwuger, M. J., & El Seoud, O. A. (2002). Vegetable oils-based microemulsions: Formation, properties, and application for “ex-situ” soil decontamination. Colloid & Polymer Science, 280, 973–983. https://doi.org/10.1007/s00396-002-0715-y.
Byrne, C., Subramanian, G., & Pillai, S. C. (2017). Recent advances in photocatalysis for environmental applications. Journal of Environmental Chemical Engineering, 0, 1. https://doi.org/10.1016/j.jece.2017.07.080.
Carolin, C. F., Kumar, P. S., Saravanan, A., Joshiba, G. J., & Naushad, M. (2017). Efficient techniques for the removal of toxic heavy metals from aquatic environment: A review. Journal of Environmental Chemical Engineering, 5, 2782–2799. https://doi.org/10.1016/j.jece.2017.05.029.
Castro Dantas, T. N., Dantas Neto, A. A., Moura, M. C. P. A., Barros Neto, E. L., Forte, K. R., & Leite, R. H. L. (2003). Heavy metals extraction by microemulsions. Water Research, 37, 2709–2717. https://doi.org/10.1016/S0043-1354(03)00072-1.
Castro Dantas, T. N., Oliveira, K. R., Dantas Neto, A. A., & Moura, M. C. P. A. (2009). The use of microemulsions to remove chromium from industrial sludge. Water Research, 43, 1464–1470. https://doi.org/10.1016/j.watres.2008.12.047.
Dantas Neto, A., Catro Dantas, T., & Moura, M. (2004). Evaluation and optimization of chromium removal from tannery effluent by microemulsion in the Morris extractor. Journal of Hazardous Materials, 114, 115–122. https://doi.org/10.1016/j.jhazmat.2004.07.007.
Fanun, M. (2012). Microemulsions as delivery systems. Current Opinion in Colloid & Interface Science, 17, 306–313. https://doi.org/10.1016/j.cocis.2012.06.001.
Flanagan, J., & Singh, H. (2006). Microemulsions: A potential delivery system for bioactives in food. Critical Reviews in Food Science and Nutrition, 46, 221–237. https://doi.org/10.1080/10408690590956710.
Hloucha, M. (2014). Microemulsions. In Ullmann’s encyclopedia of industrial chemistry (pp. 1–16). Weinheim, Germany: Wiley-VCH Verlag GmbH & KGaA. https://doi.org/10.1002/14356007.q16_q02.
Huling, S.G., Pivetz, B.E. (2006). Engineering issue in-situ chemical oxidation [WWW Document]. URL https://nepis.epa.gov/. Accessed 3 July 2018.
Karunaratne, D. N., Pamunuwa, G., & Ranatunga, U. (2017). Introductory chapter: Microemulsions. In Properties and uses of microemulsions (pp. 3–13). Rijeka: InTech. https://doi.org/10.5772/intechopen.68823.
Ma, Q., Davidson, P. M., & Zhong, Q. (2016). Antimicrobial properties of microemulsions formulated with essential oils, soybean oil, and Tween 80. International Journal of Food Microbiology, 226, 20–25. https://doi.org/10.1016/j.ijfoodmicro.2016.03.011.
McClements, D. J. (2012). Nanoemulsions versus microemulsions: Terminology, differences, and similarities. Soft Matter, 8, 1719–1729. https://doi.org/10.1039/C2SM06903B.
Mehta, S. K., & Kaur, G. (2011). Microemulsions: Thermodynamic and dynamic properties. Thermodynamics, 381–406. https://doi.org/10.5772/12954.
Melo, K. R. O., Castro Dantas, T. N., Moura, M. C. P. A., Dantas Neto, A. A., Oliveira, M. R., & Barros Neto, E. L. (2015). Chromium extraction by microemulsions in two- and three-phase systems. Brazilian Journal of Chemical Engineering, 32, 949–956. https://doi.org/10.1590/0104-6632.20150324s00002985.
Moulik, S. P., & Rakshit, A. K. (2006). Physicochemisty and applications of microemulsions. Surface Science, 22, 159–186.
Mulligan, C. N. (2005). Environmental applications for biosurfactants. Environmental Pollution, 133, 183–198. https://doi.org/10.1016/j.envpol.2004.06.009.
Muñoz Hernández, M., Ochoa Gómez, J. R., & Fernández Sánchez, C. (2005). Formación de microemulsiones inversas de acrilamida. Tecnologia y Desarro Rev Ciencia, Tecnologia y Medio Ambient, 3, 29.
Niti, C., Sunita, S., Kamlesh, K., & Rakesh, K. (2013). Bioremediation: An emerging technology for remediation of pesticides. Research Journal of Chemistry and Environment, 17, 88–105.
Quintero, J. C., Moreira, M. T., Feijoo, G., & Lema, J. M. (2005). Effect of surfactants on the soil desorption of hexachlorocyclohexane (HCH) isomers and their anaerobic biodegradation. Journal of Chemical Technology and Biotechnology, 80, 1005–1015. https://doi.org/10.1002/jctb.1277.
Robles-González, I. V., Fava, F., & Poggi-Varaldo, H. M. (2008). A review on slurry bioreactors for bioremediation of soils and sediments. Microbial Cell Factories, 7, 5. https://doi.org/10.1186/1475-2859-7-5.
Saez, J. M., Casillas García, V., & Benimeli, C. S. (2017). Improvement of lindane removal by Streptomyces sp. M7 by using stable microemulsions. Ecotoxicology and Environmental Safety, 144, 351–359. https://doi.org/10.1016/j.ecoenv.2017.06.026.
Salam, J. A., & Das, N. (2013). Enhanced biodegradation of lindane using oil-in-water bio-microemulsion stabilized by biosurfactant produced by a new yeast strain, Pseudozyma VITJzN01. Journal of Microbiology and Biotechnology, 23, 1598–1609. https://doi.org/10.4014/jmb.1307.07016.
Talegaonkar, S., Azeem, A., Ahmad, F. J., Khar, R. K., Pathan, S. A., & Khan, Z. I. (2008). Microemulsions: A novel approach to enhanced drug delivery. Recent Patents on Drug Delivery & Formulation, 2, 238–257. https://doi.org/10.2174/187221108786241679.
US EPA, U.S.E.P.A. (2011). In situ chemical reduction [WWW Document]. https://archive.epa.gov/ada/web/html/iscr.html. Accessed 3 July 2018.
Vargas-Ruiz, S., Schulreich, C., Kostevic, A., Tiersch, B., Koetz, J., Kakorin, S., von Klitzing, R., Jung, M., Hellweg, T., & Wellert, S. (2016). Extraction of model contaminants from solid surfaces by environmentally compatible microemulsions. Journal of Colloid and Interface Science, 471, 118–126. https://doi.org/10.1016/j.jcis.2016.03.006.
Wang, S., & Mulligan, C. N. (2009). Rhamnolipid biosurfactant-enhanced soil flushing for the removal of arsenic and heavy metals from mine tailings. Process Biochemistry, 44, 296–301. https://doi.org/10.1016/j.procbio.2008.11.006.
Wood, T. K. (2008). Molecular approaches in bioremediation. Current Opinion in Biotechnology, 19, 572–578. https://doi.org/10.1016/j.copbio.2008.10.003.
Worakitkanchanakul, W., Imura, T., Morita, T., Fukuoka, T., Sakai, H., Abe, M., Rujiravanit, R., Chavadej, S., & Kitamoto, D. (2008). Formation of W/O microemulsion based on natural glycolipid biosurfactant, mannosylerythritol lipid-a. Journal of Oleo Science, 57, 55–59. https://doi.org/10.5650/jos.57.55.
Zhao, B., Zhu, L., & Gao, Y. (2005). A novel solubilization of phenanthrene using Winsor I microemulsion-based sodium castor oil sulfate. Journal of Hazardous Materials, 119, 205–211. https://doi.org/10.1016/j.jhazmat.2004.12.009.
Zheng, G. (2011). Bioremediation of organochlorine pesticides contaminated soil with microemulsions. PhD thesis. Hong Kong Baptist University.
Zheng, G., & Wong, J. W. C. (2010). Application of microemulsion to remediate organochlorine pesticides contaminated soils. In: Proceedings of the Annual International Conference on Soils, Sediments, Water and Energy, Vol. 15, Article 4.
Zheng, G., Zhao, Z., & Wong, J. W. C. (2011). Role of non-ionic surfactants and plant oils on the solubilization of organochlorine pesticides by oil-in-water microemulsions. Environmental Technology, 32, 269–279. https://doi.org/10.1080/09593330.2010.496468.
Zheng, G., Selvam, A., & Wong, J. W. C. (2012a). Enhanced solubilization and desorption of organochlorine pesticides (OCPs) from soil by oil-swollen micelles formed with a nonionic surfactant. Environmental Science & Technology, 46, 12062–12068. https://doi.org/10.1021/es302832z.
Zheng, G., Selvam, A., & Wong, J. W. C. (2012b). Oil-in-water microemulsions enhance the biodegradation of DDT by Phanerochaete chrysosporium. Bioresource Technology, 126, 397–403. https://doi.org/10.1016/j.biortech.2012.02.141.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Saez, J.M., Casillas García, V., Polti, M.A., Benimeli, C.S. (2019). Microemulsions as a Novel Tool for Enhancing the Bioremediation of Xenobiotics. In: Arora, P. (eds) Microbial Metabolism of Xenobiotic Compounds. Microorganisms for Sustainability, vol 10. Springer, Singapore. https://doi.org/10.1007/978-981-13-7462-3_15
Download citation
DOI: https://doi.org/10.1007/978-981-13-7462-3_15
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-7461-6
Online ISBN: 978-981-13-7462-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)