Abstract
Essential oils (EOs) are extracted from flowers, leaves, barks, roots, and fruits of the medicinal plants using hydrodistilation or steam distillation and continuous solvent extraction. EOs are mixture of chemical constituents which have less molecular weight substances, such as alcohols, polyphenols, terpenoids, carbonyl compounds, and aliphatic compounds which provide smell and possess biological properties. EOs have been used as folk medicine throughout the history. Nowadays, EOs are widely used as an alternative medicine in varied industries such as pharmaceutical, agricultural, sanitary, and food industries due to their antibacterial, antifungal, antiviral, antiparasitical, antidiabetic, anticancer (cytotoxic), insect repellent, food industry (flavoring), aromatherapy, antioxidant, perfume, and cosmetic properties. EOs have a great demand and interest as cosmetic and pharmaceutical substances. The isolation, identification, and characterization of major components of EOs have a premier significance. Individual compounds present in EOs mixture such as thymol, camphor, limonene, α-pinene, terpinolene, menthol, menthone, etc. exhibit wide-ranging biological properties. Commercially, still synthetic chemicals are widely used as biological activities than the EOs from the plants. However, EOs from natural sources are more effective and safe for human health and the environment compared to the synthetic chemicals. The aim of the present chapter is to discuss the specific chemical compounds occurring in EOs, their medical applications, and economic importance.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
9.1 Introduction
Essential oils (EOs) are obtained from aromatic and medicinal plants as a volatile mixture of chemical compounds with strong odor. EOs are extracted from the aromatic and medicinal plants using steam or hydrodistillation or Soxhlet extraction (solvent extraction or continuous extraction) methods developed in the middle ages by Arabs (Bakkali et al. 2008; Raut and Karuppayil 2014). EOs are considered as one of the most predominant plant products in agriculture, as they exhibit antifungal, antibacterial, antioxidant, anticancer, antidiabetic, antiviral, insect repellent, and anti-inflammatory properties (Buchbauer 2010; Teixeira et al. 2013; Raut and Karuppayil 2014; Said et al. 2016; Swamy et al. 2016).
Research on artificial pharmaceutical substances reveals the significance of EOs extracted from medicinal and aromatic plants, as their therapeutic properties have numerous applications. Consequently, researchers and farmers have been motivated to expand the cultivation and market these substances (Swamy and Sinniah 2015, 2016). Presently, about 100 herbs are known for their EOs, while more than 2000 herbs scattered across 60 families, such as Umbelliferae, Lamiaceae, Lauraceae, Myrtaceae, etc., could produce medicinally valued EOs. In global markets, only 300 among 3000 known types of EOs are deemed to be of commercial importance. EOs have found application in agricultural sectors and can be potentially used in other industries, such as pharmaceuticals, drugs, food, perfumes, makeup products, sanitary products, dentistry, food preservatives, additives, cosmetics, and natural remedies (Swamy et al. 2016; Mahmoudi 2017). EOs, in perfumes, creams, soaps, in flavor and fragrance for foods, sanitary products and industrial solvents phytocompounds, such as limonene, patchoulol, geranyl acetate, etc., derived from have been widely used. Moreover, essential oil blends are used in bath products and in aromatherapy. Further, many EOs are particularly valued for their medicinal properties (Swamy and Sinniah 2015, 2016; Arumugam et al. 2016). For example, menthol EOs are used as natural bug repellent, as well as for treating joint pain, respiratory allergies, muscle pain, headache, hair growth, and fever relief, as well as in cancer treatment (menthol protects against cell death and DNA damage).
EOs or natural products are widely used as fragrances. However, their application in human health, agricultural industry, and environmental protection requires better understanding of their biological properties. Some of the EOs and their chemical constituents are viable as alternatives to the synthetic compounds, presently widely used in the chemical industry. This is because EOs are not associated with harmful side effects (Carson and Riley 2003). In nature, EOs play an important role in providing plant protection against pathogenic bacteria, viruses, and fungi and preventing the attack by insect pests. In addition, EOs can attract or repel insects when present in pollen and seeds. To protect chemical compounds’ ecological equilibrium, the use of EOs in pharmaceutical, food, bactericidal, and fungicidal is becoming more prevalent in recent times. EOs yielding medicinal and aromatic plants are normally native to warm countries, where they represent an important traditional pharmacopeia (Arumugam et al. 2016). EOs are less dense than water. They are volatile and mostly colorless, as well as soluble in organic solvents. All plant parts, such as buds, leaves, fruits, bark, root, stems, twigs, and flowers, can contain EOs.
Different methods can be applied for essential oil extraction, such as hydrodistillation, steam distillation, and solvent extraction (including liquid carbon dioxide or microwave extraction). For example, hydrodistillation or steam distillation is typically used for Citrus and Lamiaceae family members. Various factors, such as the extraction method, geographical conditions, type of soil, plant material, and harvesting stage, are being reported to influence on the occurrence of number of chemical constituents in EOs and variations in EO quality and yield (Masotti et al. 2003; Angioni et al. 2006; Swamy and Sinniah 2015; Swamy et al. 2016). In order to ensure a constant chemical composition, quality, and quantity, EOs should be extracted under the same conditions, such as using same plant organs, extraction method, harvesting period or season, and growing plants in the same soil types. Many of the EOs are commercialized and chemotyped by gas chromatography mass spectrometry (GC-MS), and the results have been published in international organizations like the ISO, WHO, EP (European pharmacopoeia), and Council of Europe (Smith et al. 2005) to protect good grade and amount of EOs.
Apiaceae, Lamiaceae, Myrtaceae, Poaceae, and Rutaceae families are of particular importance for medicinal applications. For example, some of the EOs, like anise, caraway, black caraway, clove, oregano, cumin, coriander, sage, basil, dill, lemon balm, peppermint, thyme, and tea oils, already have widespread medicinal applications. Some of the essential oil containing plant families, like Liliaceae, Fabaceae, Pinaceae, Piperaceae, Cupressaceae, and Hypericaceae, also exhibit a considerable medicinal potential (Hammer and Carson 2011). The aim of the present chapter is to discuss the specific chemical compounds occurring in EOs, their medical applications, and economic importance.
9.2 Chemical Composition of Essential Oils
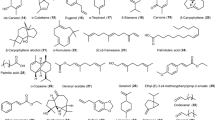
EOs are volatile liquids that are rarely colored. They are complex mixtures comprising of different concentrations, quantities, and compositions of 20–60 chemical components (Bakkali et al. 2008). Among these, two to three major chemical compounds are known to occur in prominent concentrations (20–70%), while other components are present in less concentration. For example, menthone (39.55%) and isopulegone (30.49%) are the major components of Mentha longifolia essential oil (Nagarjuna et al. 2017), while cinnamyl acetate (41.98%) is extracted from Cinnamomum zeylanicum Blume (Jayaprakash et al. 2000). Similarly, eugenol (86.02%) is obtained from Cinnamomum verum (Patel et al. 2013), whereas linalool (46.97%) and 1,8-cineole (14.97%) are the major components of Ocimum basilicum (Santoro et al. 2007a, b). Likewise, Pogostemon cablin essential oil possesses mainly the patchouli alcohol, also called as patchoulol (32–37%), a tricyclic sesquiterpene (Swamy and Sinniah 2015). While, the leaf essential oil of Plectranthus amboinicus is rich in carvacrol (43%), thymol (7%) (a phenolic monoterpenes) (Arumugam et al. 2016). Mainly, higher concentrations of chemical constituents govern the biological properties of the EOs. Most of the EOs also constitute low molecular weight chemical components, such as terpenes and terpenoids (Croteau et al. 2000; Betts 2001; Bowels 2003; Pichersky et al. 2006; Swamy and Sinniah 2015; Arumugam et al. 2016). Terpenes and terpenoids, along with other of aliphatic and aromatic chemical constituents, are shown in Fig. 9.1.
Terpenes are biosynthetically derived isoprene (2-methyl 1,3-butadiene) units. The molecular formula of isoprene unit is C5H8. Thus, the basic molecular formula of terpenes comprises of multiples of isoprene units, such as (C5H8)n, where n denotes the number of isoprene units. This is known as biogenetic rule or C5 rule. The isopentenyl diphosphate (IPP) molecule has a major role in the terpenes biosynthesis. As chains of IPP units accumulate (acyclic or cyclic), the resulting terpenes are classified based on the size into hemiterpenes (C5), monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), triterpenes (C30), and tetraterpenes (C40). Terpene that is having oxygen is called oxygenated terpenoid. EOs consist of 90% monoterpene (a combination of two isoprene units) molecules, thus allowing for a variety of structures and functions. Sesquiterpenes are also present in EOs, but they are not like monoterpenes as main. Sesquiterpenes can also assume a variety of structures and functions, as shown in Table 9.1. When a chemical constituent is optically active, the two optical isomers are frequently obtained in various plants. For example, optical isomers of (+)-α-pinene and (−)-β-pinene can be obtained from P. palustris and P. caribaea, respectively, while optical isomers of linalool obtained (−)linalol is sourced from C. sativum and (+)linalool from a few C. camphora plants. Sometimes, a racemic mixture is also encountered, whereby (±)-citronellol is very common. In particular, (+) citronellol from Eucalyptus citriodora and the rose and geranium EOs (−) citronellol form is common.
The EOs terpenes are major chemical constituents than aromatic hydrocarbons. In plants, the biosynthetic pathways of aromatic hydrocarbons (phenyl propane) and terpene derivatives are completely different. For example, cinnamaldehyde is a major compound in cinnamon and clove oil, while eugenol is a minor constituent. Aromatic hydrocarbons generally occur in plants, namely, C. sativum, S. aromaticum, P. anisum, F. vulgare, M. fragrans, P. crispum, S. albidum, and L. verum , and some plant families, such as Myrtaceae, Rutaceae, and Lamiaceae. In addition, EOs constitute aldehydes (cinnamaldehyde, cuminic aldehyde, perillaldehyde, etc.), alcohols (cinnamic alcohol, terpinenol, menthol, etc.), phenols (eugenol, carvacrol, etc.), and methoxy derivatives (anethole, estragole, etc.); compounds occur on aromatic hydrocarbons.
9.3 Biological Effects of Essential Oils
At present, around 60 plant families are known to produce EOs, which are valued in medicinal, pharmaceutical, flavor and fragrance, and agricultural industries. Several plant species belonging to the Apiaceae, Alliaceae, Asteraceae, Lamiaceae, Myrtaceae, Poaceae, and Rutaceae family produce EOs with medicinal and industrial values (Vigan 2010; Hammer and Carson 2011). Details of EOs produced from medicinal and aromatic plants and their medicinal importance are mentioned in Table 9.2. EOs are rich in terpenes, while phenylpropanoids more frequently occur in Apiaceae, Alliaceae, Lamiaceae, Myrtaceae, and Rutaceae plant families (Chami et al. 2004). These family plants are used for the commercial level manufacture of EOs. For example, patchoulol, coriander, anise, dill, and fennel EOs are extracted from P. cablin, C. sativum, P. anisum, A. graveolens and F. vulgare, respectively. These EOs are well known for their antimicrobial and anticancer activities. The plants belonging to the Lamiaceae and Apiaceae family are popular for antimicrobial, anticancer, antibacterial, antimutagenic, anti-inflammatory, and antioxidant activities (Swamy and Sinniah 2015; Swamy et al. 2016). Some of the plants from Lamiaceae family produce EOs (Burt 2004; Hammer et al. 2006; Hussain et al. 2008), such as M. piperita, R. officinalis, O. basilicum, S. officinalis, M. officinalis, S. hortensis, T. vulgaris, L. angustifolia, and O. vulgore (Swamy and Sinniah 2015; Swamy et al. 2016). Likewise, EOs from Lauraceae and Myrtaceae families also exhibit antimicrobial, antitumor, anticancer, antibacterial, and antiviral activities (Burt 2004; Hammer et al. 2006 ). Cinnamomum verum (Lauraceae) and Syzygium aromaticum (Myrtaceae) EOs are particularly rich in eugenol. Many EOs have been screened for their pharmacological potential, and in the following sections, studies showing different pharmacological activities of EOs are discussed.
9.3.1 Essential Oils as Antibacterial Agents
Many essential oils have been investigated for their antibacterial and antifungal activities, as well as their potential against Gram-positive and Gram-negative bacteria (Swamy et al. 2016). EOs show good antibacterial properties against Salmonella, Staphylococcus, and other bacterial pathogens. Thus, it is essential to study their effects as very good alternatives to antibiotics (Fujita et al. 2015; Karbach et al. 2015; Sienkiewicz et al. 2015). O. basilicum essential oil exhibits good antibacterial properties against Gram-positive bacteria (Al Abbasy et al. 2015; Avetisyan et al. 2017). In the investigations of antibacterial effects, manuka oil has been shown to exhibit good antibacterial activity. Similarly, eucalyptus, rosmarinus, Lavandula oil, and tree oil were found effective against Streptococcus mutans, S. sobrinus, Fusobacterium nucleatum, and Porphyromonas gingivalis (Takarada et al. 2004). Tea tree (Melaleuca alternifolia) oil is demonstrated to be sensitive to 15 genera of oral bacteria, indicating its potential applications in oral hygiene (Hammer et al. 2003). Pittosporum undulatum and Hedychium gardnerianum EOs show the highest antibacterial activities against Staphylococcus epidermis and S. aureus.
Despite the discovery of new antibiotics, bacterial infectious/diseases still pose a serious threat to human health, predominantly due to the appearance of antibiotic-resistant strains. In addition, as the global people continues to expand, this will result in a greater prevalence of bacterial diseases, low immunity, and increased drug resistance. Therefore, bacterial infections will be more likely to be fatal (Ahmad and Beg 2001; Hall-Stoodley et al. 2004; Swamy et al. 2016; Rudramurthy et al. 2016). To decrease the risk of infectious diseases, high concentrations of antibacterial drugs are usually employed, resulting in toxicity and adverse side effects. Hence, there is a need to explore alternative approaches and develop new molecules against human pathogenic bacteria (Galvao et al. 2012; Rudramurthy et al. 2016). In this context, plant EOs exhibit a good potential due to their proven activity against both Gram-positive and Gram-negative bacteria as shown in Table 9.3 (Edris 2007; Lang and Buchbauer 2012; Hassanshahian et al. 2014; Teixeira et al. 2013). Some EOs show potential antibacterial activity against Gram-positive bacteria only, such as Santalum album, Leptospermum scoparium, and Chrysopogon zizanioides (Hammer and Carson 2011). According to the available evidence, cinnamon, lemongrass, thyme, clove, rosewood, orange, rosemary, peppermint, bay, basil, and eucalyptus EOs exhibit the most effective antimicrobial activity. EOs are very active at ˂1% minimum inhibition concentrations (MICs). Escherichia coli exhibits zone of inhibition at 0.02, 0.04, and 0.06% concentrations against clove, grass, oregano, bay, and thyme EOs, respectively (Hammer and Carson 2011). Some EOs show less activity, but their major constituent molecules are observed to possess higher activity. For example, eugenol, carvacrol, and 4-terpinenol display greater antibacterial activity than their corresponding EOs. In extant literature, phenols and aldehydes are reported potential antimicrobial activity (Lambert et al. 2001; Ultee et al. 2002; Carson et al. 2006). A large number of the EOs have been shown to be successful against drug-resistant strains, antibiotics, and biofilms (May et al. 2000; Bozin et al. 2006; Galvao et al. 2012). EOs of A. fragrantissima, A. ligustica, A. absinthium, A. biennis, A. cana, A. dracunculus, A. longifolia, A. frigida, C. officinalis, C. sativum, C. cyminum, C. longus, D. littoralis, E. erythropapps, E. rostkoviana, F. margarita, L. nobilis, L. angustifolia, L. longifolia, J. excelsa, M. suaveolens, N. sativa, O. vulgare, T. vulgaris, O. basilicum, P. cablin, T. kotschyanus, S. cumini, T. ammi, and S. sparganophora show potential antibacterial activity against S. aureus, S. epidermidis, E. coli, S. mutans, B. thermosphacta, L. innocua, L. monocytogenes, P. putida, B. cereus, B. subtilis, N. gonorrhoeae, K. pneumoniae, C. botulinum, C. perfringens, S. sonnei, S. lutea, P. putida, M. flavus, L. innocua, E. faecalis, and S. putrefaciens (Lopes-Lutz and Alviano 2008; Maggi et al. 2009; Matasyoh et al. 2009; Begnami et al. 2010; Runyoro et al. 2010; Ait-Ouazzou et al. 2012; Bejaoui et al. 2013; Teixeira et al. 2013; Yang et al. 2013; Amatiste et al. 2014; Andrade et al. 2014; Bilcu et al. 2014; Bisht et al. 2014; Flores et al. 2014; Kasim et al. 2014; Khoury et al. 2014; Petretto et al. 2014; Pullagummi et al. 2014; Santurio et al. 2014; Singh et al. 2014;; yousefbeyk et al. 2014; Zeedan et al. 2014; Ahmadi et al. 2015; Beatovia et al. 2015; Santos et al. 2015; Ibrahim et al. 2015a, b; Novy et al. 2015).
9.3.2 Essential Oils as Antioxidant Agents
Modern era has brought about different health problems, such as noncommunicable diseases (e.g., cancer, diabetes, and Alzheimer’s, Parkinson’s, and heart diseases) which are attributed to oxidative stresses. EOs exhibit a significant antioxidant activity due to their phtocompounds, such as flavonoids, terpenoids, and phenolic compounds (McCord 2000; Tomaino et al. 2005; Edris 2007; Ferguson and Philpott 2008; Ruan et al. 2008; Miguel 2010; Cavar et al. 2012; Andrade et al. 2013; Sanchez-Vioque et al. 2013; Aleksic and Knezevic 2014; Bouzabata et al. 2015). Among many EOs, O. majorana, T. filifolia, B. monnieri, C. longa, S. cryptantha, A. millefolium, S. multicaulis, M. officinalis, M. alternifolia, Ocimum, and Mentha sp. have been reported to possess significant antioxidant activity (Mau et al. 2003; Tepe et al. 2004; Kim et al. 2004; Maheshwari et al. 2006; Maestri et al. 2006; Gulluce et al. 2007; Tripathi et al. 2007; Politeo et al. 2007; Hussain et al. 2008; Aqil et al. 2012; Mohamed et al. 2013; Toscano-Garibay et al. 2017;). Thymol and carvacrol containing EOs in particular show strong antioxidant properties (Tepe et al. 2004; Miguel 2010). Likewise, EOs of Cuminum cyminum, Petroselinum sativum, S. cumini, and Coriandrum sativum also exhibit efficient antioxidant (Romeilah et al. 2010; Eshwarappa et al. 2014). In addition, clove oil shows a much stronger antioxidant and radical scavenging activity compared to cinnamon, basil, oregano, nutmeg, and thyme EOs (Tomaino et al. 2005).
9.3.3 Essential Oils as Anticancer Agents
As cancer is a growing problem globally, many curing and preventive therapies have been developed over the years. In the human body, cancer is characterized by uncontrolled proliferation of abnormal cells. The malignant cells have the potential to be metastatic, requiring urgent treatment, such as radiotherapy, and chemotherapy. Among these, chemotherapy treatment is most challenging and can be difficult for patients to tolerate due to extreme side effects. Therefore, many alternative treatments and therapies have been explored. In both developed and developing countries, herbal medicines have been historically used for traditional medicinal treatments. For thousands of years, African and Asian populations have used medicinal plants in folk medicine. Even developed nations are starting to recognize the health benefits medicinal plants, according to the WHO. Plants identified for their anticancer properties have been chemically characterized to reveal the occurrence of many bio-active compounds, such as polyphenols, taxols, brassinosteroids, etc.
Flavonoids, tannins, curcumin resveratrol, and gallocatechins are some of the plant-derived polyphenolic compounds possessing anticancer properties. A regular intake of healthy diet can improve the human health as they are rich in natural antioxidants and can thus reduce the risk of developing cancer. For example, gallocatechins found in green tea and resveratrol found in peanuts, grapes, and red wine are effective in preventing cancer (Azmi et al. 2006; Apostolou et al. 2013). Polyphenols have been shown to regulate cancer cell growth through modifications of acetylation, methylation, or phosphorylation processes involved in the regulation of chromatin function. For example, C. longa EOs has been treated various cancer cell lines shown to suppression the tumor necrosis factor (TNF) impression along interaction with various stimuli (Gupta et al. 2014). Flavonoids, another class of plant secondary metabolites, possess therapeutic efficacy and scientifically prove to impart health benefits to humans. In traditional Chinese medicine, litchi leaf (Litchi chinensis) is used in cancer treatment (Cao et al. 2013; Wen et al. 2014). Litchi leaf is rich in flavonoids, such as flavones, flavonols, and chalcones (Wen et al. 2014). The essential oil of Dryopteris erythrosora showed potential anticancer activity against human lung cancer cells (A456 cell line) (Kloog and Cox 2004; Cao et al. 2013).
Plant-derived compounds also show potential activity against cancer cell lines. These compounds occur naturally and are easily available and nontoxic to the healthy human cells. Thus, they could be administrated to patients orally (Cornblatt et al. 2007; Amin et al. 2009). Still, there are a few exceptions, such as glycosides, lectins, saponins, lignans, lectins, and taxanes (Unnati et al. 2013). BR compounds, such as sulforaphane, isothiocyanates, isoflavones, and pomiferin, are considered histone deacetylase (HDAC) inhibitors. For example, sulforaphane has been used against breast cancer proliferation (Pledgie-Tracy et al. 2007; Seidel et al. 2012). In the studies on inhibition of cancer cell proliferation, taxols (plant molecules) were shown effective against different types of malignancies, like colon cancer, gastric cancer, breast cancer, leukemia, and human liver and pulmonary tumors (Edris 2007; Kaefer and Milner 2008; Hamid et al. 2011). In Table 9.4, details of different medicinal and aromatic plant EOs possessing anticancer properties are cited. For example, Cymbopogon martini EOs are rich in geraniol. Geraniol is used against ion homeostasis which interferes with membrane function as well as cancer cell line signaling. Atractylodes lancea oils are used for the treatment of malignant tumors (Tsuneki et al. 2005), whereas Myristica fragrans (M. fragrans) oils contain myristicin and are used for their hepatoprotective activities (Morita et al. 2003). Lemongrass oil mainly consists of citral, which is used for in vivo studies on the initial phases of hepatocarcinogenesis (Puatanachokchai et al. 2002). EOs extracted from E. ciliata shows potential anticancer activity against human glioblastoma (U87), pancreatic cancer (Panc-1), and triple negative breast cancer (MIDA-MB231) (Pudziuvelyte et al. 2017). A. fragrantissima EOs show potential anticancer activity against human breast cancer cell line (MCF-7) and colon cancer cell line (HCT116) and the IC50 (μg/ml) MCF7 for 0.51 and HCT116 for 0.62 μg/ml. Compared to solvent extracts, EOs have shown better anticancer activity (Choucry. 2017), and also A. aucheri, M. communis, and O. vulgare EOs show efficient anticancer activity against human promyelocytic leukemia cell lines (HL-60, NB4), lymphocytes, tumor HeLa cells, and Ehrlich ascites carcinoma cells (EACC) (Taherkhani 2015; Romeilah 2016). Orange peel EOs have been investigated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay against lung cancer cell line (A549) and prostate cancer cell line (22RV-1); it showed good inhibition of the proliferation of a lung and prostate cancer cell lines (Yang et al. 2017).
Individual chemical constituents in EOs show potential anticancer and antitumor activity such as D-limonene (in vivo), geraniol (in vitro and in vivo), thymol and carvacrol (in vitro), thymoquinone (in vitro and in vivo), farnesol (in vivo and in vitro), (−)-α-bisabolol (in vitro and in vivo), (−)-β-elemene (in vitro and in vivo), (−)-β-caryophyllene (in vitro), α-humulene (in vitro), nerolidol (in vitro), germacrone (in vitro), and eugenol (in vitro and in vivo) studies which show efficient anticancer activity against stomach (mice), lung (mice), breast (rats, MCF-7), prostate (PC3, mice), skin (B16F10, rats, SCC VII, A431), colon (Caco-2, mice, rats, V79), pancreas (MIAPaca-2, hamsters), kidney (rats), mouth (hamsters), bones (MG63), brain (DBTRG-05MG), liver (HepG2, Caco-2), and blood (HL60) cancer types (Malíková et al. 2008; Lesgards et al. 2014; Bayala et al. 2014; Gautam et al. 2014) respectively.
9.3.4 Essential Oils as Antifungal Agents
Many EOs have been investigated for their antifungal activities. Fungi are very difficult to target because of cellular and molecular levels, human pathogenic fungi, and eukaryotes which are very similar with their host. However, eukaryotes and human pathogenic fungi and their hosts have similarities at molecular and cellular levels (Routh et al. 2011). Some of the human fungal pathogens, such as Aspergillus spp., Cryptococcus sp., and Candida spp., are very problematic for immunocompromised patients. Hence, limited numbers of antifungal drugs are available against fungi (Kathiravan et al. 2012). Currently, the prescribed drugs are resistant to fungal strains and may lead to cause biofilm infections and adverse side effects. Consequently, fungal infections are associated with high morbidity and mortality rates (Sardi et al. 2013; Swamy et al. 2016). Plant EOs that are effective against human pathogenic fungi, plant fungi, and yeast are mentioned in Table 9.5. Based on the EO efficiency, the zone of inhibition to different the targeted organisms varies. For instance, EOs of plants, such as coriander, anise, and fennel, though belonging to the same family, i.e., Apiaceae, show differences in their antifungal activity against Candida albicans with MICs (minimum inhibitory concentrations) of 0.25%, 0.5%, and 1%, respectively. Among the EOs, Japanese mint, ginger grass, cinnamon, lemongrass, clove, anise, and geranium oils are particularly encouraging against C. albicans and the essential concentration range between 0.01% and 0.15% (Devkatte et al. 2005; Hammer and Carson 2011). EOs can rapidly inhibit growth of dermatophytes and their spores. This is an attribute to the occurrence of high levels of phytocompounds, i.e., α-bisabolol (an alcohol) and eugenol (a phenylpropanoid), in their EOs (Bajpai et al. 2009; Maxia et al. 2009; Pragadheesh et al. 2013). C. citratus EOs show a potential activity against many filamentous fungi at the concentration range of 0.006–0.03%. Also, it inhibits the growth of Aspergillus niger, A. flavus, P. chrysogenum, and P. verrucosum below 1% concentration (Viuda-Martos et al. 2008). Eucalyptus oil rich in citral, geraniol, geranyl acetate, and citronelol components was found to inhibit the growth of C. albicans by blocking the S phase of its life cycle (Zore et al. 2011a). All chemical constituents of tea tree (Melaleuca alternifolia) oil, except β-myrcene, exhibit in vitro antifungal activity. Tea tree oils show potential antifungal activity against dermatophytes and filamentous fungi (Hammer et al. 2003). Likewise, the growth of A. niger was significantly by the EOs of Melaleuca ericifolia fresh leaves. EOs from various plants that are generally used for the flavor and fragrance including Mentha piperita, Brassica niger, Angelica archangelica, and Cymbopogon citratus have been tested for their antifungal activity and found that they exhibit very strong antifungal activity. EOs extracted from A. marmelos, C. sativum, D. foetidum, E. erythropappus, E. rostkoviana, F. vulgare, G. spathulata, M. alternifolia, M. pulegium, M. communis, N. sativa, O. vulgare, P. graveolens, P. cablin, R. officinalis, S. sclarea, and S. aromaticum show efficient antifungal activity of C. albicans, A. niger, F. oxysporum, C. gattii, C. neoformans, S. cerevisiae, A. alternate, F. oxysporum, A. flavus, T. rubrum, E. floccosum, C. neoformans, M. furfur, M. canis, M. sympodialis, M. gypseum, R. rubra, T. rubrum, T. tonsurans, C. zemplinina, K. apiculata, T. phaffii, F. moniliforme, F. graminearum, P. viridicatum, T. violaceum, B. cinerea, P. oryzae, C. tropicalis, C. krusei, and C. glabrata (Hammer et al. 2002; Matasyoh et al. 2009; Begnami et al. 2010; Hammer and Carson 2011; Berka-zougali et al. 2012; Wang et al. 2012; Santos et al. 2015; Hammer et al. 2012; Hristova et al. 2013; Kocevski et al. 2013; Petretto et al. 2014; Pullagummi et al. 2014; Singh et al. 2014; Ibrahim et al. 2015a, b; Latifah-Munirah et al. 2015; Novy et al. 2015; Papajani et al. 2015;; Venturi et al. 2015; Souza et al. 2016). Most research on the antifungal activity is in the initial phases of clinical trials. Thus, EOs are functioning as an alternative for the existing antifungal drugs (Samber et al. 2015).
9.3.5 Essential Oils and Their Antiviral Activity
In addition to antimicrobial and anticancer activity, EOs also exhibit antiviral activity (see Table 9.6). EOs show potential inhibition against viral replication, as they consist of monoterpenes, sesquiterpenes, and phenylpropanoid chemical constituents (Astani et al. 2011). Eucalyptus, thyme, and M. alternifolia (tea tree oil) EOs show potential antiviral activity against herpes virus. Their activity has also been established against viral envelope structures (Carson et al. 2001; Reichling et al. 2005; Schnitzler et al. 2007, 2011). For example, oregano oils exhibit potential antiviral activity against herpes simplex virus (HSV) and yellow fever virus (Meneses et al. 2009). Monoterpenes of EOs, such as isoborneol, have been shown effective against HSV-1 virus (Armaka et al. 1999). HSV-2 virus is more delicate than HSV-1 virus to the pine, tea tree, manuka, lemon balm, and santolia EOs in small concentrations, i.e., in the range between 0.0001 and 0.0009% of the IC50 value (Garcia et al. 2003; Saddi et al. 2007; Koch et al. 2008; Schnitzler et al. 2011). In a report published by Benencia and Courreges (2000), clove oil eugenol was used against HSV-induced keratitis. Antiviral activity through plaque reduction assay against African green monkey, EOs such as Melaleuca armillaris (M. armillaris), Melaleuca ericifolia (M. ericifolia), and Melaleuca styphelioides (M. styphelioides) showed 99%, 91.5% and 92% effectiveness, respectively (Deans and Ritchie 1987). A. fragrantissima, A. arborescens, F. margarita, G. marifolia, H. mutabilis, L. salviifolia, M. officinalis, M. mollis, O. campechianum, P. cablin, and T. ammi EOs show potential antiviral activity against ORF virus (a parapox virus), HSV-I, avian influenza A virus (H5N1), HSV-1, HSV-2, avian influenza virus (AIV), subtype (H9N2), influenza A (H2N2) virus, and Japanese encephalitis virus (JEV) (Allahverdiyev et al. 2004; Sinico et al. 2005; Wu et al. 2011, 2013; Kiyohara et al. 2012; Zeedan et al. 2014; Ibrahim et al. 2015a, b; Venturi et al. 2015 Roy et al. 2015; Brand et al. 2016).
9.3.6 Essential Oils as Antidiabetic Agents
Diabetes mellitus (DM), generally known as diabetes, is a metabolic disorder that is becoming increasing prevalent in modern society due to unhealthy lifestyle. Insulin-dependent diabetes is called Type-I diabetes, its causes do not produce insulin, and it damages the pancreas. Type-II diabetes is non-insulin diabetes, it causes insulin resistance in the liver, peripheral tissues, and reduced β-cell mass (Srinivasan and Ramarao 2007; Matthaei et al. 2000), respectively. Diabetes causes changes in metabolism of carbohydrates, fats, and proteins, which results in hyperglycemia, glycosuria, and hyperlipidemia (Baradaran et al. 2013; Behradmanesh et al. 2013; Mirhoseini et al. 2013). Diabetes can be successfully managed with a proper diet and consuming drugs. Also, the use of traditional medicines can effectively control the risk of diabetes with reduced side effects. The essential oil of Vaccinium arctostaphylos containing high levels of anthocyanoside myrtillin is used in the traditional medicine for diabetes control (Murray 1997). In mouse models, Securigera securidaca essential oil had significantly found effective in reducing the blood glucose level (Hisseinzadeh et al. 2002). Likewise, Gymnema sylvestre essential oil has been reported to be effective against both Type 1and Type 2 diabetes. It influenced the absorption of glucose in the digestive track and regenerated and proliferated β-cells (Lirussi et al. 2002; Amini et al. 2012; Madihi et al. 2013; Nasri et al. 2013; Nasri and Shirzad 2013; Rafieian-Kopaei et al. 2013). Similarly, Atriplex halimus has been used for Type 2 diabetes treatment in animal models, as it contains fibers, proteins, and other trace elements, such as chromium. Consumption of A. halimus, 3 grams dried leaves can reduce blood sugars in Type 2 diabetes patients (Bahmani et al. 2014). Camellia sinensis seed oil containing flavonoids like catechin, epicatechin, epigallocatechin, and gallocatechin can increase insulin levels, and their polyphenolic compounds act as antioxidants (Asadi et al. 2013; Parsaei et al. 2013). EOs or natural products have had major impact on diabetes, whereby flavonoids, metformin, anthocyanin, catechin, quercetin, flavone, phenylpropanoids, lipoic acids, and coumarin metabolites are particularly effective (Arabbi et al. 2004; Rafieian-Kopaei et al. 2013; Singab et al. 2014).
Foeniculum vulgare (F. vulgare) EOs show potential antidiabetic activity in rates corrected the hyperglycemia from 162.5 ± 3.19 mg/dl to 81.97 ± 1.97 mg/dl and also high activity of serum glutathione peroxide from 59.72 ± 2.78 U/g Hb to 99.60 ± 6.38 U/g Hb (El-Soud et al. 2011). Yen et al. (2015) from Taiwan described that different families of commercial EOs purchased from local market such as (Lamiaceae, Rutaceae, Myrtaceae, Cupressaceae, Piperaceae, Burseraceae, Zingiberaceae, Geraniaceae, Apiaceae, Asteraceae, Pinaceae, and Lauraceae) show potential antidiabetic activity after 24 hrs in culture medium of 3 T3-L1 adipocytes. Alpha amylase inhibition assay shows efficient antidiabetic activity with S. aromaticum and C. cyminum EOs (Tahir et al. 2016).
9.3.7 Essential Oils as Insect Repellents
Insect repellent is a substance applied to the surface of skin or on clothing to prevent insect bites (Blackwell et al. 2003; Choochote et al. 2007; Nerio et al. 2010). Generally, repellents work as vapor barriers, preventing the insects from coming into contact with the surface. Currently, many synthetic chemicals are used to control insects and arthropods; however, they are causing concerns regarding human health and environmental pollution. Plant molecules or plant EOs are an alternative to this and are used as insect and arthropod repellants. Because of their natural origin, they are relatively safe for human health and environmental friendly. These insect repellent plant molecules have been isolated from a large number of plants, mainly from their essential oils. Some of them have been commercialized in certain formulations as insect repellents (Chaubey 2007). Synthetic insect repellents are widely used to prevent infestation of stored grains, fruits, and other cellulosic materials by different pests, mostly arthropods. Similar circumstances occur for animals and human health. To control insects different insecticides have been used; these insecticides transmit to human pathogens. These days many of these insects are resistant to the chemicals, and it should be applied to larger amounts, due to the temperature changes, global warming, etc.; actually global warming has moved the mosquitoes to transmit malaria, dengue, and yellow fever into high altitude, some temperatures affecting these diseases. EOs are volatile and complex mixtures of hydrocarbons (monoterpenes and sesquiterpenes) with different functional groups (ethers, alcohols, aldehydes, esters, ketones, phenols, and phenol ethers). However, these chemicals can act as insect repellents, in particular if combined with other natural products. For example, to increase protection time, vanillin could be used with C. winterianus EOs. Among plant families, Ocimium spp., Eucalyptus spp., and Cymbopogon spp. are widely used as insect repellents. Similarly, some major compounds, such as citronellol, camphor, thymol, α-pinene, and limonene, have shown good insect repellent activity. Among the plant families that contain about 3000 EOs, approximately 10% of these EOs have commercial importance in pharmaceutical, food, and cosmetics industries. The United States Food and Drug Administration (FDA) considers EOs as insect repellents that are safe for human health and environmentally friendly (Trongtokit et al. 2005; Nerio et al. 2010). EOs from a large number of plant families showing the potential insect repellent activity are shown in Table 9.7.
Worldwide, Cymbopogon spp. produce the most widely used natural insect repellent. In tropical or forest regions, these families are used as mosquito repellents (Trongtokit et al. 2005; Moore et al. 2007). EOs extracted from these plant families have been tested against the arthropod, Cymbopogon excavates. The results showed that EOs were 100% efficient for insect repellent activity up to 2 hrs. Likewise, when applied against Anopheles arabiensis, the repellent efficacy of these oils was decreased by 59.3% after 4 h (Govere et al. 2000). Vanillin (5%) mixed with C. winterianus EOs shows 100% efficacy up to 6 hrs against A. aegypti, C. quinquefasciatus, and A. dirus (Tawatsin et al. 2001). However, C. nardus and C. flexuosus oils were inactive against Cydia pomonella (Lepidoptera) and Lasioderma serricorne (cigarette beetle) (Landolt et al. 1999). Eucalyptus oils show high repellent activity against Mansonia mosquitoes, Pediculus humanus capitis, Ixodes ricinus, and Aedes albopictus (Hadis et al. 2003; Yang and Ma 2005; Jaenson et al. 2006; Toloza et al. 2008), as well as moderate activity against A. aegypti and C. pomonella (Trongtokit et al. 2005; Gillij et al. 2008), but no activity against L. serricorne (Hori 2003). Ocimum spp. EOs are also found as an efficient insect repellent (Padilha de Paula et al. 2003). The insect pests, such as A. aegypti, A. dirus, and C. quinquefasciatus, were potentially repelled by the O. americanum) essential oil (Tawatsin et al. 2001). Similarly, O. selloi, O. basilicum, and O. gratissimum EOs were potentially effective repellents against A. braziliensis (Padilha de Paula et al. 2003) while exhibiting no repellent activity against L. serricorne and C. pomonella (Landolt et al. 1999; Hori 2003). Fresh leaves of EOs extracted from O. sanctum, M. piperita, E. globulus, and P. amboinicus oils show potential insect repellent against Aedes aegypti (Lalthazuali and Mathew 2017). Some of the EOs such as J. procera, C. citrates, C. zeylanicum, R. officinalis, Z. officinale, A. marmelos, L. acidissima, S. indicus, S. amaranthoides, C. odorata, D. elata, C. longa, P. heyneanus, and Z. limonella show against Anopheles arabiensis, Culex tritaeniorhynchus, Anopheles subpictus, Culex quinquefasciatus (mosquitoes), Aedes aegypti (mosquitoes), Anopheles stephensi (malaria), and Aedes albopictus (mosquitos) strong insect repellent activity in laboratory level, respectively (Govindarajan 2011; Govindarajan et al. 2015; Karunamoorthi et al. 2014; Reegan et al. 2015; Das et al. 2015).
9.3.8 Antimutagenic Properties of Essential Oils
Antimutagenic properties arise due to the inhibition of diffusion of the mutagens into the cells, antioxidant and direct radical scavenging activity, inactivation of mutagens and produced by a mutagen, antioxidant activity of enzyme cell activation, and inhibition of metabolic conversation of P450 of promutagens into mutagens, for instance, by plant extracts (Sharma et al. 2001; Ipek et al. 2005). Plant extract constituents, such as superoxide dismutase (enzyme), glutathione, N-acetylcysteine, retinoids, carotenoids, flavonoids, and other polyphenols, are known to function as reactive oxygen species (ROS) scavengers that can prevent mutagenesis (Racchi 2013; Toscano-Garibay et al. 2017). EOs or their individual components, such as α-bisabolol, aflatoxin B1, 2-aminoanthracene, benzo-a-pyrene, and 2-aminofluorene, potentially inhibit induced mutagenesis and moderate N-oxide, 4-nitroquinoline, and 2-nitrofluorene induced mutagenesis while having less or no induced mutagenesis for sodium azide and nitro-o-phenylenediamine (Gomes-Carneiro et al. 2005). The antimutagenic effect of α-bisabolol is due to the interaction of it with promutagen biotransformation enzymes. Salvia officinalis EOs show the potential inhibition of UVC-induced mutagenesis in Salmonella typhimurium, E. coli, and Saccharomyces cerevisiae (Dudai et al. 2005; Vukovic-Gacic et al. 2006). In an experiment, Idaomar et al. (2002) treated Drosophila melanogaster, with EOs of Ledum groenlandicum, Ravensara aromatica, and Helichrysum italicum significantly reduced the induced mutation frequency. Likewise, Origanum compactum EOs showed a potential antimutagenic effect against the mutagen, urethane (Mezzoug et al. 2007). O. majorana, C. sinensis, C. latifolia, A. aucheri, A. ciniformis, and J. leptoloba EOs show potential antimutagenic activity against S. typhimurium strains TA97a, TA98, TA100, TA100, and TA1535 (Fernandesa et al. 2015; Taherkhani. 2015, 2016; Dantas et al. 2016; Toscano-Garibay et al. 2017).
9.3.9 Phototoxicity
Plant molecules, such as furanocoumarins and coumarins present in grapefruit peel oil and citrus plants oils, are photoactive in nature. For example, citrus EOs contain psoralen (a furocoumarin) that binds with DNA under ultraviolet A (UVA) light, causing it to become highly cytotoxic and mutagenic due to the formation of mono and diadducts in DNA (Lang and Buchbauer 2012; Raut and Karuppayil 2014). However, in dark conditions, cytotoxic and mutagenic activity is not detected (Dijoux et al. 2006; Bakkali et al. 2008). Cytotoxicity and phototoxicity depend on the EOs which contain the chemical constituents that produce free radicals according to the sunlight exposure. Wood oil (Fusanus spicatus) is cytotoxic but not phototoxic (Dijoux et al. 2006; Bakkali et al. 2008; Raut and Karuppayil 2014). EOs producing reactive oxygen species (ROS) destruct the cellular and organelle membranes, prooxidants on proteins, and DNA. Under sunlight, oxygen singlets occur, due to reactive oxygen species producing energy on excitation. This may be due to the destruction of the polysaccharides, nucleic acids proteins, and enzymes and, in some times, causes the formation of adducts with DNA and lipid membranes. With or without light, free radical generation depends on the chemical constituents present in the EOs. Citrus aurantium and Cymbopogon citratus EOs show potential cytotoxic and phototoxic activity (Dijoux et al. 2006). Thus, these photoactive compounds find their application in biomedicine fields including photochemotherapy.
9.3.10 Carcinogenicity of Essential Oils
EOs are potentially cytotoxic without being mutagenic. Thus, carcinogenicity of EOs and their constituents are considered carcinogenic as they are involved in the metabolic activation of secondary carcinogens (Guba 2001). EOs like S. sclarea and M. quinquenervia produce estrogen secretions, which could induce estrogen-dependent cancer. Major chemical compounds, such as flavins, porphyrins, hydrocarbures, and cyanins, are photosensitive molecules that could cause skin cancer. Under ultraviolet A radiation, EOs containing psoralen are also photosensitive to light and therefore could induce cancer and DNA adducts (Lesgards et al. 2014; Romeilah. 2016; Choucry. 2017). EOs of Mentha species containing pulegone as one of the major constituents is known to induce carcinogenicity through metabolism generating glutathione (Zhou et al. 2004). Sassafras albidum and Ocotea pretiosa EOs contain safrole, and Laurus nobilis and Melaleuca leucadendron EOs contain methyl eugenol as the major constituent. Safrole and methyl eugenol (phenylpropenes) could induce carcinogenic metabolites in rodents (Burkey et al. 2000; Liu et al. 2000; Gautam et al. 2014). D-limonene from citrus and estragole from O. basilicum EOs could induce carcinogenic mutations in male rats and mouse models (Anthony et al. 1987; Miller et al. 1983; Bayala et al. 2014; Chen et al. 2017).
9.3.11 Essential Oils as Antiprotozoal Agents
Different protozoan diseases are very important to public health, such as malaria, trichomoniasis, giardiasis, and leishmaniasis caused by Plasmodium sp., Entamoeba histolytica, and Trypanosoma cruzi species, respectively. Availability of antiprotozoal drugs is limited, and their prolonged use causes side effects (Sauter et al. 2012). Hence, plant extracts and EOs could be a safer treatment alternative for protozoal diseases (Sauter et al. 2012). For example, Thymus vulgaris essential oil, thymol, is the major component that inhibits trypanosomal parasite through damage of plasma membrane (Santoro et al. 2007a, b; Saeidnia and Gohari 2012). Compared to T. vulgaris, C. citrates and O. gratissimum EOs show a better antitrypanosomal activity. Terpenoids like thymol, carvacrol, and linalool are known to inhibit Entamoeba histolytica. EOs from M. alternifolia, C. copticum, and L. angustifolia show potential protozoal effects (Carson et al. 2006; Mansoor et al. 2011). C. citrates, Origanum spp., L. multiflora, O. gratissimum, and S. thymbra EOs exhibit a potential antimalarial activity (Tchoumbougnang et al. 2005; El babili et al. 2011). Specifically, C. cajucara, C. citrates, O. gratissimum, A. millefolium, A. abrotanum, C. ambrosioides, P. caribaea, and Piper spp. EOs show antileishmanial activity (Santin et al. 2009; Santos et al. 2010; Ahmed et al. 2011; Tariku et al. 2011).
9.4 Economic Importance of Essential Oils
In the global markets, EOs and their derived molecules are widely used, such as in perfumes and cosmetics, as well as in food, pharmaceutical, and agricultural industries. Throughout Europe, Africa, and Asia, as well as in the USA, EOs have been used in cosmetics (skin creams, body lotions, soaps, perfumes, shampoos, etc.), medicinal industry (pharmaceutical and bulk drug industry, aromatherapy products, and medicinal supplements), and food industry (herbs, spices, and additives) (Nakatsu et al. 2000; Hussain et al. 2008; Teixeria et al. 2013; Swamy and Sinniah 2015, 2016). Essential oil production has exceeded 70,000 tons per annum, and the main producers are the USA, Brazil, China, India, Australia, Indonesia, Malaysia, Thailand, Sri Lanka, South Africa, Italy, Russia, Nepal, Bangladesh, Germany, and Pakistan. For example, clove, celery, basil, and lemongrass EOs are mainly produced in India; rosemary and lavender EOs are usually grown in Spain and France; geranium and rose geranium EOs are endemic to Africa; and tea oils are grown mostly in Australia and South Wales (Bedi and Tanuja 2010). Among 3000 EOs, only 10% have been commercially exploited (Djilani and Dicko 2012). EOs such as basil, orange oil, corn mint, peppermint, eucalyptus, citronella, lemon, clove, camphor, and cumin oils are medicinally important worldwide (Hussain et al. 2008; Bedi and Tanuja 2010). Based on purity, composition, and material sources, their market value can vary considerably. Globally, more than 80% of the people are depending on the plant-based traditional medicine (Akhtar et al. 2014; Arumugam et al. 2016; Swamy and Sinniah 2015; Swamy et al. 2012). Generally, anise and coriander oil cost $20 to $30 per pound, while thyme, dill, and calendula might cost >$100. Moreover, sweet basil, fennel, clary sage, lavender, and caraway EOs could cost $50–$80 per pound. World wide EOs in global markets estimate more than 62 billion USD per year, and it is imagined by the year 2050 to grow up to 5 trillion USD per year. World wide, plant-based (Natural Products) molecules have high demand in the food industry, perfumes, and cosmetic and pharmaceutical substances. Internationally, more than 250 EOs trade at the value of 1.2 billion USD per year (Akhtar et al. 2014; Arumugam et al. 2016; Bhattacharya et al. 2014; Swamy et al. 2016). Still there are wide differences between low- and high-income peoples in the worldwide annual economic demand of pharmaceutical substances. An average spending by each person on pharmaceutical substances per year in low-income countries, such as India, Nigeria and Sri Lanka is about 0.75 US$, 1.2 US$, 0.58 US$, respectively. While, the same in high-income countries like Japan, the USA, and Germany is 38.5 US$, 35.10 US$, and 53.4 US$, respectively. This indicates clearly that there is a significant difference in the expenditure for pharmaceutical products between low- and high-income nations. This is because people in low-income nations largely depend on herb-based medicines (Lu et al. 2011; Bukar et al. 2016).
9.5 Conclusions and Future Prospects
The EOs could act as antimicrobial agents in personal hygiene, air purification, internal use, insecticides and preservations of crops, and food products because of its non-genotoxic risks. However, some EOs showed antimutagenic, anticarcinogenic, photosensitive, and antidiabetic activities. Recent studies on EOs and their chemical constituents, such as polyphenols, flavonoids, and alcohols, showed their potential in reducing tumor cell proliferation, and murine leukemia. They have also been used in cosmetic, food, and pharmaceutical industries, chemotherapy, and prevention of drug resistance against infectious and noninfectious diseases. Although lot of works have been done on the EOs, but still future researches are needed to optimize their doses in combination with existing drugs for the safer use as a medicine for patients.
References
Agarwal V, Lal P, Pruthi V (2008) Prevention of Candida albicans biofilm by plant oils. Mycopathologia 165:13–19
Ahmad I, Beg AZ (2001) Antimicrobial and phytochemical studies on 45 Indian medicinal plants against multi-drug resistant human pathogens. J Ethnopharmacol 74:113–123
Ahmadi R, Alizadeh A, Ketabchi S (2015) Antimicrobial activity of the essential oil of Thymus kotschyanus grown wild in Iran. Int J Biosci 6:239–248
Ahmed SBH, Sghaier RM, Guesmi F, Kaabi B, Mejri M, Attia H, Laouini D, Smaali I (2011) Evaluation of antileishmanial, cytotoxic and antioxidant activities of essential oils extracted from plants issued from the leishmaniasis- endemic region of Sned (Tunisia). Nat Prod Res 12:1195–1201
Ait-Ouazzou A, Lor’an S, Arakrak A, Laglaoui A, Rota C, Herrera A, Pagán R, Conchello P (2012) Evaluation of the chemical composition and antimicrobial activity of Mentha pulegium, Juniperus phoenicea, and Cyperus longus essential oils from Morocco. Food Res Int 45:313–319
Akhtar MS, Degaga B, Azam T (2014) Antimicrobial activity of essential oils extracted from medicinal plants against the pathogenic microorganisms: a review. Biol Sci Pharm Res 2:1–7
Al Abbasy WD, Pathare N, Al-Sabahi NJ, Khan AS (2015) Chemical composition and antibacterial activity of essential oil isolated from Omani basil (Ocimum basilicum Linn). Asian Pac J Trop Dis 5:645–649
Aleksic V, Knezevic P (2014) Antimicrobial and antioxidative activity of extracts and essential oils of Myrtus communis L. Microbiol Res 169:240–254
Allahverdiyev A, Duran N, Ozguven M, Koltas S (2004) Antiviral activity of the volatile oils of Melissa officinalis L. against Herpes simplex virus type-2. Phytomed 11:657–661
Alviano WS, Mendonca-Filho RR, Alviano DS, Bizzo HR, Souto-Padron T, Rodrigues ML, Bolognese AM, Alviano CS, Souza MMG (2005) Antimicrobial activity of Croton cajucara Benth linalool rich essential oil on artificial biofilms and planktonic microorganisms. Oral Microbiol Immunol 20:101–105
Amatiste S, Sagrafoli D, Giacinti G, Rosa G, Carfora V, Marri N, Tammaro A, Bovi E, Rosati R (2014) Antimicrobial activity of essential oils against Staphylococcus aureus in fresh sheep cheese. Italian J Food Saf 3:1696
Amin A, Gali-Muhtasib H, Ocker M, Schneider-Stock R (2009) Overview of major classes of plant-derived anticancer drugs. Int J Biomed Sci 5:1–11
Amini GF, Rafieian-Kopaei M, Nematbakhsh M, Baradaran A, Nasri H (2012) Ameliorative effects of metformin on renal histologic and biochemical alterations of gentamicin-induced renal toxicity in Wistar rats. J Res Med Sci 17:621–625
Andrade MA, Cardoso MG, de Andrade J, Silva LF, Teixeira ML, Resende JMV, Figueiredo ACS, Barroso JG (2013) Chemical composition and antioxidant activity of essential oils from Cinnamodendron dinisii Schwacke and Siparuna guianensis Aublet. Antioxidants 2:384–397
Andrade BFMT, Barbosa LN, Probst IS, Junior AF (2014) Antimicrobial activity of essential oils. J Essent Oil Res 26:34–40
Angioni A, Barra A, Coroneo V, Dessi S, Cabras P (2006) Chemical composition, seasonal variability, and antifungal activity of Lavandula stoechas L. ssp. stoechas essential oils from stem/leaves and flowers. J Agric Food Chem 54:4364–4370
Ansari MA, Vasudevan P, Tandon M, Razdan RK (2000) Larvicidal and mosquito repellent action of peppermint (Mentha piperita) oil. Bioresour Technol 71:267–271
Anthony A, Caldwell G, Hutt AG, Smith RL (1987) Metabolism of estragole in rat and mouse and influence of dose size on excretion of the proximate carcinogen 10-hydroxyestragole. Food Chem Toxicol 25:799–806
Apostolou A, Stagos D, Galitsiou E, Spyrou A, Haroutounian S, Portesis N, Trizoglou I, Hayes AW, Tsatsakis AM, Kouretas D (2013) Assessment of polyphenolic content, antioxidant activity, protection against ROS-induced DNA damage and anticancer activity of Viti vinifera stem extracts. Food Chem Toxicol 61:60–68
Aqil F, Gupta A, Munagala R, Jeyabalan J, Kausar H, Sharma RJ, Singh IP, Gupta RC (2012) Antioxidant and antiproliferative activities of anthocyanin/ellagitannin-enriched extracts from Syzygium cumini L. (Jamun, the Indian Blackberry). Nutr Cancer 64:428–438
Arabbi PR, Genoves MI, Lajolo FM (2004) Flavonoids in vegetable foods commonly consumed in Brazil and estimated ingestion by the Brazilian population. J Agric Food Chem 52:1124–1131
Armaka M, Papanikolaou E, Sivropoulou A, Arsenakis M (1999) Antiviral properties of isoborneol, a potent inhibitor of herpes simplex virus type 1. Antivir Res 43:79–92
Arumugam G, Swamy MK, Sinniah UR (2016) Plectranthus amboinicus (Lour.) Spreng: Botanical, phytochemical, pharmacological and nutritional significance. Molecules 21:369. https://doi.org/10.3390/molecules21040369
Asadi Y, Karimi M, Parsaei P, Ezzati S, Khadivi R, Zamiri A, Rafieian-Kopaei M (2013) Effects of Camellia sinensis ethanolic extract on histometric and histopathological healing process of burn wound in rat. Middle-East J Sci Res 13:14–19
Astani A, Reichling J, Schnitzler P (2011) Screening for antiviral activities of isolated compounds from essential oils. Evidence-Based Compl Altern Med 2011:253643. https://doi.org/10.1093/ecam/nep187
Avetisyan A, Markosian A, Petrosyan M, Sahakyan N, Babayan A, Aloyan S, Trchounian A (2017) Chemical composition and some biological activities of the essential oils from basil Ocimum different cultivars. BMC Compl Altern Med 17:60. https://doi.org/10.1186/s12906-017-1587-5
Azmi AS, Bhat SH, Hanif S, Hadi SM (2006) Plant polyphenols mobilize endogenous copper in human peripheral lymphocytes leading to oxidative DNA breakage: A putative mechanism for anticancer Properties. FEBS Lett 580:533–538
Baananou S, Bouftira I, Mahmoud A, Boukef K, Marongiu B, Boughattas NA (2013) Antiulcerogenic and antibacterial activities of Apium graveolens essential oil and extract. Nat Prod Res 27:1075–1083
Bahmani M, Golshahi H, Saki K, Rafieian-Kopaei M, Delfan B, Mohammadi T (2014) Medicinal plants and secondary metabolites for diabetes mellitus control. Asian Pac J Trop Dis 4:S687–S692
Bajpai VK, Yoon JI, Kang SC (2009) Antifungal potential of essential oil and various organic extracts of Nandina domestica Thunb., against skin infectious fungal pathogens. Appl Microbiol Biotechnol 83:1127–1133
Bakkali F, Averbeck S, Averbeck D, Idaomar M (2008) Biological effects of essential oils-a review. Food Chem Toxicol 46:446–475
Bansod S, Rai M (2008) Antifungal activity of essential oils from Indian medicinal plants against human pathogenic Aspergillus fumigatus and A. niger. World J Med Sci 3:81–88
Baradaran A, Madihi Y, Merrikhi A, Rafieian-Kopaei M, Nasri H (2013) Serum lipoprotein (a) in diabetic patients with various renal function not yet on dialysis. Pak J Med Sci 29:354–357
Bayala B, Bassole IHN, Scifo R, Gnoula C, Morel L, Lobaccaro JMA, Simpore J (2014) Anticancer activity of essential oils and their chemical components-a review. Am J Cancer Res 4:591–607
Beatovic D, Krstic-Milosevic D, Trifunovic S, Šiljegovic J, Glamoclija J, Ristic M, Jelacic S (2015) Chemical composition, antioxidant and antimicrobial activities of the essential oils of twelve Ocimum basilicum L. cultivars grown in Serbia. Rec Nat Prod 9:62–75
Bedi S, Tanuja VSP (2010) A handbook of aromatic and essential oil plants: Cultivation, chemistry, processing and uses. Agrobios, Jodhpur
Begnami AF, Duarte MCT, Furletti V, Rehder VLG (2010) Antimicrobial potential of Coriandrum sativum L. against different Candida species in vitro. Food Chem 118:74–77
Behradmanesh S, Horestani MK, Baradaran A, Nasri H (2013) Association of serum uric acid with proteinuria in type 2 diabetic patients. J Res Med Sci 18:44–46
Bejaoui A, Chaabane H, Jemli M, Boulila A, Boussaid M (2013) Essential oil composition and antibacterial activity of Origanum vulgare subsp. glandulosum Desf. at different phonological stages. J Med Food 16:1115–1120
Benencia F, Courreges MC (2000) In vitro and in vivo activity of eugenol on human herpes virus. Phytother Res 14:495–500
Benkeblia N (2004) Antimicrobial activity of essential oil extracts of various onions (Allium cepa) and garlic (Allium sativum). LWT-Food Sci Technol 37:263–268
Berka-Zougali B, Ferhat MA, Hassani A, Chemat F, Allaf KS (2012) Comparative study of essential oils extracted from Algerian Myrtus communis L. leaves using microwaves and hydrodistillation. Int J Mol Sci 13:4673–4695
Betts TJ (2001) Chemical characterization of the different types of volatile oil constituents by various solute retention ratios with the use of conventional and novel commercial gas chromatographic stationary phases. J Chromatogr A 936:33–46
Bhattacharya R, Reddy KRC, Mishra AK (2014) Export strategy of Ayurvedic products from India. Int J Ayurved Med 5:125–128
Bilcu M, Grumezescu AM, Oprea AE, Popescu RC, Mogoanu GD, Hristu R, Stanciu GA, Mihailescu DF, Lazar V, Bezirtzoglou E, Chifiriuc MC (2014) Efficiency of vanilla, patchouli and ylang essential oils stabilized by iron oxide@C14 nanostructures against bacterial adherence and biofilms formed by Staphylococcus aureus and Klebsiella pneumoniae clinical strains. Molecules 19:17943–17956
Bisht DS, Menon KRK, Singhal MK (2014) Comparative antimicrobial activity of essential oils of Cuminum cyminum L. and Foeniculum vulgare Mill. seeds against Salmonella typhimurium and Escherichia coli. J Essent Oil-Bearing Plants 17:617–622
Blackwell A, Stuart AE, Estambale BA (2003) The repellant and antifeedant activity of Myrica gale oil against Aedes aegypti mosquitoes and its enhancement by the addition of salicyluric acid. Proc R Colloid Phys 33:209–214
Bouzabata A, Cabral C, Goncalves MJ, Cruz MT, Bighelli A, Cavaleiro C, Casanova J, Tomi F, Salgueiro L (2015) Myrtus communis L. as source of a bioactive and safe essential oil. Food Chem Toxicol 75:166–172
Bowles EJ (2003) Chemistry of Aromatherapeutic Oils. Allen & Unwin, Crows Nest
Bozin B, Mimica-Dukic N, Simin N, Anackov G (2006) Characterization of the volatile composition of essential oils of some Lamiaceae spices and the antimicrobial and antioxidant activities of the entire oils. J Agric Food Chem 54:1822–1828
Brand YM, Roa-Linares VC, Betancur-Galvis LA, Duran-Garcıa DC, Stashenko E (2016) Antiviral activity of Colombian Labiatae and Verbenaceae family essential oils and monoterpenes on human herpes viruses. J Essent Oil Res 28:130–137
Buchbauer G (2010) Biological activities of essential oils. In: Baser KHC, Buchbauer G (eds) Handbook of essential oils: Science, technology, and applications. CRC Press, Boca Raton, pp 235–280
Bukar BB, Dayom DW, Uguru MO (2016) The growing economic importance of medicinal plants and the need for developing countries to harness from it: A mini review. IOSR J Pharma 6:42–52
Burkey JL, Sauer JM, McQueen CA, Sipes IG (2000) Cytotoxicity and genotoxicity of methyleugenol and related congeners – a mechanism of activation for methyleugenol. Mutat Res 453:25–33
Burt S (2004) Essential oils: their antibacterial properties and potential applications in foods–a review. Int J Food Microbiol 94:223–253
Calcabrini A, Stringaro A, Toccacieli L, Meschini S, Marra M, Colone M, Salvatore G, Mondello F, Arancia G, Molinari A (2004) Terpinen-4-ol,themain component of Melaleuca alternifolia (tea tree)oil inhibits the in vitro growth of human melanoma cells. J Invest Dermatol 122:349–360
Cao J, Xia X, Chen X, Xiao J, Wang Q (2013) Characterization of flavonoids from Dryopteris erythrosora and evaluation of their antioxidant, anticancer and acetylcholinesterase inhibition activities. Food Chem Toxicol 51:242–250
Carnesecchi S, Bras-Goncalves R, Bradaia A, Zeisel M, Gosse F, Poupon MF, Raul F (2004) Geraniol, a component of plant essential oils, modulates DNA synthesis and potentiates 5-fluorouracil efficacy on human colon tumor xenografts. Cancer Lett 215:53–59
Carson CF, Riley TV (2003) Non-antibiotic therapies for infectious diseases. Commun Dis Intell 27:S143–S146
Carson CF, Ashton L, Dry L, Smith DW, Riley TV (2001) Melaleuca alternifolia (tea tree) oil gel (6%) for the treatment of recurrent herpes labialis. J Antimicrob Chemother 48:450–451
Carson CF, Hammer KA, Riley TV (2006) Melaleuca alternifolia (Tea Tree) oil: a review of antimicrobial and other medicinal properties. Clin Microbiol Rev 19:50–62
Cavalieri E, Mariotto S, Fabrizi C, De Prati AC, Gottardo R, Leone S, Berra LV, Lauro GM, Ciampa AR, Suzuki H (2004) α-Bisabolol, a nontoxic natural compound, strongly induces apoptosis in gliomacells. Biochem Biophys Res Commun 315:589–594
Cavar S, Maksimovic M, Vidic D, Paric A (2012) Chemical composition and antioxidant and antimicrobial activity of essential oil of Artemisia annua L. from Bosnia. Ind Crop Prod 37:479–485
Cermelli C, Fabio A, Fabio G, Quaglio P (2008) Effect of eucalyptus essential oil on respiratory bacteria and viruses. Curr Microbiol 56:89–92
Cetin B, Ozer H, Cakir A, Li D (2009) Chemical composition of hydrodistilled essential oil of Artemisia incana (L.) Druce and antimicrobial activity against food borne microorganisms. Chem Biodivers 6:2302–2310
Chami F, Chami N, Bennis S, Trouillas J, Remmal A (2004) Evaluation of carvacrol and eugenol as prophylaxis and treatment of vaginal candidiasis in an immunosuppressed rat model. J Antimicrob Chemother 54:909–914
Chaubey MK (2007) Insecticidal activity of Trachyspermum ammi (Umbelliferae), Anethum graveolens (Umbelliferae) and Nigella sativa (Ranunculaceae) essential oils against stored product beetle Tribolium castaneum Herbst (Coleoptera: Tenebrionidae). Afr J Agric Res 2:596–600
Chen L, Liu P, Evans TC Jr, Ettwiller LM (2017) DNA damage is a pervasive cause of sequencing errors, directly confounding variant identification. Science 355:752–756
Cheng S, Chang H, Wu C, Chang S (2007) Anti-termitic activities of essential oils from coniferous trees against Coptotermes formosanus. Bioresour Technol 98:456–459
Choochote W, Chaithong U, Kamsuk K, Jitpakdi A, Tippawangkosol P, Tuetun B, Champakaew D, Pitasawat B (2007) Repellent activity of selected essential oils against Aedes aegypti. Fitoterapia 78:359–364
Choucry MA (2017) Chemical composition and anticancer activity of Achillea fragrantissima (Forssk.) Sch. Bip. (Asteraceae) essential oil from Egypt. J Pharmacogn Phytother 9:1–5
Cornblatt BS, Ye L, Dinkova-Kostova AT, Erb M, Fahey JW, Singh K, Chen MA, Stierer T, Garrett-Mayer E, Argani P, Davidson NE, Talalay P, Kensler TW, Visvanathan K (2007) Preclinical and clinical evaluation of sulforaphane for chemoprevention in the breast. Carcinogenesis 28:1485–1490
Croteau R, Kutchan TM, Lewis NG (2000) Natural products (secondary metabolites). In: Buchanan B, Gruissem W, Jones R (eds) Biochemistry and Molecular Biology of Plants. Wiley, Blackwell, Chichester
Dantas AD, Klein-Júnior LC, Machado MS, Guecheva TN, Dos Santos LD, Zanette RA, de Mello FB, Pêgas Henriques JA, de Mello JR (2016) Origanum majorana essential oil lacks mutagenic activity in the Salmonella/Microsome and Micronucleus Assays. Sci World J 2016:3694901
Das NG, Dhiman S, Talukdar PK, Rabha B, Goswami D, Veer V (2015) Synergistic mosquito-repellent activity of Curcuma longa, Pogostemon heyneanus and Zanthoxylum limonella essential oils. J Infect Public Health 8:323–328
De Logu A, Loy G, Pellerano ML, Bonsignore L, Schivo ML (2000) Inactivation of HSV-1 and HSV-2 and prevention of cell-to-cell virus spread by Santolina insularies essential oil. Antivir Res 48:177–185
De Sousa AC, Alviano DS, Blank AF, Alves PB, Alviano CS, Gattass CR (2004) Melissa officinalis L. essential oil: antitumoral and antioxidant activities. J Pharm Pharmacol 56:677–681
Deans SG, Ritchie G (1987) Antibacterial properties of plant essential oils. Int J Food Microbiol 5:165–180
Delaquis PJ, Stanich K, Girard B, Mazza G (2002) Antimicrobial activity of individual and mixed fractions of dill, cilantro, coriander and eucalyptus essential oils. Int J Food Microbiol 74:101–109
Desam NR, Al-Rajab AJ, Sharma M, Mylabathula MM, Gowkanapalli RR, Mohammed A (2017) Chemical composition, antibacterial and antifungal activities of Saudi Arabian Mentha longifolia L. essential oil. J Coast Life Med 5:441–446
Devkatte A, Zore GB, Karuppayil SM (2005) Potential of plant oils as inhibitors of Candida albicans growth. FEMS Yeast Res 5:867–873
Dijoux N, Guingand Y, Bourgeois C, Durand S, Fromageot C, Combe C, Ferret PJ (2006) Assessment of the phototoxic hazard of some essential oils using modified 3T3 neutral red uptake assay. Toxicol In Vitro 20:480–489
Djenane D, Aider M, Yanguela J, Idir L, Gomez D, Roncales P (2012) Antioxidant and antibacterial effects of Lavandula and Mentha essential oils in minced beef inoculated with E. coli O157: H7and S. aureus during storage at abuse refrigeration temperature. Meat Sci 92:667–674
Djilani A, Dicko A (2012) The therapeutic benefits of essential oils. In: Bouayed J, Bohn T (eds) Nutrition, Well-being and Health. InTech, Rijeka, pp 155–178
Dorman HJD, Deans SG (2000) Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J Appl Microbiol 88:308–316
Dryden MS, Dailly S, Crouch M (2004) A randomized, controlled trial of tea tree topical preparations versus a standard topical regimen for the clearance of MRSA colonization. J Hosp Infect 58:86–87
Dudai N, Weinstein Y, Krup M, Rabinski T, Ofir R (2005) Citral is a new inducer of caspase-3 in tumor cell lines. Planta Med 71:484–488
Edris AE (2007) Pharmaceutical and the rapeutic potentials of essential oils and their individual volatile constituents: A review. Phytother Res 21:308–323
El Babili F, Bouajila J, Souchard JP, Bertrand C, Bellvert F, Fouraste I, Valentin A (2011) Oregano: chemical analysis and evaluation of its antimalarial, antiox idant, and cytotoxic activities. J Food Sci 76:C512–C518
El-Soud NA, El-Laithy N, El-Saeed G, Wahby MS, Khalil M, Morsy F, Shaffie N (2011) Antidiabetic activities of Foeniculum Vulgare Mill. essential oil in Streptozotocin-induced diabetic rats. Maced J Med Sci 4:139–146
Erler F, Ulug I, Yalcinkaya B (2006) Repellent activity of five essential oils against Culex pipiens. Fitoterapia 77:491–494
Eshwarappa RSB, Iyer RS, Subbaramaiah SR, Richard SA, Dhananjaya BL (2014) Antioxidant activity of Syzygium cumini leaf gall extracts. Bio Impacts 4:101–107
Fabio A, Cermelli C, Fabio G, Nicoletti P, Quaglio P (2007) Screening of the antibacterial effects of a variety of essential oils on microorganisms responsible for respiratory infections. Phytother Res 21:374–377
Ferguson LR, Philpott M (2008) Nutrition and mutagenesis. Annu Rev Nutr 28:313–329
Fernandesa FH, Guterresb ZDR, Violantec IMP, Lopesb TFS, Garceza WS, Garceza FR (2015) Evaluation of mutagenic and antimicrobial properties of brown propolis essential oil from the Brazilian Cerrado biome. Toxicol Rep 2:1482–1488
Flores CR, Pennec A, Nugier-Chauvin C, Daniellou R, Herrera-Estrella L, Chauvin AL (2014) Chemical composition and antibacterial activity of essential oils extracted from plants cultivated in Mexico. J Mex Chem Soc 58:452–455
Fontenelle ROS, Morais SM, Brito EHS, Brilhante RSN, Cordeiro RA, Nascimento NRF, Rocha MFG, Sidrim JJC (2008) Antifungal activity of essential oils of Croton species from the Brazilian Catinga biome. J Appl Microbiol 104:1383–1390
Fujita KI, Chavasiri W, Kubo I (2015) Anti-salmonella activity of volatile compounds of Vietnam coriander. Phytother Res 29:1081–1087
Galvao LCDC, Furletti VF, Bersan SMF, Da Cunha MG, Ruiz ALTG, Carvalho JED, Sartoratto A, Rehder VLG, Figueira GM, Teixeira Duarte MC, Ikegaki M, DeAlencar SM, Rosalen PL (2012) Antimicrobial activity of essential oils against Streptococcus mutans and their anti-proliferative effects. Evidence-Based Compl Altern Med 2012:751435. https://doi.org/10.1155/2012/751435
Garcia CC, Talarico L, Almeida N, Colombres S, Duschatzky C, Damonte EB (2003) Virucidal activity of essential oils from aromatic plants of San Luis, Argentina. Phytother Res 17:1073–1075
García M, Donadel OJ, Ardanaz CE, Tonn CE, Sosa ME (2005) Toxic and repellent effects of Baccharis salicifolia essential oil on Tribolium castaneum. Pest Manag Sci 61:612–618
Garozzo A, Timpanaro R, Bisignano B, Furneri PM, Bisignano G, Castro A (2009) In vitro antiviral activity of Melaleuca alternifolia essential oil. Lett Appl Microbiol 49:806–808
Garozzo A, Timpanaro R, Stivala A, Bisignano G, Castro A (2011) Activity of Melaleuca alternifolia (tea tree)oil on Influenza virus A/PR/8:study on the mechanism of action. Antivir Res 89:83–88
Gautam N, Mantha AK, Mittal S (2014) Essential oils and their constituents as anticancer agents: A mechanistic view. BioMed Res Int 2014:154106
Gill CI, Boyd A, Mc Dermott E, Mc Cann M, Servili M, Selvaggini R, Taticchi A, Rowland I (2005) Potential anticancer effects of virgin olive oil phenols on colorectal carcinogenesis models in vitro. Int J Cancer 117:1–7
Gillij YG, Gleiser RM, Zygadlo JA (2008) Mosquito repellent activity of essential oils of aromatic plants growing in Argentina. Bioresour Technol 99:2507–2515
Goel D, Goel R, Singh V, Ali M, Mallavarapu G, Kumar S (2007) Composition of the essential oil from the root of Artemisia annua. J Nat Med 61:458–461
Gomes-Carneiro MR, Dias DM, De-Oliveira ACAX, Paumgartten FJ (2005) Evaluation of mutagenic and antimutagenic activities of α-bisabolol in the Salmonella microsome assay. Mut Res 585:105–112
Govere J, Durrheim DN, Du TN, Hunt RH, Coetzee M (2000) Local plants as repellents against Anopheles arabiensis, in Mpumalanga Province, South Africa. Afr J Med 46:213–216
Govindarajan M (2011) Larvicidal and repellent properties of some essential oils against Culex tritaeniorhynchus Giles and Anopheles subpictus Grassi (Diptera: Culicidae). Asian Pac J Trop Med 4:106–111
Govindarajan M, Rajeswary M, Sivakumar R (2015) Repellent properties of Delonix elata (L.) Gamble (Family: Fabaceae) against malaria vector Anopheles stephensi (Liston) (Diptera: Culicidae). J Saudi Soc Agric Sci 14:128–133
Guba R (2001) Toxicity myths–essential oils and their carcinogenic potential. Int J Aromather 11:76–83
Gulluce M, Sahin F, Sokmen M, Ozer H, Daferera D, Sokmen A, Polissiou M, Adiguzel A, Ozkan H (2007) Antimicrobial and antioxidant properties of the essential oils and methanol extract from Mentha longifolia L. sp. longifolia. Food Chem 103:1449–1456
Gupta SC, Tyagi AK, Deshmukh-Taskar P, Hinojosa M, Prasad S, Aggarwal BB (2014) Down regulation of tumor necrosis factor and other proinflammatory biomarkers by polyphenols. Arch Biochem Biophys 559:91–99
Hadis M, Lulu M, Mekonnen Y, Asfaw T (2003) Field trials on the repellent activity of four plant products against mainly Mansonia population in Western Ethiopia. Phytother Res 17:202–205
Hall-Stoodley L, Costerton JW, Stoodley P (2004) Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol 2:95–108
Hamid AA, Aiyelaagbe OO, Usman LA (2011) Essential oils: its medicinal and pharmacological uses. Int J Curr Res 33:86–98
Hammer KA, Carson CF (2011) Antibacterial and antifungal activities of essential oils. In: Thormar H (ed) Lipids and Essential Oils as Antimicrobial Agents. Wiley, Chichester, pp 255–306
Hammer KA, Carson CF, Riley TV (2002) In vitro activity of Melaleuca alternifolia (tea tree) oil against dermatophytes and other filamentous fungi. J Antimicrob Chemother 50:195–199
Hammer KA, Dry L, Johnson M, Michalak EM, Carson CF, Riley TV (2003) Susceptibility of oral bacteria to Melaleuca alternifolia (tea tree) oil in vitro. Oral Microbiol Immunol 18:389–392
Hammer KA, Carson CF, Riley TV, Nielsen JB (2006) A review of the toxicity of Melaleuca alternifolia (tea tree) oil. Food Chem Toxicol 44:616–625
Hammer KA, Carson CF, Rileya TV (2012) Effects of Melaleuca alternifolia (tea tree) essential oil and the major monoterpene component terpinen-4-ol on the development of single and multistep antibiotic resistance and antimicrobial susceptibility. Antimicrob Agents Chemother 56:909–915
Hassanshahian M, Bayat Z, Saeidi S, Shiri Y (2014) Antimicrobial activity of Trachyspermum ammi essential oil against human bacterial. Int J Adv Biol Biom Res 2:18–24
Hata T, Sakaguchi I, Mori M, Ikeda N, Kato Y, Minamino M, Watabe K (2003) Induction of apoptosis by Citrus paradisi essential oil in human leukemic(HL-60) cells. In Vivo 17:553–559
Hirulkar NB, Agrawal M (2010) Antimicrobial activity of rose petals extract against some pathogenic bacteria. Int J Pharm Biol Arch 1:478–484
Hisseinzadeh H, Ramezani M, Danaei AR (2002) Antihyperglycaemic effect and acute toxicity of Securigera secuidaca L. seed extracts in mice. Phytother Res 16:745–747
Hong EJ, Na KJ, Choi IG, Choi KC, Jeung EB (2004) Antibacterial and antifungal effects of essential oils from coniferous trees. Biol Pharm Bull 27:863–866
Hori M (2003) Repellency of essential oils against the cigarette beetle, Lasioderma serricorne (Fabricius) (Coleoptera: Anobiidae). Appl Entomol Zool 38:467–473
Hristova Y, Gochev V, Wanner J, Jirovetz L, Schmidt E, Girova T, Kuzmanov A (2013) Chemical composition and antifungal activity of essential oil of Salvia sclarea L. from Bulgaria against clinical isolates of Candida species. J Biosci Biotech 2:39–44
Hussain AI, Anwar F, Hussain Sherazi ST, Przybylski R (2008) Chemical composition, antioxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depends on seasonal variations. Food Chem 108:986–995
Ibrahim NA, El-Hawary SS, Mohammed MMD, Farid MA, Abdel-Washed NAM, Ali MA, El-Abd EAW (2015a) Chemical composition, antiviral against avian influenza (H5N1) virus and antimicrobial activities of the essential oils of the leaves and fruits of Fortunella margarita, lour. swingle, growing in Egypt. J Appl Pharma Sci 5:6–12
Ibrahim NA, El-Sakhawy FS, Mohammed MMD, Farid MA, Abdel-Wahed NAM, Deabes DAH (2015b) Chemical composition, antimicrobial and antifungal activities of essential oils of the leaves of Aegle marmelos (L.) Correa growing in Egypt. J Appl Pharma Sci 5:1–5
Idaomar M, El Hamss R, Bakkali F, Mezzoug N, Zhiri A, Baudoux D, Munoz-Serrano A, Liemans V, Alonso-Moraga A (2002) Genotoxicity and antigenotoxicity of some essential oils evaluated by wing spot test of Drosophila melanogaster. Mutat Res 513:61–68
Ioannou E, Poiata A, Hancianu M, Tzakou O (2007) Chemical composition and in vitro antimicrobial activity of the essential oils of flower heads and leaves of Santolina rosmarinifolia L. from Romania. Nat Prod Res 21:18–23
Ipek E, Zeytinoglu H, Okay S, Tuylu BA, Kurkcuoglu M, Husnu Can Baser K (2005) Genotoxicity and antigenotoxicity of Origanum oil and carvacrol evaluated by Ames Salmonella/microsomal test. Food Chem 93:551–556
Irkin R, Korukluoglu M (2009) Effectiveness of Cymbopogon citratus L. essential oil to inhibit the growth of some filamentous fungi and yeasts. J Med Food 12:193–197
Iwalokun BA, Gbenle GO, Adewole TA, Smith SI, Akinsinde KA, Omonigbehin EO (2003) Effects of Ocimum gratissimum L. essential oil at sub-inhibitory concentrations on virulent and multidrug-resistant Shigella strains from Lagos, Nigeria. APMIS 111:477–482
Jaenson TG, Palsson K, Borg-Karlson AK (2006) Evaluation of extracts and oils of mosquito (Diptera: Culicidae) repellent plants from Sweden and Guinea-Bissau. J Med Entomol 43:113–119
Jayaprakasha GK, Jagan Mohan Rao L, Sakariah KK (2000) Chemical composition of the flower oil of Cinnamomum zeylanicum blume. J Agric Food Chem 48:4294–4295
Juliao LS, Bizzo HR, Souza AM, Lourenco MC, Silva PE, Tavares ES, Rastrelli L, Leitao SG (2009) Essential oils from two Lantana species with antimycobacterial activity. Nat Prod Comm 4:1733–1736
Kaefer CM, Milner JA (2008) The role of herbs and spices in cancer prevention. J Nutr Biochem 19:347–361
Karbach J, Ebenezer S, Warnke PH, Behrens E, Al-Nawas B (2015) Antimicrobial effect of Australian antibacterial essential oils as alternative to common antiseptic solutions against clinically relevant oral pathogens. Clin Lab 61:61–68
Karunamoorthi K, Girmay A, Hayleeyesus SF (2014) Mosquito repellent activity of essential oil of Ethiopian ethnomedicinal plant against Afro-tropical malarial vector Anopheles arabiensis. J King Saud Univer Sci 26:305–310
Kasim LS, Olaleye KO, Fagbohun AB, Ibitoye SF, Adejumo OE (2014) Chemical composition and antibacterial activity of essential oils from Struchium sparganophora Linn. Ktze asteraceae. Adv Biol Chem 4:246–252
Kathiravan MK, Salake AB, Chothe AS, Dudhe PB, Watode RP, Mukta MS, Gadhwe S (2012) The biology and chemistry of antifungal agents: A review. Bioorg Med Chem 20:5678–5698
Khosravi AR, Minooeianhaghighi MH, Shokri H (2011) The potential inhibitory effect of Cuminum cyminum, Ziziphora clinopodioides and Nigella sativa essential oils on the growth of Aspergillus fumigatus and Aspergillus flavus. Braz J Microbiol 42:216–224
Khoury M, El Beyrouthy M, Ouaini N, Iriti M, Eparvier V, Stien D (2014) Chemical composition and antimicrobial activity of the essential oil of Juniperus excelsa M. Bieb. growing wild in Lebanon. Chem Biodivers 11:825–830
Kim EY, Baik IH, Kim JH, Kim SR, Rhyu MR (2004) Screening of the antioxidant activity of some medicinal plants. Kor J Food Sci Technol 36:333–338
Kiyohara H, Ichino C, Kawamura Y, Nagai T, Sato N, Yamada H (2012) Patchouli alcohol: in vitro direct anti-influenza virus sesquiterpene in Pogostemon cablin Benth. J Nat Med 66:55–61
Kloog Y, Cox AD (2004) Prenyl-binding domains: potential targets for Ras inhibitors and anticancer drugs. Semin Cancer Biol 14:253–261
Kocevski D, Du M, Kan J, Jing C, Lacanin I, Pavlovic H (2013) Antifungal effect of Allium tuberosum, Cinnamomum cassia, and Pogostemon cablin essential oils and their components against population of Aspergillus species. J Food Sci 78:M731–M737
Koch C, Reichling J, Schneele J, Schnitzler P (2008) Inhibitory effect of essential oils against herpes simplex virus type 2. Phytomedicine 15:71–78
Koo JY, Kim HJ, Jung KO, Park KY (2004) Curcumin inhibits the growth of AGS human gastric carcinoma cells in vitro and shows synergism with5-fluorouracil. J Med Food 7:117–121
Kordali S, Kotan R, Mavi A, Cakir A, Ala A, Yildirim A (2005) Determination of the chemical composition and antioxidant activity of the essential oil of Artemisia dracunculus and of the antifungal and antibacterial activities of Turkish Artemisia absinthium, A. dracunculus, Artemisia santonicum, and Artemisia spicigera essential oils. J Agric Food Chem 53:9452–9458
Koschier E, Sedy K (2003) Labiate essential oils affecting host selection and acceptance of Thrips tabaci lindeman. Crop Prot 22:929–934
Kumar R, Mishra AK, Dubey NK, Tripathi YB (2007) Evaluation of Chenopodium ambrosioides oil as a potential source of antifungal, antiaflatoxigenic and antioxidant activity. Int J Food Microbiol 115:159–164
Lalthazuali, Mathew N (2017) Mosquito repellent activity of volatile oils from selected aromatic plants. Parasitol Res 116:821–825
Lambert RJW, Skandamis PN, Coote PJ, Nychas GJE (2001) A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J Appl Microbiol 91:453–462
Landolt PJ, Hofstetter RW, Biddick LL (1999) Plant essential oils as arrestants and repellents for neonate larvae of the codling moth (Lepidoptera: Tortricidae). Environ Entomol 28:954–960
Lang G, Buchbauer G (2012) A review on recent research results (2008–2010) on essential oils as antimicrobials and antifungals: areview. Flav Frag J 27:13–39
Latifah-Munirah B, Himratul-Aznita WH, Zain NM (2015) Eugenol, an essential oil of clove, causes disruption to the cell wall of Candida albicans (ATCC 14053). Front Life Sci 8:231–240
Lee BK, Kim JH, Jung JW, Choi JW, Han ES, Lee SH, Kwang HK, Ryu JH (2005) Myristicin-induced neuro toxicity in human neuroblastoma SK-N-SHcells. Toxicol Lett 157:49–56
Lesgards JF, Baldovini N, Vidal N, Pietri S (2014) Anticancer activities of essential oils constituents and synergy with conventional therapies: A review. Phytother Res 28:1423–1446
Li Y, Li MY, Wang L, Jiang ZH, Li WY, Li H (2004) Induction of apoptosis of cultured hepatocarcinoma cell by essential oil of Artemisia Annul L. J Sichuan Univ Med Sci Ed 35:337–339
Lirussi F, Beccarello A, Zanette G, De Monte A, Donadon V, Velussi M, Crepaldi G (2002) Silybin-beta-cyclodextrin in the treatment of patients with diabetes mellitus and alcoholic liver disease. Efficacy study of a new preparation of an anti-oxidant agent. Diabetes Nutr Metab 15:222–231
Liu CJ, Chen CL, Chang KW, Chu CH, Liu TY (2000) Safrole in betel quid may be a risk factor for hepatocellular carcinoma: case report. Can Med Ass J 162:359–360
Lo Cantore P, Iacobellis NS, De Marco A, Capasso F, Senatore F (2004) Antibacterial activity of Coriandrum sativum L. and Foeniculum vulgare Miller Var. vulgare (Miller) essential oils. J Agric Food Chem 52:7862–7866
Lopes-Lutz D, Alviano DS, Alviano CS. Kolodziejczyk PP (2008) Screening of chemical composition, antimicrobial and antioxidant activities of Artemisia essential oils. Phytochemistry 69:1732–1738
Lopez P, Sanchez C, Batlle R, Nerin C (2005) Solid and vapor phase antimicrobial activities of six essential oils: susceptibility of selected food borne bacterial and fungal strains. J Agric Food Chem 53:6939–6946
Lopez P, Sanchez C, Batlle R, Nerin C (2007) Vapor-phase activities of cinnamon, thyme, and oregano essential oils and key constituents against food borne microorganisms. J Agric Food Chem 55:4348–4356
Lu Y, Hernandez P, Abegunde D, Edejer T (2011) Medicine Expenditure. In: World medicine situation. Geneva, pp 43–76
Luk SU, Lee TKW, Liu J, Lee DTW, Chiu YT, Ma S, Ng IOL, Wong YC, Chan FL, Ling MT (2011) Chemopreventive effect of PSP through targeting of prostate cancer stem cell-like population. PLoS One 6:e19804
Madihi Y, Merrikhi A, Baradaran A, Rafieian-Kopaei M, Shahinfard N, Ansari R, Baradaran J (2013) Impact of sumac on postprandial high-fat oxidative stress. Pak J Med Sci 29:340–345
Maestri DM, Nepote V, Lamarque AL, Zygadlo JA (2006) Natural products as antioxidants. In: Filippo I (ed) Phytochemistry: Advances in research. Signpost, Trivandrum, pp 105–135
Maggi F, Bramucci M, Cecchini C, Coman MM, Cresci A, Cristalli G, Lupidi G, Papa F, Quassinti L, Sagratini G, Vittori S (2009) Composition and biological activity of essential oil of Achillea ligustica All. (Asteraceae) naturalized in central Italy: ideal candidate for anti-cariogenic formulations. Fitoterapia 80:313–319
Maheshwari RK, Singh AK, Gaddipati J, Srimal RC (2006) Multiple biological activities of curcum: a short review. Life Sci 78:2081–2087
Mahmoudi R (2017) Application of medicinal plants: From past to present. MOJ Biol Med 1:16
Malíková J, Swaczynová J, Kolář Z, Strnad M (2008) Anticancer and antiproliferative activity of natural brasinosteroids. Phtyochemistry 69:418–426
Manosroi J, Dhumtanom P, Manosroi A (2006) Anti-proliferative activity of essential oil extracted from Thai medicinal plants on KB and P388 cell lines. Cancer Lett 235:114–120
Mansoor A, Ibrahim MA, Zaidi MA, Ahmed M (2011) Antiprotozoal activities of Vincetoxicum stocksii and Carum copticum. Bangladesh J Pharmacol 6:51–54
Mansour MA, Ginawi OT, El-Hadiyah T, El-Khatib AS, Al-Shabanah OA, Al-Sawaf HA (2001) Effects of the volatile oil constituents of Nigella sativa on carbon tetrachloride induced hepatotoxicity in mice: evidence for antioxidant effects of thymoquinone. Res Commun Mol Pathol Pharmacol 110:239–251
Masotti V, Juteau F, Bessie’re JM, Viano J (2003) Seasonal and phenological variations of the essential oil from the narrow endemic species Artemisia molinieri and its biological activities. J Agric Food Chem 51:7115–7121
Matasyoh JC, Maiyo ZC, Ngure RM, Chepkorir R (2009) Chemical composition and antimicrobial activity of the essential oil of Coriandrum sativum. Food Chem 113:526–529
Matthaei S, Stumvoll M, Kellerer M, Häring HU (2000) Pathophysiology and pharmacological treatment of insulin resistance. Endocr Rev 2:585–618
Mau JL, Lai EY, Wang NP, Chen CC, Chang CH, Chyau CC (2003) Composition and antioxidant activity of the essential oil from Curcuma zedoaria. Food Chem 82:583–591
Maxia A, Marongiu B, Piras A, Porcedda S, Tuveri E, Goncalves MJ, Cavaleiro C, Salgueiro L (2009) Chemical characterization and biological activity of essential oils from Daucus carota L. subsp. Carota growing wild on the Mediterranean coast and on the Atlantic coast. Fitoterapia 80:57–61
May J, Chan C, King A, Williams L, French GL (2000) Time kill studies of tea tree oils on clinical isolates. J Antimicrob Chemother 45:639–643
McCord JM (2000) The evolution of free radicals and oxidative stress. Am J Med 108:652–659
Meneses R, Ocazionez RE, Martinez JR, Stashenko EE (2009) Inhibitory effect of essential oils obtained from plants grown in Colombia on yellow fever virus replication in vitro. Ann Clin Microbiol Antimicrob 8:8. https://doi.org/10.1186/1476-0711-8-8
Mezzoug N, Elhadri A, Dallouh A, Amkiss S, Skali NS, Abrini J, Zhiri A, Baudoux D, Diallo B, El Jaziri M, Idaomar M (2007) Investigation of the mutagenic and antimutagenic effects of Origanum compactum essential oil and some of its constituents. Mutat Res 629:100–110
Miguel MG (2010) Antioxidant and anti-inflammatory activities of essential oils: A short review. Molecules 15:9252–9287
Miller EC, Swanson AB, Phillips DH, Fletcher TL, Liem A, Miller JA (1983) Structure activity studies of the carcinogenicities in the mouse and rat of some naturally occurring and synthetic alkenylbenzene derivatives related to safrole and estragole. Cancer Res 43:1124–1134
Milner JA (2001) A historical perspective on garlic and cancer. J Nutr 131:S1027–S1031
Mimica-Dukic N, Bozin B, Sokovic M, Simin N (2004) Antimicrobial and antioxidant activities of Melissa officinalis L. (Lamiaceae) essential oil. J Agric Food Chem 52:2485–2489
Minami M, Kita M, Nakaya T, Yamamoto T, Kuriyama H, Imanishi J (2003) The inhibitory effect of essential oils on herpes simplex virus type-1 replication in vitro. Microbiol Immunol 47:681–684
Mirhoseini M, Baradaran A, Rafieian-Kopaei M (2013) Medicinal plants, diabetes mellitus and urgent needs. J Herb Med Pharmacol 2:53–54
Mkaddem M, Bouajila J, Ennajar M, Lebrihi A, Mathieu F, Romdhane M (2009) Chemical composition and antimicrobial and antioxidant activities of Mentha (longifolia L. and viridis) essential oils. J Food Sci 74:M358–M363
Mohamed A, Ali SI, El-Baz FK (2013) Antioxidant and antibacterial activities of crude extracts and essential oils of Syzygium cumini Leaves. PLoS One 8(4):e60269
Moore SJ, Hill N, Ruiz C, Cameron MM (2007) Field evaluation of traditionally used plant-based insect repellents and fumigants against the malaria vector Anopheles darlingi in Riberalta, Bolivian Amazon. J Med Entomol 44:624–630
Morita T, Jinno K, Kawagishi H, Arimoto Y, Suganuma H, Inakuma T, Sugiyama K (2003) Hepatoprotective effect of myristicin from nutmeg (Myristica fra grans) on lipopolysaccharide/d-galactosamine-induced liver injury. J Agric Food Chem 51:1560–1565
Moteki H, Hibasami H, Yamada Y, Katsuzaki H, Imai K, Komiya T (2002) Specific induction of apoptosis by 1,8-cineole in two human leukemia cell lines, but not a in human stomach cancer cell line. Oncol Rep 9:757–760
Murray M (1997) Bilberry (Vaccinium myrtillus). Am J Nat Med 4:18–22
Nakatsu T, Lupo AT, Chinn JW, Kang RKL (2000) Biological activity of essential oils and their constituents. Stud Nat Prod Chem 21:571–631
Nasri H, Shirzad H (2013) Toxicity and safety of medicinal plants. J Herb Med Plarmacol 2:21–22
Nasri H, Baradaran A, Ardalan MR, Mardani S, Momeni A, Rafieian-Kopaei M (2013) Bright reno protective properties of metformin: beyond blood glucose regulatory effects. Iran J Kidney Dis 7:423–428
Nerio SL, Olivero-Verbel J, Stashenko E (2010) Repellent activity of essential oils: A review. Bioresour Technol 101:372–378
Novy P, Davidova H, Serrano-Rojero CS, Rondevaldova J, Pulkrabek J, Kokoska L (2015) Composition and antimicrobial activity of Euphrasia rostkoviana hayne essential oil. Evidence-Based Compl Altern Med 2015:734101. https://doi.org/10.1155/2015/734101
Odalo JO, Omolo MO, Malebo H, Angira J, Njeru PM, Ndiege IO, Hassanali A (2005) Repellency of essential oils of some plants from the Kenyan coast against Anopheles gambiae. Acta Trop 95:210–218
Omolo MO, Okinyo D, Ndiege IO, Lwande W, Hassanali A (2004) Repellency of essential oils of some Kenyan plants against Anopheles gambiae. Phytochemistry 65:2797–2802
Oyedele AO, Gbolade AA, Sosan MB, Adewoyin FB, Soyelu OL, Orafidiya OO (2002) Formulation of an effective mosquito-repellent topical product from Lemongrass oil. Phytomedicine 9:259–262
Ozbek H, Ugras S, Dulger H, Bayram I, Tuncer I, Ozturk G, Ozturk A (2003) Hepatoprotective effect of Foeniculum vulgare essential oil. Fitoterapia 74:317–319
Ozcan MM, Chalchat JC (2008) Chemical composition and antifungal activity of rosemary (Rosmarinus officinalis L.) oil from Turkey. Int J Food Sci Nutr 59:691–698
Ozek G, Demirci F, Ozek T, Tabanca N, Wedge DE, Khan SI, Baser KHC, Duran A, Hamzaoglu E (2010) Gas chromatographic–mass spectrometric analysis of volatiles obtained by four different techniques from Salvia rosifolia Sm. and evaluation for biological activity. J Chromatogr A 1217:741–748
Padilha de Paula J, Gomes-Carneiro MR, Paumgartten FJR (2003) Chemical composition, toxicity and mosquito repellency of Ocimum selloi oil. J Ethnopharmacol 88:253–260
Papachristos DP, Stamopoulos DC (2002) Repellent, toxic and reproduction inhibitory effects of essential oil vapours on Acanthoscelides obtectus (Say) (Coleoptera: Bruchidae). J Stored Prod Res 38:117–128
Papajani V, Haloci E, Goci E, Shkreli R, Manfredini S (2015) Evaluation of antifungal activity of Origanum vulgare and Rosmarinus officinalis essential oil before and after inclusion in β-cyclodextrine. Int J Pharm Pharm Sci 7:270–273
Parsaei P, Karimi M, Asadi SY, Rafieian-Kopaei M (2013) Bioactive components and preventive effect of green tea (Camellia sinensis) extract on post laparotomy intra-abdominal adhesion in rats. Int J Surg 11:811–815
Pascual M, Ballesta M (2003) Chemical variation in an Ocimum basilicum germplasm collection and activity of the essential oils on Callosobruchus maculatus. Biochem Syst Ecol 31:673–679
Patel K, Ali S, Sotheeswaran S, Dufour JP (2013) Composition of the leaf essential oil of Cinnamomum verum (Lauraceae) from Fiji Islands. J Essent Oil Bearing Plants 10:374–377
Peighami-Ashnaei S, Farzaneh M, Sharifi Tehrani A, Behboudi K (2008) Effect of essential oils in control of plant diseases. Commun Agric Appl Biol Sci 74:843–847
Pepeljnjak S, Kosalec I, Kalodera Z, Blazevic N (2005) Antimicrobial activity of juniper berry essential oil (Juniperus communis L., Cupressaceae). Acta Pharma 55:417–422
Petretto GL, Fancello F, Zara S, Foddai M, Mangia NP, Sanna ML, Omer EA, Menghini L, Chessa M, Pintore G (2014) Antimicrobial activity against beneficial microorganisms and chemical composition of essential oil of Mentha suaveolens ssp. insularis grown in Sardinia. J Food Sci 79:M369–M377
Pichersky E, Noel JP, Dudareva N (2006) Biosynthesis of plant volatiles: nature’s diversity and ingenuity. Science 311:808–811
Pinto E, Vale-Silva L, Cavaleiro C, Salgueiro L (2009) Antifungal activity of the clove essential oil from Syzygium aromaticum on Candida, Aspergillus and dermatophyte species. J Med Microbiol 58:1454–1462
Pledgie-Tracy A, Sobolewski MD, Davidson NE (2007) Sulforaphane induces cell type-specific apoptosis in human breast cancer cell lines. Mol Cancer Ther 6:1013–1021
Politeo O, Jukic M, Milos M (2007) Chemical composition and antioxidant capacity of free volatile a glycones from basil (Ocimum basilicum L.) compared with its essential oil. Food Chem 101:379–385
Pragadheesh VS, Saroj A, Yadav A, Chanotiya CS, Alam M, Samad A (2013) Chemical characterization and antifungal activity of Cinnamomum camphora essential oil. Ind Crop Prod 49:628–633
Prajapati V, Tripathi AK, Aggarwal KK, Khanuja SPS (2005) Insecticidal, repellent and oviposition-deterrent activity of selected essential oils against Anopheles stephensi, Aedes aegypti and Culex quinquefasciatus. Bioresour Technol 96:1749–1757
Puatanachokchai R, Kishida H, Denda A, Murata N, Konishi Y, Vinitketkumnuen U, Nakae D (2002) Inhibitory effects of lemongrass Cymbopogon citrates extract on the early phase of hepatocarcinogenesis after initiation with diethyl-nitrosamine in male Fischer 344 rats. Cancer Lett 183:9–15
Pudziuvelyte L, Stankevicius M, Maruska A, Petrikaite V, Ragazinskiene O, Draksiene G, Bernatoniene J (2017) Chemical composition and anticancer activity of Elsholtzia ciliata essential oils and extracts prepared by different methods. Ind Crop Prod 107:90–96
Pullagummi C, Rao NB, Singh BCS, Bheemagani AJ, Kumar P, Venkatesh K, Rani AR (2014) Comparitive studies on antibacterial activity of Patchouli [Pogostemon cablin (Blanco) Benth] and Geranium (Pelargonium graveolens) aromatic medicinal plants. Afr J Biotechnol 13:2379–2384
Pushpanathan T, Jebanesan A, Govindarajan M (2006) Larvicidal, ovicidal and repellent activities of Cymbopogan citratus Stapf. (Graminae) essential oil against the filarial mosquito Culex quinquefasciatus (Say) (Diptera: Culicidae). Trop Biomed 23:208–212
Pushpanathan T, Jebanesan A, Govindarajan M (2008) The essential oil of Zingiber officinalis Linn. (Zingiberaceae) as a mosquito larvicidal and repellent agent against the filarial vector Culex quinquefasciatus Say (Diptera: Culicidae). Parasitol Res 102:1289–1291
Rabadia AG, Kamat S, Kamat D (2012) Antifungal activity of essential oils against fluconazole resistant fungi. Int J Phytomed 3:506–510
Racchi ML (2013) Antioxidant Defenses in Plants with Attention to Prunus and Citrus spp. Antioxidants 2:340–369
Rafieian-Kopaei M, Baradaran A, Merrikhi A, Nematbakhsh M, Madihi Y, Nasri H (2013) Efficacy of co-administration of garlic extract and metformin for prevention of gentamicin-renal toxicity in wistar rats: A biochemical study. Int J Prev Med 4:258–264
Rafii F, Shahverdi AR (2007) Comparison of essential oils from three plants for enhancement of antimicrobial activity of nitrofurantoin against entero bacteria. Chemotherapy 53:21–25
Rajkumar S, Jebanesan A (2005) Repellency of volatile oils from Moschosma polystachyum and Solanum xanthocarpum against filarial vector Culex quinquefasciatus Say. Trop Biomed 22:139–142
Rasooli I, Fakoor MH, Yadegarinia D, Gachkar L, Allameh A, Rezaei MB (2008) Antimycotoxigenic characteristics of Rosmarinus officinalis and Trachyspermum copticum L. essential oils. Int J Food Microbiol 122:135–139
Rather MA, Dar BA, Dar MY, Wani BA, Shah WA, Bhat BA, Bashir AG, Bhat KA, Anand R, Qurishi MA (2012) Chemical composition, antioxidant and antibacterial activities of the leaf essential oil of Juglans regia L. andits constituents. Phytomed 19:1185–1190
Raut JS, Karuppayil SM (2014) A status review on the medicinal properties of essential oils. Ind Crop Prod 62:250–264
Razzaghi-Abyaneh M, Shams-Ghahfarokhi M, Yoshinari T, Rezaee MB, Jaimand K, Nagasawa H, Sakuda S (2008) Inhibitory effects of Satureja hortensis L. essential oil on growth and aflatoxin production by Aspergillus parasiticus. Int J Food Microbiol 123:228–233
Reegan AD, Gandhi MR, Paulraj MG, Ignacimuthu S (2015) Ovicidal and oviposition deterrent activities of medicinal plant extracts against Aedes aegypti L. and Culex quinquefasciatus Say mosquitoes (Diptera: Culicidae). Osong Public Health Res Perspect 6:64–69
Reichling J, Koch C, Stahl-Biskup E, Sojka C, Schnitzler P (2005) Virucidal activity of a beta-triketone-richess entail oil of Leptospermum scoparium (manuka oil) against HSV-1and HSV-2 in cell culture. PlantaMed 71:1123–1127
Roller S, Ernest N, Buckle J (2009) The antimicrobial activity of high-necrodane and other lavender oils onmethicillin-sensitive and-resistant Staphylococcus aureus (MSSA and MRSA). J Altern Compl Med 15:275–279
Romeilah RM (2016) Chemical compositions, antioxidant, anticancer activities and biological effects of Myrtus communis L. and Origanum vulgare essential oils. Asian J Biochem 11:104–107
Romeilah RM, Fayed SA, Mahmoud GI (2010) Chemical compositions, antiviral and antioxidant activities of seven essential oils. J Appl Sci Res 6:50–62
Rosato A, Vitali C, De Laurentis N, Armenise D, Antonietta MM (2007) Antibacterial effect of some essential oils administered alone or in combination with norfloxacin. Phytomedicine 14:727–732
Rota C, Carraminana JJ, Burillo J, Herrera A (2004) In vitro antimicrobial activity of essential oils from aromatic plants against selected food borne pathogens. J Food Prot 67:1252–1256
Routh MM, Raut JS, Karuppayil SM (2011) Dual properties of anticancer agents: an exploratory study on the in vitro anti-Candida properties of thirty drugs. Chemotherapy 57:372–380
Roy S, Chaurvedi P, Chowdhary A (2015) Evaluation of antiviral activity of essential oil of Trachyspermum Ammi against Japanese encephalitis virus. Pharm Res 7:263–267
Ruan ZP, Zhang LL, Lin YM (2008) Evaluation of the antioxidant activity of Syzygium cumini leaves. Molecules 13:2545–2556
Rudramurthy GR, Swamy MK, Sinniah UR, Ghasemzadeh A (2016) Nanoparticles: alternatives against drug-resistant pathogenic microbes. Molecules 21:836
Runyoro D, Ngassapaa O, Vagionas K, Aligiannis N, Graikou K, Chinou I (2010) Chemical composition and antimicrobial activity of the essential oils of four Ocimum species growing in Tanzania. Food Chem 119:311–316
Saddi M, Sanna A, Cottiglia F, Chisu L, Casu L, Bonsignore L, DeLogu A (2007) Antiherpevirus activity of Artemisia arborescens essential oil and inhibition of lateral diffusion in vero cells. Ann Clin Microbiol Antimicrob 6:10. https://doi.org/10.1186/1476-0711-6-10
Saeidnia S, Gohari AR (2012) Trypanocidal monoterpenes: lead compounds to design future trypanocidal drugs. Stud Nat Prod Chem 37:173–189
Said ZB, Haddadi-Guemghar H, Boulekbache-Makhlouf L, Rigou P, Remini H, Adjaoud A, Khoudja NK, Madani K (2016) Essential oils composition, antibacterial and antioxidant activities of hydro distillated extract of Eucalyptus globulus fruits. Ind Crop Prod 89:167–175
Saidana D, Mahjoub MA, Boussaada O, Chriaa J, Chéraif I, Daami M, Mighri Z, Helal AN (2008) Chemical composition and antimicrobial activity of volatile compounds of Tamarix boveanai (Tamaricaceae). Microbiol Res 163:445–455
Saikia D, Khanuja SPS, Kahol AP, Gupta SC, Kumar S (2001) Comparative antifungal activity of essential oils and constituents from three distinct genotypes of Cymbopogon spp. Curr Sci 80:1264–1265
Salim EI, Fukushima S (2003) Chemopreventive potential of volatile oil from black cumin (Nigella sativa L.) seeds against rat colon carcinogenesis. Nutr Cancer 45:95–202
Samber N, Khan A, Varma A, Manzoor N (2015) Synergistic anticandidal activity and mode of action of Mentha piperita essential oil and its major components. Pharm Biol 53:1496–1504
Sanchez-Vioque R, Polissiou M, Astraka K, Mozos-Pascual M, Tarantilis P, Herraiz-Penalver D, Santana-Meridas O (2013) Polyphenol composition and antioxidant and metal chelating activities of the solid residues from the essential oil industry. Ind Crop Prod 49:150–159
Santin RM, Oliveira SA, Nakamura VC, Dias Filho BP, Ferreira ICP, Ueda Nakamura T (2009) In vitro activity of the essential oil of Cymbopogon citrates. Parasitol Res 105:1489–1496
Santoro GF, Cardoso MG, Guimarães LG, Mendonça LZ, Soares MJ (2007a) Trypanosoma cruzi: Activity of essential oils from Achillea millefolium L., Syzygium aromaticum L. and Ocimum basilicum L. on epimastigotes and trypomastigotes. Exp Parasitol 116:283–290
Santoro GF, Cardoso MG, Guimares LGL, Mendonca LZ, Soares MJ (2007b) Effect of oregano(Origanum vulgare L.) and thyme (Thymus vulgare L.) essential oils on Trypanosoma cruzi (Protozoa: Kinetoplastida) growth and ultrastructure. Parasitol Res 100:783–790
Santos AO, Santin AC, Yamaguchi MU, Cortez LER, Ueda Nakamura T, Dias Filho BP, Nakamura CV (2010) Antileishmanial activity of an essential oil from the leaves and flowers of Achillea millefolium. Ann Trop Med Parasitol 104:475–483
Santos NO, Mariane B, Lago JH, Sartorelli P, Rosa W, Soares MG, Da Silva AM, Lorenzi H, Vallim MA, Pascon RC (2015) Assessing the chemical composition and antimicrobial activity of essential oils from Brazilian plants-Eremanthus erythropappus (Asteraceae), Plectrantuns barbatus, and P. amboinicus (Lamiaceae). Molecules 20:8440–8452
Santurio DF, De Jesus FPK, Zanette RA, Schlemmer KB, Fraton A, Fries LLM (2014) Antimicrobial activity of the essential oil of thyme and of thymol against Escherichia coli strains. Acta Sci Vet 42:1–4
Sardi JCO, Scorzoni L, Bernardi T, Fusco Almeida AM, Giannini MM (2013) Candida species: current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J Med Microbiol 62:10–24
Sartorelli P, Marquioreto AD, Amaral-Baroli A, Lima MEL, Moreno PRH (2007) Chemical composition and antimicrobial activity of the essential oils from two species of Eucalyptus. Phytother Res 21:231–233
Sauter IP, Rossa GE, Lucas AM, Cibulski SP, Roehe PM, Da Silva LAA, Rott MB, Vargas RMF, Cassel E, Von Poser GL (2012) Chemical composition and amoebicidal activity of Piper hispidinervum (Piperaceae) essential oil. Ind Crop Prod 40:292–295
Schnitzler P, Schon K, Reichling J (2001) Antiviral activity of Australian tea tree oil and eucalyptus oil against herpes simplex virus in cell culture. Pharmazie 56:343–347
Schnitzler P, Koch C, Reichling J (2007) Susceptibility of drug-resistant clinical herpes simplex virus type 1 strains to essential oils of ginger, thyme, hyssop, and sandalwood. Antimicrob Agents Chemother 51:1859–1862
Schnitzler P, Astani A, Reichling J (2011) Antiviral effects of plant-derived essential oils and pure oil components. In: Thormar H (ed) Lipids and essential oils as antimicrobial agents. Wiley, Chichester, pp 239–254
Schuhmacher A, Reichling J, Schnitzler P (2003) Virucidal effect of peppermint oil on the enveloped viruses, herpes simplex virus type1and type 2 in vitro. Phytomedicine 10:504–510
Seidel C, Florean C, Schnekenburger M, Dicato M, Diederich M (2012) Chromatin-modifying agents in anti-cancer therapy. Biochimie 94:2264–2279
Shah WA, Dar MY, Zagar MI, Agnihotri VK, Qurishi MA, Singh B (2013) Chemical composition and antimicrobial activity of the leaf essential oil of Skimmia laureola growing wild in Jammu and Kashmir, India. Nat Prod Res 27:1023–1027
Shan B, Cai YZ, Brooks JD, Corke H (2007) The in vitro antibacterial activity of dietary spice and medicinal herb extracts. Int J Food Microbiol 117:112–119
Sharma N, Trikha P, Athar M, Raisuddin S (2001) Inhibition of benzo(a)pyrene- and cyclophosphamide-induced mutagenicity by Cinnamomum cassia. Mutat Res 480:179–188
Sienkiewicz M, Głowacka A, Poznanska-Kurowska K, Kaszuba A, Urbaniak A, Kowalczyk E (2015) The effect of clary sage oil on Staphylococci responsible for wound infections. Postepy Dermatol Alergol 32:21–26
Singab AN, Youssef FS, Ashour ML (2014) Medicinal plants with potential antidiabetic activity and their assessment. Med Aromat Plants 3:151. https://doi.org/10.4172/2167-0412.1000151
Singh G, Kapoor IPS, Pandey SK, Singh UK, Singh RK (2002) Studies on essential oils: part 10; antibacterial activity of volatile oils of some spices. Phytother Res 16:680–682
Singh G, Marimuthu P, De Heluani CS, Catalan CA (2006) Antioxidant and biocidal activities of Carum nigrum (seed) essential oil, oleoresin, and their selected components. J Agric Food Chem 54:174–181
Singh G, Kapoor IPS, Singh P, De Heluani CS, De Lampasona MP, Catalan CA (2008) Chemistry, antioxidant and antimicrobial investigations on essential oil and oleoresins of Zingiber officinale. Food Chem Toxicol 46:3295–3302
Singh P, Shukla R, Kumar A, Prakash B, Singh S, Dubey NK (2010) Effect of Citrus reticulata and Cymbopogon citratus essential oils on Aspergillus flavus growth and aflatoxin production on Asparagus racemosus. Mycopathologia 170:195–202
Singh S, Das SS, Singh G, Schuff C, De Lampasona MP, Catalan CAN (2014) Composition, in vitro antioxidant and antimicrobial activities of essential oil and oleoresins obtained from black cumin seeds (Nigella sativa L.). Bio Med Res Int 2014:918209
Sinico C, De Logu A, Lai F, Valenti D, Manconi M, Loy G, Fadda AM (2005) Liposomal incorporation of Artemisia arborescens L. essential oil and in vitro antiviral activity. Eur J Pharm Biopharm 59:161–168
Smith RL, Cohen SM, Doull J, Feron VJ, Goodman JI, Marnett LJ, Portoghese PS, Waddell WJ, Wagner BM, Hall RL, Higley NA, Lucas-Gavin C, Adams TB (2005) A procedure for the safety evaluation of natural flavor complexes used as ingredients in food: essential oils. Food Chem Toxicol 43:345–363
Sonboli A, Babakhani B, Mehrabian AR (2006) Antimicrobial activity of six constituents of essential oil from Salvia. Z Naturforsch 61:160–164
Souza CM, Pereira Junior SA, Moraes Tda S, Damasceno JL, Amorim Mendes S, Dias HJ, Stefani R, Tavares DC, Martins CH, Crotti AE, Mendes-Giannini MJ, Pires RH (2016) Antifungal activity of plant-derived essential oils on Candida tropicalis planktonic and biofilms cells. Med Mycol 54:515–523
Srinivasan K, Ramarao P (2007) Animal models in type 2 diabetes research: an overview. Indian J Med Res 125:451–472
Swamy MK, Sinniah UR (2015) A comprehensive review on the phytochemical constituents and pharmacological activities of Pogostemon cablin Benth.: an aromatic medicinal plant of industrial importance. Molecules 20:8521–8547
Swamy MK, Sinniah UR (2016) Patchouli (Pogostemon cablin Benth.): Botany, agrotechnology and biotechnological aspects. Ind Crop Prod 87:161–176
Swamy MK, Sudipta KM, Lokesh P, Neeki MA, Rashmi W, Bhaumik SH, Darshil SH, Vijay R, Kashyap NSS (2012) Phytochemical screening and in vitro antimicrobial activity of Bougainvillea spectabilis flower extracts. Int J Phytomed 4:375–379
Swamy MK, Sinniah UR, Akhtar MS (2016) Antimicrobial properties of plant essential oils against human pathogens and their mode of action: An updated review. Evidence-Based Compl Altern Med 2016:3012462. https://doi.org/10.1155/2016/3012462
Sylvestre M, Legault J, Dufour D, Pichette A (2005) Chemical composition and anticancer activity of leaf essential oil of Myrica gale L. Phytomedicine 12:299–304
Sylvestre M, Pichette A, Longtin A, Nagau F, Legault J (2006) Essential oil analysis and anticancer activity of leaf essential oil of Croton flavens L. from Guadeloupe. J Ethnopharmacol 103:99–102
Taherkhani M (2015) Anticancer, cytotoxic activity, mutagenic and anti-mutagenic activities of Artemisia aucheri essential oil. TEOP 18:1329–1337
Taherkhani (2016) Chemical constituents, antimicrobial, cytotoxicity, mutagenic and antimutagenic effects of Artemisia ciniformis. Iranian J Pharma Res 15:471–481
Tahir HU, Sarfraz RA, Ashraf A, Adil S (2016) Chemical composition and antidiabetic activity of essential oils obtained from two spices (Syzygium aromaticum and Cuminum cyminum). Int J Food Propert 19:2156–2164
Takarada K, Kimizuka R, Takahashi N, Honma K, Okuda K, Kato T (2004) A comparison of the antibacterial efficacies of essential oils against oral pathogens. Oral Microbiol Immunol 19:61–64
Tariku Y, Hymete A, Hailu A, Rohloff J (2011) In vitro evaluation of antileishmanial activity and toxicity of essential oils of Artemisia absinthium and Echinops kebericho. Chem Biodivers 8:614–623
Tavares AC, Goncalves MJ, Cavaleiro C, Cruz MT, Lopes MC, Canhoto J, Salgueiro LR (2008) Essential oil of Daucus carota subsp. Halophilus: composition, antifungal activity and cytotoxicity. J Ethnopharmacol 119:129–134
Tawatsin A, Wratten SD, Scott RR, Thavara U, Techandamrongsin Y (2001) Repellency of volatile oils from plants against three mosquito vectors. J Vector Ecol 26:76–82
Tchoumbougnang F, Zollo PH, Dagne E, Mekonnen Y (2005) In vivo antimalarial activity of essential oils from Cymbopogon citratus and Ocimum gratissimum on mice infected with Plasmodium berghei. Planta Med 71:20–23
Teixeira B, Marques A, Ramos C, Neng NR, Nogueira JMF, Saraiva JA, Nunes ML (2013) Chemical composition and antibacterial and antioxidant properties of commercial essential oils. Ind Crop Prod 43:587–595
Tepe B, Donmez E, Unlu M, Candan F, Daferera D, Vardar-Unlu G, Polissiou M, Sokmen A (2004) Antimicrobial and antioxidative activities of the essential oils and methanol extracts of Salvia cryptantha (Montbret et Aucherex Benth.) and Salvia multicaulis (Vahl). Food Chem 84:519–525
Tohidpour A, Sattari M, Omidbaigi R, Yadegar A, Nazemi J (2010) Antibacterial effect of essential oils from two medicinal plants against methicillin-resistant Staphylococcus aureus (MRSA). Phytomed 17:142–145
Tolouee M, Alinezhad S, Saberi RR, Eslamifar A, Zad SJ, Jaimand K, Taeb J, Razzaghi-Abyaneh M (2010) Effect of Matricaria chamomilla L. flower essential oil on the growth and ultra-structure of Aspergillus niger van Tieghem. Int J Food Microbiol 139:127–133
Toloza AC, Zygadlo J, Mougabure Cueto G, Biurrun F, Zerba E, Picollo MI (2006) Fumigant and repellent properties of essential oils and component compounds against permethrin-resistant Pediculus humanus capitis (Anoplura: Pediculidae) from Argentina. J Med Entomol 43:889–895
Toloza AC, Lucia A, Zerba E, Masuh H, Picollo MI (2008) Interspecific hybridization of eucalyptus as a potential tool to improve the bioactivity of essential oils against permethrin-resistant head lice from Argentina. Bioresour Technol 99:7341–7347
Tomaino A, Cimino F, Zimbalatti V, Venuti V, Sulfaro V, De Pasquale A, Saija A (2005) Influence of heating on antioxidant activity and the chemical composition of some spice essential oils. Food Chem 89:549–554
Toscano-Garibay JD, Arriaga-Alba M, Sánchez-Navarrete J, Mendoza-García M, Flores-Estrada JJ, Moreno-Eutimio MA, Espinosa-Aguirre JJ, González-Ávila M, Ruiz-Pérez NJ (2017) Antimutagenic and antioxidant activity of the essential oils of Citrus sinensis and Citrus latifolia. Sci Rep 7:11479
Tripathi R, Mohan H, Kamat JP (2007) Modulation of oxidative damage by natural products. Food Chem 100:81–90
Trongtokit Y, Rongsriyam Y, Komalamisra N, Apiwathnasorn C (2005) Comparative repellency of 38 essential oils against mosquito bites. Phytother Res 19:303–309
Tsuneki H, Ma EL, Kobayashi S, Sekizaki N, Maekawa K, Sasaoka T, Wang MW, Kimura I (2005) Antiangiogenic activity of β-eudesmol in vitro and in vivo. Eur J Pharmacol 512:105–115
Tunón H, Thorsell W, Mikiver A, Malander I (2006) Arthropod repellency, especially tick (Ixodes ricinus), exerted by extract from Artemisia abrotanum and essential oil from flowers of Dianthus caryophyllum. Fitoterapia 77:257–261
Ultee A, Bennik MHJ, Moezelaar R (2002) The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl Environ Microbiol 68:1561–1568
Unnati S, Ripal S, Sanjeev A, Niyati A (2013) Novel anticancer agents from plant sources. Chin J Nat Med 11:16–23
Venturi CR, Danielli LJ, Klein F, Apel MA, Montanha JA, Bordignon SA, Roehe PM, Fuentefria AM, Henriques AT (2015) Chemical analysis and in vitro antiviral and antifungal activities of essential oils from Glechon spathulata and Glechon marifolia. Pharm Biol 53:682–688
Vigan M (2010) Essential oils: renewal of interest and toxicity. Eur J Dermatol 20:685–692
Viuda-Martos M, Ruiz-Navajas Y, Fernandez-Lopez J, Perez-Alvarez J (2008) Antifungal activity of lemon (Citrus lemon L.), mandarin (Citrus reticulata L.), grapefruit (Citrus paradisi L.) and orange (Citrus sinensis L.) essential oils. Food Cont 19:1130–1138
Vukovic-Gacic B, Nikcevic S, Beric-Bjedov T, Knezevic-Vukcevic J, Simic D (2006) Antimutagenic effect of essential oil of sage (Salvia officinalis L.) and its monoterpenes against UV-induced mutations in Escherichia coli and Saccharomyces cerevisiae. Food Chem Toxicol 44:1730–1738
Wang J, Zhua F, Zhoua XM, Niua CY, Lei CL (2006) Repellent and fumigant activity of essential oil from Artemisia vulgaris to Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). J Stored Prod Res 42:339–347
Wang GS, Deng JH, Ma YH, Shi M, Li B (2012) Mechanisms, clinically curative effects, and antifungal activities of cinnamon oil and pogostemon oil complex against three species of Candida. J Tradit Chin Med 32(1):19–24
Wen L, Wu D, Jiang Y, Prasad KN, Lin S, Jiang G, He J, Zhao M, Luo W, Yang B (2014) Identification of flavonoids in litchi (Litchi chinensis Soon.) leaf and evaluation of anticancer activities. J Funct Foods 6:555–563
Wu H, Li B, Wang X, Jin M, Wang G (2011) Inhibitory effect and possible mechanism of action of patchouli alcohol against influenza a (H2N2) virus. Molecules 16:6489–6501
Wu XL, Ju DH, Chen J, Yu B, Liu KL, He JX, Dai CQ, Wu S, Chang Z, Wang YP, Chen XY (2013) Immunologic mechanism of patchouli alcohol anti-H1N1 influenza virus may through regulation of the RLHsignal pathway in vitro. Curr Microbiol 67:431–436
Yang P, Ma Y (2005) Repellent effect of plant essential oils against Aedes albopictus. J Vector Ecol 30:231–234
Yang X, Zhang X, Yang SP, Liu WQ (2013) Evaluation of the antibacterial activity of patchouli oil. Iran J Pharm Res 12:307–316
Yang C, Chen H, Chen H, Zhong B, Luo X, Chun J (2017) Antioxidant and anticancer activities of essential oil from Gannan navel orange peel. Molecules 22:1391
Yen HF, Hsieh CT, Hsieh TJ, Chang FR, Wang CK (2015) In vitro anti-diabetic effect and chemical component analysis of 29 essential oils products. J Food Drug Anal 23:124–129
Yoo CB, Han KT, Cho KS, Ha J, Park HJ, Nam JH, Kil UH, Lee KT (2005) Eugenol isolated from the essential oil of Eugenia caryophyllata induces a reactive oxygen species mediated apoptosis in HL-60 human promyelocytic leukemia cells. Cancer Lett 225:41–52
Yousefbeyk F, Gohari AR, Sourmaghi MHS, Amini M, Jamalifar H, Amin M, Golfakhrabadi F, Ramezani N, Amin G (2014) Chemical composition and antimicrobial activity of essential oils from different parts of Daucus littoralis Smith subsp. Hyrcanicus Rech. f. J Essent Oil-Bearing Plants 17:570–576
Zeedan GSG, Abdalhamed AM, Ottai ME, Abdelshafy S, Abdeen E (2014) Antimicrobial, antiviral activity and GC-MS analysis of essential oil extracted from Achillea fragrantissima plant growing in Sinai Peninsula, Egypt. J Microbiol Biochem Technol S8:006. https://doi.org/10.4172/1948-5948.S8-006
Zhou S, Koh HL, Gao Y, Gong ZY, Lee EJ (2004) Herbal bioactivation: the good, the bad and the ugly. Life Sci 74:935–968
Zore GB, Thakre AD, Jadhav S, Karuppayil SM (2011a) Terpinoids inhibit Candida albicans growth by affecting membrane integrity and arrest of cell cycle. Phytomed 18:1181–1190
Zore GB, Thakre AD, Rathod V, Karuppayil SM (2011b) Evaluation of anti-Candida potential of geranium oil constituents against clinical isolates of Candida albicans differentially sensitive to fluconazole: inhibition of growth, dimorphism and sensitization. Mycoses 54:e99–e109
Zuzarte M, Maria JG, Carlos C, Canhoto J, Vale-Silva L, Silva MJ, Pinto E, Salgueiro L (2011) Chemical composition and antifungal activity of the essential oils of Lavandula viridis L’Her. J Med Microbiol 60:612–618
Zuzarte M, Goncalves MJ, Cruz MT (2012) Lavandula luisieri essential oil as a source of antifungal drugs. Food Chem 135:1505–1510
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Reddy, D.N. (2019). Essential Oils Extracted from Medicinal Plants and Their Applications. In: Akhtar, M., Swamy, M., Sinniah, U. (eds) Natural Bio-active Compounds. Springer, Singapore. https://doi.org/10.1007/978-981-13-7154-7_9
Download citation
DOI: https://doi.org/10.1007/978-981-13-7154-7_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-7153-0
Online ISBN: 978-981-13-7154-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)