Abstract
Artificial joint implants have been widely applied in clinical surgery of joint replacement for those patients whose natural joints suffer from trauma, disease, or overuse. Typical artificial joints consist of a metallic component integrated with bone and a polymer component that facilitates movement. The requirements for the polymer include biocompatibility, toughness, wear resistance, lubrication, etc. Over the past 50 years, ultrahigh-molecular-weight polyethylene (UHMWPE) has been used to fabricate artificial hips, knees, shoulders, and other joints. In this chapter, a historical development of artificial joint implants for arthroplasty is briefly reviewed. The fundamental physicochemical properties and processing of UHMWPE are summarized to demonstrate its superiority in the application of artificial joint implants. In view of clinical outcomes, main challenges of conventional UHMWPE joint implants are finally discussed, such as oxidation degradation, sterilization, wear, and debris.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Ultrahigh-molecular-weight polyethylene
- Artificial joints
- Joint implants
- Clinical application
- Wear-resisting
- Anti-oxidation
1.1 The History of Artificial Joint Implants for Arthroplasty
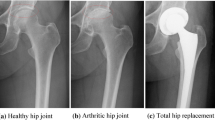
When natural joints fail due to trauma, disease, or overuse, replacement with artificial ones is needed to maintain the joint function and relieve the pain of patients. Joint replacement, also called joint arthroplasty, is a surgery that removes parts of an arthritic or damaged joint and replaces it with artificial implants or prosthesis comprised of metal, plastic, or ceramic devices. Prosthesis is designed to replicate the movement function of a normal and healthy joint. Nowadays, roughly 570,000 primary total hip and knee replacements are performed in the United States each year [1, 2]. According to the American Academy of Orthopedic Surgeons, the procedures performed in the United States are expected to increase to at least 750,000 per year by 2030 (Fig. 1.1) [3].
The projected number of primary (a) and revision (b) total hip arthroplasty (THA) and total knee arthroplasty (TKA) procedures in the United States from 2005 to 2030. (Reprinted from [3] with permission from Wolters Kluwer Health, Inc.)
Despite the long-lasting demands for artificial joints, it has taken about two centuries for human beings to establish the modern joint implant designs as well as optimal materials for joint implants. The earliest attempt of hip replacement occurred in 1891. Gluck presented the first use of ivory to replace femoral heads for a patient whose hip joints had been destroyed [4]. Since then, worldwide, surgeons carried out the interpositional arthroplasty from the late nineteenth to early twentieth century, which involved placing a variety of tissues between articulating surfaces of a remodeled joint, such as the fascia lata, skin, and pig bladder submucosa [5]. The first mold arthroplasty out of glass was performed by an American surgeon Marius Smith-Petersen in 1925. This mold consisted of a hollow hemisphere that can fit the femoral head and give rise to a new smooth surface required by the joint movement. However, the glass failed to endure great forces through the hip joint during movements. A British surgeon, George McKee, was the first to use a metal-on-metal prosthesis on a regular basis in 1953. He used the modified Thompson stem, which was a cemented hemiarthroplasty used for the neck of femur fracture. That implant showed a 28-year survival rate of 74% [6]. Unfortunately, that prosthesis was more and more unpopular in the 1970s because the local effect of metal particles was found during the revision surgery for prosthetic failures [7]. An orthopedic surgeon, Sir John Charnley, proposed low-friction arthroplasty in the early 1960s and thus created an era of modern total joint arthroplasty. This design is still used today as a golden standard. It is comprised of three parts: (1) a metal femoral stem, (2) a polyethylene acetabular component, and (3) an acrylic bone cement. Thus, a small femoral head is used to reduce wear due to its small surface area [8, 9].
Currently, a growing number of successful joint replacement operations have been achieved every year using different artificial joint materials. Scientists and engineers have been struggling to find the best combination that yields the fewest complications and excellent long-term survival, as highly motivated by the clinical applications and challenges of joint implants.
Metal-on-metal (MOM) prostheses are usually fabricated from cobalt, chromium, and molybdenum (CoCrMo) alloys. In the MOM total hip implants, a large metal femoral head on a standard femoral stem articulates against a metal acetabular cup or liner. Metal articulating surfaces could be engineered to be extremely smooth, which is beneficial to reduce the wear rate. A low wear rate is expected to prolong the lifetime of the implant and thus delay the need for any revision surgery. Thus, MOM hip replacements have traditionally been used for young patients. These alloys exhibit excellent hardness and strength but may raise concerns about long-term metal ion release [10]. Besides, during the long-term use, MOM hip implants have been estimated to release many wear particles because the particles tend to be smaller and smaller [11]. The CoCrMo particles may be more easily engulfed by cells and migrate into body fluids. It was found that patients with MOM hip implants often have an elevated level of chromium and cobalt in their blood, urine, and hair [12]. The long-term effect of elevated metal ions residual in vivo on the human beings’ health remains unknown.
Since the first introduction by Pierre Boutin, a French surgeon, in 1970, ceramic heads have accounted for half of the hip arthroplasties in Europe. To address the friction and wear that frequently emerged in other materials, the ceramic materials used in orthopedics include either alumina or zirconia. Compared with metals, ceramics usually induce reduced osteolysis, which is favorable for joints or joint surface materials. Moreover, their facile processing and forming and superior mechanical properties endow some ceramics as bone substitute materials. The ceramic-on-ceramic (COC) (Fig. 1.2) prostheses exhibit a high level of hardness, scratch resistance, inert nature, good biocompatibility, and stability in physiological conditions [13]. Besides, the hydrophilicity of these prostheses improves the lubrication and thus leads to a low coefficient of friction and excellent wear resistance [14]. However, the cost of ceramics is often too high, and the risk of fracture should be taken into account because ceramics are brittle materials. Once the fracture occurs, it is a complex task to revise because ceramic femoral heads typically break into multiple fragments that are hard to clean from surrounding tissues [15]. Squeaking is another issue for COC joint prosthesis. It is defined as an audible sound of 20–20,000 Hz during movement of the hard-on-hard bearings. The squeaking rate in MoM and CoC articulation has been reported to be 0.3% to 24.6%. Unfortunately, the origination of squeaking behavior is not fully understood yet. It has been widely accepted that patient, implant, and surgical factors play a role in the squeaking phenomenon [16].
Representative modern joint prosthesis. (a) Metal-on-metal, (b) ceramic-on-ceramic, (c) metal-on-polyethylene, and (d) ceramic-on-polyethylene. (Reprinted from [17] with open access)
Since Charnley first utilized ultrahigh-molecular-weight polyethylene (UHMWPE) in 1962, the most widely accepted implant configuration contains a metal component articulating against a polymeric component prepared from UHMWPE. Nowadays, such metal-on-UHMWPE joint implants have become an international standard for curing degenerative joint disorders. Total hip replacement for both femoral and acetabular surfaces became more and more popular during the 1980s and then grew steadily thereafter. These joints initially had a UHMWPE cup cemented into the acetabulum articulating against a metal head. The metal head was then attached to a stem inserted into the femur. Over the past 20 years, cement-free designs have been adopted, which involves a polymer component fixed to the pelvis by a metal shell and articulating against a metal or ceramic femoral head. On average, more than 90% of all total joint replacements that use UHMWPE-bearing surfaces survive for more than 10 years, according to the Swedish hip registry [18]. Such joints tend to be revised at a rate of about 1% per year in the first decade after implantation. Data show that implant survivorship reduces after 10 years, especially for patients who are younger than 55 years [18].
This chapter presents a general introduction to the clinical applications of UHMWPE in joint implants, which consists of four sections including (1) fundamentals to UHMWPE, (2) conventional UHMWPE for joint implants, (3) toughened UHMWPE for joint implants, and (4) clinical outcomes of conventional UHMWPE joint implants.
1.2 Fundamentals to UHMWPE
The medical grade UHMWPE used in total joint implants is a kind of linear homopolymer. Before giving the definition of UHMWPE, it is necessary to have a general concept about polymer. A polymer is defined as a big molecule constructed by numerous small molecules via covalent bonds. The small molecules used as the basic building blocks for these large molecules are known as monomers. The monomer segments can all be the same to generate a homopolymer. If there are more than two monomers involved, a copolymer can be obtained. The illustration of homopolymer and copolymer is shown in Fig. 1.3. The UHMWPE used for joint implants are homopolymers. Here, we focus on the synthesis, structure, and property of UHMWPE.
Polyethylene (PE), the largest tonnage plastic material, has one of the simplest molecular structures ([CH2CH2]n) formed from ethylene (Fig. 1.4). It was first commercially produced in 1939 for the use in electric insulation. There are four different industrial routes to produce PE including (1) high-pressure processes, (2) Ziegler processes, (3) the Phillips process, and (4) the Standard Oil (Indiana) process. A pressure of 1000–3000 atm and a temperature of 80–300 °C are often used for the high-pressure process. Free-radical initiators, such as benzoyl peroxide or oxygen, are generally used, and conditions need to be carefully controlled to prevent a runaway reaction. Generally, the high-pressure process yields low-density PE, typically in the range 0.915–0.945 g cm−3, with relatively low molar mass. Ziegler processes are based on coordination reactions catalyzed by metal alkyl systems. A typical Ziegler–Natta catalyst is the complex prepared from titanium tetrachloride and triethylaluminum. It is fed into the reaction vessel first, and subsequently ethylene is added. The reaction is carried out at low pressures and low temperatures, typically no more than 70 °C, with rigorous exclusion of air and moisture. The PE products are of intermediate density of about 0.945 g cm−3 with molecular weights ranging from 5 to 10 million. In the Phillips process, ethylene monomers are dissolved in cyclohexane and then polymerized by a supported metal oxide (mainly CrO3) catalyst at 130–160 °C and 1.4–3.5 MPa. The resulting commercial products have a melt flow index of only 0.2–5 and the highest density (0.96 g cm−3). The process invented by Standard Oil (Indiana) is based on the use of a supported transition metal oxide combined with a promoter. The reaction temperature is in the range of 230–270 °C and the pressures are 40–80 atm.
The size of a polymer molecule can be defined either by its molecular weight (Mw) or by the number of repeat units in the molecule (degree of polymerization, DP). The relative molar mass of the polymer is thus the product of the relative MW of the repeat unit and the DP. Due to the methods used to prepare polymers, the distribution of the MW is heterogeneous for polymers rather than the exact value of MW for small molecules. In principle, there are several ways to calculate such average MW. The most straightforward is the simple arithmetic mean, usually called the number average molar mass, Mn, which is defined by Eq. 1.1:
where Mi is the molar mass of the molecular species i and Ni is the number of molecules of i in the sample.
Another definition is the weight-average molar mass (Mw), which is scaled to Mi2 by Eq. 1.2:
For a polymer consisting of molecules of the same molar mass Mn = Mw, but in all other cases, Mw is greater than Mn. We can thus use the ratio of Mw to Mn as an indication of molecular dispersity in a particular polymer sample. This ratio is called the polydispersity index (PDI), where the sample with PDI = 1 is said to be homo- or monodispersed.
There are various methods to determine the Mw of polymers including end-group analysis, measurement of colligative properties, light scattering, ultracentrifugation, and measurement of dilute solution viscosity. In principle, absolute values of MW can be obtained by those methods except viscosity measurement. The viscosity method relies on prior calibration using standard polymers with known molar mass.
UHMWPE is a linear homopolymer of ethylene. The term “ultrahigh” refers to an average MW larger than 1.5 million g/mol. UHMWPE is a unique polymer with excellent physical and mechanical properties. Moreover, UHMWPE exhibits the best sliding wear resistance and impact toughness and thus has been used to replace steel in many applications requiring wear resistance [19]. Since 1962, UHMWPE has been used in orthopedics as a bearing material for joint implants. Nowadays, around 1.4 million joint replacement procedures are performed worldwide. More than 90% of all total hip implants worldwide in the past decade have used UHMWPE. Despite the great success of restorative procedures, UHMWPE implants have a finite lifetime due to the wear and damage.
UHMWPE was first synthesized by Ziegler in the early 1950s and then commercially produced in 1955. A highly reactive organotitanate catalyst was used to polymerize the ethylene gas. The resultant polymer chains are remarkably linear with measured branching of less than 3%.
As shown in Fig. 1.5, the Ziegler–Natta mechanism is usually used to depict the polymerization of ethylene. Generally, the catalyst consists of titanium and aluminum alkyls. During the reaction of catalyst stereochemistry, the aluminum alkyls form a titanium alkyl bond. Ethylene is then inserted into this chemical bond to generate a titanium ethylene alkyl. The chain grows by inserting more ethylene molecules into this titanium carbon bond. A chain transfer agent such as molecular hydrogen is typically employed to control molecular weight because it reacts cleanly, leaves no residue, and is low cost. A chain transfer agent terminates a growing chain without deactivating the catalyst.
Ziegler–Natta mechanism for the polymerization of ethylene. (Reprinted from [19] with permission from Taylor & Francis)
Several parameters such as reaction temperature, activation of the catalyst, and concentration of alkyls affect the polymerization of UHMWPE. Ziegler–Natta polymerization does not easily allow for secondary reactions. In situ modification of UHMWPE is not practiced. The Ziegler–Natta reaction can be seen as an inherent “clean” strategy, and the product requires no purification beyond removal of the slurry diluent by staged dryers. The crude polymer is normally white and opaque due to the scattering by the polyethylene crystallites. The average particle diameters of the commercial product usually range from 50 to 250 microns.
The melt viscosity of UHMWPE is very high due to the ultrahigh molecular weight. It cannot flow like low MW polyethylene when the temperature is above its melting temperature. No processing aids or additives are allowed for medical-grade UHMWPE for clinical applications. Therefore, conventional thermoplastic processing techniques such as injection molding, screw extrusion, and blow molding are not suitable for UHMWPE. Semi-manufactures of UHMWPE are typically used by compression molding and ram extrusion under high temperatures and pressures.
A typical compression molding press installed at Poly Hi Solidur Meditech in Germany was designed by Hoechst in 1970s for the production of CHIRULEN. There are two sheets (1 m × 2 m) molded in a single press cycle: one is pressed between the upper and middle platens, and the second is produced between the middle and lower ones. In this way, the heating and loading systems are totally controlled by computer. The entire press is performed in a clean room to prevent the introduction of extraneous matter into the sheet.
The ram extrusion of UHMWPE was developed by converters in the United States in 1970s. Similar to the compression molding facilities, a medical-grade extruder is typically used in a clean room to reduce and avoid extraneous matters into the UHMWPE. The schematic illustration of ram extrusion is shown in Fig. 1.6, where UHMWPE powder is first fed into an extruder comprised of a hopper that allows the powder to enter into a receiving chamber, a horizontal reciprocating ram, a heated die, and an outlet successively. The UHMWPE is maintained under pressure by the ram and the back pressure of the melting UHMWPE. Beyond the outlet, the rod of UHMWPE is slowly cooled in a series of electric heating mantles.
Medical-grade UHMWPE is a semicrystalline polymer that can be described as many ordered regions (crystalline lamellae) embedded in a disordered amorphous matrix (Fig. 1.7a). The degree of crystalline is very important for the properties of UHMWPE. With a high crystallinity, the polymer shows a high elastic modulus, superior yield strength, improved resistance to creep deformation, and enhanced fatigue resistance, which are critical for joint implants. The degree and orientation of crystalline regions within a polyethylene strongly depend on various factors such as MW, processing conditions, and external loading.
The crystalline morphology of UHMWPE can be visualized by using transmission electron microscopy (TEM) on ultrathin slices. The amorphous phase can be selectively stained and turn gray in the micrograph, while the lamellae appear as light domains with gray outlines (Fig. 1.7b). The composite structures of UHMWPE are featured as an interconnected network of amorphous and crystalline regions.
UHMWPE shows outstanding tensile properties. Tensile testing is commonly conducted to characterize the mechanical properties of UHMWPE. A dumbbell-shaped specimen is punched out and stretched at a specified crosshead speed in a tensile load frame. The load (F) and displacement (L) are converted to stress and strain. Moreover, Young’s modulus, yield stress, ultimate tensile stress, and elongation at break are all determined from the stress–strain curve (Fig. 1.8). Upon loading, the spherulites of UHMWPE start to deform, showing linear elastic deformation at low strain. Once the load becomes adequate to break the spherulites, the spherulites rupture and the polymer undergoes plastic deformation, that is, the polymer yields. After yielding, the stress decreases and then gradually increases when the lamellae start to deform along the elongation, with a gradual orientation. During this procedure, the amorphous chains and lamellae are gradually stretched, resulting in strain hardening. At very high strain, the stress abruptly increases until the sample fails. Thus, the ultimate stress, yield strength, elongation at break, and fracture toughness are determined.
Typical “dogbone” tensile specimen (a) and a typical engineering tensile stress–strain curve of UHMWPE (b). (Reprinted from [20] with permission from Elsevier)
Fatigue testing is a method to determine the crack propagation in a specimen subjected to an oscillating tensile load. The numbers of oscillations (N) used in these tests are typically several million cycles. The purpose of the test is to monitor the crack propagation resistance under cyclic loadings. In this test, a compact specimen, as used in the J-integral tests (Fig. 1.9a), is placed in a hydraulic load frame and then oscillated at either a fixed displacement or between fixed loads. During the test, the crack length (Δa) is periodically measured. The stress intensity factor (K), which depends on the load range used, sample geometry, and crack length, is computed from the raw data (a vs N), which are then used to prepare a curve of log da/dN versus log ΔK (Fig. 1.9b).
Specimens with various notch geometries used for fatigue analysis (a) and fatigue crack propagation rate (da/dN) data as a function of the stress intensity range (ΔK) for virgin UHMWPE (UHMWPE, black), cross-linked (75 kGy) and remelted UHMWPE (RXLPE (RXLPE, blue) and vitamin E (0.1 wt%) blended and cross-linked (75 kGy) UHMWPE (VXLPLE, orange). (Reprinted from [21] with permission from Elsevier)
1.3 Conventional UHMWPE for Joint Implants
In the late 1950s, Dr. John Charnley, a joint replacement pioneer, found that natural joints have a low friction coefficient due to the synovial fluid between the contacting surfaces as it is compressed. The pressurized fluid film protects the cartilage from wear. Arthritis and other joint diseases can cause the cartilage to lose its ability to lubricate joints. Therefore, the design of artificial joints aims to mimic such lubrication, which is dependent on the boundary lubrication of the materials in contact with each other. Polytetrafluoroethylene (PTFE) was Charnley’s earliest polymeric-bearing material for artificial hip joints in 1958. The earliest design had a PTFE acetabular element articulating against a PTFE femoral component.
Although PTFE implants initially restored pain-free mobility to those suffering from joint disease, they were worn out rapidly. Many debris from PTFE wear led to inflammation and pain, and thus there was an urgent need to revise nearly all of the implanted joints just 2–3 years after implantation. In 1962, Charnley used UHMWPE to increase joint longevity. Initially, it was available only to a limited number of surgeons trained personally by Charnley. Since then, it had become more widely used during the 1970s. Total hip replacement for both femoral and acetabular surfaces experienced explosive popularity during the 1980s and then grew steadily thereafter. These joints initially had a UHMWPE cup cemented into the acetabulum articulating against a metal head. The metal head was then attached to a stem inserted into the femur.
From the standpoint of medical application, UHMWPE orthopedic components should be sterilized before clinical use. High-energy radiation was the most commonly used sterilization technique. The source of γ radiation is the decay of an unstable60Co nucleus, while electron beams are generated from a thermally excited tungsten filament accelerated by electric fields. The electron beam is easier to control and requires a very short period of treatment (usually in seconds). Usually, UHMWPE components are stored on the shelf for long durations prior to implantation (6 months or longer). In addition, UHMWPE inserts of total joint replacements have historically been packaged in air and thereafter sterilized by γ radiation. However, it is well established that such ionizing irradiation can result in chain scission and degradation of PE. A large number of radicals are thus formed to cause unexpected cross-linking, which have detrimental effects on the morphology, mechanical properties, and wear of UHMWPE [22,23,24].
Once a polymer material is exposed to strong energy environments, it is likely to form free radicals due to the bond scission, and thus the mechanical properties of polymer reduces, as induced by the chain fragmentation. In orthopedics, this degradation is mainly associated with the radiation and electron beams commonly used during sterilization mentioned above. If oxygen is present when the degradation occurs, it is called oxidation. The oxidation process cannot be interrupted if it has been initiated, and the oxidation rate increases continuously with a series of reactions that involve free radicals and oxygen. The extent of oxidation is strongly dependent on the amount of oxygen. The origin of oxygen is very complicated including (1) from atmosphere present at sterilization, (2) penetrated by diffusion into the polymer during processing and storage, and (3) being used in vivo. Therefore, the oxidation can continue during storage and in vivo after implantation [22].
In order to solve these oxidation issues, some manufacturers sterilize UHMWPE using non-radiation-based methods, such as ethylene oxide (EtO) or gas plasma sterilization. EtO is widely used for sterilization of UHMWPE components sealed in gas permeable packages. UHMWPE sterilized with EtO does not undergo any variation in chemical or physical structures. Gas plasma (GP) is a surface sterilization strategy based on the action of ionized gas (i.e., hydrogen peroxide or peracetic acid), which deactivates biological organisms. Commercially available GP sterilization methods are usually performed at low temperatures (< 50 °C) and have no significant effects on the physical, chemical, and mechanical properties of UHMWPE. In response to long-term postirradiation aging and oxidation, some manufacturers further developed the sterilization system with high-energy radiation performed in vacuum or under inert gases (N2 or Ar).
Wear is the process of removing parts of a material from the surface during reciprocal movement along another surface with greater hardness. In artificial joint components, the UHMWPE is removed because it is soft and relatively weak compared with the metal or ceramic materials in femoral head and femoral knee components. During the wear process, the removed PE particles can migrate to the prosthetic tissues and thus induce aseptic loosening, through a mechanism involving the formation of reactive tissue and consequent osteolysis, which has been recognized as the main reason for the failure of implants based on conventional UHMWPE [25]. Unfortunately, the exact mechanism of immune reaction that occurs in periprosthetic osteolysis of joint replacements is still unclear. Up to now, there are several types of immune processes that are believed to be relevant to osteolysis. A foreign-body and granulomatous response to UHMWPE wear particles may denote a nonspecific chronic inflammatory reaction involving activated mononucleated macrophages and fibroblasts but few T lymphocytes [26]. The activation of macrophages is strongly related to the size, shape, volume, and number of radiation-sterilized UHMWPE debris particles [27]. Especially those 0.3–10 μm in size are phagocytable and are therefore the most biologically active [28]. In addition, the influence of the chemical composition of the UHMWPE particles has recently been suggested. The reactivity might be related to the composition of the surfaces of the particles themselves and in particular to the level of oxidation of the UHMWPE [29, 30]. It is found that oxidized particles from γ-irradiated UHMWPE would be more effective in activating the macrophages than the non-oxidized ones from EtO-sterilized UHMWPE. The properties of the absorbed molecules such as the hydrophilic/hydrophobic feature and the release of radicals also affect the surface reactivity of the particles [30]. In fact, although catastrophic failures due to supreme wear and heavy oxidation are quite infrequent, wear is also dependent on time. The abrasion and the production of abrasive particles therefore remain a severe issue for young and active patients with long life expectancies. Cross-linked UHMWPE appears to be the solution to the wear issue.
1.4 Toughened UHMWPE for Joint Implants: Lessons Learned
In order to strengthen and toughen UHMWPE, composites can be engineered by blending UHMWPE powders with micro- or nano-objectives (particles or fibers) before consolidation. In the 1970s, carbon fiber-reinforced (CFR) UHMWPE composites were first used for orthopedic implants and were commercially produced as Poly II in Zimmer, Inc., Warsaw, USA. However, the short-term clinical failures eventually resulted in the abandonment of Poly II [31]. The CFR UHMWPE was reinforced by randomly oriented carbon fibers during the direct compression molding of UHMWE matrix [32]. Initially, the incorporated carbon fibers were deemed to be the reason for reinforcement [33]. However, further investigation confirmed that the poor fiber–matrix interface decreased the ductility and crack resistance [34]. In practice, there are many difficulties in the blending process, resulting in incomplete consolidation of the UHMWPE powders and carbon fibers. Therefore, severe issues such as wear, fracture, and extensive delamination made the composites hazardous for clinical applications. Moreover, carbon fibers with very high modulus, once exposed from the UHMWPE matrix, may severely damage the femoral head.
Alternative self-reinforced composites, also called homocomposites, are another strategy to reinforce UHMWPE. In this way, the matrix and reinforced component all come from the same material (i.e., UHMWPE). In the 1990s, self-reinforced UHMWPE composites were widely studied, which were developed both by sintering oriented fibers together and by reinforcing a polymer matrix with UHMWPE fibers. Within the composites, UHMWPE fibers provide much higher tensile modulus (around 113 GPa) and ultimate tensile strength (2–4 GPa) compared with bulk UHMWPE consolidated from powders [35, 36]. Moreover, the melting temperature of UHMWPE fibers is 10 °C higher than the powders, which could be compression molded either by themselves or by combining with powders during a narrow temperature range to keep the initial orientation [37]. This self-reinforcement strategy was first used to fabricate oriented UHMWPE composites for orthopedic bearings by sintering compressed continuous Spectra 1000 fibers at Zimmer [38]. The schematic presentation is shown in Fig. 1.10. The sintered UHMWPE fibers are oriented orthogonal to the bearing surface. The obtained UHMWPE composites exhibit dramatically increased modulus and strength by an order of magnitude when tested along the direction of fiber orientation. However, self-reinforced UHMWPE homocomposites have never been commercialized for total joint replacement applications due to the main challenge of the processing compared with radiation-processed materials.
Schematic presentation of self-reinforced UHMWPE composites. (Reprinted from [39] with permission from Elsevier)
1.5 Clinical Outcomes of Conventional UHMWPE Joint Implants
Joint replacement surgeries have witnessed a rapid development over the past decades. An obvious trend has been observed that people aged 45–60 years are increasingly opting for joint replacement surgeries. Technological advancements have resulted in better prosthetics, longer lifespan, and more comfort for the patients. Increasing geriatric population in several countries, such as the United States, Canada, and Japan, is expected to drive the demand for medical UHMWPE over the long term. According to a new report by Grand View Research, Inc. [40], the global medical-grade UHMWPE market demand was 60.9 kilotons in 2015 and is expected to reach 204.8 kilotons by 2024, growing at a CAGR of 15% from 2016 to 2024. The market value is expected to reach 1.36 billion USD by 2024.
Although UHMWPE has achieved a success in total joint arthroplasty for over 30 years, we are still facing many challenges in clinical applications due to the material failures which are typically caused by the gamma irradiation-induced oxidative degradation and the high cyclic stress environment of the joints. With early joint replacement components, gamma irradiation in air at a dose level of approximately 25 kGy was used as a major sterilization method due to its low cost. However, oxidative degradation can take place when UHMWPE is exposed to gamma radiation in the presence of air. More seriously, such oxidative degradation can continue, and the number of oxidation products will increase without any further irradiation once there is an oxygen source [41]. Clinical investigations have shown that the oxidative degradation has a negative impact on the performance of acetabular hip components [42,43,44] and tibial knee components [45, 46] in terms of wear and fracture resistance.
To minimize the oxidation and its subsequent effects on wear and mechanical properties of UHMWPE, orthopedic implant manufacturers have turned to modified sterilization protocols, such as gamma radiation sterilization in vacuum packaging or inert gas packaging with reduced amount of oxygen. These strategies can greatly reduce or eliminate the potential of oxidation due to the lack of oxygen source. But the free radicals still remain in vivo. The oxidation of UHMPE components sterilized under low oxygen conditions was, in fact, investigated, and the highest oxidation indices tend to occur at stress concentrations inherent in the UHMWPE component design [47, 48].
The gross fracture and component cracking of conventional UHMWPE total joint replacement components has been found [42, 49, 50]. All of these design features are due to inherent stress concentrators. Oxidation of these regions may further increase the susceptibility to fracture. For example, a recent study of retrieved conventional UHMWPE acetabular liners indicated that the percentage of retrieved liners that showed cracking increased with increasing the oxidation level [49] (Fig. 1.11).
(a) Highly oxidized liners with extensive rim cracks extending onto articular surface. (b) Liner with multiple subsurface rim cracks demonstrated by transillumination. (c) Liner revised after complete mechanical failure secondary to fragmentation. (d) Liner with extensive oxidation and impingement damage leading to extensive cracking and separation of the rim. (Reprinted from [49] with permission from Elsevier)
Another significant issue in the clinic is the abrasion wear (Fig. 1.12). It frequently results in the removal of a partial component of one material from the surface during reciprocal movement along another surface with greater hardness. In orthopedic joint components, the UHMWPE is easily removed because the interactions of its chains are relatively weak compared to those between the metal and ceramic materials. Such removed PE particles induce aseptic loosening through a mechanism involving the formation of reactive tissue and consequently osteolysis [25]. The surface wear mechanism is able to produce relatively small particles with the size less than 1 μm typically, while the fatigue wear mechanism usually generates larger particles. The smaller particles can cause an osteolytic response more seriously than the larger ones [51]. The existence of such particles could cause the immune reaction that occurs in periprosthetic osteolysis of joint replacements, but the mechanism is still unclear. It could be related to some types of immune processes. For example, the granulomatous response to UHMWPE particles is a nonspecific chronic inflammatory reaction involving activated mononucleated macrophages and fibroblasts but few T lymphocytes [26].
(a) A clinically retrieved total hip acetabular component. (b) A clinically retrieved total knee tibial insert. (Reprinted from [52] with permission from Wolters Kluwer Health, Inc.)
1.6 Conclusions
To date, UHMWPE has been a popular joint implant material because of its low coefficient of friction, low wear over the long term, and stability and biocompatibility in the body. Since it has been placed in the knee and hip joints in the 1960s, UHMWPE bearings have also been used in shoulder, elbow, wrist, ankle, and great toe replacements. The development of UHMWPE material continuously breaks the bottleneck in surgical outcome. It is expected to see a significant decrease in revisions because of UHMWPE-related failures such as particle-induced osteolysis and delamination-related instability. Radiation cross-linking has appeared as a standard bearing surface to achieve enhanced wear resistance of UHMWPE and is likely to decrease the incidence of osteolysis. With the development of polyethylene technologies including synthesis, processing, and fabrication, it is expected to offer more flexibility in implant design such that more anatomical reconstruction of joints can be performed with the least amount of invasive interference to improve the life quality of joint implant patients.
References
Kurtz S, Mowat F, Ong K, Chan N, Lau E, Halpern M (2005) Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. J Bone Joint Surg Am 87A(7):1487–1497
Maradit KH, Larson DR, Crowson CS, Kremers WK, Washington RE, Steiner CA, Jiranek WA, Berry DJ (2015) Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am 97(17):1386–1397
Kurtz S, Ong K, Lau E, Mowat F, Halpern M (2007) Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am 89(4):780–785
Speed JS, Smith H (1940) Arthroplasty – a review of the past ten years. Surg Gynecol Obstet 70:224–230
Learmonth ID, Young C, Rorabeck C (2007) The operation of the century: total hip replacement. Lancet 370(9597):1508–1519
Brown SR, Davies WA, DeHeer DH, Swanson AB (2002) Long-term survival of McKee-Farrar total hip prostheses. Clin Orthop Relat Res 402:157–163
McKellop H, Park SH, Chiesa R, Doorn P, Lu B, Normand P, Grigoris P, Amstutz H (1996) In vivo wear of 3 types of metal on metal hip prostheses during 2 decades of use. Clin Orthop Relat Res 329:S128–S140
Charnley J (1961) Arthroplasty of the hip: a new operation. Lancet 277(7187):1129–1132
Charnley J (1973) Arthroplasty of the hip: a new operation. Clin Orthop Relat Res 95:4–8
MacDonald SJ, Brodner W, Jacobs JJ (2004) A consensus paper on metal ions in metal-on-metal hip arthroplasties. J Arthroplast 19(8):12–16
Firkins PJ, Tipper JL, Saadatzadeh MR, Ingham E, Stone MH, Farrar R, Fisher J (2001) Quantitative analysis of wear and wear debris from metal-on-metal hip prostheses tested in a physiological hip joint simulator. Biomed Mater Eng 11(2):143–157
Hallab NJ, Anderson S, Caicedo M, Skipor A, Campbell P, Jacobs JJ (2004) Immune responses correlate with serum-metal in metal-on-metal hip arthroplasty. J Arthroplast 19(8):88–93
Katti KS (2004) Biomaterials in total joint replacement. Colloids Surf B Biointerfaces 39(3):133–142
Christel PS (1992) Biocompatibility of surgical-grade dense polycrystalline alumina. Clin Orthop Relat Res 282:10–18
Hannouche D, Nich C, Bizot P, Meunier A, Nizard RM, Sedel L (2003) Fractures of ceramic bearings – history and present status. Clin Orthop Relat Res 417:19–26
Mai K, Verioti C, Ezzet KA, Copp SN, Walker RH, Colwell CW Jr (2010) Incidence of ‛squeaking’ after ceramic-on-ceramic total hip arthroplasty. Clin Orthop Relat Res 468(2):413–417
Schwartsmann CR, Boschin LC, Gonçalves RZ, Yépez AK, de Freitas Spinelli L (2012) New bearing surfaces in total hip replacement. Rev Bras Ortop 47(2):154–159
Malchau H (2002) Prognosis of total hip replacement: update of results and risk-ratio analysis for revision and re-revision from the Swedish National Hip Arthroplasty Register 1979–2000. In: 69th annual meeting of American Academy of Orthopedic Surgeons, Dallas, Texas
Kelly JM (2002) Ultra-high molecular weight polyethylene. J Macromol Sci C Polym Rev C42(3):355–371
Spiegelberg S (2009) Chapter 24 – Characterization of physical, chemical, and mechanical properties of UHMWPE A2 – Kurtz, Steven M. In: Kurtz S (ed) UHMWPE biomaterials handbook, 2nd edn. Academic, Boston, pp 355–368
Ansari F, Gludovatz B, Kozak A, Ritchie RO, Pruitt LA (2016) Notch fatigue of ultrahigh molecular weight polyethylene (UHMWPE) used in total joint replacements. J Mech Behav Biomed Mater 60:267–279
Bracco P, del Prever EMB, Cannas M, Luda MP, Costa L (2006) Oxidation behaviour in prosthetic UHMWPE components sterilised with high energy radiation in a low-oxygen environment. Polym Degrad Stab 91(9):2030–2038
Birkinshaw C, Buggy M, Daly S, Oneill M (1989) The effect of gamma-radiation on the physical structure and mechanical-properties of ultrahigh molecular-weight polyethylene. J Appl Polym Sci 38(11):1967–1973
Bostrom MP, Bennett AP, Rimnac CM, Wright TM (1994) The natural-history of ultra-high-molecular-weight polyethylene. Clin Orthop Relat Res 309:20–28
Harris WH (2001) Wear and periprosthetic osteolysis – the problem. Clin Orthop Relat Res 393:66–70
Goodman SB (2007) Wear particles, periprosthetic osteolysis and the immune system. Biomaterials 28(34):5044–5048
Ingham E, Fisher J (2005) The role of macrophages in osteolysis of total joint replacement. Biomaterials 26(11):1271–1286
Chiba J, Schwendeman LJ, Booth RE, Crossett LS, Rubash HE (1994) A biochemical, histologic, and immunohistologic analysis of membranes obtained from failed cemented and cementless total knee arthroplasty. Clin Orthop Relat Res 299:114–124
Fubini B (1997) Surface reactivity in the pathogenic response to particulates. Environ Health Perspect 105:1013–1020
Brach del Prever EM, Bistolfi A, Costa L, Bracco P, Linari A, Botto Micca F, Crova M, Gallinaro P (2003) The biological reaction to polyethylene wear debris can be related with oxidation of the UHMWPE cups. Chir Organi Mov 88:291–303
Wright TM, Astion DJ, Bansal M, Rimnac CM, Green T, Insall JN, Robinson RP (1988) Failure of carbon fiber-reinforced polyethylene total knee-replacement components. A report of two cases. J Bone Joint Surg Am 70(6):926–932
Farling GM (1976) Human body implant of graphitic carbon fiber reinforced ultra-high molecular weight polyethylene. USA Patent, 4055862
Ainsworth RFG, Bardos D (1977) An improved bearing material for joint replacement prostheses: carbon fiber-reinfored UHMW polyethylene. Trans Third Soc Biomater 3:119
Connelly GM, Rimnac CM, Wright TM, Hertzberg RW, Manson JA (1984) Fatigue crack propagation behavior of ultrahigh molecular weight polyethylene. J Orthop Res 2(2):119–125
Deng M, Shalaby SW (1997) Properties of self-reinforced ultra-high-molecular-weight polyethylene composites. Biomaterials 18(9):645–655
Suh NP, Mosleh M, Arinez J (1998) Tribology of polyethylene homocomposites. Wear 214(2):231–236
Mosleh M (1998) An UHMWPE homocomposite for joint prostheses. In: Jacobs JJ, Craig TL (eds) Alternative bearing surfaces in total joint replacement. American Society for Testing and Materials, West Conshohoken, pp 256–265
Price H, Lin S, Hawkins M, Parr J (1997) Reinforced polyethylene for articular surfaces. USA Patent, 5609638
Siskey R, Smelt H, Boon-Ceelen K, Persson M (2016) UHMWPE homocomposites and fibers. In: Kurtz S (ed) UHMWPE biomaterials handbook, 3rd edn. William Andrew Publishing, Oxford, pp p398–p411
Grand View Research (2016) Medical grade ultra high molecular weight polyethylene (uhmwpe) market analysis by application (total hip replacement, knee replacement, shoulder replacement, ankle replacement, small joints) and segment forecast to 2024
Costa L, Bracco P (2004) Mechanisms of crosslinking, oxidative degradation and stabilization of UHMWPE. In: Kurtz S (ed) UHMWPE biomaterials handbook. Elsevier Academic Press, Boston, pp 309–323
Kurtz S, Rimnac C, Hozack W, Turner J, Marcolongo M, Goldberg V, Kraay M, Edidin A (2005) In vivo degradation of polyethylene liners after gamma sterilization in air. J Bone Joint Surg 87(4):815–823
McKellop H, Shen F, Lu B, Campbell P, Salovey R (2000) Effect of sterilization method and other modifications on the wear resistance of acetabular cups made of ultra-high molecular weight polyethylene. J Bone Joint Surg 82A(12):1708–1725
Sutula LC, Collier JP, Saum KA, Currier BH, Currier JH, Sanford WM, Mayor MB, Wooding RE, Sperling DK, Williams IR, Kasprzak DJ, Surprenant VA (1995) Impact of gamma-sterilization on clinical-performance of polyethylene in the hip. Clin Orthop Relat Res 319:28–40
Won C, Rohatgi S, Kraay M, Goldberg V, Rimnac C (2000) Effect of resin type and manufacturing method on wear of polyethylene tibial components. Clin Orthop Relat Res 376:161–171
Williams IR, Mayor MB, Collier JP (1998) The impact of sterilization method on wear in knee arthroplasty. Clin Orthop Relat Res 356:170–180
Kurtz S, Rimnac C, Rimnac C, Hozack W, Turner J, Marcolongo M, Goldberg V, Kraay M, Edidin A (2005) In vivo degradation of polyethylene liners after gamma sterilization in air. J Bone Joint Surg 87(4):815–823
Currier B, Currier J, Mayor M, Lyford K, Van Citters D, Collier J (2007) In vivo oxidation of gammabarrier-sterilized ultra-high-molecular-weight polyethylene bearings. J Arthroplast 22(5):721–731
Birman M, Noble P, Conditt M, Li S, Mathis K (2005) Cracking and impingement in ultra-high-molecular-weight polyethylene acetabular liners. J Arthroplast 20(Suppl. 3):87–92
Chiu Y-S, Chen W-M, Huang C-K, Chiang C-C, Chen T-H (2004) Fracture of the polyethylene tibial post in a NexGen posterior-stabilized knee prosthesis. J Arthroplast 19(8):1045–1049
Green T, Fisher J, Matthews J, Stone M, Ingham E (2000) Effect of size and close on bone resorption activity of macrophages by in vitro clinically relevant ultra high molecular weight polyethylene particles. J Biomed Mater Res 53(5):490–497
Greenwald A, Bauer T, Ries M (2001) New polys for old: contribution or caveat? J Bone Joint Surg 83:27–31
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Science+Business Media Singapore
About this chapter
Cite this chapter
Chen, J., Gao, G., Fu, J. (2019). Clinical Applications of UHMWPE in Joint Implants. In: Fu, J., Jin, ZM., Wang, JW. (eds) UHMWPE Biomaterials for Joint Implants. Springer Series in Biomaterials Science and Engineering, vol 13. Springer, Singapore. https://doi.org/10.1007/978-981-13-6924-7_1
Download citation
DOI: https://doi.org/10.1007/978-981-13-6924-7_1
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-6923-0
Online ISBN: 978-981-13-6924-7
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)