Abstract
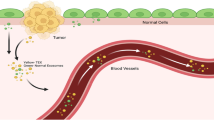
Extracellular vesicles (EVs) are membrane-surrounded structures secreted by cells, which involve exosomes, microvesicles, apoptotic bodies, and many others. Recent studies indicated that cancer-associated EVs play pivotal roles in constructing favorable microenvironments for cancer cells through communication with various surrounding or remote cells. In fact, they induce immunosuppression, angiogenesis, and epithelial-mesenchymal transition (EMT) via transport of functional nucleic acids, proteins, and metabolites. They also transport tumor-associated antigens to antigen-presenting cells. Since the cargoes of cancer-derived EVs retain the molecular properties of their sources and cancer cells actively release EVs into bodily fluids that are easy to access, EVs are considered to be attractive resources for cancer biomarker development. In the following chapter, we describe the biology of EVs, as well as methods and issues relevant for purifying EVs and measuring EV biomarkers. We also provide an overview of reported EV biomarker molecules and discuss the feasibility of EV-based cancer liquid biopsy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Extracellular vesicles
- Exosome
- Cancer
- Liquid biopsy
- Liquid biopsy
- Protein
- Tumor-associated antigen
- Proteome
1 Introduction
Each year, approximately 14 million people are diagnosed with cancer worldwide. The development of cancer biomarkers as diagnostic tools for the screening or management of cancers may play a crucial role in reducing cancer-related mortality. Indeed, early detection is one of the most effective ways to reduce cancer death rates. Early detection may be accomplished by screening the general population using blood-based biomarkers. Liquid biopsy using blood-based biomarkers may also enable patient-specific targeted therapy or the monitoring of drug resistance acquisition by tumor cells [1]. Circulating tumor cells (CTCs) and cell-free DNA (cfDNA) are promising materials for liquid biopsy; however, several issues remain to be solved, particularly regarding sensitivity [2].
EVs are membranous vesicles released by any types of cells in our body. The functions of EVs include maintaining cellular homeostasis and presenting intracellular antigens [3, 4]. Multiple kinds of stresses, such as hypoxia, pH alteration, oxidative stress, shear stress, or radiation, trigger secretion of EVs [5]. Cancer cells actively secrete EVs even during the early phase of the disease [6]. EVs contain biomolecular cargoes that are protected from degradative enzymes in body fluids [7, 8]. For example, cfDNAs are intensively degraded in blood, which are typically detected as short fragments with less than 100 bp [8]. In contrast, DNA fragments in EVs range from 100 bp to 17 kb in size, indicating that wider range of genetic information would be accessible in EV-DNAs [9]. Protection of RNA cargoes from RNase in biofluids also significantly enhances the depth of transcriptomic analysis by next-generation sequencers [10]. In addition, EV cargoes directly reflect the characteristics of their sources. Indeed, KRAS, TP53, NOTCH1, and BRCA2 mutations were detected in biofluid EVs from patients with pancreatic cancer and ampullary cancer [10]. Cancer-associated EVs also contain cancer-specific protein cargoes. NYO-ESO-1 and TP53 were detected in EVs from cancer patients’ sera, which could be effective targets for cancer liquid biopsy [11].
Recent studies of EVs as cancer biomarkers have mainly focused on nucleic acids and proteins. Reports indicated that the diagnostic performance of nucleic acids is excellent, but several major problems remain unresolved. These issues fundamentally come from unfixed EV purification methods. On the other hand, reports on EV proteins are accumulating at a steady rate. These EV proteins are now cataloged in public databases and can be utilized in meta-analyses [12]. For detection technologies, enzyme-linked immunosorbent assay (ELISA), EV microarray, and ExoScreen are widely used in measurements of EV protein biomarkers [13, 14]. These methods have a significant advantage in clinical application of EV protein biomarkers because EV protein concentrations can be directly measured without EV purification, allowing quick, low-cost, and reproducible tests. In this chapter, the reported EV protein biomarkers (Table 1.1) are overviewed, followed by consideration of clinical values for EV-based liquid biopsy (Table 1.2).
2 General Remarks on EVs as Biomarkers
2.1 Molecular Characteristics of EVs
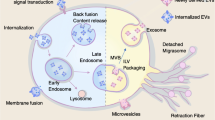
EVs are classified into three main groups according to biogenesis processes and particle sizes. (i) Exosomes are 30–200 nm in size and formed as intraluminal vesicles (ILVs) within the lumen of multivesicular bodies (MVBs). MVBs fuse with the plasma membrane to release ILVs. Exosomes from cancer cells contain tumor-associated antigens, and these antigens can transform dendritic cells [15]. (ii) Microvesicles, which are 100–1000 nm in diameter, are formed when cell membranes are partially pinched off and released directly from the originating cells. (iii) Apoptotic bodies are relatively large particles (500–2000 nm) and formed during the late stage of apoptosis. Smaller types of apoptotic bodies (approximately 500 nm in diameter) also contain intracellular antigens [16]. EV subtypes have different cargo profiles, suggesting that their roles may also differ [17].
Members of tetraspanin family (CD9, CD63, and CD81) are generally used as exosome markers. However, these factors are also expressed in apoptotic bodies and microvesicles. These proteins are also expressed on the cell surface and are thus expressed in other types of EVs that are generated by direct budding from the plasma membrane. Different reports have shown that CD9, CD63, and CD81 are abundant not only in exosomes but also in microvesicles or apoptotic bodies; thus, additional factors are needed to discriminate among these different types of EVs [18].
2.2 Purification and Detection Methods for EV Biomarkers
Measurement of nucleic acids, such as RNA or DNA, in EVs needs to be accompanied with appropriate EV purification steps. An issue that arises is that the obtained EV population differs according to the used purification methods [19]. The available EV purification methods, such as ultracentrifugation, chromatography, antibody-based capture, and the microfluidic system have advantages and disadvantages. Thus, it is necessary to optimize the method that yields the highest purity of the target population [18]. Proteome analysis plays a vital role in the identification of biomarkers among protein targets. A new analytical strategy has been developed for proteome analysis. This technique uses EVs directly secreted from surgically resected fresh tissues as the subject rather than EVs obtained from patient blood samples or cultured cell lines. This method enabled to deal with high-purity organ-specific EVs and allowed to in-depth OMICS-wide analysis of EVs [19]. For measurement of protein targets expressed on EVs, immunoassays are often employed using a pair of antibodies. After capturing EVs by an anti-tetraspanin antibody, such as an anti-CD9, anti-CD63, or anti-CD81 antibody, a target-specific antibody can detect the targeted EV surface biomarker protein (EV sandwich ELISA). This method enables detection of biomarkers expressed on EVs directly from body fluid samples in a high-throughput manner without any purification processes. This point is a great advantage for clinical application [20].
2.3 EV Protein Biomarkers and Liquid Biopsy for Cancer Diagnosis
EV biomarker studies have been accumulated for lung cancer. Ueda et al. [21] isolated exosomes from the serum of lung cancer patients, conducted proteome analysis, and identified 1369 proteins. The investigators successfully distinguished between lung adenocarcinoma patients and controls (healthy donors and benign lung diseases) using Exo-CD91 antigen. Jakobsen et al. [22] and Sandfeld-Paulsen et al. [11] analyzed protein biomarkers in plasma samples from lung cancer patients using EV microarrays and reported an association between NY-ESO-1 and prognosis. As a liquid biopsy of lung cancer, Mohrmann et al. screened nucleic acids in exosomes (exoNA) and detected driver mutations of BRAFV600, KRASG12/G13, and EGFRexon19del/L858R. These mutations were detected more sensitively in exoNA than in cfDNA [23]. Additionally, Castellanos-Rizaldos et al. reported successful detection of EGFR T790M mutation in exoNA, which is responsible for EGFR TKI drug resistance [24].
DEL-1, which is identified via LC-MS/MS analysis of serum from breast cancer patients, was confirmed to have clinical value in a retrospective study using ELISA. The detection of DEL-1 on circulating EVs facilitated early-stage breast cancer diagnosis and discrimination of breast cancer from benign breast disease [25]. Salma et al. suggested that EV-survivin may be useful in breast cancer diagnosis [26]. Kibria et al. suggested that EV-CD47 may be a possible breast cancer biomarker [27]. Toth et al. compared blood samples from breast cancer patients and healthy subjects using fluorescence-activated cell sorting (FACS). They reported that CD45+ leukocyte-derived microparticles had levels of sensitivity and specificity similar to those of the existing biomarker, CA15-3 [28]. GSTP1 and TRPC5 may be useful as negative predictive factors for anthracycline/taxane-based chemotherapy regimens in breast cancer patients [29, 30]. Fang et al. reported that HER2-positive exosomes in plasma from breast cancer patients correlate positively with HER2 expression in breast cancer tissue [31].
Yoshioka et al. reported the utility of ExoScreen and CD147 as EV protein biomarkers for colorectal cancer [14]. Allenson et al. reported the detection of mutated KRAS in exoDNA from early-stage pancreatic cancer patients [32].
Jingushi et al. identified AZU1 as a biomarker candidate via proteome analysis of renal cell carcinoma tissue-exudative EVs [33]. Mass spectrometric analysis of tumor-derived exosomes identified TYRP2, VLA-4, and HSP70 as biomarker candidates [34]. Kharaziha et al. conducted proteome analysis of prostate cancer tumor cell-derived exosomes to identify predictive factors for docetaxel therapy. Their results suggested that MDR-1, MDR-3, endophilin-A2, and PABP4 may be biomarker candidates [35].
3 Summary
EVs are attractive resources for cancer liquid biopsies. Some reports already showed favorable results in development of EV-based cancer diagnosis technologies. However, there are still great challenges for clinical application including standardization of methods for EV purification and an incomplete understanding of the characteristics and molecular composition of EVs. With overcoming these issues, screening the general population with EV biomarker may achieve early detection of cancer and reduce cancer death rates.
References
Ahronian LG, Corcoran RB. Strategies for monitoring and combating resistance to combination kinase inhibitors for cancer therapy. Genome Med. 2017;9(1):37. https://doi.org/10.1186/s13073-017-0431-3.
Merker JD, Oxnard GR, Compton C, Diehn M, Hurley P, Lazar AJ, et al. Circulating tumor DNA analysis in patients with cancer: American society of clinical oncology and college of American pathologists joint review. J Clin Oncol. 2018;36(16):1631–41. https://doi.org/10.1200/JCO.2017.76.8671.
Takahashi A, Okada R, Nagao K, Kawamata Y, Hanyu A, Yoshimoto S, et al. Exosomes maintain cellular homeostasis by excreting harmful DNA from cells. Nat Commun. 2017;8:15287. https://doi.org/10.1038/ncomms15287.
Smith VL, Cheng Y, Bryant BR, Schorey JS. Exosomes function in antigen presentation during an in vivo Mycobacterium tuberculosis infection. Sci Rep. 2017;7:43578. https://doi.org/10.1038/srep43578.
Kucharzewska P, Belting M. Emerging roles of extracellular vesicles in the adaptive response of tumour cells to microenvironmental stress. J Extracell Vesicles. 2013;2:20304. https://doi.org/10.3402/jev.v2i0.20304.
Abak A, Abhari A, Rahimzadeh S. Exosomes in cancer: small vesicular transporters for cancer progression and metastasis, biomarkers in cancer therapeutics. PeerJ. 2018;6:e4763. https://doi.org/10.7717/peerj.4763.
Huang X, Yuan T, Tschannen M, Sun Z, Jacob H, Du M, et al. Characterization of human plasma-derived exosomal RNAs by deep sequencing. BMC Genomics. 2013;14:319. https://doi.org/10.1186/1471-2164-14-319.
Ridder K, Keller S, Dams M, Rupp AK, Schlaudraff J, Del Turco D, et al. Extracellular vesicle-mediated transfer of genetic information between the hematopoietic system and the brain in response to inflammation. PLoS Biol. 2014;12(6):e1001874. https://doi.org/10.1371/journal.pbio.1001874.
Kalluri R, LeBleu VS. Discovery of double-stranded genomic DNA in circulating exosomes. Cold Spring Harb Symp Quant Biol. 2016;81:275–80. https://doi.org/10.1101/sqb.2016.81.030932.
San Lucas FA, Allenson K, Bernard V, Castillo J, Kim DU, Ellis K, et al. Minimally invasive genomic and transcriptomic profiling of visceral cancers by next-generation sequencing of circulating exosomes. Ann Oncol. 2016;27(4):635–41. https://doi.org/10.1093/annonc/mdv604.
Sandfeld-Paulsen B, Aggerholm-Pedersen N, Baek R, Jakobsen KR, Meldgaard P, Folkersen BH, et al. Exosomal proteins as prognostic biomarkers in non-small cell lung cancer. Mol Oncol. 2016;10(10):1595–602. https://doi.org/10.1016/j.molonc.2016.10.003.
Rosa-Fernandes L, Rocha VB, Carregari VC, Urbani A, Palmisano G. A perspective on extracellular vesicles proteomics. Front Chem. 2017;5:102. https://doi.org/10.3389/fchem.2017.00102.
Jorgensen M, Baek R, Pedersen S, Sondergaard EK, Kristensen SR, Varming K. Extracellular vesicle (EV) array: microarray capturing of exosomes and other extracellular vesicles for multiplexed phenotyping. J Extracell Vesicles. 2013;2:20920. https://doi.org/10.3402/jev.v2i0.20920.
Yoshioka Y, Kosaka N, Konishi Y, Ohta H, Okamoto H, Sonoda H, et al. Ultra-sensitive liquid biopsy of circulating extracellular vesicles using ExoScreen. Nat Commun. 2014;5:3591. https://doi.org/10.1038/ncomms4591.
Wolfers J, Lozier A, Raposo G, Regnault A, Thery C, Masurier C, et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med. 2001;7(3):297–303. https://doi.org/10.1038/85438.
Schiller M, Bekeredjian-Ding I, Heyder P, Blank N, Ho AD, Lorenz HM. Autoantigens are translocated into small apoptotic bodies during early stages of apoptosis. Cell Death Differ. 2008;15(1):183–91. https://doi.org/10.1038/sj.cdd.4402239.
Crescitelli R, Lässer C, Szabó TG, Kittel A, Eldh M, Dianzani I, et al. Distinct RNA profiles in subpopulations of extracellular vesicles: apoptotic bodies, microvesicles and exosomes. J Extracell Vesicles. 2013;2:20677. https://doi.org/10.3402/jev.v2i0.20677.
Willms E, Cabanas C, Mager I, Wood MJA, Vader P. Extracellular vesicle heterogeneity: subpopulations, isolation techniques, and diverse functions in cancer progression. Front Immunol. 2018;9(738):738. https://doi.org/10.3389/fimmu.2018.00738.
Buschmann D, Kirchner B, Hermann S, Märte M, Wurmser C, Brandes F, et al. Evaluation of serum extracellular vesicle isolation methods for profiling miRNAs by next-generation sequencing. J Extracell Vesicles. 2018;7(1):1481321. https://doi.org/10.1080/20013078.2018.1481321.
Li W, Li C, Zhou T, Liu X, Liu X, Li X, et al. Role of exosomal proteins in cancer diagnosis. Mol Cancer. 2017;16:145. https://doi.org/10.1186/s12943-017-0706-8.
Ueda K, Ishikawa N, Tatsuguchi A, Saichi N, Fujii R, Nakagawa H. Antibody-coupled monolithic silica microtips for highthroughput molecular profiling of circulating exosomes. Sci Rep. 2014;4:6232. https://doi.org/10.1038/srep06232.
Jakobsen KR, Paulsen BS, Baek R, Varming K, Sorensen BS, Jorgensen MM. Exosomal proteins as potential diagnostic markers in advanced non-small cell lung carcinoma. J Extracell Vesicles. 2015;4:26659. https://doi.org/10.3402/jev.v4.26659.
Mohrmann L, Huang HJ, Hong DS, Tsimberidou AM, Fu S, Piha-Paul SA, et al. Liquid biopsies using plasma exosomal nucleic acids and plasma cell-free DNA compared with clinical outcomes of patients with advanced cancers. Clin Cancer Res. 2018;24(1):181–8. https://doi.org/10.1158/1078-0432.CCR-17-2007.
Castellanos-Rizaldos E, Grimm DG, Tadigotla V, Hurley J, Healy J, Neal PL, et al. Exosome-based detection of EGFR T790M in plasma from non-small cell lung cancer patients. Clin Cancer Res. 2018;24(12):2944–50. https://doi.org/10.1158/1078-0432.CCR-17-3369.
Moon PG, Lee JE, Cho YE, Lee SJ, Jung JH, Chae YS, et al. Identification of developmental endothelial locus-1 on circulating extracellular vesicles as a novel biomarker for early breast cancer detection. Clin Cancer Res. 2016;22(7):1757–66. https://doi.org/10.1158/1078-0432.CCR-15-0654.
Khan S, Bennit HF, Turay D, Perez M, Mirshahidi S, Yuan Y, et al. Early diagnostic value of survivin and its alternative splice variants in breast cancer. BMC Cancer. 2014;14:176. https://doi.org/10.1186/1471-2407-14-176.
Kibria G, Ramos EK, Lee KE, Bedoyan S, Huang S, Samaeekia R, et al. A rapid, automated surface protein profiling of single circulating exosomes in human blood. Sci Rep. 2016;6:36502. https://doi.org/10.1038/srep36502.
Toth B, Nieuwland R, Liebhardt S, Ditsch N, Steinig K, Stieber P, et al. Circulating microparticles in breast cancer patients: a comparative analysis with established biomarkers. Anticancer Res. 2008;28(2A):1107–12.
Yang SJ, Wang DD, Li J, Xu HZ, Shen HY, Chen X, et al. Predictive role of GSTP1-containing exosomes in chemotherapy-resistant breast cancer. Gene. 2017;623:5–14. https://doi.org/10.1016/j.gene.2017.04.031.
Wang T, Ning K, Lu TX, Sun X, Jin L, Qi X, et al. Increasing circulating exosomes-carrying TRPC5 predicts chemoresistance in metastatic breast cancer patients. Cancer Sci. 2017;108(3):448–54. https://doi.org/10.1111/cas.13150.
Fang S, Tian H, Li X, Jin D, Li X, Kong J, et al. Clinical application of a microfluidic chip for immunocapture and quantification of circulating exosomes to assist breast cancer diagnosis and molecular classification. PLoS One. 2017;12(4):e0175050. https://doi.org/10.1371/journal.pone.0175050.
Allenson K, Castillo J, San Lucas FA, Scelo G, Kim DU, Bernard V, et al. High prevalence of mutant KRAS in circulating exosome-derived DNA from early-stage pancreatic cancer patients. Ann Oncol. 2017;28(4):741–7. https://doi.org/10.1093/annonc/mdx004.
Jingushi K, Uemura M, Ohnishi N, Nakata W, Fujita K, Naito T, et al. Extracellular vesicles isolated from human renal cell carcinoma tissues disrupt vascular endothelial cell morphology via azurocidin. Int J Cancer. 2018;142(3):607–17. https://doi.org/10.1002/ijc.31080.
Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18(6):883–91. https://doi.org/10.1038/nm.2753.
Kharaziha P, Chioureas D, Rutishauser D, Baltatzis G, Lennartsson L, Fonseca P, et al. Molecular profiling of prostate cancer derived exosomes may reveal a predictive signature for response to docetaxel. Oncotarget. 2015;6(25):21740–54. https://doi.org/10.18632/oncotarget.3226.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Sumazaki, M., Ueda, K. (2019). Liquid Biopsy Diagnostics Using Extracellular Vesicles. In: Shimada, H. (eds) Biomarkers in Cancer Therapy. Springer, Singapore. https://doi.org/10.1007/978-981-13-7295-7_1
Download citation
DOI: https://doi.org/10.1007/978-981-13-7295-7_1
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-7294-0
Online ISBN: 978-981-13-7295-7
eBook Packages: MedicineMedicine (R0)