Abstract
Hydroxyapatite (HA), a mineral component of bones and teeth, has been widely studied for various medical applications. The purpose of this research is to compare the HA from diverse bovine sources and chemical synthetic in the respectively physical and chemical powder properties such as grain size, morphology, crystallinity, phase stability and chemical functional groups. Bovine HA (B-HA) were extracted from the fresh femur bones of adult bovine, calf and bovine bone bio-waste. Synthesized HA (S-HA) were prepared by chemical precipitation method with the pH 6.0 and 12.0 of mother liquor. All of HA samples then were calcined at 800 °C. The TEM observation illustrated that particle shapes and sizes of HA differed depending on their bovine sources. In addition, XRD and FT-IR results implied that pure HA have been successfully obtained in B-HA group while S-HA with high pH value of 12.0 occurred the phrase transformation after thermal treatment.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
HA is an abundant mineral part of human hard tissue with its chemical formula of Ca5(PO4)3(OH)2 and molar ratio Ca/P of 1.67 [1, 2]. HA can directly bond with native bone without formation of collagen interface layer, which usually appear in cases of many bio-inert material implantations [3]. Due to these properties, HA has been used for decades as an alternative biomaterial for bone grafting [4, 5]. In fact, HA can either be synthesized from the chemical reactions or derived from the natural sources [6]. The laboratory synthesized HA can be obtained by using precipitation [7], ultrasonic [8], spray drying [9], sol-gel [10], multiple emulsion [11] and microwave assistant [12]. It is worth noting that the structural and the mechanical properties of synthetic HA can be modified by varying the processing method. However, these synthesis procedures might be complicated because of dependence on many factors such as the starting chemicals, concentration of the initial solution, pH maintenance and synthetic method. About the natural HA, it can be directly taken from common bio-waste sources such as eggshell [13], porcine [14], bovine [15], fish bone [16] by the one of the following methods as thermal decomposition [17], subcritical water process [18] or alkaline hydrolysis [19]. Besides, HA extracted from animal bone could be a non-stoichiometric material due to the presence of other calcium phosphate (CaP) phases or the trace ions such as Na, Mg, and Zn [20, 21].
Therefore, the manufacturing stage of HA from diverse sources and its final powder properties have been investigated as crucial factors to produce the desired biomaterials. There was some evidence that demonstrated the powder properties of nature HA corresponding to the different strains of animals. However, the factors influencing animal physique such as age have not been considered. Consequently, the primary purpose of this research is to compare the HA from different bovine sources in the respective physical and chemical properties such as grain size, morphology, crystallinity, phase stability, and chemical functional groups. The natural HA powders were obtained from fresh femur bones of adult bovine (HA-A), calf (HA-C) and bovine bones bio-waste (HA-W). In addition, the synthetic HA group with influencing factor of varying pH value at 6.0 and 12.0 were also prepared for the comparison.”
2 Materials and Methods
2.1 Materials
Calcium hydroxide (Ca(OH)2, 98%) and phosphoric acid (H3PO4, 85–87%) was purchased from GuangDong GuangHua Sci-Tech Co., Ltd, China. The fresh femur bones of adult bovine (2–3 years old) and calf (10–12 months old) were collected from a slaughterhouse while the bovine bone bio-waste was from a local animal boneyard
2.2 Methods
Hydroxyapatite synthesized by chemical reaction. S-HA samples were obtained from chemical substances through a 4-step process included stirring, pH adjustment, microwave irradiation and calcination. Firstly, Ca(OH)2 was mixed with H3PO4 at 1.67 of Ca/P ratio to produce a precursor precipitation. Throughout the mixing process for 2 h, the pH of the system was maintained at pH 6.0 and 12.0. After that, these mixtures were placed in a micro-wave at the power of 750 W for 25 min and then calcined at 800 °C for 3 h under ambient condition.
Hydroxyapatite derived from bovine bone. HA was extracted from bovine bone by the same method in the previous research [22]. Briefly, the fresh femur bones were washed and went to the 5-h boiling process in ambient condition and then 2-h boiling process under pressure. The boiling procedure was repeated three times. After that, they were calcined at 800°C for 3 h. The HA obtained from femur of adult bovine, calf, and waste bone was labeled as HA-A, HA-C, HA-W in turn.
Characterization. Firstly, the morphology and particle size of HA samples were analyzed by transmission electron microscopy (TEM) observation. Then, the crystal structure of each sample was characterized by X-ray diffractometer (XRD). Data were collected over the diffraction angles (2θ) from 20° to 80° with scanning speed of 2°/min. Fourier transform infrared (FT-IR) spectra of the samples were obtained with wave number from 4000 to 400 cm−1.
3 Results and Discussion
In the Fig. 1, the TEM images show the size and morphology of HA particles with the magnification of 50.0 k times. HA at pH 6.0 and 12.0 had non-uniform shape distribution with early high agglomeration by forming neck between HA particles, bonding them together. It was, therefore, difficult to distinguish separate grain and the size of S-HA particles could not temporarily be estimated. In contrast with S-HA group, HA particles in B-HA groups had uniform spherical shape with their diameters varied according to the different bovine sources. In specific, the particle diameters of HA-A, HA-C and HA-W were approximately 200, 100, and 170 nm, respectively.
From these results, they suggested that S-HA would be easy to achieve the interconnection in microstructure under the thermal treatment. About the B-HA, the bovine sources clearly had an impact on the calcination behavior such as the grain sizes of HA particles, which required for further investigations. Besides, the uniform spherical shape of HA particles, along with particle size, could be one of the important effect on their interactions with live cells [23].
The successful synthesis of HA from chemical reaction and bovine bone was confirmed by XRD and FT-IR analysis. In Fig. 2, the XRD patterns of all HA samples calcined at 800 °C exhibited in sharp diffraction peaks, indicating a high crystallinity, and well matched with standard peaks of HA (JCDS File No.9-432) [24]. Briefly, the peaks of pure HA at diffraction angles as 2θ of 21°, 25°, 28°, 29°, 31°, 32°, 35°, 36°, 37°, 39°, 40°, 42°, 43°, 45°, 46°, 48°, and 49° were fully showed in the spectra in both S-HA and B-HA. However, those of HA with pH 12.0 appeared strange peaks at the diffraction angle 2θ of 29.2°, 43°, 47.3°, and 48.3°, which suggested the generation of tri-calcium phosphate (TCP) as by-product after the calcination.
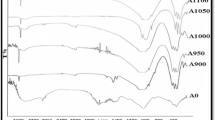
In Fig. 3, B-HA presents the FT-IR spectra of chemical functional groups in HA structure, which consists of hydroxyl \( ( {\text{OH}}^{ - } ) \) and phosphate (PO43−) band. For the \( ( {\text{OH}}^{ - } ) \) band, two peaks at 3572 cm−1 and 631 cm−1 represented for hydroxyl stretching mode and vibrational mode (v1), respectively. Next, for the PO43− group consists of four vibrational modes v1, v2, v3, and v4. In detail, a peak at 954 cm−1 represented for v1 vibration, a weak peak at 471 cm−1 represented for v2 vibration, two peaks at 1087 and 1046 cm−1 represented for v3 vibration, and a set of peaks at 638, 599, and 574 cm−1 represented for v4 vibration. In addition, the carbonate (CO32−) group can also be seen in the infrared spectrum. For v2 and v3 vibrations of CO32− group, a peak at 875 cm−1 indicated for v2 vibration and two peaks at 1650 and 1300 cm−1 indicated for v3 vibration [25]. The peak for v4 vibration of this group had very low intensity and hence it was not able to be seen. Beside the peaks of the main structure of HA but lack of \( ({\text{OH}}^{ - }) \) peak at 3572 cm−1 as indicated by the red ellipse, the spectrum of HA with pH 12.0 noticeably presented the bands of PO43− stretch ν1 at 960 cm−1 and PO43− bend ν3 at 1122 cm−1 corresponding to TCP [26].
From the XRD and the FT-IR results, HA derived from bovine bones had no signs of HA phase decomposition into secondary phases such as α-TCP, β-TCP, TTCP or CaO after calcination. In opposition to S-HA, although the temperature of 800 °C had been carefully chosen to transform the precursor precipitation into HA and prevent the appearances of other calcium phosphate (CaP) phases, these results indicated that decomposition into other CaP phrases in S-HA group has still considerably occurred. Thus, this was not in agreement with some reports those had mentioned the decomposition of HA into secondary phases proceeded above 1100 °C [27], thereby suggesting that HA synthesized with high pH is thermodynamically less stable than bovine HA.
4 Conclusion
In this study, the different routes of HA was a critical factor influencing grain size, formation of microstructure and phase stability. Under the heat treatment, TEM images illustrated that S-HA group was accessible to achieve the microstructure by the grain interconnection while HA particles in B-HA group varied their uniform spherical shape according to the different bovine sources. XRD and FI-IR demonstrated that B-HA was beneficial in producing thermally stable phase of stoichiometric HA whereas the phrase decomposition took place in S-HA after calcination below 1000 °C. Thus, these primary results in this study suggest that the unique characterizations of natural and synthetic HA must be significantly considered to achieve the desired HA properties and the suitable stability sources for biomedical applications.
References
Suchanek, W., Yoshimura, M: Processing and properties of hydroxyapatite-based biomaterials for use as hard tissue replacement implants. J. Mater. Res. 13(1), 94–117 (1998)
Ferraz, M.P., Monteiro, F.J., Manuel, C.M.: Hydroxyapatite nanoparticles: a review of preparation methodologies. J. Appl. Biomater. Biomech. 2(2), 74–80 (2004)
Ducheyne, P., Radin, S., King, L.: The effect of calcium phosphate ceramic composition and structure on in vitro behavior, I. Dissolution J. Biomed. Mater. Res. Part A 27(1), 25–34 (1993)
Damien, C.J., Parsons, J.R.: Bone graft and bone graft substitutes: a review of current technology and applications. J. Appl. Biomater. 2(3), 187–208 (1991)
Suneelkumar, C., Datta, K., Srinivasan, M.R., Kumar, S.T.: Biphasic calcium phosphate in periapical surgery. J Conservative Dent. JCD 11(2), 92 (2008)
Sánchez-Salcedo, S., Vila, M., Diaz, A., Acosta, C., Barton, I., Escobar, A., Vallet-Regí, M.: Synthesis of HA/β-TCP bioceramic foams from natural products. J. Sol-Gel. Sci. Technol. 79(1), 160–166 (2016)
Mobasherpour, I., Heshajin, M.S., Kazemzadeh, A., Zakeri, M.: Synthesis of nanocrystalline hydroxyapatite by using precipitation method. J. Alloy. Compd. 430(1–2), 330–333 (2007)
Cao, L.Y., Zhang, C.B., Huang, J.F.: Synthesis of hydroxyapatite nanoparticles in ultrasonic precipitation. Ceram. Int. 31(8), 1041–1044 (2005)
Bastan, F.E., Erdogan, G., Moskalewicz, T., Ustel, F.: Spray drying of hydroxyapatite powders: The effect of spray drying parameters and heat treatment on the particle size and morphology. J. Alloy. Compd. 724, 586–596 (2017)
Padmanabhan, S.K., Balakrishnan, A., Chu, M.C., Lee, Y.J., Kim, T.N., Cho, S.J.: Sol–gel synthesis and characterization of hydroxyapatite nanorods. Particuology 7(6), 466–470 (2009)
Kimura, I.: Synthesis of hydroxyapatite by interfacial reaction in a multiple emulsion. Adv. Mater. Sci.Eng. (2007)
Hassan, M.N., Mahmoud, M.M., El-Fattah, A.A., Kandil, S.: Microwave-assisted preparation of Nano-hydroxyapatite for bone substitutes. Ceram. Int. 42(3), 3725–3744 (2016)
Sah, M.K., Rath, S.N.: Soluble eggshell membrane: A natural protein to improve the properties of biomaterials used for tissue engineering applications. Mater. Sci. Eng. C 67, 807–821 (2016)
Qiao, W., Liu, Q., Li, Z., Zhang, H., Chen, Z.: Changes in physicochemical and biological properties of porcine bone derived hydroxyapatite induced by the incorporation of fluoride. Sci. Technol. Adv. Mater. 18(1), 110–121 (2017)
Niakan, A., Ramesh, S., Ganesan, P., Tan, C.Y., Purbolaksono, J., Chandran, H., Teng, W.D.: Sintering behaviour of natural porous hydroxyapatite derived from bovine bone. Ceram. Int. 41(2), 3024–3029 (2015)
Sunil, B.R., Jagannatham, M.: Producing hydroxyapatite from fish bones by heat treatment. Mater. Lett. 185, 411–414 (2016)
Hosseinzadeh, E., Davarpanah, M., Nemati, N. H., Tavakoli, S.A.: Fabrication of a hard tissue replacement using natural hydroxyapatite derived from bovine bones by thermal decomposition method. Int. J. Org. Transplant. Med. 5(1), 23 (2014)
Barakat, N.A., Khil, M.S., Omran, A.M., Sheikh, F.A., Kim, H.Y.: Extraction of pure natural hydroxyapatite from the bovine bones bio waste by three different methods. J. Mater. Process. Technol. 209(7), 3408–3415 (2009)
Venkatesan, J., Lowe, B., Manivasagan, P., Kang, K.H., Chalisserry, E.P., Anil, S., Kim, S.K.: Isolation and characterization of nano-hydroxyapatite from salmon fish bone. Materials 8(8), 5426–5439 (2015)
Akram, M., Ahmed, R., Shakir, I., Ibrahim, W.A.W., Hussain, R.: Extracting hydroxyapatite and its precursors from natural resources. J. Mater. Sci. 49(4), 1461–1475 (2014)
Rey, C., Combes, C., Drouet, C., Glimcher, M.J.: Bone mineral: update on chemical composition and structure. Osteoporos. Int. 20(6), 1013–1021 (2009)
Tram, B.N.T., Nguyen, T.H., Van Toi, V.: Synthesis and characterization of hydroxyapatite biomaterials from bio wastes. In: 5th International Conference on Biomedical Engineering in Vietnam pp. 336–338. Springer, Cham (2015)
Shang, L., Nienhaus, K., Nienhaus, G.U.: Engineered nanoparticles interacting with cells: size matters. J. Nanobiotechnol. 12(5), b26 (2014)
Rahavi, S.S., Ghaderi, O., Monshi, A., Fathi, M.H.: A comparative study on physicochemical properties of hydroxyapatite powders derived from natural and synthetic sources. Russ. J. Non-Ferrous Met. 58(3), 276–286 (2017)
Rehman, I., Bonfield, W.: Characterization of hydroxyapatite and carbonated apatite by photo acoustic FTIR spectroscopy. J. Mater. Sci. - Mater. Med. 8(1), 1–4 (1997)
White, J.M., Goodis, H.: In vitro evaluation of an hydroxyapatite root canal system filling material. J. Endod. 17(11), 561–566 (1991)
Ramesh, S., Tan, C.Y., Tolouei, R., Amiriyan, M., Purbolaksono, J., Sopyan, I., Teng, W.D.: Sintering behavior of hydroxyapatite prepared from different routes. Mater. Des. 34, 148–154 (2012)
Acknowledgements
This research is funded by International University, Vietnam National University Ho Chi Minh under grant number SV2017-BME-08.
Conflict of Interest The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Dang, NT.N., Le, HP., Van Toi, V., Nguyen, TH. (2020). A Comparative Study on Hydroxyapatite Derived from Bovine Bones and Synthetic Sources. In: Van Toi , V., Le, T., Ngo, H., Nguyen, TH. (eds) 7th International Conference on the Development of Biomedical Engineering in Vietnam (BME7). BME 2018. IFMBE Proceedings, vol 69. Springer, Singapore. https://doi.org/10.1007/978-981-13-5859-3_29
Download citation
DOI: https://doi.org/10.1007/978-981-13-5859-3_29
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-5858-6
Online ISBN: 978-981-13-5859-3
eBook Packages: EngineeringEngineering (R0)