Abstract
In this study, we used functional near-infrared spectroscopy to measure the brain hemodynamic responses during a verbal fluency task in both healthy controls (HC) and mild cognitive impairment patients (MCI). We found a greater amplitude of oxy-hemoglobin and deoxy-hemoglobin changes, and a significantly higher value of maximum slope calculated from oxy-hemoglobin change in MCI compared to HC during the task. Our experimental results suggest the potential of using the cerebral hemodynamic responses, especially the maximum slope of oxy-hemoglobin change, as a biomarker for MCI.
Minhee Kim and Thien Nguyen—These authors contributed equally to this work.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Functional near-infrared spectroscopy
- Alzheimer’s disease
- Semantic verbal fluency task
- Phonemic verbal fluency task
1 Introduction
Mild cognitive impairment (MCI) is a progressive stage of dementia, which reduces the cognitive and memory functions more than the normal aging but not serious enough to disturb the patient’s daily life [1]. Because there is a high risk for MCI to develop into Alzheimer’s disease (AD), it is important to diagnose MCI in the early stage. Several imaging techniques have been used to develop a diagnostic method for MCI such as fMRI, PET, and EEG [2,3,4]. PET/fMRI provide whole brain images with high spatial resolution but PET uses radioactive agents, fMRI restricts people with metal-implanted devices, and both PET/fMRI systems are extremely expensive. EEG has a high temporal resolution, but its signal is not localized. In addition, the above-mentioned techniques are highly sensitive to movement noises. As a result, diagnosing MCI with these techniques is still challenging.
Hence, we suggest using functional near-infrared spectroscopy (fNIRS) to investigate MCI. fNIRS is a noninvasive optical technique to measure brain hemodynamic changes. It is a low-cost system that can be applied harmlessly on everyone. Additionally, it is relatively stable with motion-induced artifacts. Currently, fNIRS has been used for researches to diagnose MCI using dual-task [5], working memory task [6], and verbal fluency task (VFT) [7,8,9]. In this study, we measure fNIRS signals during a VFT. We hypothesize that the compensatory mechanism may alter the cerebral hemodynamic responses in MCI during the task. We also compare our results with the other fNIRS studies using VFT. Because fNIRS can measure the brain hemoglobin oxygenation without any restrictions, we believe that it will play an important role in diagnosing MCI.
2 Method
2.1 Participant
A total of 69 right-handed elderly subjects living in Gwangju and adjacent cities (Republic of Korea) participated in this study. Based on the AD diagnosis criteria at Chonnam National University Hospital (Gwangju, Republic of Korea), subjects were divided into two groups: a healthy control (HC) group and a MCI group. The HC group has subjects without any psychiatric disorders (39 subjects, 18 males, ages: 72.2 ± 5.6 years). The MCI group includes subjects who were diagnosed as MCI due to AD (30 subjects, 21 males, ages: 76.0 ± 3.5 years). Before the experiment, all subjects signed an agreement to participate in the experiment after a technician explained the experimental procedure. This study was approved by Institutional Review Board at GIST.
2.2 fNIRS Device
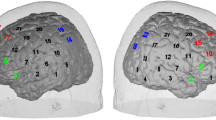
A lab-built four channels fNIRS device was designed to measure signals from the subject’s forehead. Channel 1 and 2 were located at the right forehead and channel 3 and 4 were at the subject’s left forehead. Our fNIRS device has been tested and applied in several previous studies [10, 11]. The device in this study consists of 2 LEDs (OE-MV7385-P, OptoENG), which emit light at 730 nm and 850 nm and 3 photodiodes (OPT101, Texas Instruments). The separation between a LED and a photodiode was 3 cm (Fig. 1). The device sampling rate was 8 Hz and intensity of the LED light was 5 mW. The LED and photodiodes were attached to the subject’s forehead using a medical double layer tape. The ambient light was shielded using a black-colored hairband covering the entire forehead.
2.3 Experimental Protocol
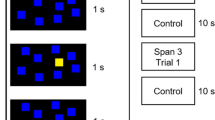
Verbal fluency task (VFT) comprised of two different tasks, phonemic and semantic and 30 s of rest period between them. In a phonemic task (30 s), the subjects were asked to speak words beginning with a certain letter (e.g. letter “P” with pig, power, paper, etc.), and in a semantic task (30 s), the subjects had to tell words belonging to a certain category (e.g. “animal” category with dog, cat, lion, etc.). Including the baseline period, total experimental time was less than 3 min (Fig. 2). During the experiment, subjects were asked not to move their body to prevent motion artifact.
2.4 Processing Method
Firstly, relative concentration changes of oxy-hemoglobin \(\left( {\left[ {\Delta {\text{OHb}}} \right]} \right)\) and deoxy-hemoglobin \(\left( {\left[ {\Delta {\text{RHb}}} \right]} \right)\) were calculated from the light intensity using modified Beer-Lambert’s law [12]. Unit of \(\left[ {\Delta {\text{OHb}}} \right]\) and \(\left[ {\Delta {\text{RHb}}} \right]\) is μM/DPF where DPF is the differential path length factor. Secondly, \(\left[ {\Delta {\text{OHb}}} \right]\) and \(\left[ {\Delta {\text{RHb}}} \right]\) were band-pass filtered with 2nd order Butterworth at 0.01–0.1 Hz. Thirdly, the maximum increase of \(\left[ {\Delta {\text{OHb}}} \right]\) and the minimum decrease of \(\left[ {\Delta {\text{RHb}}} \right]\) within a 35-second window (30 s during the task and 5 s after the task) were computed. Both \(\left[ {\Delta {\text{OHb}}} \right]\) and \(\left[ {\Delta {\text{RHb}}} \right]\) were corrected to 0 μM/DPF when the task started and there was no other normalization process. After that, the highest positive slope was calculated from the 1st derivative of \(\left[ {\Delta {\text{OHb}}} \right]\). Finally, an unpaired two-sample t-test was applied to the computed features to check the significance of the difference between two groups.
3 Result
3.1 Behavioral Results
In the phonemic task, the HC group produced 6.6 ± 2.8 words, and the MCI group produced 5.5 ± 3.1 words. The HC and MCI groups in the semantic task generated 9.6 ± 3.1 and 8.8 ± 3.2 words, respectively. In both tasks, HC group spoke more words compared to MCI group, but not statistically significant (p > 0.1).
3.2 fNIRS Results
Figure 3 plot the averaged time traces of \(\left[ {\Delta {\text{OHb}}} \right]\) and \(\left[ {\Delta {\text{RHb}}} \right]\) from channel 1 in 50 s (10 s before and after the task). During the task, \(\left[ {\Delta {\text{OHb}}} \right]\) increased in both groups, but \(\left[ {\Delta {\text{OHb}}} \right]\) of MCI reached its maximum earlier than the one in HC. In contrast, \(\left[ {\Delta {\text{RHb}}} \right]\) was constant in HC, while it decreased in MCI.
The maximum increase in \(\left[ {\Delta {\text{OHb}}} \right]\) in MCI group was higher than HC group for all the channels from both tasks, but there was no significance due to large variation within a group (Fig. 4a). In agreement with the increase in \(\left[ {\Delta {\text{OHb}}} \right]\), the maximum decrease in \(\left[ {\Delta {\text{RHb}}} \right]\) of MCI group was greater than the one in the HC group. A significant difference was found during a phonemic task in channels 1 and 4 (p < 0.05), while there was no significant difference during a semantic task (Fig. 4b). The maximum slope of \(\left[ {\Delta {\text{OHb}}} \right]\) was significantly higher in the MCI group than HC group during the phonemic tasks (p < 0.05), and especially, channel 1 showed p < 0.001 (Fig. 4c).
4 Discussion
The cerebral hemodynamic responses were monitored during a process of generating words, mainly related to the memory function. Most previous studies showed an increased \(\left[ {\Delta {\text{OHb}}} \right]\) and a decreased \(\left[ {\Delta {\text{RHb}}} \right]\) during a VFT [7,8,9]. In agreement with former studies, our study showed a similar trend in both groups but with a higher amplitude of \(\left[ {\Delta {\text{OHb}}} \right]\) and \(\left[ {\Delta {\text{RHb}}} \right]\) in MCI patients. This result can be explained by the compensation theory [13]. The compensation theory is a hypothesis to explain a natural aging process in elderly. When dealing with the same amount of workload, the elders’ brain recruits more energy than the youngers’ one. The former study revealed that MCI also followed this theory showing a higher level of activation [8]. Hence, even with age-matched, MCI group required more energy than HC group to perform the VFT.
Since oxy-hemoglobin is a major factor to represent regional cerebral blood flow (rCBF) [14], the slope of \(\left[ {\Delta {\text{OHb}}} \right]\) represents how fast the oxygen is carried to the brain through the blood. The higher the slope is, the more blood supply to the brain is. Therefore, we focused on analyzing the slope of \(\left[ {\Delta {\text{OHb}}} \right]\) rather than the one of \(\left[ {\Delta {\text{RHb}}} \right]\). In addition, depending on the study purpose, the selection of window size for processing the data affects the result of the group analysis. The slope change when the subjects start to generate words has been studied and showed a significantly lower value in AD compared to HC and MCI [8]. In this study, we extracted the maximum slope from 35 s window to observe brain activity during the whole task and this parameter was able to provide a significantly higher value in MCI compared to HC. Furthermore, we noticed that MCI subjects tended to speak words without any restriction at the beginning of the task, but they felt difficulties from the middle of the task. It may be an evidence that the greater neural activity was recruited when workload became larger during VFT. These results imply that MCI patients tried to come up with matching words during verbal fluency tasks, which is shown by an increased \(\left[ {\Delta {\text{OHb}}} \right]\) slope but failed to speak as many words as HC group.
In summary, we performed VFT study to assess altered hemodynamic responses in MCI. Compared to previous studies, our study has two new aspects. Firstly, instead of focusing on one type of VFT, we assessed the prefrontal hemodynamic changes using both alphabet and category VFT. In general, both tasks successfully tested the cognitive function by observation of the prefrontal activation. However, because the mechanism to generate the words from the phonemic and semantic conditions is different, it is valuable to compare the performance and hemodynamic response between two groups in each condition. In fact, the minimum amplitude of \(\left[ {\Delta {\text{RHb}}} \right]\) was found to be significant between two groups in phonemic (channel 1 and 4) but not semantic. Secondly, rather than computing \(\left[ {\Delta {\text{OHb}}} \right]\) slope during first several seconds of the task, we calculated the maximum \(\left[ {\Delta {\text{OHb}}} \right]\) slope during the whole task (35 s). By doing so, we were able to reveal the significant difference between MCI and HC groups in term of \(\left[ {\Delta {\text{OHb}}} \right]\) slope. From our experimental results, we suggest using the slope of \(\left[ {\Delta {\text{OHb}}} \right]\) during a VFT as a biomarker to characterize MCI.
5 Conclusion
We investigated the cerebral hemodynamic responses in normal healthy controls and mild cognitive impairment patients. We found a higher increase in the oxy-hemoglobin concentration, a larger decrease in the deoxy-hemoglobin concentration, and a significantly greater slope of oxy-hemoglobin change in MCI during a verbal fluency task. These results suggest that the maximum slope of oxy-hemoglobin change during a verbal fluency task may be a promising biomarker to diagnose MCI due to the AD when further studies are conducted to compare MCI from AD and MCI from other causes.
References
Albert, M.S., et al.: The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. J. Alzheimers Assoc. 7(3), 270–279 (2011)
Petrella, J.R., Prince, S.E., Wang, L., Hellegers, C., Doraiswamy, P.M.: Prognostic value of posteromedial cortex deactivation in mild cognitive impairment. PLOS ONE 2(10), e1104 (2007)
Jelic, V., Kowalski, J.: Evidence-based evaluation of diagnostic accuracy of resting EEG in dementia and mild cognitive impairment. Clin. EEG Neurosci. 40(2), 129–142 (2009)
Marcus, C., Mena, E., Subramaniam, R.M.: Brain PET in the diagnosis of Alzheimer’s disease. Clin. Nucl. Med. 39(10), e413–e426 (2014)
Doi, T., et al.: Brain activation during dual-task walking and executive function among older adults with mild cognitive impairment: a fNIRS study. Aging Clin. Exp. Res. 25(5), 539–544 (2013)
Niu, H.-J., Li, X., Chen, Y.-J., Ma, C., Zhang, J.-Y., Zhang, Z.-J.: Reduced frontal activation during a working memory task in mild cognitive impairment: a non-invasive near-infrared spectroscopy study. CNS Neurosci. Ther. 19(2), 125–131 (2013)
Arai, H., et al.: A quantitative near-infrared spectroscopy study: a decrease in cerebral hemoglobin oxygenation in Alzheimer’s disease and mild cognitive impairment. Brain Cogn. 61(2), 189–194 (2006)
Yap, K.H., et al.: Visualizing hyperactivation in neurodegeneration based on prefrontal oxygenation: a comparative study of mild Alzheimer’s disease, mild cognitive impairment, and healthy controls. Front. Aging Neurosci. 9, 287 (2017)
Yeung, M.K., et al.: Altered frontal lateralization underlies the category fluency deficits in older adults with mild cognitive impairment: a near-infrared spectroscopy study. Front. Aging Neurosci. 8, 59 (2016)
Ahn, S., Nguyen, T., Jang, H., Kim, J.G., Jun, S.C.: Exploring neuro-physiological correlates of drivers’ mental fatigue caused by sleep deprivation using simultaneous EEG, ECG, and fNIRS data. Front. Hum. Neurosci. 10, 219 (2016)
Nguyen, T., Ahn, S., Jang, H., Jun, S.C., Kim, J.G.: Utilization of a combined EEG/NIRS system to predict driver drowsiness. Sci. Rep. 7, 43933 (2017)
Phillips, Z., Kim, E., Kim, J.G.: Preliminary study of gender-based brain lateralization using multi-channel near-infrared spectroscopy. J. Opt. Soc. Korea 19(3), 284–296 (2015)
Reuter-Lorenz, P.A., Cappell, K.A.: Neurocognitive aging and the compensation hypothesis. Curr. Dir. Psychol. Sci. 17(3), 177–182 (2008)
Hoshi, Y., Kobayashi, N., Tamura, M.: Interpretation of near-infrared spectroscopy signals: a study with a newly developed perfused rat brain model. J. Appl. Physiol. 90(5), 1657–1662 (2001)
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Kim, M. et al. (2020). Investigation of Cerebral Hemodynamic Changes in Mild Cognitive Impairment Due to Alzheimer’s Disease During a Verbal Fluency Task. In: Van Toi , V., Le, T., Ngo, H., Nguyen, TH. (eds) 7th International Conference on the Development of Biomedical Engineering in Vietnam (BME7). BME 2018. IFMBE Proceedings, vol 69. Springer, Singapore. https://doi.org/10.1007/978-981-13-5859-3_67
Download citation
DOI: https://doi.org/10.1007/978-981-13-5859-3_67
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-5858-6
Online ISBN: 978-981-13-5859-3
eBook Packages: EngineeringEngineering (R0)