Abstract
Brassinosteroids (BS), a class of polyhydroxylated steroidal plant hormones were collectively named as ‘Brassins’ after their initial discovery from the pollen grains of Brassica napus. They occur in whole plant kingdom and almost all plant parts. Pollen and immature seeds are the richest sources of BS. A spectrum of physiological, biochemical and molecular responses in plants have been attributed to BS, which include shoot and root growth, fertility and seed germination, cell elongation, vascular differentiation, xylem formation in epicotyls, and also in the regulation of expression of several genes involved in xylem development. They also affect cotyledon growth, root elongation, leaf formation and growth, and plant biomass. Ethylene production is another important physiological response in plant that has been attributed to BS activity. They have also been found to protect plants from various abiotic and biotic stress factors, such as salt, temperature, water, heavy metals and pathogens. BS also enhance the yield of several cereals, legumes, oilseed crops and crops of horticultural importance. In horticultural crops, they favour fruit production and quality of the fruits. This chapter describes various studies wherein BS have been exploited to enhance the productivity of different horticultural crops. Most importantly, they are naturally occurring and eco-friendly, thus they can easily replace the hazardous chemicals.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Brassinolide
- Brassinosteroids
- 24-Epibrassinolide
- Ethylene
- Flowering
- Fruits quality
- 28-Homobrassinolide
- Horticulture
1 Introduction
Brassinosteroids (BS), a recently recognized new class of plant hormones (Clouse and Sasse 1998; Khripach et al. 1999) is also called “polyhydroxylated steroidal plant hormone” (Fariduddin et al. 2014) and a new and unique class of plant growth regulators (Sirhindi 2013). Their occurrence was first noted in the pollen grains of Brassica napus. They were subsequently called “Brassinosteroids”, although they were initially named as “Brassins”. They occur in whole plant kingdom as well as all plant parts. However, their quantity varies from one part to another. Their quantity is higher in young growing tissues than mature tissues. Highest levels (1–100 μg kg−1 fresh tissue) are found in pollen and immature seeds, whereas shoots and leaves usually, possess lower amounts i.e. 0.01–0.1 μg kg−1 (fresh tissue).
Initially they were found associated with reproductive development of a plant (Clouse and Sasse 1998). However, later research broadened their role in a wide spectrum of growth and developmental events. The developmental processes affected by BS include cell division and cell elongation in stems and roots, photo-morphogenesis, reproductive development, leaf senescence, and also in stress responses (Ali et al. 2007; Sirhindi 2013; Fariduddin et al. 2014). Their essentiality in normal growth and development was proved beyond doubt by some eminent worker such as Clouse and Sasse (1998) and Sasse (2003). The essentiality of BS in plant growth and development has been proved in different studies wherein “brassinazole” an inhibitor of BS biosynthesis has been used. The other processes influenced by BS include shoot and root growth, fertility and seed germination, cell elongation, vascular differentiation, xylem formation in epicotyls, and also in the regulation of expression of several genes involved in xylem development (Clouse and Sasse 1998; Taiz and Zeiger 2004). They also affect cotyledon growth, root elongation, leaf formation and growth, and plant biomass. Exogenous application of BS also improves the activities of different enzymes such as carbonic anhydrase, nitrate reductase (Ali et al. 2006; Alam et al. 2007), rubisco (Yu et al. 2004) and those involved in Calvin cycle (Fedina et al. 2008). In addition to this, BS have a great potential to confer resistance to plants against various biotic and abiotic stresses, such as salinity (Ali et al. 2007), water stress (Vardhini and Rao 2002), temperature extremes (Sirhindi 2013), and heavy metals (Hayat et al. 2007; Ali et al. 2008a, b; Yusuf et al. 2012). Besides these key roles, BS have also been found to affect whole physiology of the plant, starting from seed germination to harvest or seed maturation. Application of BS has been found to enhance the seed germination in chickpea (Ali et al. 2005), Indian mutard (Sirhindi 2013) and tobacco (Lubner-Metzger 2001). Furthermore, the exogenous application of BS has been found to enhance the yield of a number of crop plants such as Brassica juncea, Arachis hypogeal, Vigna radiata (Vardhini and Rao 2002), Lycopersicon esculentum (Ali et al. 2006) and Cicer arietinum (Ali et al. 2007), both under stress and stress free conditions. However, few treatments have been performed in the field, under real growing condition. Most of the studies have been conducted with plants grown under controlled environmental conditions in the laboratory. Many BS and BS-analogues that showed high biological activity in bioassays or controlled-environment experiments failed to stimulate plants grown under field conditions (Hola et al. 2010). This can be explained by various reasons such as the timing of BS application (Nunez et al. 2003), duration of exposure and the BS treatment, frequency of BS treatment and the dose, type and mode of BS can also substantially affect the growth/yield promoting activity of these compounds (Hola et al. 2010). However, more accurate studies on dosage, mode and time of application, fit brassinosteroid suitability for the plant or cultivar, and association with other phytohormones are needed.
BS increase crop yield and show anti-stress effects on several plants at very low doses. Besides this, they are easily metabolized (Adam and Schneider 1999; Schneider 2002) and are also eco-friendly (Kang and Guo 2011) with a huge potential of increasing agricultural and horticultural productivity. In order to make them cost-effective many types of BS analogues have been prepared (Zullo and Adam 2002). The analogues include BB6 and MH5, DI-31 (BB16) and DI-100.
The phenomenon of plant growth, development and productivity is determined both by exogenous and endogenous factors. Phytohormones play very a critical role among the endogenous factors. Therefore, they are extensively exploited in order to improve crop performance/yield (Montoya et al. 2005). Although, it is well established that BS have a beneficial effect on the growth and productivity of many agricultural and horticultural crops. However, these are very costly and cannot be afford by the farmers of developing countries. To make them cost effective, some commercial analogues of many BS have been synthesised and are used in many countries.
2 Occurrence
Brassinosteroid (BS) analogues, brassinolide (BL) and castasterone (CS) occur in whole plant kingdom. The brassinosteroids has been isolated and characterised almost from every plant part, which includes pollen grains, flower buds, fruits, seeds, vascular cambium, leaves, shoots and roots. They occur both in free as well as conjugated form (specifically with sugars and fatty acids). Sixty-nine BS analogues have been isolated from different plants/parts so far (Bajguz and Tretyn 2003). They also occur in galls of Castanea crenata, Distylium racemosum and Catharanthus roseus. Their quantity is higher in young growing tissues than mature tissues. Highest levels (range of 1–100 μg kg−1 fresh tissue) are found in pollen and immature seeds, whereas shoots and leaves usually possess lower amounts of BS i.e. 0.01–0.1 μg kg−1 (fresh tissue). The group wise number of plants which possess at least one BS include 53 angiosperms (12 monocotyledons and 41 dicotyledons), 6 gymnosperms, 1 pteridophyte (Equisetum arvense), 1 bryophyte (Marchantia polymorpha) and 3 algae (Chlorella vulgaris, Cystoseira myrica and Hydrodictyon reticulatum) (Bajguz and Tretyn 2003).
3 Structure
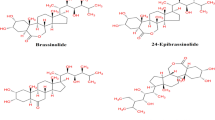
Plant sterols are converted to BL via teasterone, typhasterol and castasterone, are synthesised by an isoprenoid biosynthetic pathway, including acetyl CoA, mevalonate, isopentenyl pyrophosphate, geranyl pyrophosphate and farnesyl pyrophosphate (Clouse and Sasse 1998; Symons et al. 2008). Brassinosteroids are polyhydroxy steroid lactone with the structure of brassinolide (BS) (Fig. 12.1) and the structure of steroids (Fig. 12.2) having the same carbon skeleton of animal steroids as cholestane, ergostane, and stigmastane. However, the chemists and plant physiologists used an approach in which the most active and first identified representative of this class of compounds, i.e., brassinolide (BL), is taken as the basic structure of the system. A great diversity in the basic structure at cyclic and side chain is found which is responsible for important metabolic transformations to form two other highly active analogues of BS namely 24-Epibrassinolide (EBL) and 28-Homobrassinolide (HBL) (Fig. 12.2). Furthermore, BS are nontoxic (Esposito et al. 2011) and environmental friendly hormones (Kang and Guo 2011).
4 Outline Biosynthesis Pathway (Fig. 12.3)
The schematic pathways of BS biosynthesis. Arrows correspond to conversion steps, fat arrows denote reactions which, based on enzymological data, constitute the main synthesis routes (highlighted by gray background). The numbering of the important substituted carbon atoms is shown in the structure of campesterol (CR). Other steroid compounds are: 22-hydroxycampesterol (22-OHCR), 22,23-dihydroxycampesterol (22,23-diOHCR), (22S,24R)-hydroxyergost-4-en-3-one (4-en-3-one), (22S,24R)-22-hydroxyergost-4-en-3-one (22-OH-4-en-3-one), (22S,24R)-22,23-dihydroxyergost-4-en-3-one (22,23-diOH-4-en-3-one), (22S,24R)-hydroxyergost-3-one (3-one), (22S,24R)-22-hydroxyergost-3-one (22-OH-3-one), 3-epi-6-deoxocathasterone (3-epi-dCT), campestanol (CN), 6-oxocampestanol (6-oxoCN), 6-deoxocathasterone (dCT), cathasterone (CT), 6-deoxoteasterone (dTE), teasterone (TE), 3-dehydro-6-deoxoteasterone (dDT), 3-dehydroteasterone (DT), 6-deoxotyphasterol (dTY), typhasterol (TY), 6-deoxocastasterone (dCS), castasterone (CS), brassinolide (BL). The Arabidopsis enzymes with in vitro confirmed functions are the C-22 hydroxylase DWARF 4 (DWF4)/CYP90B1 (At3g50660), the C-23 hydroxylases ROTUNDIFOLIA 3 (ROT3)/CYP90C1 (At4g36380) and CYP90D1 (At3g13730), the steroid 5α-reductase DE-ETIOLATED 2 (DET2; At2g38050), the C-6 oxidase CYP85A1 (At5g38970), and the C-6-oxidase, BL synthase CYP85A2 (At3g30180). (Figure adopted from Hategan et al. 2010)
5 Brassinosteroid Signalling (Fig. 12.4)
The BR (=BS) signal transduction pathway in Arabidopsis. (a) Inactive BR pathway. In the absence of BRs, BRI1 is inactive and associates with BKI1. The BRI1-bound BSK1 and BSU1 are inactive, and consequently BIN2 is active. BIN2 phosphorylates BZR1 and BZR2/BES1, which cannot bind DNA and are retained in the cytoplasm by the 14-3-3 proteins to finally be degraded by the proteasome. (b) Active BR pathway. In the presence of BRs, BRI1 is activated through dissociation of BKI1 andoligomerization/transphosphorylation with BAK1. Activated BRI1 phosphorylates BSK1, which activates BSU1. The activated BSU1 inhibits BIN2 through dephosphorylation and, hence, BZR1 and BZR2/BES1 are dephosphorylated, possibly with the help of an unknown phosphatase (PPase). The unphosphorylated BZR1and BZR2 accumulate in the nucleus and regulate the BR responses. (Picture adapted from Tang et al. 2010)
6 Application of BS in Horticulture
Horticultural plants are the garden crops such as fruits, nuts, vegetables, culinary herbs and spices, beverage crops and medicinal as well as ornamental plants. The edible horticultural crops are used entirely as human food and are often used in the living state, are highly processed, are often used as animal feed and usually contain high percentage of dry matter. Show great diversity with respect to flower or fruit colour, shape and value. This is well established fact that the production of horticultural plants requires intense management, high management cost, environmental control, significant technology use and high risk. Different ways such as cultivation of high yielding cultivars/hybrids, fertilizers, pesticides, insecticides and other chemicals have been exploited to enhance the productivity of the horticultural crops to feed the ever increasing human population. However, repeated and excessive use of these chemicals deteriorates the environment and the ecosystem. On the other hand, application of the plant growth regulators in general and BS in particular are highly beneficial for plant productivity and eco-friendly too.
6.1 Effects on Tomato
Brassinosteroids have been applied to tomato plants at different stages and through different modes such as pre-sowing seed soaking, root dipping and foliar spray. Pre-sowing seed soaking treatment of tomato for 4 h in 1 ppm solution enhanced the yield of tomato plants under greenhouse conditions (Takematsu and Izumi 1985). The application of 22,23,24-triepibrassinolide and 28-homobrassinolide increased tomato fruit setting by 43–111%, whereas in response to 28-homobrassinolide, this increase was of the magnitude of 118–129% (Mori et al. 1986). Likewise, tomato sprayed with EBL exhibited an increase of 10–18% in their fruit yield (Savelieva et al. 1997). In some other studies, the treatment of tomato plants with BS, at flowering stage led to the enhancement of the number and weight of tomato (Balmush et al. 1995). The highest crop enhancement, in field conditions was obtained when tomato and cucumber plants were treated with EBL twice, first the seed soaking followed by spraying at flowering stage (Churikova and Derevshchukov 1997). Supplementation of tomato plantlets through root dipping, at the time of transplantation, with varied concentrations of HBL for 15, 30 and 45 min caused an increase in the number, size and weight of the fruits (Fig. 12.5a, b) and the improvement in the quality characters like lycopene and β-carotene content were also reported (Fig. 12.6a, b; Ali et al. 2006).
Effect HBL on the number of fruits (a) and fruit yield (b) in Lycopersicon esculentum Mill. (Ali et al. 2006)
Effect HBL on the lycopene content (a) and β-carotene content (b) of ripe fruits of Lycopersicon esculentum Mill. (Ali et al. 2006)
Vardhini and Rao (2002) also observed that BS application increased the lycopene and carbohydrate levels and ethylene production, whereas the level of chlorophyll and ascorbic acid decreased, which was consistent with accelerated ripening, mediated by the ethylene production. In a different study, Montoya et al. (2005) also found that biosynthesis of BS was enhanced in the developing fruits of tomato.
The expression of different genes is also altered by BS application. These genes include golden 2-like (LeGLK2), phytoene synthase 1 (LePSY1), ripening-related ACC synthase 2 (LeACS2), ripening-related ACC synthase 4 (LeACS4), 1-aminocyclopropane-1-carboxylate oxidase 1 (LeACO1) and 1-aminocyclopropane-1-carboxylate oxidase 4 (LeACO4) involved in lycopene and ascorbic acid biosynthesis which showed a declining trend. Moreover, the expression of LeACS2, LeACS4, LeACO1, LeACO4 and LePSY1 was increased by a followup treatment with brassinolide treatment, while the expression of LeGLK2 was reduced. However, fruit treated with brassinazole showed the opposite effects, where tomato fruit ripening was delayed. These findings suggest that brassinosteroids are involved in the development of fruit quality attributes and ethylene-mediated fruit ripening of tomato. These authors concluded that postharvest application of brassinolide significantly promoted lycopene synthesis but suppressed chlorophyll synthesis via regulating transcript levels of LePSY1 and LeGLK2. Moreover, ethylene production was obviously increased by brassinolide treatment through inducing the expression of ethylene biosynthesis related genes, including LeACS2, LeACS4, LeACO1 and LeACO4. This effect of brassinosteroids might be due to the promotion of ethylene synthesis to some extent, which contributed to LePSY1 and LeGLK2 changing (Lisso et al. 2006; Zhu et al. 2010; Liu et al. 2014). The application of BS also reduce the electrolyte leakage and malanaldehyde content and enhance phenol and proline content, thereby preventing the fruit damage caused by oxidative stress, thus enhance the shelf life of the fruits (Aghdam et al. 2012).
6.2 Effects on Pepper, Spinach, Sugarbeet and Cabbage
24-epibrassinolide (10−6 M) application at different stages (vegetative, buds formation and early fruiting) improved flower number, fruit number and yield per plant, but was without affecting fruit mass and size. The response was proportionate to the growth stage of the plant and the application frequency of the hormone (Samira et al. 2012). Similarly, other BS analogues such as s DI-31 and DI-100 at the rate of 4, 8 and 12 ppm concentration together with a seaweed extract and amino acid mixture called Tomex Amin (2.5 l/ha) also enhanced the pepper quality, such as fresh weight, height/diameter (h/d) ratio, lobe number/fruit, firmness, colour and ripening index. Moreover, antioxidant activity and phenolic content was higher in pepper treated plants than control (Serna et al. 2012).
Epibrassinolide (EBL, 10−2 ppm) treatment of spinach for 8 h enhanced its germination from 54% to 72% (Ikekawa and Akutsu 1987) and crop yield of cabbage (Asatova 1991). Genma (1987) observed 18% increase in the yield of sugarbeet by BS treatment and Vedeneev et al. (1995) observed an increase of 26–33%. In a different study, Kurganskii (1993) observed an increase of 10–13% in crop yield and the sugar content in sugarbeet under stress free condition and Schilling et al. (1991) observed 8% increase under stress conditions, in response to BS treatment.
6.3 Effects on Potato
Treating potato tubers with EBL solution induced/prolonged their dormancy and inhibited sprouting, by increasing production of ethylene and ABA (Korableva et al. 2002). Genma (1987) also observed that 0.3 g ha−1 BS application enhanced the tuber fraction by 24%. Similarly, Savelieva et al. (1997) also observed an increase in the size of potato tubers in response to BS application. In a different study, spraying the potato plants with BL (10−2–10−4 ppm) three times, at the interval of 1 week increased the mean tuber weight from 100 to 145 g mediated by changes in abscisic acid and ethylene level in the treated tubers (Korableva et al. 1998). It was noted that brassinolide promoted potato tuber development, inhibited its germination during storage and increased resistance to infections by Phytophthora infestans and Fusarium sulfureum (Kazakova et al. 1991).
6.4 Effects on Cucumber
Horticultural crop productivity largely depends on the number of the female flowers and successful pollination. EBL treatment of cucumber plants increased female flower production, mediated by BS-induced ethylene production. Comparing the response of cucumber, melon and zucchini to the exogenous treatment of BS, cucumber was more sensitive than zucchini, which was reflected as reduction in the number of male flowers in the initial phase of development and promoting the initiation of the female flower in the main shoot (Papadopoulou and Grumet 2005). BS also play an important role during early fruit development which was demonstrated by using cucumber cultivars with different parthenocarpic capacities (Fu et al. 2008). BS triggered active cell division together with increased transcripts of cell cycle-related genes, especially that of cyclin D3 genes. These results strongly suggest that BS play an import role during early fruit development in cucumber (Fu et al. 2008).
6.5 Effects on Watermelon, Strawberry, Cranberry, Gooseberry, Apple, Cherry, Citrus and Peach
Productivity of a fruit crop is greatly influenced by fruit setting. EBL treatment of melon enhanced the fruit yield by 10–20% (Ikekawa and Nagai 1987; Wang et al. 1994). The improved yield was mediated by an increase in the fruit setting, number of flowers and delayed senescence. Khripach et al. (1999) also attributed the BS mediated improvement in the quality and yield of strawberries, cranberry, gooseberry, apple, cherry, citrus and peach the increase in the fruit set, prevention of the premature fall of young fruits, delayed senescence and other factors involved in the fruit yield and quality. Moreover, molecular biotechnology has also proved the involvement of BS in the strawberry fruit ripening (Bombarely et al. 2010). Chai et al. (2013) also explained its possible mechanism of action. They analysed BS content and BS receptor gene FaBRI1 expression during ‘Akihime’ strawberry fruit development. It was found that BS levels increased during the later developmental stages, and the mRNA expression levels of FaBRI1increased rapidly from white to initial red stages, suggesting that BS is associated with fruit ripening. This was further confirmed by exogenous application of BS and its inhibitor brassinazole (BZ) to big-green fruit, which significantly promoted and inhibited strawberry fruit ripening, respectively. More importantly, down-regulation of FaBRI1 expression in de-greening fruit markedly retarded strawberry red-colouring.
6.6 Effects on Grape, Berry and Mango
There are intriguing evidences which suggest that increase in endogenous BS levels are associated with ripening in grapes. Exogenous application of EBL to grape berries, significantly promoted their ripening, while brassinazole (Brz), an inhibitor of BS biosynthesis, significantly delayed fruit ripening (Symons et al. 2006; Lisso et al. 2006). A significant increase in endogenous BS levels in grapes stimulates BS receptor gene brassinosteroid insensitive 1 expression that was consistent with observed at the onset of fruit ripening. Symons et al. (2006) also observed an increase in the expression of BS biosynthesis enzyme gene, brassinosteroid-6-oxidase demonstrating that BRs are involved in grape berry ripening.
Zaharah et al. (2012) demonstrated that the exogenous application of EBL promoted fruit ripening in mango. There was a marked accumulation of BS analogues, castasterone and brassinolide. However, the castasterone level was slightly higher than that of BL, on day 8 of the study (0.13 ng g−1 FW). Moreover, the exogenous application of EBL treatments (45 and 60 ng g−1 FW) significantly advanced the onset of the climacteric peak of ethylene production and respiration rate by 2 and 1 day(s), respectively. Both of these treatments also had a higher climacteric ethylene production peak (4.81 and 5.74 nmol C2H4kg−1 h−1) and respiration rate (4.87 and 5.06 mmol CO2kg−1 h−1) compared with the control. Furthermore, the exogenous applications of EBL also promoted fruit softening, particularly between days 3 and 7 of the ripening.
6.7 Effects on Passion Fruit
The application of BB-16, a BS analogue 3 weeks after flowering, increased in the estimated yield of the passion (Passiflora edulis flavicarpa) fruit by 65%. The yield parameters were number of fruits plant−1 and the mean mass of each fruit, corresponding to an estimated production of 20.1 t ha−1, compared to that of the control (12.6 tons ha−1). The BS analogue was considered more efficient when applied for three consecutive weeks after the appearance of the first flower due to the great increase in yield and soluble solids contents in passion fruit (Gomes et al. 2006).
6.8 Effects on Orange
Brassinolide (BL) treatment of orange trees during flowering increased their fruit setting. However, when applied during fruit growth it decreased the physiological drop of fruits, causing an increased number of fruits per plant, accompanied by an increase in the average fruit weight. BS treatment also enhanced juice production in Citrus unshiu together with a higher brix/acidity ratio (Kuraishi et al. 1991) and also prevented fruit abscission in Citrus madurensis Lour. (Iwahori et al. 1990).
6.9 Effects on Litchi, Passiflora and Jujube Fruits
BS treatment improved fruit yield and quality in litchi in terms of increased the activities of pectin methyesterase and polygalacturonase and the content of water-soluble pectin, protopectin and calcium in the fruit pericarp, and reduced fruit cracking rate thereby increasing the commercial value of the fruit (Peng et al. 2004). Likewise, BS treatment also reduced postharvest decay caused by Penicillium expansum in jujube fruit. Besides this, BS application also delayed fruit senescence thereby increasing the life span of the fruit.
6.10 Effects on Tea and Coffee
Foliar spray of summer tea plants with 24-epibrassinolide (EBL), a bioactive BS analogue, promoted photosynthesis in a concentration-dependent manner. EBL also increased concentrations of tea polyphenols and amino acids. Furthermore, concentrations of catechins and theanine increased, while that of caffeine remained unaltered following treatment with the BS. EBL also improved activity of phenylalanine ammonia-lyase (PAL) and glutamine: 2-oxoglutarate aminotransferase (GOGAT) enzymes involved in catechins and theanine biosynthesis, respectively. These favourable metabolic changes consequently improved the quality of summer tea (Li et al. 2016). Mazzafera and Zullo (1990) demonstrated that EBL or 24-epicastasterone treatment of coffee showed no significant effect on seed setting, seed size or yield. However, Coffea stenophyllacalli grew up to 237% between 60 and 130 days of culturing in the presence of 24-epibrassinolide (Ramos et al. 1987).
6.11 Reproductive Growth
Horticultural crop productivity primarily depends upon the successful pollination and subsequent fertilization. Relatively higher levels of BS in pollen and seed reflect a critical role of BS in reproduction. BS also influence branching and flower formation via modulating metabolic pathways and relative nutrient allocation or interacting with other signalling pathways. BS also affect fertilization via the stimulation of filament and pollen growth, and modify pollen properties (Mussig 2005). Cuttings grown in a nutrient medium containing BL, EBL and HBL analogues of BS increased the yield of cuttings suitable for planting by 25–50% depending on the cultivar and finally increased crop yield up to 50% (Bobrick 1995).
6.12 Effects on Flowering
Flower formation, survival and maturation of the flower, pollination and the subsequent fruit setting and maturation are the main factors that determine the fruit productivity of a horticultural crop. Application of BB-6 and BB-16 (BS analogues) to the foliage of the Cactus pear hastened vegetative buds formation both under greenhouse and field conditions (Aristeo-Cortes et al. 2003). However, a concentration and the method of BS application dependent decrease in the number of flowers was observed in Pharbitis nil in response to BL and castasterone (CS) treatment where 1 and 10 μM of BL caused a complete inhibition of flower formation (Kesy et al. 2003). Contrary to this, BS treatment increased the number of flowers in strawberry and grape fruits (Vardhini and Rao 2002).
6.13 Effects on Micropropagation of Horticultural Plants
Potential of regulation of growth and development in plants by BS has also been efficiently utilised in the micropropagation of various plants of horticultural importance such as cassava (Manihot esculenta Crantz), yam (Dioscorea alata L.) and pineapple (Ananas comosus L. Merril). BS analogues, 28-homocastasterone or 3β-acetyl-28-homoteasterone have been used successfully for this purpose (Bieberach et al. 2000). 5α-fluoro-28-homocastasterone (5F-HCTS), another BS analogue facilitated the apple rootstock multiplication rate up to 112% mediated by an increase in the number of primary and secondary lateral branches (Schaefer et al. 2002). 5F-HCTS also stimulated branch elongation in in vitro-grown shoots of Malus prunifolia that was mediated by the manipulation of endogenous BS levels (Pereira-Netto et al. 2006; Kang and Guo 2011). BS in culture medium also stimulated adventitious bud formation in cauliflower and coconut. In coconut, plumule explants efficiently formed initial callus, embryogenic callus and somatic embryos in presence of BS in culture medium (Azpeitia et al. 2003).
7 Conclusion and Future Prospects
-
1.
Brassinosteroids is a group of naturally occurring steroidal plant hormones which is represented by several analogues. Out of them, the stable ones are epibrassinolide, homobrassinolide and brassinolide. Besides their stability, they are also non-toxic and eco-friendly.
-
2.
Their exogenous application can enhance the productivity of tomato, potato, mango, straw berry, litchi, passiflora, grapes, watermelon etc. However, the studies are very less and the field is very vast which has remained almost untouched. Keeping in view, the great potential of BS, they can be exploited in the enhancement of vegetables productivity.
-
3.
Since BS are also known for their role in protection of plants from different stress situations including biotic stress such as the attack of different pathogens. Therefore, it can easily and efficiently replace different pesticides and fungicides, which otherwise have health hazards and also degrade environment.
-
4.
Micropropagation is an unconventional method of plant propagation wherein a large number of plantlets are generated from a small explant. BS have been exploited in a limited number of studies. This field is still open and potential of BS can also be exploited to fulfill the food requirements of increasing population reducing both time and labour.
References
Adam, G., & Schneider, B. (1999). Uptake, transport and metabolism. In A. Sakurai, T. Yokota, & S. D. Clouse (Eds.), Brassinosteroids – Steroidal plant hormones (pp. 113–136). Tokyo: Springer.
Aghdam, M. S., Asghari, M., Farmani, B., Mohayeji, M., & Moradbeygi, H. (2012). Impact of postharvest brassinosteroids treatment on PAL activity in tomato fruit in response to chilling stress. Scientia Horticulturae, 144, 116–120.
Alam, M. M., Hayat, S., Ali, B., & Ahmad, A. (2007). Effect of 28-homobrassinolide treatment on nickel in Brassica juncea. Photosynthetica, 45, 139–142.
Ali, B., Hayat, S., & Ahmad, A. (2005). Response of germinating seeds of Cicer arietinum to 28-homobrassinolide and/or potassium. General and Applied Plant Physiology, 31, 55–63.
Ali, B., Hayat, S., Hasan, S. A., & Ahmad, A. (2006). Effect of root applied 28-homobrassinolide on the performance of Lycopersicon esculentum. Scientia Horticulturae, 110, 267–273.
Ali, B., Hayat, S., & Ahmad, A. (2007). 28-Homobrassinolide ameliorates the saline stress in chickpea (Cicer arietinum L). Environmental and Experimental Botany, 59, 217–223.
Ali, B., Hasan, S. A., Hayat, S., Hayat, Q., Yadav, S., Fariduddin, Q., & Ahmad, A. (2008a). A role for brassinosteroids in the amelioration of aluminium stress through antioxidant system in mung bean (Vigna radiata L. Wilczek). Environmental and Experimental Botany, 62, 153–159.
Ali, B., Hayat, S., Fariduddin, Q., & Ahmad, A. (2008b). r24-Epibrassinolide protects against the stress generated by salinity and nickel in Brassica juncea. Chemosphere, 72, 1387–1392.
Aristeo-Cortes, P., Terrazas, T., Colinas León, T., & Larqué-Saavedra, A. (2003). Brassinosteroid effects on the precocity and yield of cladodes of cactus pear (Opuntia ficus-indica (L) Mill.). Scientia Horticulturae, 97, 65–73.
Asatova, S. S. (1991). Effect of epibrassinolide on growth and development of vegetables. In Conference on brassinosteroids (2nd ed.). Minsk.
Azpeitia, A., Chan, J., Saenz, L., & Oropeza, C. (2003). Effect of 22(S), 23(S)-homobrassinolide on somatic embryogenesis in plumule explants of Cocos nucifera (L.) cultured in vitro. The Journal of Horticultural Science and Biotechnol, 78, 591–596.
Bajguz, A., & Tretyn, A. (2003). The chemical characteristic and distribution of brassinosteroids in plants. Phytochemistry, 62, 1027–1046.
Balmush, G. T., Russu, M. M., & Karabdzhak. (1995). Effect of epibrassinolide on tomato growth and development. In Brassinosteroids – Biorational ecologically safe regulators of growth and productivity of plants (4th ed., pp. 22–23). Minsk.
Bieberach, C. Y., León, B., Centurión, O. T., Ramírez, J. A., Gros, E., & Galagovsky, L. (2000). Estudios preliminares sobre el efecto de dos brasinoesteroides sintéticos sobre el crecimiento in vitro de yuca, ñame y piña. Anales de la Asociación Química Argentina, 88, 1–7.
Bobrick, A. O. (1995). Application of brassinosteroids in potato breeding. In Brassinosteroids – Biorational ecologically safe regulators of growth and productivity of plants (4th ed., p. 23). Minsk.
Bombarely, A., Merchante, C., Csukasi, F., Cruz-Rus, E., Caballero, J. L., Medina-Escobar, N., Blanco-Portales, R., Botella, M. A., MunozBlanco, J., Sanchez-Sevilla, J. F., & Valpuesta, V. (2010). Generation and analysis of ESTs from strawberry (Fragaria×ananassa) fruits and evaluation of their utility in genetic and molecular studies. BMC Genomics, 11, 503–510.
Chai, Y. M., Zhang, Q., Tian, L., Li, C. L., Ling, Y. X., Qin, L., & Shen, Y. Y. (2013). Brassinosteroid is involved in strawberry fruit ripening. Journal of Plant Growth Regulation, 69, 63–69.
Churikova, V. V., & Derevshchukov, S. N. (1997). Registration trials of the growth regulator “Epin” on tomato and cucumber. Technical report of Voronezh State University.
Clouse, S. D., & Sasse, J. M. (1998). Brassinosteroids: Essential regulators of plant growth and development. Annual Review of Plant Physiology and Plant Molecular Biology, 49, 427–451.
Esposito, D., Komarnytsky, S., Shapses, S., & Raskin, I. (2011). Anabolic effect of plant brassinosteroid. The FASEB Journal, 25, 3708–3719.
Fariduddin, Q., Yusuf, M., Ahmad, I., & Ahmad, A. (2014). Brassinosteroids and their role in response of plants to abiotic stresses. Biologia Plantarum, 58, 9–17.
Fedina, E. O., Karimova, F. G., Tarchevsky, I. A., & Khripach, V. A. (2008). Effect of epibrassinolide on tyrosine phosphorylation of the Calvin cycle enzymes. Russian Journal of Plant Physiology, 55, 193–200.
Fu, F. Q., Mao, W. H., Shi, K., Zhou, Y. H., Asami, T., & Yu, J. Q. (2008). A role of brassinosteroids in early fruit development in cucumber. Journal of Experimental Botany, 59, 2299–2308.
Genma, T. (1987). Methods of cultivating potatos with brassinolide-containing yield enhancer. PCT Int Appl WO 88 04, 890 [C.a. 110, 187813].
Gomes, M. M. A., Campostrini, E., Leal, N. R., Viana, A. P., Ferraz, T. M., Siqueira, L. N., Rosa, R. C. C., Netto, A. T., Nunez-Vazquez, M., & Zullo, M. A. T. (2006). Brassinosteroid analogue effects on the yield of yellow passion fruit plants (Passiflora edulis f. flavicarpa). Science Horticulture, 110, 235–240.
Hategan, L., Godza, B., & Szekeresm. (2010). Regulation of brassinosteroids signalling. In S. Hayat & A. Ahmad (Eds.), Brassinosteroids: A class of plant hormone (pp. 57–82). Dordrecht: Springer.
Hayat, S., Ali, B., Hasan, S. A., & Ahmad, A. (2007). Brassinosteroid enhanced the level of antioxidants under cadmium stress in Brassica juncea. Environmental and Experimental Botany, 60, 33–41.
Hola, D., Rothova, O., Kocova, M., Kohout, L., & Kvasnic, M. (2010). The effect of brassinosteroids on the morphology, development and yield of field-grown maize. Plant Growth Regulation, 61, 29–43.
Ikekawa, N., & Akutsu, T. (1987). Culturing method for spinach using brassinosteroid as growth promoters. Jpn. Kokai Tokkyo Koho. JP 63,239,201 [88,239,201] [C.A. 111, 52465].
Ikekawa, N., & Nagai, T. (1987). Brassinosteroids fruiting hormones for melons. Jpn. Kokai Tokkyo Koho. JP 63,243,001 [88,243,0201] [C.A. 111, 129007].
Iwahori, S., Tominaga, S., & Higuchi, S. (1990). Retardation of abscission of citrus leaf and fruitlet explants by brassinolide. Plant Growth Regulation, 9, 119–125.
Kang, Y. Y., & Guo, S. R. (2011). Role of brassinosteroids in horticultural crops. In S. Hayat & A. Ahmad (Eds.), Brassinosteroids: A class of plant hormone (pp. 269–288). Dordrecht: Springer.
Kazakova, V. N., Karsunkina, N. P., & Sukhova, L. S. (1991). Effect of brassinolide and fusicoccin on potato productivity and tuber resistance to fungal diseases under storage. Izvestiia Timiryazevskoi sel’skokhoziaistvennoi Akademii, 0, 82–88.
Kesy, J., Trzaskalsky, A., Galoch, E., & Kopcewicz, J. (2003). Inhibitory effect of brassinosteroids on the flowering of the short-day plant Pharbitis nil. Biologia Plantarum, 47, 597–600.
Khripach, V. A., Zhabinski, V. N., & de Groot, A. E. (1999). Practical applications and toxicology. In Brassinosteroids: A new class of plant hormones (pp. 325–345). London: Academic.
Korableva, N. P., Platonova, T. A., & Dogonadze, M. Z. (1998). Changes in ethylene biosynthesis in the meristems of potato tubers (Salanum tuberosum L.) under the action of brassinolide. Dokl. Akad. Nauk. Russia.
Korableva, N. P., Platonova, T. A., Dogonadze, M. Z., & Evsunina, A. S. (2002). Brassinolide effect on growth of apical meristems, ethylene production, and abscisic acid content in potato tubers. Biologia Plantarum, 45, 39–43.
Kuraishi, S., Sakurai, N., Eun, J. S., & Sugiyama, K. (1991). Effect of brassinolide on level of indoleacetic acid and abscisic acid in squash hypocotyls. In H. G. Cuttler, T. Yokota, & G. Adam (Eds.), Brassinosteroids: Chemistry, bioactivity and application (ACS symposium series) (Vol. 474, pp. 312–319). Washington, DC: American chemical Society.
Kurganskii, N. P. (1993). Application of “Apin” on sugar beet in 1991–1992. Technical report of experimental station on sugar beet. Belarus.
Leubner-Metzger, G. (2001). Brassinosteroids and gibberellins promote tobacco seed germination by distinct pathway. Planta, 213, 758–763.
Li, X., Ahammed, G. J., Li, Z. X., Zhang, L., Wei, J. P., Shen, C., Yan, P., Zhang, L. P., & Han, W. Y. (2016). Brassinosteroids improve quality of summer tea (Camellia sinensis L.) by balancing biosynthesis of polyphenols and amino acids. Frontiers in Plant Science, 7, 1304. https://doi.org/10.3389/fpls.2016.01304.
Lisso, J., Altmann, T., & Müssig, C. (2006). Metabolic changes in fruits of the tomatodx mutant. Phytochemistry, 67, 2232–2238.
Liu, L., Jia, C., Zhang, M., Chen, D., Chen, S., Guo, R., & Wang, Q. (2014). Ectopic expression of a BZR1-1D transcription factor in brassinosteroid signalling enhances carotenoid accumulation and fruit quality attributes in tomato. Plant Biotechnology Journal, 12, 105–115.
Mazzafera, P., & Zullo, M. A. T. (1990). Brassinosteroids on coffee. Bragantia, 49, 37–41.
Montoya, T., Nomura, T., Yokota, T., Farrar, K., Harrison, K., Jones, J. G. D., Kaneta, T., Kamiya, Y., Szekeres, M., & Boshop, G. J. (2005). Patterns of dwarf expression and brassinosteroid accumulation in tomato reveal the importance of brassinosteroid synthesis during fruit development. The Plant Journal, 42, 262–269.
Mori, K., Takematsu, T., Sakakibara, M., & Oshio, H. 1986. Homobrassinolide, and its production and use. US Patent. 4: 604,240.
Mussig, C. (2005). Brassinosteroid-promoted growth. Plant Biology, 7, 110–117.
Nunez, M., Mazzafera, P., Mazorra, L. M., Siqueira, W. J., & Zullo, M. A. T. (2003). Influence of a brassinosteroid analogue on antioxidant enzymes in rice grown in culture medium with NaCl. Biologia Plantarum, 47, 67–70.
Papadopoulou, E., & Grumet, R. (2005). Brassinosteriod-induced femaleness in cucumber and relationship to ethylene production. Horticultural Science, 40, 1763–1767.
Peng, J., Tang, X., & Feng, H. (2004). Effects of brassinolide on the physiological properties of litchi pericarp (Litchi chinensis cv. nuomoci). Scientia Horticulturae, 101, 407–416.
Pereira-Netto, A., Cruz-Silva, C., Schaefer, S., Ramirez, J., & Galagovsky, L. (2006). Brassinosteroid-stimulated branch elongation in the marubakaido apple rootstock. Trees, 20, 286–291.
Ramos, L. C. S., Zullo, M. A. T., & Teixeira, J. P. F. (1987). Efeito de 24-epibrassinolídio em calos de Coffea stenophylla. In Proceedings of the 140 Congresso Brasileiro de Pesquisas Cafeeiras/1o Congresso Latinoamericano de Tecnologia Cafeeira (pp. 82–83). Campinas.
Samira, I. M., Mansour-Gueddes, S. B., Dridi-Mouhandes, B., & Denden, M. (2012). 24-epibrassinolide enhances flower and fruit production of pepper (Capsicum annuum L.) under salt stress. Journal of Stress Physiology Biochemistry, 8, 224–233.
Sasse, J. M. (2003). Physiological actions of brassinosteroids: An update. Plant Growth Regulation, 22, 276–288.
Savelieva, E. A., Goncharov, V. M., & Tseiko, Z. E. (1997). Effect of “Epin” on the crop and disease resistace of tomatoin green houses. Technical report of Gomel agricultural station, Belarus.
Schaefer, S., Medeiro, S., Ramirez, J., Galagovsky, L., & Pereira-Netto, A. (2002). Brassinosteroid-driven enhancement of the in vitromultiplication rate for the marubakaido apple rootstock [Malus prunifolia(Willd.) Borkh]. Plant Cell Reports, 20, 1093–1097.
Schilling, G., Schiller, C., & Otto, S. (1991). Influence of brassinosteroids on organ relations and enzyme activities in sugar beet plants. In H. G. Cuttler, T. Yokota, & G. Adam (Eds.), Brassinosteroids: Chemistry, bioactivity and application (ACS Symposium Series) (Vol. 474, pp. 208–219). Washington, DC: American chemical Society.
Schneider, B. (2002). Pathways and enzymes of brassinosteroid biosynthesis. In K. Esser, U. Lüttge, W. Beyschlag, & F. Hellwig (Eds.), Progress in botany (Vol. 63, pp. 286–306). Berlin: Springer.
Serna, M., Hernandez, F., Coll, F., Coll, Y., & Amoro, A. (2012). Brassinosteroid analogues effects on the yield and quality parameters of greenhouse-grown pepper (Capsicum annuum L.). Plant Growth Regulation, 68, 333–342.
Sirhindi, G. (2013). Brassinosteroids: Biosynthesis and role in growth, development, and thermotolerance responses. In G. R. Rout & A. B. Das (Eds.), Molecular stress physiology of plants (pp. 309–329). New Delhi: Springer.
Symons, G. M., Davies, C., Shavrukov, Y., Dry, I. B., Reid, J. B., & Thomas, M. R. (2006). Grapes on steroids: Brassinosteroids are involved in grape berry ripening. Plant Physiology, 140, 150–158.
Symons, G. M., Ross, J. J., Jager, C. E., & Reid, J. B. (2008). Brassinosteroid transport. Journal of Experimental Botany, 59, 17–24.
Taiz, L., & Zeiger, E. (2004). Plant physiology (pp. 607–611). Sunderland: Sinauer Associates.
Takematsu, T., & Izumi, K. (1985). Acceleration of plant growth in cultured soil. Jpn Kokai Tokkyo Koho JP 62 04,205 [87 04,205] [C.A. 107, 72876].
Tang, W., Deng, Z., & Wang, Z.-Y. (2010). Proteomics shed light on the brassinosteroid signaling mechanisms. Current Opinion in Plant Biology, 13, 27–33.
Vardhini, B. V., & Rao, S. S. R. (2002). Acceleration of ripening of tomato pericarp discs by brassinosteroids. Phytochemistry, 61, 843–847.
Vedeneev, A. N., Deeva, V. P., & Khripach, V. A. (1995). Effect of epibrassinolide on sugar beet. In Brassinosteroids – Biorational ecologically safe regulators of growth and productivity of plants (4th ed., p. 24). Minsk.
Wang, Y. Q., Luo, W. H., & Zhao, Y. J. (1994). Effect of epibrassinolide on growth and fruit quality of water melon. Zhiwu Shenglixue Tangxun, 30, 423–425.
Yu, J. Q., Huang, L. F., Hu, W. H., Zhou, Y. H., Mao, W. H., Ye, S. F., & Nogues, S. (2004). A role for brassinosteroids in the regulation of photosynthesis in Cucumis sativus. Journal of Experimental Botany, 55, 1135–1143.
Yusuf, M., Fariduddin, Q., & Ahmad, A. (2012). 24-Epibrassinolide modulates growth, nodulation, antioxidant system, and osmolyte in tolerant and sensitive varieties of Vigna radiata under different levels of nickel: A shotgun approach. Plant Physiology and Biochemistry, 57, 143–153.
Zaharah, S. S., Singh, Z., Symons, G. M., & Reid, J. B. (2012). Role of brassinosteroids, ethylene, abscisic acid, and indole-3-acetic acid in mango fruit ripening. Journal of Plant Growth Regulation, 31, 363–372.
Zhu, Z., Zhang, Z., Qin, G., & Tian, S. (2010). Effects of brassinosteroids on postharvest disease and senescence of jujube fruit in storage. Postharvest Biology and Technology, 56, 50–55.
Zullo, M. A. T., & Adam, G. (2002). Brassinosteroid phytohormones – Structure, bioactivity and applications. Brazilian Journal of Plant Physiology, 14, 143–181.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Ali, B. (2019). Brassinosteroids: The Promising Plant Growth Regulators in Horticulture. In: Hayat, S., Yusuf, M., Bhardwaj, R., Bajguz, A. (eds) Brassinosteroids: Plant Growth and Development. Springer, Singapore. https://doi.org/10.1007/978-981-13-6058-9_12
Download citation
DOI: https://doi.org/10.1007/978-981-13-6058-9_12
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-6057-2
Online ISBN: 978-981-13-6058-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)