Abstract
At present, most silver nanoparticles (AgNPs) conductive inks on the market are in high-temperature sintering modes. In order to increase the application range of printed electronics, it is necessary to prepare conductive inks with low-temperature sintering and good conductivity. In this study, AgNPs and RGO/AgNPs nanocomposites were prepared with glucose as the green reducing agent. The morphology and structural characteristics of the material were analyzed by XRD, SEM and TEM, which were shown that the average particle size of AgNPs was about 30 nm, and when the mass ratio of graphene oxide(GO) and silver nitrate is 1:3, the average particle size of AgNPs loaded on RGO was about 18 nm. The conductive ink was prepared by mixing RGO/AgNPs nanocomposite and AgNPs as conductive filler. After sintering at 100 °C at low temperature, the conductive property was excellent, and its resistivity could reach to 2.4 μΩ cm.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Conductive inks are the essential material for Printed electronics. As silver has good electrical conductivity and oxidation resistance, it is reported that silver conductive inks are the most widely used in the market [1, 2]. However, at present, most silver-based conductive inks are metallized by high-temperature sintering and drying mode [3, 4], which restricts the application of the ink at low temperatures such as PET, coated paper, and other printing substrates [5, 6]. The properties of nanomaterials are highly dependent on the size [7], and some scholars have ever reduced the sintering temperature by preparing small-sized nanosilver particles. For example, Moon et al. [8] used silver nanoparticle with an average particle size of 50 nm to prepare a conductive ink. After sintering at 150 °C, the resistivity of the coating reached 18.4 μΩ cm. Although the sintering temperature of the ink is related to the melting point of the particles, the melting point depends on the size of the particles: the smaller the particle size, the lower the melting point [9]. However, when the particle size is less than 10–15 nm, that is, the particle size is smaller than the mean free path of electrons, diffuse reflection of electrons occurs on the surface, and the resistivity suddenly rises and metal characteristics are lost. Therefore, the nano-silver metal characteristics cannot be lost while preparing small-size nano-silver.
Graphene has been widely used as a printed electronics field [10] due to its excellent performances,such as unique mechanical rigidity and excellent electrical properties. Comparing with the single structure of graphene or AgNP, their composites show new synergistic properties [11] such as excellent conductivity, optical transparency and good stability [12, 13]. Therefore, the RGO/AgNPs nanocomposite material can have a strong market application prospect in the low-temperature sintering conductive ink.
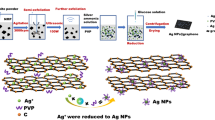
This article uses a simple and rapid method to prepare a conductive ink with low temperature sintering and good conductivity, as shown in Fig. 1. First, AgNPs with the small particle size was prepared. Secondly, the mass ratio of GO to silver nitrate during the initial reaction was studied (1:1, 1:2, 1:3, 1:4, 1:5). The effect of AgNPs size on graphene was studied, and RGO/AgNPs nanocomposite with smaller diameters were prepared. Finally, RGO/AgNPs nanocomposite and AgNPs were mixed to prepare RGO/AgNPs nanocomposite conductive ink which has excellent electrical conductivity after sintering at low temperatures.
2 Experimental
2.1 Materials
Absolute ethanol (C2H6O, AR, Tianjing in Fuyu), ammonia (NH3 H2O, AR, Tianjing in Fuyu), polyvinylpyrrolidone (PVP, K30, AR, shanghai in lanji), glucose (C6H12O7, AR, Tianjing in Kermel), and silver nitrate (AgNO3, AR, ≥99.8%, Tianjing in yingda) are all analytically pure and can be directly use, Graphene oxide [14], waterborne polyurethane/polyacrylate [15] and deionized water were all made in the laboratory.
2.2 Synthesis of AgNPs
Under dark conditions, 0.1 M silver nitrate ethanol solution was formulated into a silver ammonia solution with pH = 10. A certain amount of PVP (mPVP: mAgNO3 = 1.5:1) was added to the silver ammonia solution and stirred at 60 °C for 0.5 h when the solution brownish yellow. Using a constant pressure separatory funnel, the configured 1 M glucose-ethanol solution was slowly added dropwise to the brown-yellow solution and incubated at a constant temperature of 60 °C for 1 h. Centrifuge with ethanol, 10,000 rpm, 10 min, repeat 4–5 times. Removing the lower colloid and adding anhydrous ethanol to re-disperse ultrasonically to obtain a pure nano silver colloid.
2.3 Synthesis of RGO/AgNPs
A certain amount of silver nitrate was formulated into a silver ammonia solution having a pH = 9 and mixed with a 1 mg/mL graphene oxide dispersion. 0.1 g of polyvinylpyrrolidone was added and ultrasonically dispersed for 0.5 h. A 0.3 M solution of glucose in ethanol was added dropwise to the mixed solution, and the mixture was heated and stirred for 1 h. After cooling to room temperature, the mixture was centrifuged with deionized water at 8000 rpm for 10 min, centrifuged three times, and centrifuged three times with ethanol to obtain a stable graphene/nano-silver composite material dispersed in ethanol. The RGO/AgNPs nanocomposite named as A1, A2, A3, A4, and A5 correspond respectively to a mass ratio of graphene oxide to silver nitrate of 1:1, 1:2, 1:3, 1:4, 1:5.
2.4 Preparation of Conductive Ink
A1, A2, A3, A4, A5 RGO/AgNPs nanocomposite and AgNPs were dispersed in 1:4 ratio in deionized water, glycerol solvent, and absolute ethanol in a volume ratio of 1:2:3 and ensured that the conductive filler content was 60%. In addition, 10% waterborne polyurethane/polyacrylate was added, ultrasonic dispersion was performed for 30 min, and the conductive ink was prepared after 0.5 h of stirring. The ink was evenly coated on the coated paper. Each long coated sample was printed on the coated paper and vacuum heat treated at 100 °C for 0.5 h.
2.5 Characterization
The X-ray diffraction (XRD) of GO, RGO/AgNPs nanocomposite and AgNPs was characterized by XRD-7000 diffractometer. The SEM images of the graphene/nano-silver composite and the conductive ink after sintering at low temperature were obtained with a SU-8010 scanning electron microscope. The JEM-3001 transmission electron microscope characterizes the A1–A5 graphene/nano-silver composites. The conductivity of the conductive ink was measured with an RTS-9 digital four-probe tester.
3 Results and Discussion
3.1 Characterization of AgNPs
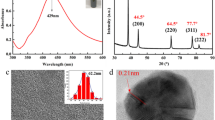
The XRD of preparing AgNPs at low temperature using glucose as a reducing agent and PVP as a protective agent is shown in Fig. 2a. The four diffraction peaks with 2θ of 38.10°, 44.28°, 64.42°, and 77.38° correspond to the (111), (200), (220), and (311) crystal plane diffraction in the face centered cubic system [16]. According to Scherrer’s equation [17], the average crystal size of AgNPs is about 28 nm. After analyzing the XRD of AgNPs, it was shown that the nanoparticle with small particle size was successfully prepared. In general, the lens directly measures the diameter of the particles. It is greater than or equal to the XRD pattern. The calculated crystal size is also accurate. Therefore, the size is obtained by the TEM of AgNPs as shown in Fig. 2b. From the figure, it can be seen that using glucose as a reducing agent and PVP as a protective agent, the particle size distribution of AgNPs prepared at a low temperature is relatively uniform, indicating that AgNPs with a small particle diameter and an average particle diameter of 30 nm are successfully prepared.
3.2 Characterization of RGO/AgNPs Nanocomposite
The XRD of GO and RGO/AgNPs nanocomposite is shown in Fig. 3a. The RGO/AgNPs nanocomposite has no diffraction peak (001) of GO, indicating that GO is successfully reduced to graphene. It can be clearly seen from the figure that XRD patterns of RGO/AgNPs nanocomposite have three well-defined diffraction peaks at 38.14°, 44.33°, and 64.45° at 2θ, corresponding to the three diffraction peaks of nanosilver (111), (200) and (220). This also shows that nanosilver is successfully loaded on the surface of graphene. From the SEM of the RGO/AgNPs nanocomposite material, the graphene surface is flat and less wrinkled as shown in Fig. 3b. This is because nanosilver is successfully loaded on the surface of graphene, preventing agglomeration of graphene. This also shows that the morphology of the RGO/AgNPs nanocomposite produced by glucose reduction is good.
The TEM of the composite prepared by GO and silver nitrate at different mass ratios is shown in Fig. 4. From Figure a, the transparent lamellar structure of graphene can be seen without obvious agglomeration. However, there is a large difference in the size and distribution of AgNPs loaded on the graphene sheet, which is influenced by the mass ratio of GO and silver nitrate during the initial reaction. With the increase of the mass ratio, AgNPs loaded on the graphene increase and the particle size decreases. When the mass ratio of GO and silver nitrate is 1:3, AgNPs loaded on the graphene have the best morphology, the average particle size is 18 nm, and the distribution is uniform. However, as the mass ratio increases, AgNPs aggregate with each other, making the particle size larger and the distribution uneven. Therefore, the increase in the mass ratio of GO and silver nitrate will tend to produce AgNPs with smaller particle sizes with in a certain range. If the mass ratio exceeds a certain range, agglomerates will occur between particles, and the particle size will increase.
3.3 Resistivity of Conductive Ink
The results of using A1-A5 RGO/AgNPs nanocomposite materials and AgNPs mixed as conductive fillers for the resistivity of the conductive ink are shown in Fig. 5. Because the resistivity of the A5 group conductive ink is too large, it does not appear in the figure. The third set of conductive inks had the lowest resistance and the resistivity reached 2.4 μΩ cm. This is because in the A5 group of conductive inks have a large particle size of AgNPs loaded on the graphene, and the sintering temperature is too low to form a network of conductive paths. The conductive ink of group A3 was heat-treated and cured at a temperature of 100 °C. After SEM analysis, the results are shown in Fig. 6. It can be seen that the solvent of the ink volatilized after sintering, the conductive fillers melt and contacted with each other to form a dense conductive channel, so that the ink has conductivity.
4 Conclusions
In this paper, low-temperature sintering conductive inks are prepared by preparing AgNPss with small particle diameters. Using glucose as a green reducing agent, AgNPs with an average particle size of 30 nm were prepared at a low temperature. When the mass ratio of GO and silver nitrate is 1:3, AgNPs supported on the graphene is uniformly distributed and the particles are small (average particle size is 18 nm). RGO/AgNPs nanocomposite and AgNPs were mixed as conductive filler. RGO/AgNPs nanocomposite-based conductive ink was prepared by adding binder and organic solvent. The coating resistance after sintering at 100 °C was 2.4 μΩ cm. Replacing part of the nanosilver with graphene/silver nanocomposites not only reduces the cost of the nanosilver ink, but also expands the application range of printed electronics.
References
Stewart, I. E., Kim, M. J., & Wiley, B. J. (2017). Effect of morphology on the electrical resistivity of silver nanostructure films. ACS Applied Materials & Interfaces, 9(2), 1870–1876.
Wang, F., Mao, P., & He, H. (2016). Dispensing of high concentration ag nano-particles ink for ultra-low resistivity paper-based writing electronics. Scientific Reports, 6, 21398.
Nie, X., Wang, H., & Zou, J. (2012). Inkjet printing of silver citrate conductive ink on pet substrate. Applied Surface Science, 261(8), 554–560.
Zhang, Z., Zhang, X., Xin, Z., Deng, M., Wen, Y., & Song, Y. (2011). Synthesis of monodisperse silver nanoparticles for ink-jet printed flexible electronics. Nanotechnology, 22(42), 425601.
Yu, H., Li, L., & Zhang, Y. (2012). Silver nanoparticle-based thermal interface materials with ultra-low thermal resistance for power electronics applications. Scripta Materialia, 66(11), 931–934.
Ogura, H., Maruyama, M., Matsubayashi, R., Ogawa, T., Nakamura, S., Komatsu, T., et al. (2010). Carboxylate-passivated silver nanoparticles and their application to sintered interconnection: a replacement for high temperature lead-rich solders. Journal of Electronic Materials, 39(8), 1233–1240.
Hornyak, G. L., Tibbals, H. F., & Dutta, J. (2008). Introduction to nanoscience. Introduction to nanoscience: CRC Press.
Moon, Y. J., Kang, H., Kang, K., Moon, S. J., & Hwang, J. Y. (2015). Effect of thickness on surface morphology of silver nanoparticle layer during furnace sintering. Journal of Electronic Materials, 44(4), 1192–1199.
Amp, P. R. C., & Jesser, W. A. (1977). Thermodynamic theory of size dependence of melting temperature in metals. Nature, 269(5628), 481–483.
Majee, S., Liu, C., Wu, B., Zhang, S. L., & Zhang, Z. B. (2017). Ink-jet printed highly conductive pristine graphene patterns achieved with water-based ink and aqueous doping processing. Carbon, 114, 77–83.
Zhang, Z., Xu, F., Yang, W., Guo, M., Wang, X., Zhang, B., et al. (2011). A facile one-pot method to high-quality Ag-graphene composite nanosheets for efficient surface-enhanced raman scattering. Chemical Communications, 47(22), 6440–6442.
Myekhlai, M., Lee, S., Lee, T., Chung, H., & Jeong, H. (2015). A facile and eco-friendly synthesis of graphene–silver hybrid materials for transparent conductive films. Ceramics International, 41(1), 983–989.
Chamoli, P., Das, M. K., & Kar, K. K. (2017). Green synthesis of silver-graphene nanocomposite-based transparent conducting film. Physica E: Low-dimensional Systems and Nanostructures, 90, 76–84.
Neto, A. H. C. (2010). The electronic properties of graphene. Physica Status Solidi, 244(11), 4106–4111.
Wang, X., Shen, Y., & Lai, X. (2014). Micromorphology and mechanism of polyurethane/polyacrylate membranes modified with epoxide group. Progress in Organic Coatings, 77(1), 268–276.
Yang, J., Zang, C., Sun, L., Zhao, N., & Cheng, X. (2011). Synthesis of graphene/ag nanocomposite with good dispersibility and electroconductibility via solvothermal method. Materials Chemistry and Physics, 129(1), 270–274.
Shen, J., Shi, M., Yan, B., Ma, H., Li, N., & Ye, M. (2011). One-pot hydrothermal synthesis of ag-reduced graphene oxide composite with ionic liquid. Journal of Materials Chemistry, 21(21), 7795–7801.
Acknowledgements
This work was supported key laboratory project of Shaanxi provincial department of education (No.16JS082).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Zou, Q., Cao, C., Zhu, H., Hou, C. (2019). Preparation of Low Temperature Sintered Graphene/Silver Nanocomposite-Based Conductive Ink. In: Zhao, P., Ouyang, Y., Xu, M., Yang, L., Ren, Y. (eds) Advances in Graphic Communication, Printing and Packaging. Lecture Notes in Electrical Engineering, vol 543. Springer, Singapore. https://doi.org/10.1007/978-981-13-3663-8_101
Download citation
DOI: https://doi.org/10.1007/978-981-13-3663-8_101
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-3662-1
Online ISBN: 978-981-13-3663-8
eBook Packages: EngineeringEngineering (R0)