Abstract
Even 30 years after the discovery of magainins, biophysical and structural investigations on how these peptides interact with membranes can still bear surprises and add new interesting detail to how these peptides exert their antimicrobial action. Early on, using oriented solid-state NMR spectroscopy, it was found that the amphipathic helices formed by magainins are active when being oriented parallel to the membrane surface. More recent investigations indicate that this in-planar alignment is also found when PGLa and magainin in combination exert synergistic pore-forming activities, where studies on the mechanism of synergistic interaction are ongoing. In a related manner, the investigation of dimeric antimicrobial peptide sequences has become an interesting topic of research which bears promise to refine our views how antimicrobial action occurs. The molecular shape concept has been introduced to explain the effects of lipids and peptides on membrane morphology, locally and globally, and in particular of cationic amphipathic helices that partition into the membrane interface. This concept has been extended in this review to include more recent ideas on soft membranes that can adapt to external stimuli including membrane-disruptive molecules. In this manner, the lipids can change their shape in the presence of low peptide concentrations, thereby maintaining the bilayer properties. At higher peptide concentrations, phase transitions occur which lead to the formation of pores and membrane lytic processes. In the context of the molecular shape concept, the properties of lipopeptides, including surfactins, are shortly presented, and comparisons with the hydrophobic alamethicin sequence are made.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Magainin
- PGLa
- Cecropin
- LL37
- Surfactin
- Alamethicin
- Membrane topology
- Membrane pore
- Membrane macroscopic phase
- SMART model
- Carpet model
- Toroidal pore model
- Peptide-lipid interactions
- Molecular shape concept
1 Introduction
The innate immune system of higher organisms provides a first line of defense against a multitude of pathogenic microorganisms, where the release of antimicrobial peptides (AMP) is a powerful and fast response to fight bacterial and fungal infections (Boman 1995; Zasloff 2002). A multitude of AMPs belonging to different classes have been detected in the plant and animal kingdom, including in the human body (Agerberth et al. 1995). These complement a multitude of antibiotic peptides produced by microorganisms that have early on been identified and investigated (Leitgeb et al. 2007; Rautenbach et al. 2016a, b). The corresponding databases encompass thousands of sequences (Pirtskhalava et al. 2016; Wang et al. 2016), and early on several of them have been investigated by cell biological and biophysical approaches in order to reveal their mechanisms of action (Sansom 1991; Bechinger 1997). Magainins from the African clawed frog were among the first of those peptides found in the animal kingdom, where the antimicrobial activity has been described about 30 years ago (Zasloff 1987), thereby adding a new exciting perspective to earlier publications describing peptides from toads and frogs (Kiss and Michl 1962; Giovannini et al. 1987).
The very first attempts to develop these peptides into commercial products were unsuccessful which resulted in a temporary decline in interest on this topic around the turn of the millennium. However, the rapid increase in multiresistant pathogens (Chang et al. 2003) has again stimulated research activities on AMPs because they promise to be paradigms for new classes of antibiotics with mechanisms of action that are less prone to be neutralized by microbial resistance (Zasloff 2002). To protect such polypeptides from proteases, special formulations inside nanostructures, attachment to surfaces, or the use of unnatural amino acids is explored (Yang et al. 2014; Yuksel and Karakecili 2014; Reijmar et al. 2016). Furthermore, mimetics in the shape of small molecules or foldamers have also been developed (Violette et al. 2006; Arnusch et al. 2012; Ghosh and Haldar 2015; Ghosh et al. 2018).
Magainin and other antimicrobial peptides discussed in this paper are thought to interfere with the barrier function of bacterial membranes rather than specific membrane receptors (Bechinger 2015). Whereas molecules whose interactions are with proteinaceous receptors can be made inefficient by one or a few changes in amino acid sequence, polypeptides that act by disrupting the lipid bilayer physicochemical properties are less likely to become inactivated by resistance (Rollins-Smith et al. 2002). Indeed the amphipathic nature of AMPs has been found essential and can be achieved by helical (Sansom 1991; Bechinger 1997), cyclic (Cao et al. 2018; Laurencin et al. 2018; Tsutsumi et al. 2018; Zhao et al. 2018), and/or β-sheet arrangements (Hong and Su 2011; Salnikov et al. 2011; Rautenbach et al. 2016a, b; Sychev et al. 2018; Usachev et al. 2017). Thus the insights gained from the studies of cationic amphipathic antimicrobial peptides have stimulated the design of a number of small amphipathic molecules (Arnusch et al. 2012; Ghosh et al. 2014), pseudopeptides (Porter et al. 2002; Patch and Barron 2003; Kuroda and DeGrado 2005; Violette et al. 2006; Makovitzki et al. 2008; Scott et al. 2008; Rotem and Mor 2009; Palermo and Kuroda 2010; Laurencin et al. 2018), and polymers (Rank et al. 2017) with potent antimicrobial properties.
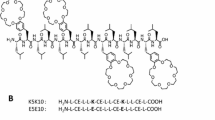
In this review, some of the laboratory-based biophysical techniques that have provided valuable information on the interactions of peptides shall be introduced and the corresponding data presented (Lear et al. 1988; Killian et al. 1998; Harzer and Bechinger 2000; Bechinger 2011) including work on the synergistic activities between PGLa and magainin 2 two members of the magainin family (see Table 4.1 for amino acid sequences). Even after 30 years of biophysical studies on how these AMPs interact with lipid bilayers, new details are revealed, and as a consequence, exiting new research directions open up (Salnikov et al. 2010; Hong and Su 2011). It is now clear that such amphipathic membrane-active peptides are very dynamic in nature and can adopt a large diversity of conformations and topologies whose exchange and interactions are governed by multiple equilibria (Bechinger 2015). These cationic linear peptides are random coil in solution and adopt their three-dimensional amphipathic helical structure only when interacting with membranes. By disrupting the integrity of bacterial and fungal membranes, they inhibit the growth of microorganisms and/or enter into the cell interior (Roversi et al. 2014). This results in complex patterns of metabolic reactions by the bacterial cells (Kozlowska et al. 2014; Cardoso et al. 2017). Because many peptides have been shown to also modulate the immune response of the host organisms, they are also called “host defense peptides” (McCafferty et al. 1999’ Holzl et al. 2008; Diamond et al. 2009; Steinstraesser et al. 2010). Such cell biological studies shall not be part of this review.
2 Electrophysiological Recordings
Electrophysiological experiments provide an interesting method to observe and characterize membrane interactions of peptides and proteins. One approach is to measure the ionic conductivities across small patches of a lipid bilayer (ranging from several micrometers up to hundreds of micrometers) separating two electrodes connected to a voltage-clamp amplifier (Montal and Mueller 1972). The electrodes can be positioned in different chambers or constitute the inside and outside of dedicated pipettes. When lipids alone are used, a tight electric seal (gigaseal) is established; thus no ions can flow across the lipid bilayer. In the presence of small amounts of pore-forming polypeptides, a decrease in ohmic resistance occurs (“membrane openings”), and ionic current can be recorded, when at the same time the transmembrane voltage is electronically regulated to remain constant (“voltage clamp”). Ideally, single events are observed and characterized in frequency, duration, and conductivity. More recent chip technology working with arrays of freestanding lipid bilayers has simplified the handling and provides an increased throughput for such single-channel measurements (del Rio Martinez et al. 2015).
Notably, discrete single-channel openings/events are rather difficult to observe for magainins and cecropins because most of the time, these peptides lyse the membranes. However, in some electrophysiological experiments, discrete multilevel conductivities have been recorded (Christensen et al. 1988; Duclohier et al. 1989; Cruciani et al. 1991; Watanabe and Kawano 2016), which early on was taken as an indicator for transmembrane helical bundle formation (Tieleman et al. 2002). However, unlike the well-defined ion channels formed by alamethicin, those recorded in the presence of cationic amphipathic peptides are erratic and characterized by large variations (Christensen et al. 1988; Duclohier et al. 1989; Cruciani et al. 1991). Therefore, despite some similarities, electrophysiological recordings between these peptides exhibit rather pronounced differences as do the physicochemical properties of their primary sequences, i.e., number of charges and overall hydrophobicity (Table 4.1).
3 Fluorescence Spectroscopy
3.1 Fluorophore Release
An alternative method to monitor the formation of membrane openings has been established based on fluorescence spectroscopic approaches. In these experiments vesicles are prepared in the presence of high concentrations of fluorophore such as calcein. At a concentration of some tens of millimolar, the dye molecules are close to each other resulting in the quenching of their radiative emission. Once unilamellar vesicles have been formed, the fluorophore on the outside is exchanged by passage through a gel filtration column. Using this method, LUVs and the encapsulated dye can be easily separated from the outside buffer. Care should be taken that the osmolarities of the buffer solutions at the interior and exterior of the liposomes match each other to assure that there is no pressure gradient across the membrane (Marquette et al. 2008). Under these precautions, vesicles are stable for days or even weeks keeping the dye encapsulated and ready for future experiments.
Upon addition of peptide, the formation of pores can be measured by monitoring the increase in the fluorescence intensity due to the escape of the dye from the vesicles. Fluorescence is continuously measured while irradiating the sample with monochromatic light tuned to the most intense absorption band of the chromophore. Variants of the technique have been developed to measure the kinetics in more detail and to distinguish between all-or-nothing and graded release. For example, in the fluorescence quenching assay, the fluorophore is encapsulated with a quencher. Upon all-or-nothing release, both leak out without changes in fluorescence inside the vesicles. In contrast, a graded release results in a fluorescence increase when the quencher inside the vesicles is diluted (Ladokhin et al. 1995; Clark et al. 2011). Alternatively, due to an about tenfold difference in fluorescence lifetime between entrapped and free dye, their respective proportions can be estimated using biexponential fits. This has been used to develop an approach to distinguish all-or-nothing and graded release (Patel et al. 2014). Yet another method is based on the direct observation of giant unilamellar vesicles under the microscope which can thus be studied one-by-one individually (Tamba and Yamazaki 2005).
Using fluorescence spectroscopy, the formation of magainin pores was investigated, and the kinetics of calcein release from individual giant unilamellar vesicles (GUVs) made from DOPC/DOPG at different molar ratios was monitored (Islam et al. 2014). Membrane pore formation from DOPC/DOPG GUVs sets in at peptide concentrations of 0.7 mole% (Tamba and Yamazaki 2009). After the addition of peptide, it takes minutes before the release of fluorophores sets in, but then the vesicles, which are several micrometers in diameter, empty within only 30 s. The fast release is suggestive that magainin pore formation in GUVs follows an all-or-nothing mechanism rather than gradual diffusion through the pore. Similar conclusions were drawn from calcein release experiments from suspensions of large unilamellar vesicles made from POPC and POPG (Gregory et al. 2008). However, the topic turns out more complex because the mechanism is lipid dependent and a graded release is observed when the POPG content is reduced from 50 to 20 mole% (Gregory et al. 2008). Once the pore has formed, the subsequent fluorophore release is a two-stage process where an initial fast release is due to an unbalance of the bilayer because as a first step magainin solely associates with the outside monolayer (Tamba et al. 2010). The movement of the peptide from the outer to the inner leaflet results in the transient formation of very large pores concomitant with an equilibration of the peptide density (Tamba et al. 2010). In the following, a slower release of fluorophore sets in, but even these persistent openings are large enough to allow for the passage of molecules with a hydrodynamic radius of 3 nm (Tamba et al. 2010). In follow-up investigations, it was shown that antimicrobial action was associated with a preferential interfacial localization (rather than insertion into the hydrophobic core of the membrane) and correlated to the Gibbs free energy of membrane association rather than membrane insertion (Clark et al. 2011).
3.2 Fluorescence: Natural Chromophores and Membrane Partitioning
The intrinsic emission properties of AMPs containing chromophores naturally, by mutagenesis or by chemical attachment, can be exploited to gain insight into peptide-lipid interactions. The amino acid residues phenylalanine, tyrosine, and tryptophan absorb and emit light in the near-UV region. Intensity modulation of emission spectra and/or wavelength shift is generally observed when the amino acids change environments, for example, when they move from aqueous buffers into a lipid membrane. This fluorescence-based approach has the great advantage of being nondestructive for biological samples and require only small amounts of material.
Among the three naturally occurring amino acid chromophores, tryptophan emission has the longest wavelength emission and the highest absorption and emission yields, thereby being most sensitive to measure. Several groups have taken advantage of measuring the blue shift and/or intensity changes in the emission spectrum to quantify an apparent binding constant between peptide and a large variety of model membranes (Matos et al. 2010; Zanin et al. 2013; Michalek et al. 2014). To monitor the membrane-induced changes in fluorescence, a solution of peptide is continuously excited at a fixed wavelength, while the dispersed emission spectra are recorded after each addition of aliquot of vesicle suspension. The wavelength of the maximum emission intensity and/or its amplitude is then plotted against the lipid concentration, and a fitting procedure is applied to extract apparent peptide-to-lipid binding constants. Antimicrobial peptide association to membranes of different composition was used using this method (Vogt and Bechinger 1999), including for magainin 2 and its interactions with PGLa within lipid bilayers (Matsuzaki et al. 1998).
3.3 Fluorescence: Depth of Membrane Insertion
Acrylamide and free radicals are known to quench tryptophan fluorescence, and this property has been used to monitor the depth of insertion of AMPs into membranes (Caputo and London 2003). Collisional quenching is enhanced with the contact frequency between the fluorophore and its environment and so with the distance between the fluorescent amino acids and the position of the quencher. Whereas acrylamide is water soluble, the hydrophobic alkyl chain-bound doxyl radicals are positioned within the membrane interior. This method has been calibrated by studying the emission properties of tryptophan residues of different mutants of a membrane-spanning helix (Caputo and London 2003). The quenching efficiency of the emission by acrylamide molecules in solution was found to be decreasing with the distance from the membrane surface; the one by the membrane-inserted radical depends on the penetration depth of the tryptophan. The quenching efficiencies can thus serve as an atomics-scale ruler to localize the position of the tryptophan relative to the membrane surface.
Brome has also been reported as an efficient short-range fluorescence quencher. Brominated phospholipids have been introduced from the beginning of the 1990s to estimate the position of tryptophan residues within the membrane through the use of different labeling positions along the phospholipid fatty acyl chains (Bolen and Holloway 1990). As an example of the method, the membrane penetration depth of tryptophans within the amino-terminal domain of huntingtin was investigated taking advantage of lipids dibrominated at either positions 6 and 7, 9 and 10, or 11 and 12 (Michalek et al. 2014). Fluorescence quenching in the presence of paramagnetic agents allowed the determination of an in-planar alignment of Phe to Trp mutants of magainin 2, where all three tryptophans investigated are localized at about 1 nm from the bilayer center (Matsuzaki et al. 1994).
3.4 Fluorescence Self-Quenching
A fluorescence self-quenching effect has been developed to quantitatively evaluate if peptides distribute randomly at the membrane surface or in a more heterogeneous manner (Fig. 4.1). Many models of peptide organization in membranes assume that there are oligomeric structures of peptide monomers. For example, the toroidal pore model assumes the formation of well-defined transmembrane multimers (Ludtke et al. 1996; Matsuzaki 1998). In contrast, the carpet (Shai 1999) or the SMART model (Bechinger 2015) postulate a looser and less well-defined arrangement of the peptides at the membrane surface. Nevertheless, these concepts do not exclude that high local order and/or nonrandom peptide assemblies play an important role in enabling and modulating the membrane-disruptive function. Indeed, for the designed antibiotic peptide LAH4, the formation of nematic phases at the membrane surface has been demonstrated which can be modulated by the charge of the lipids and the salt concentration of the surrounding buffer (Fig. 4.1c) (Aisenbrey and Bechinger 2014). Self-quenching of fluorescent molecules acts on a length scale of nanometers and is therefore sensitive to the packing of peptides on the membrane surface at high concentration. Self-quenching is explored by dilution of the label (replacing labeled peptide by unlabeled peptide, Fig. 4.1a), and distances are estimated by fitting the data with a Poisson distribution. Ongoing research indicates an important role of mesophases and clustering for the function of magainin 2 and PGLa (manuscript in preparation).
Fluorescence self-quenching to analyze the peptide distribution. The fluorescence signal is measured at a fixed peptide-to-lipid ratio, where the fraction of fluorophore-carrying peptide is increased in a stepwise manner (a). When the fluorophore-labeled peptides are distributed randomly (illustrated in panel b), a linear increase in fluorescence is observed (black data points and line in a). In the presence of peptide aggregates or when peptides are localized in more confined domains (c), adding more of the labeled peptide is accompanied in self-quenching of the fluorescence and a nonlinear fluorescence increase (red data points and line in a)
3.5 Förster Resonance Energy Transfer (FRET)
FRET is another fluorescence technique widely used in the field of biophysics that can reveal the proximity between two systems of interest. Most often the proteins and/or peptides have to be labeled with soluble and non-perturbative dyes, so-called acceptor and donor for those who are prone to absorb and emit light, respectively. When they encounter in close vicinity, the excited states of both fluorescent molecules mix resulting in the emission of the acceptor when the donor is excited with light. Because the emission wavelengths of the acceptor are longer than the ones of the donor, this phenomenon is sometimes associated with a considerable shift in the emission spectrum. Detecting the quenching of the emission of the donor and/or the enhancement of the fluorescence of the acceptor reveals the proximity of the dyes. These kinds of experiments can be performed within a conventional spectrofluorometer or under the lens of a microscope which adds spatial resolution to the spectroscopic information. Because of the high absorption coefficients of some well-chosen dyes with almost unity emission yields, single molecules can be detected when highly sensitive instruments are used.
The interaction of PGLa with magainin 2 inside membranes was probed by FRET (Marquette et al. 2015). The peptides were labeled with NBD, and rhodamine fluorophores and LUVs made of POPC/POPS or of POPE/POPG were chosen as model bilayers. At high peptide-to-lipid ratio, FRET is indeed observed because even their random distribution assures that they find neighbors regularly within the Förster radius (≈56 Å for the NBD-rhodamine pair, Medintz and Hildebrandt 2013). However, upon dilution of the peptides in the lipid bilayer, the FRET effect disappears indicating that the peptides do not interact strongly and specifically but distribute in a more stochastic manner along the membrane surface (Marquette et al. 2015).
3.6 Fluorescence Imaging
Fluorescence imaging aims to get a deeper understanding in the mechanisms of AMP action by making direct observations on microscopic living or nonliving systems. The main advantage of imaging techniques lies in the fact that temporal and spatial information are made accessible at the same time and sometimes on very dilute systems as low as single fluorescent molecules. When probing bacteria, imaging techniques can provide subcellular spatial resolution and/or discern heterogeneities between cells within a sample population. Over the last decades, time-resolved/high-resolution imaging assays have brought a new dimension to the description and understanding mode of action of AMPs (reviewed in Choi et al. 2016).
To perform time-resolved imaging on single cells with resolution at the millisecond time scale, a time-resolved microscope equipped with high-magnification objectives and phase-contrast detection system has been used. Different schemes of chromophore labeling including visualizing the AMPs themselves have proven their potential in revealing when and where key mechanistic events occur within individual cells (Choi et al. 2016). It is believed that in the near future improvement of time resolution combined with superresolution imaging systems could provide an even more comprehensive picture of how AMPs halt growth and/or kill bacteria.
The binding of antimicrobial peptides to live bacteria was monitored using microscopic imaging revealing a spatiotemporal sequence of events including membrane permeabilization. These images reveal that the human LL37 peptide (Table 4.1) attacks septating E. coli cells. The peptide distributes unevenly and preferentially binds to the septum and the curved regions of the outer membrane (Barns and Weisshaar 2013). In non-septating cells, it is found to associate with one of the endcaps. When the AMPs enter the periplasmic space, the cells shrink which is probably due to an osmotic effect. The outer membrane loses the barrier function first, and after a short delay, permeabilization of the cytoplasmatic membrane is observed. The openings of the outer and cytoplasmic membranes monitored in the presence of LL37 are localized and persistent rather than global and transient (Rangarajan et al. 2013). Although many events observed in this manner follow a related schedule, important details vary with the antimicrobial compound when cationic polymers and longer or shorter peptides such as LL37, cecropin A, or melittin, are compared to each other (Yang et al. 2018). From mutagenesis experiments and a comparison of E. coli cells grown under aerobic or anaerobic conditions, it has been concluded that LL37 specifically affects the electron transport chain (Choi et al. 2017). On the other hand, human β-defensins tend to concentrate in a few foci that localize at the septum of cell division sites of Enterococcus faecalis where they colocalize with PG, CL, Sec A, and sortases, interfering with the activities of the latter proteins (Kandaswamy et al. 2013). In contrast, alamethicin causes a different series of permeabilization events (Barns and Weisshaar 2016). Thus, the detailed cellular response depends on the peptide, the bacterial species, and the growth conditions. Notably, this reflects experiments with membrane model systems where the very details varied with the lipid composition of the bilayers under investigation (Gregory et al. 2008; Cheng et al. 2011).
4 Circular Dichroism Spectroscopy
Peptides exhibit multiple chiral centers that are optically active in the mid-ultraviolet spectral range. This can be investigated by circular dichroism where the absorption spectra exhibit pronounced features correlating to the dihedral angles of the polypeptide backbone and thereby the secondary structure (Sreerama and Woody 2000; Miles and Wallace 2006). For example, α-helical folds exhibit two characteristic minima at 208 and 222 nm, while the spectral intensities of random coil structures show a single minimum at 195 nm. Thus, the CD line shape of polypeptides can be deconvoluted into contributions from helices, sheets, turns, and random coil conformations (Sreerama and Woody 2000; Miles and Wallace 2006). When peptides are reconstituted into oriented membranes, different CD line shapes are obtained that provide information on the peptide alignment in membranes (Wu et al. 1990; Perrone et al. 2014). In this context, it should be mentioned that ATR FTIR spectra of oriented membranes were also analyzed to study the bilayer topologies of antimicrobial peptides including magainin 2 (Bechinger et al. 1999).
A refined analysis of CD spectra has even been reported to probe the monomer-dimer equilibrium of a transmembrane helix (Loudet et al. 2005); however, when reproducing such experiments, care should be taken during analysis because related changes can also be due to light scattering.
Because membrane polypeptides exhibit a high local concentration and reside in vesicles that are close to the size of the absorption wavelengths, distortions due to light scattering and absorption flattening artifacts have to be taken into consideration (Wallace and Moa 1984; Miles and Wallace 2016). Recently a new approach has been presented which allows one to quantify and correct for light scattering artifacts (Vermeer et al. 2016). Finally, uncertainties in the determination of peptide concentration due to the presence of counterions and salts or errors in weighing the sample propagate into errors in the secondary structure determination by these algorithms. Therefore, relative intensities have also been used to estimate structural transitions between random coil and helical secondary structure (Bruch et al. 1991).
Because pronounced spectral changes occur when membrane association is accompanied by structural transitions, it is possible to quantitatively determine membrane association constants by titrating vesicles step by step to a peptide solution, also of magainin AMPs (Wieprecht et al. 2000a, b; Voievoda et al. 2015). Furthermore, valuable information on the association kinetics of magainin to whole cells and lipopolysaccharides has been obtained using CD spectroscopy (Avitabile et al. 2014).
As an illustration of the technique, we show in Fig. 4.2 the CD spectra and the corresponding association isotherm of the PGLa peptide to SUVs made of POPE/POPG 3/1 mole/mole at neutral pH. A fitting procedure was applied to each spectrum to estimate the percentage of peptides bound to the membrane as a function of peptide-to-lipid ratio. Based on a simple membrane insertion model (Vogt and Bechinger 1999), an apparent association constant of K ass ≈ 1680 M−1 can be calculated, but clearly the experimental data do not correlate well with such an asymptotic binding isotherm. Indeed, it should be noted that this apparent value covers the electrostatic interaction to the membrane surface and the hydrophobic partitioning into the bilayer interface. Therefore, the membrane surface concentration of the positively charged PGLa (nominal charge +4 to +5) is much increased in the proximity of the anionic POPE/POPG bilayer. However, membrane association of the cationic peptide neutralizes the charges of the PG lipids and can even result in repulsive interactions. A more refined analysis separating electrostatic and hydrophobic contributions has been reported in the literature for the binding of PGLa to POPC/POPG 3/1 mole/mole SUVs in the presence of 100 mM NaCl (Wieprecht et al. 2000a, b). Indeed, whereas the apparent membrane association is 50-fold increased for the anionic lipid mixture when compared to pure POPC, a hydrophobic surface partition equilibrium with K p = 800–1500 M−1 was obtained for both membranes.
Titration of 50 μM PGLa with increasing amounts of SUVs (POPE/POPG 3/1) in 5 mM Tris-HCl, pH = 7, at 25 °C. The changes in the circular dichroism spectra upon membrane association (a) have been used to quantify the percentage of membrane-associated peptide and are reported on the vertical axis of the lower frame panel (b)
Therefore, an alternative analysis was performed for the data shown in Fig. 4.2 where focus is on electrostatic interactions. Under these conditions, it was assumed that the amount of peptide that binds to the POPE/POPG 3/1 mole/mole membranes is governed by electrostatic interactions and stops when charge neutrality is reached. Indeed, Fig. 4.2b exhibits a linear increase in bound peptide up to about 1.4 mM total lipid. Under these conditions, the negative charge contribution corresponds to 350 μM POPG; however, almost half of the charges reside in the inner leaflet of the vesicles and during the titration may not or only partially be accessible to the PGLa peptide. At this lipid concentration, 50 μM peptides are used up in the binding reaction, contributing 200–250 μM in positive charges. The P/L ratio at this lipid concentration is about 3.6 mole%.
Such structural investigations show that the random coil structure of magainins in aqueous solution becomes helical once the peptide inserts into membrane environments (Bechinger 1999). This conformational transition has been identified to be a driving force of membrane association (Wieprecht et al. 1999a). Oriented CD spectroscopy confirmed solid-state NMR data obtained at peptide-to-lipid ratios <3 mole% showing that the helix is oriented parallel to the membrane surface (cf. below) and suggested an insertion when the concentration is increased (Ludtke et al. 1994). However, it should be noted that at higher concentrations the membrane supramolecular architecture exhibits pronounced changes probably by a transition to bicellar fragments (Bechinger 2005).
5 Isothermal Titration Calorimetry
Isothermal titration calorimetry (ITC) is a widely used technique when thermodynamic parameters describing the interactions between two systems need to be investigated. The method is based upon the measurement of the heat absorbed or released during a reaction such as binding between a ligand and a reactant molecule. The reactant is placed in an isolated reactor, while the ligand is added in a stepwise manner, allowing the determination of enthalpy, binding constants, stoichiometry, and entropy changes, thereby providing a complete thermodynamic picture of the system. The technique is particularly well suited to study water-soluble molecules, but the method was also developed for the determination of the binding parameters between membranes and lipophilic biomolecules such as membrane peptides (Seelig 2004).
The association of magainin 2 and/or PGLa with membranes has been studied by ITC under different conditions and provided valuable insight into their reversible interaction with phospholipid bilayers (Wenk and Seelig 1998; Wieprecht et al. 1999a, b, 2000a, b, 2002). The ITC data reveal apparent partitioning constants for magainin 2 in the 12,000 M–1 range for POPC/POPG 3/1 mole/mole. When separated from the electrostatic attractive term, a hydrophobic partitioning of 55 M−1 remains (Wenk and Seelig 1998). For pure POPC, values of 2000 M−1 were obtained at 30 °C (Wieprecht et al. 1999b). Notably, when 100 nm LUVs and SUVs were compared to each other, the enthalpic and entropic contributions were much different, whereas the Gibbs free energy and therefore the binding constants hardly changed (Wieprecht et al. 2000a, b). By investigating double D-amino acid replacement sequences of magainin 2, the random coil to helix transition at the membrane surface was found to contribute about −0.6 kJ/mole per residue and thereby about 50% of the driving force of the magainin 2 membrane association (Wieprecht et al. 1999a). The hydrophobic contribution of PGLa membrane association is 800–1500 M−1 when at the same time the apparent partitioning to POPC/POPG 3/1 mole/mole membranes is 50-fold increased (Wieprecht et al. 2000a, b).
When PGLa or magainin was titrated into LUV suspensions made of POPE/POPG 3/1 at pH 7, the peptide membrane association was characterized by endothermic reaction enthalpies (ΔH) (Marquette and Bechinger 2018). These are relatively small when compared to previous investigations with 30 nm SUVs of different lipid compositions (Wenk and Seelig 1998; Wieprecht et al. 1999a, b, 2000a, 2002). In the presence of both peptides, an additional enthalpy contribution of −8 kJ/mole was observed which correlates with the formation of larger complexes, probably vesicle agglutination (Marquette and Bechinger 2018).
6 Solid-State NMR Spectroscopy
6.1 Solid-State NMR Investigations of Polypeptides
Nuclear magnetic resonance is a powerful technique to investigate the structure, topology, dynamics, and interactions of biomolecules. Whereas peptides have been investigated by multidimensional 1H-1H solution NMR spectroscopy in membrane-mimetic micelles made from deuterated DPC (Brown 1979; Georgescu et al. 2010), this technique is unsuitable for the investigation of peptides associated with large peptide-bilayer complexes. However, NMR spectroscopy becomes much more powerful when polypeptides can be prepared either by bacterial overexpression and biochemical purification or by chemical synthesis such that stable isotopic labels such as 15N, 13C, and/or 2H were introduced.
For polypeptides associated with large complexes that rotate slowly on the NMR time scales, solid-state NMR spectroscopy has been developed. Because chemical shifts, dipolar and quadrupolar interactions are all dependent on the molecular alignment relative to the magnetic field and the dipolar interactions between nuclei can be strong for immobilized molecules, in a static sample, broad overlapping line shapes are observed that hamper a detailed analysis.
One approach to obtain well-resolved solid-state NMR spectra is to subject the sample to fast rotation around the magic angle. This method results in NMR spectra where only the isotropic chemical shifts remain which resemble those observed in solution, and similar concepts for assignment and structural analysis are used (Das et al. 2015; Eddy et al. 2015; Gopinath and Veglia 2015; Jaipuria et al. 2017; Visscher et al. 2017; Naito et al. 2018).
A second approach relies on exploiting the anisotropies of interactions inherent to solid-state NMR spectra rather than averaging them (Fig. 4.3). When membranes are uniaxially oriented relative to the magnetic field of the NMR spectrometer, a unique molecular alignment is retained and spectral resolution is recovered. The resulting anisotropic chemical shifts, dipolar and quadrupolar interactions provide valuable information about the orientation of bonds, protein domains, and polypeptides as a whole. Thus, the corresponding spectra can be used to analyze the structure, dynamics, and topology of membrane-associated polypeptides (Das et al. 2015; Gopinath et al. 2015; Itkin et al. 2017; Salnikov et al. 2018).
The figure shows the 2H (a) and 15N solid-state NMR spectra (b) of an amphipathic helical model peptide (Aisenbrey and Bechinger 2004) labeled with 2H3-alanine and a 15N label at a single peptide bond (c). The 2H quadrupolar splitting and 15N chemical shift obtained from these solid-state NMR spectra are a function of the alignment of the alanine Cα-Cβ bond and of a vector close to parallel to the amide 15N-1H bond, respectively, relative to the magnetic field of the NMR spectrometer (Bo). The bonds are highlighted in red and blue in the helical structure (c). This alignment dependence can be used to obtain orientational constraints for the peptide. (d) The tilt/pitch angular pairs of the helix that agree with the 2H quadrupolar splitting are shown in red and those that agree with the 15N chemical shift in blue. Both NMR parameters have to agree with the real peptide alignment which leaves only five possible topologies where the two restraints intersect. Data from well-chosen additional positions results in a unique solution for the peptide topology (Bechinger et al. 2011)
Whereas the 15N chemical shift alone provides an approximate tilt angle of helical domains (Bechinger and Sizun 2003), the combination with 2H solid-state NMR spectra from methyl-deuterated alanines results in accurate tilt and pitch angle information (Fig. 4.3) (Bechinger et al. 2011; Salnikov et al. 2018).
Only few years after the discovery of magainins solid-state NMR experiments on these peptides reconstituted into uniaxially oriented lipid bilayers indicated for the very first time that they adopt stable alignments parallel to the membrane surface (Bechinger et al. 1990, 1991a, b, 1992, 1993). This topology has been confirmed for magainin 2 in all lipid compositions investigated so far (Bechinger 2011), for magainin analogues (Ramamoorthy et al. 2006; Mason et al. 2009), and for several other linear cationic antimicrobial peptides (Resende et al. 2009, 2014; Hayden et al. 2015; Bechinger and Gorr 2017; Sani and Separovic 2018). Furthermore, oriented CD spectra agree with such an alignment of the magainin helix (Ludtke et al. 1994). Fluorescence quenching experiments not only confirm the alignment parallel to the surface but also reveal an interfacial localization of the magainin 2 helix (Matsuzaki et al. 1994). A parallel alignment has also been detected for cecropin P1 (Table 4.1) using ATR FTIR, a topology which was associated with the term “carpet model” (Gazit et al. 1996).
This topology assures that the peptide association is reversible (Bechinger 2011) and contrasts findings for alamethicin which is much more hydrophobic and forms stable transmembrane helical bundles when investigated in DMPC or POPC membranes (North et al. 1995; Bak et al. 2001; Milov et al. 2009; Salnikov et al. 2009b, 2016b). Notably, alamethicin also exhibits pronounced differences in electrophysiological recordings where the single-channel events are well defined and reproducible (Sansom 1993; Bechinger 1997). Notably, even for alamethicin, conditions can be found where it adopts in-plane alignments (He et al. 1996; Salnikov et al. 2010) which underlines the dynamic nature of antimicrobial peptide-lipid interactions involving multiple equilibria (Bechinger 2015). Whereas the alignment of magainin 2 has been found parallel to the membrane surface regardless of membrane lipid composition (Matsuzaki et al. 1994; Bechinger 2011), its relative PGLa (Table 4.1) adopts a much wider range of alignments but only when investigated in bilayers composed of fully saturated fatty acyl chains (Tremouilhac et al. 2006a, b; Salnikov and Bechinger 2011). In DMPC the PGLa tilt angle depends on peptide-to-lipid ratio and membrane hydration (Tremouilhac et al. 2006a, b; Salnikov and Bechinger 2011). A continuous range of tilt angles was observed as a function of hydrophobic thickness in fully saturated PC bilayers (Tremouilhac et al. 2006a, b; Salnikov and Bechinger 2011). However, when studied in phospholipid bilayers carrying unsaturations (such as palmitoyl-oleoyl-phospholipids), also this peptide remains stably aligned parallel to the bilayer surface (Bechinger et al. 1991a, 1998; Bechinger 2011; Salnikov and Bechinger 2011; Strandberg et al. 2012).
6.2 Solid-State NMR Spectroscopy of Lipids
Notably, solid-state NMR spectroscopy has been used not only to investigate the structure, dynamics, and topology of membrane-associated polypeptides (see ultra) but also provides valuable information on the lipids (Bechinger and Salnikov 2012). Because the lipid packing and phase properties are modulated by interactions with peptides, this information has been particularly valuable when the mechanism of antimicrobial peptides has been investigated. 31P solid-state NMR spectra provide information on the macroscopic phase properties of phospholipid membranes or the orientational order of lipid bilayers. They are particularly straightforward to obatin due to 100% natural abundance of this nucleus (Bechinger and Salnikov 2012). Furthermore, 2H solid-state NMR spectroscopy of deuterated fatty acyl chains has been used to characterize the order parameters (Fig. 4.4), hydrophobic thickness, and packing of lipid bilayers (Harmouche and Bechinger 2018). Finally, conformational changes of the phospholipid head groups have been monitored by 2H and 31P solid-state NMR spectroscopy and thereby allowed to quantitatively follow electrostatic interactions at the membrane interface (Scherer and Seelig 1989).
(a) Phosphatidylcholine (POPC) where all 1H of the palmitoyl chain have been exchanged with 2H. Upon hydration, the lipid self-assembles into liposomes that tumble slowly in the NMR magnetic field. (b) The 2H solid-state NMR spectra are composed of quadrupolar splittings from individual CD2 functional groups that sum up into a spectrum of superimposed resonances. (c) The mobility of the CD2 segments increases from the bilayer interface to the hydrophobic membrane interior and results in a decrease in quadrupolar splitting and the order parameters S CD
An amphipathic helix that inserts into the bilayer interface with an alignment parallel to the membrane surface needs to expand the surface at the level of the lipid head groups and in the glycerol region (Matsuzaki et al. 1994). This is paralleled by a loosening of the packing of the hydrophobic region and an increased disorder of the fatty acyl chains (Salnikov et al. 2009a, b; Bortolus et al. 2014). This effect can be even more pronounced when the peptides initially associate with only the outer monolayer, which subsequently allows flip-flop of lipids and peptides across the membrane, thereby relieving the asymmetry-related tension of the outer monolayer (Matsuzaki et al. 1996; Karal et al. 2015; Hasan et al. 2018). The increased disorder at the level of the lipid fatty acyl chains results in the reduction of the membrane thickness (Ludtke et al. 1995; Kim et al. 2009). In other words, while the total volume of the lipid remains constant, in average it expands over an increased area of the bilayer which results in a reduction in hydrophobic thickness.
Pronounced decreases in order parameters especially in the bilayer interior have indeed been observed upon addition of magainin 2, PGLa, and other amphipathic peptides using 2H solid-state NMR of deuterated lipids (Hallock et al. 2002; Salnikov et al. 2009a, b; Grage et al. 2016; Harmouche and Bechinger 2018). Such bilayer disruptive properties have been estimated to extend over 10 nm in diameter (Chen et al. 2003; Mecke et al. 2005).
7 Molecular Dynamics Simulations
Molecular dynamics (MD) simulations is a computer-based numerical method for calculating the motions of atoms and molecules. Based on an empirical potential energy function, the atoms and molecules are allowed to interact for short time intervals and change positions as a result of the instantaneous forces. The trajectories are calculated by numerically solving Newton’s equations of motion for a given system of interacting particles. The empirical potential energy function, also known as a molecular mechanics force field, uses physics-based models to represent the forces that act between the particles, including bonding (bonds, angle, dihedrals) and nonbonded (van der Waals and electrostatic) terms. Using this approach, the conformational space and the time evolution of the system can be visualized at atomic resolution if a force field representing all atoms is used. An alternative is to use a coarse-grained representation, where atoms are grouped into larger entities. This approach permits long simulations of very large systems (Kmiecik et al. 2016; Harpole and Delemotte 2018).
Molecular dynamics simulations can therefore provide atomistic views of how the molecules change conformation over time. With increasing computer power, lower resolution (coarse grain), and better algorithms for exploiting today’s computer architectures, they also reveal how molecules interact and how supramolecular complexes form and evolve. MD simulations have been used, for example, to follow the insertion of hydrophobic peptides into the membrane, where they assemble into transmembrane helical bundles (Tieleman et al. 2002) and how magainin interacts with lipopolysaccharides (Smart et al. 2017). Furthermore, they permitted the visualization of the deformation of the lipid bilayer in the presence of in-plane oriented amphipathic peptides. From the conformational details given by the simulations, it was shown that the positioning of side chains is important for reaching across a bilayer leaflet and to contribute to the formation of water-filled openings (Vacha and Frenkel 2014; Farrotti et al. 2015; Pino-Angeles et al. 2016).
A 5–9 μs all-atom MD calculation shows that the starting structure of tetrameric transmembrane helical bundles of magainin or PGLa is unstable when simulated in 80–120 lipids of DMPC or DMPC/DMPG 3/1(P/L ratio of 3.3–5 mole%) (Pino-Angeles et al. 2016). The peptides exhibit tilted configurations, thereby better representing the topologies and aggregation states found during various biophysical experiments (Matsuzaki et al. 1994; Bechinger 2011). In a related manner, all-atom 100 ns simulations of 1, 2, or 8 peptides in 512 lipids (POPE/POPG 3/1; P/L, 0.5–1.6 mole%) confirm stable in-plane topologies of magainin and pleurocidin (Amos et al. 2016). Although some oligomerization occurs, the formation of pores or supramolecular rearrangement could not be observed within this relatively short time frame.
Finally, the possibility of a double-belt arrangement of peptides oriented parallel to the bilayer plane has been suggested from coarse-grain MD simulations of schematic amphipathic helices (Vacha and Frenkel 2014). Such a model resembles an inversion of the double-belt model that is discussed for apolipoproteins AI particles or related nanoparticles (Gogonea 2015).
From the molecular dynamics point of view, the magainin membrane interactions are characterized by peptides that adopt many different conformations and membrane alignments. Transmembrane alignments have been found unstable, and little direct interactions between the peptides have been observed. Openings can form by stochastic rearrangements of peptides and lipids rather than through well-defined supramolecular assemblies, which could explain the early observations during electrophysiological recordings (Christensen et al. 1988; Duclohier et al. 1989; Cruciani et al. 1991; Watanabe and Kawano 2016). In order to visualize local or global changes in the membrane macroscopic phase or membrane lysis, including the all-or-nothing release observed in dye release experiments (Gregory et al. 2008; Tamba et al. 2010), larger systems would need to be simulated over longer time scales.
Furthermore, a symmetric antiparallel dimer of PGLa has been preassembled and simulated for up to 2 μs using all-atom MD (Ulmschneider et al. 2012). Indeed, dimer formation of membrane-associated antimicrobial peptides is an interesting hypothesis but so far lacks experimental proof, for example, by solid-state NMR distance measurements. Although the GxxxG sequence within the PGLa sequence is suggestive for peptide-peptide interactions, this motif requires a hydrophobic environment to drive dimerization (Russ and Engelman 2000) rather than its experimental orientation along the membrane interface or within a water-filled channel.
8 Lipopeptide Biosurfactants with Antimicrobial Properties
Before establishing a more elaborate model for the action of antimicrobial peptides, it is interesting to mention a range of lipopeptides whose amphiphilic character is assured by a long fatty acyl chain attached to a polar peptidic structures (Ines and Dhouha 2015; Otzen 2017; Wu et al. 2017; Zhao et al. 2017). These surface-active compounds are produced by a wide variety of bacteria, fungi, and yeast and exhibit a high structural diversity (Ines and Dhouha 2015). Furthermore, following their natural templates, ultrashort lipopeptides with antimicrobial activities have been engineered (Mangoni and Shai 2011). In particular, the lipopeptides produced by Bacillus are small cyclic structures of 7–10 amino acids and a β-hydroxy fatty acid with 13–19 carbon atoms (Zhao et al. 2017). According to the peptidic ring structure, the Bacillus lipopeptides are divided into the surfactin, fengycin, and iturin family. They are widely used in agriculture, food, medicine, and feed production due to their antifungal, antibacterial, antitumor, antiviral, and anti-inflammatory activities (Wu et al. 2017; Zhao et al. 2017). Furthermore, they interact with biofilms, have been suggested to be useful in thrombolytic and Alzheimer therapies or to be used to create nanostructures for drug delivery (Wu et al. 2017; Zhao et al. 2017). Many of these activities are thought to be due to the interactions of these biosurfactants with biological membranes (Carrillo et al. 2003; Heerklotz and Seelig 2007). In the context of the molecular shape model, we will focus to present some biophysical work obtained on surfactins and related lipopeptides.
Surfactin is made of seven amino acids (with L- or D-conformation) linked to a fatty acyl chain of C12–C14 that closes the peptide ring by a β-lactone (Wu et al. 2017). Many lipopeptides have been shown to exhibit antimicrobial activities against a range of bacteria (Ines and Dhouha 2015). Importantly, they are some of the most potent and most popular antifungal agents and have been investigated for their anticancer activities (Ines and Dhouha 2015; Wu et al. 2017).
The hydrophobic alkyl chain and the more polar peptidic portion confer an amphiphilic character and thus, in cases where the fatty acyl chain adopts an extended conformation, a pronounced cone shape to the molecule (Otzen 2017). Therefore, these compounds form micelles in aqueous buffer and exhibit surfactant activities such as monolayer formation at the air-water interface (Maget-Dana and Peypoux 1994; Otzen 2017). It should be noted that in contrast to chemical detergents, biosurfactants exhibit a more mosaic-like amphipathic structure (Otzen 2017).
Calcein release from POPC vesicles by surfactin is a cooperative process (index 1.82) (Carrillo et al. 2003). The membrane-perturbing effect of surfactin, measured by calcein release, is attenuated in POPE or cholesterol-containing membranes but accentuated when 25% of DPPC has been mixed into the POPC bilayers (at 25 °C) (Carrillo et al. 2003). Notably as with detergents, the surfactin behavior in aqueous solution has been characterized in terms of CMC (7.5 μM), onset of membrane solubilization (comparatively low when compared to other detergents, Heerklotz and Seelig 2001), and aggregation number (20) (Otzen 2017). The imbalance in the lateral pressure profile at the interface and the hydrophobic portion of the lipid bilayer in the presence of surfactin and chemical detergents has been investigated by 2H solid-state NMR of specifically deuterated POPC membranes (Heerklotz et al. 2004). Whereas C12EO6 and C12EO8 cause the expected disordering of the membrane fatty acyl chains, in the presence of surfactin, the fatty acyl chains tilt, and the head group reorients to accommodate the bulky heptapeptide ring. This difference is probably related to the amphipathic but predominantly hydrophobic character of the peptide moiety which results in a deeper insertion of the peptide ring when compared to the polar groups of the detergents (Heerklotz et al. 2004). To resolve ambiguities about the mechanism how surfactin causes membrane leakage, a series of experiments was performed at non-lytic concentrations correlating data from ITC, 31P solid-state NMR, and leakage assays (Heerklotz and Seelig 2007). This systematic analysis reveals three different mechanisms depending on the surfactin-to-lipid ratio R b. Leakage starts at R b = 0.05 probably by a mechanism where the surfactin accumulates at the outer membrane leaflet and opens pores transiently to equilibrate with the inner side of the bilayer (bilayer couple mechanism). At R b = 0.15, it is suggested that surfactin-rich clusters form which cause leaks and stabilize the hydrophobic etches of those. Finally, membrane solubilization and micelle formation are observed between R b = 0.22 and 0.42. The same experiments provide a membrane partitioning coefficient of 20,000 M−1 (Heerklotz and Seelig 2007). Furthermore, fengycins, lipopeptides sold together with iturins and surfactin for agricultural applications, were investigated in fluorescence lifetime efflux measurements (Patel et al. 2011).
The length of the fatty acyl chain, hydrophobicity, and membrane association of surfactin are directly correlated to its anticancer activities (Wu et al. 2017). Using measurement on lipid monolayers and CD, spectroscopic investigations reveal changes in the membrane insertion process and the proteic structure in the presence of Ca2+ (Maget-Dana and Ptak 1995). Furthermore, these investigations demonstrate that electrostatic contributions and the space occupied by the head group play an important role in surfactin membrane insertion (Maget-Dana and Ptak 1995) although the hydrophobic interactions of the fatty acyl chains as well as other factors remain of major importance (Wu et al. 2017). The gel-to-liquid phase transition of DMPG has been shown to be significantly broadened by surfactin an effect that is enhanced in the presence of Ca2+ (Grau et al. 1999). Because of this observation, a deeper penetration into the membrane upon complexation of Ca2+ with the Glu-1 and Asp-5 residues of the peptide sequence has been suggested (Grau et al. 1999). Surfactin has been incorporated in a wide variety of nanoformulations for materials and biomedical applications (Wu et al. 2017).
Surfactin, iturins, and the closely related bacillomycins and mycosubtilin are of similar built. Iturins are made of circular peptides made of seven L- and D-amino acids connected to a β-amino fatty acid of 14–17 C-atoms. They exhibit strong antifungal but little antibacterial activities. Interestingly, iturin A has been shown to specifically interact with cholesterol and suggested to form specific phospholipid/peptide/cholesterol complexes that are responsible for the measured electrophysiological properties. The latter are strongly dependent on the lipid composition, the physical state of the lipids, and the detailed peptide structure. An interesting observation is that the CMC and the MIC follow the same trend suggesting that large iturin A aggregates are the active component in biological membranes (Maget-Dana and Peypoux 1994). When traces of iturin A are added to black lipid membranes, stepwise conductances, whose characteristics evolve over time, are observed. At P/L ratios above 10−7, the membranes break. Notably iturin A is coproduced with surfactin, and both peptides together exhibit synergistic activities in biological assays (citations in Maget-Dana and Peypoux 1994).
Owing to their membrane activities, several surfactants including iturin A have been shown to exhibit hemolytic and anti-clot-forming activities (Ines and Dhouha 2015). An overview over many more membrane-active surfactants’ interesting biomedical and technical activities is presented in Ines and Dhouha (2015).
9 The Molecular Shape Concept Explains the Many Different Supramolecular Arrangements of AMPs and Lipids
A number of seemingly contradictory models have been suggested to explain the mechanism of action and the interaction of antimicrobial peptides with membranes. The most cited are the formation of toroidal pores (Ludtke et al. 1996; Matsuzaki 1998), a dense “carpet” of peptides covering the membrane surface that causes lysis (Shai 1999), or small aggregates without specific structure within the membrane (Jenssen et al. 2006). Whereas on the one hand, occasionally channel-like events are recorded in electrophysiological experiment (Christensen et al. 1988; Duclohier et al. 1989; Cruciani et al. 1991), at high peptide concentrations, worm-like structures, disk-shaped particles, or micelles have been observed (Hallock et al. 2002; Bechinger and Lohner 2006; Wolf et al. 2017). This wide variety of observations can be taken into consideration by the differential shape of lipids (Fig. 4.5) and the resulting supramolecular phases which are a function of peptide concentration in the membrane and other environmental parameters (Bechinger 2009). The concept has been developed early on to rationalize the macroscopic phase transitions of lipids (Israelachvili et al. 1980). Because the phosphatidylcholine head groups and two fatty acyl chains expand laterally over an area that is about equivalent at the level of the membrane interface and the hydrophobic interior, the PC molecules when part of a membrane can be described by a cylindrical shape (Fig. 4.5a). In contrast, the head group of phosphatidylethanolamine is much smaller which results in a truncated inverted cone-shaped molecule (Fig. 4.5b), whereas lysolipids with only one fatty acyl chain (or detergents) are best represented by a cone (Fig. 4.5c). For a more quantitative treatment, the critical packing parameter comparing the optimal surface area at the carbon-water interface (a o), the optimal chain length (l c), and the hydrocarbon volume (v) is related by the packing parameter v/l c a o (Israelachvili et al. 1980). When these geometrical shapes are assembled into supramolecular aggregates, the PC cylinders line up side by side in a phospholipid bilayer (Fig. 4.5f), the cones form micellar assemblies (Fig. 4.5i), and the PE lipids at higher temperatures tend to form hexagonal II phases (Fig. 4.5h). When PE or detergents are forced to be part of a planar lipid bilayer, they are under curvature elastic stress where interactions with the opposite monolayer maintain the bilayer arrangement. However, a spontaneous curvature has been defined for each lipid which takes into account the differences in lateral cross section at the membrane interface when compared to the hydrophobic interior (Kollmitzer et al. 2013). Thus, the intrinsic curvature of POPC is around 0, of POPE it is −0.32, and for lyso-PE, a value of +0.18 has been determined (Kollmitzer et al. 2013; Leber et al. 2018). It should be noted that the shape is not only determined by the van der Waals contacts but can be modulated by other interactions. For example, repulsive electrostatic interactions at the head group level increase its optimal surface area, resulting in a more cone-shaped molecule and a positive curvature (Israelachvili et al. 1980).
The molecular shape concept and how molecules assemble into supramolecular arrangements. When the lateral cross sections of the lipid head groups and at the level of the fatty acyl chains are compared to each other, phosphatidylcholines and phosphatidylglycerol adopt cylindrical shapes (a), phosphatidylethanolamine with its reduced head group size corresponds to a truncated inverted cone (b), and lysolipids and detergents resemble a cone (c). The corresponding intrinsic curvatures J o of these lipids are zero, negative, and positive, respectively (Kollmitzer et al. 2013; Leber et al. 2018). (d) A highly charged amphipathic peptide partitions into the head group region without filling the hydrophobic region. Thereby it resembles a truncated cone and exerts pronounced positive curvature strain. (e) Next to such an amphipathic peptide, the lipids adjust their shape by adding gauche conformations within the alkyl chain, thereby increasing the disorder of their fatty acyl chains. The comparison with unperturbed cylinders illustrates how these conformational changes are accompanied by membrane thinning. (f) Cylindrical lipids self-assemble into stable bilayers where by being soft they can adjust to peptides or other external stimuli (Bechinger 2015). (g) When the concentration of cone-shaped molecules increases locally, phase transitions and membrane openings occur. (h) Inverted cone-shaped molecules self-assemble into hexagonal II phases. (i) Cone shapes self-assemble into micelles. In panels D–G, the helices are schematized as yellow/orange cylinders (side view) and circles (front view)
An amphipathic helix that resides at the membrane interface with the long axis approximately parallel to the membrane surface (Bechinger 2009) or surfactin, a cyclic peptide with a long fatty acyl chain (Zhao et al. 2018), occupies a much larger area at the level of the interface without filling the corresponding space at the level of the fatty acyl chain (Fig. 4.5d). This induces considerable positive curvature strain and requires major rearrangement of the lipids in compensation. This shape helps to create the line tension required for membrane pores to form (Hall et al. 2014; Henderson et al. 2016).
Thus, in contrast to the transmembrane alamethicin helix, which is modeled by a cylinder, the shape of these amphipathic cationic peptides has the properties of a large truncated cone (Fig. 4.5d). Notably, the presence of PE, which has intrinsically an inverted cone-shaped and negative curvature (Fig. 4.5b), compensates for the wedge-like properties of the in-plane oriented helices, thereby stabilizing the lipid bilayer arrangement (Batenburg et al. 1988; Hallock et al. 2002). Recently the difference in “molecular shape” of magainin and melittin has been included in a model that explains the distinct activities of these peptides (Paterson et al. 2017).
10 The “Molecular Shape” of Membrane Constituents Adapts
When interacting with the membranes, many amphipathic peptides, including magainins, fold into helical conformations and intercalate into the lipid head group region. In this manner, they act as a spacer pushing apart the lipids. This results in positive membrane curvature strain and has been rationalized by a cone molecular shape of the in-plane oriented amphipathic helix (Fig. 4.5d) (Bechinger 2009). However, the peptide does not fill the hydrophobic region completely; thus the lipids respond by increased trans-gauche isomerization or chain interdigitation (Bechinger and Lohner 2006). The details of the conformational changes of the lipids are a function of the detailed peptide alignment and penetration depth. The latter depend on the three-dimensional distribution of charges, hydrophobic side chains, i.e., the resulting hydrophobic moment and amino acid distribution. Because both the peptide and the lipids exhibit considerable conformational flexibility, the molecular shape of these membrane constituents is not fully determined, but they adjust to external forces within the supramolecular assembly. Thus, in the neighborhood of the cone of an amphipathic peptide, a PC which is cylindrical in a pure lipid bilayer adopts a more inverted cone-shaped structure, thereby stabilizing the bilayer (Fig. 4.5e). Furthermore, the peptides are not stiff helices and they can adjust their penetration depths. Their alignment relative to the membrane surface can be modulated by a wide range of interaction contributions (Bechinger 1996; Harmouche and Bechinger 2018). All these adjustments correspond to modulations of their “molecular shape” (Fig. 4.5e). The very details of the peptide-lipid supramolecular arrangement thus depend on the peptide sequence, its conformation and resulting hydrophobic moment, the peptide-to-lipid ratio, as well as the detailed lipid composition (Bechinger and Lohner 2006; Bechinger 2009), which are in a delicate balance making predictions on how the macroscopic ensemble behaves rather difficult.
11 Soft Membranes Adapt and Respond, Also Transiently
Not only the peptides are highly dynamic with considerable conformational and topological freedom (Cheng et al. 2009, 2011), but also the liquid crystalline lipid bilayer has the capacity to compensate for external influences before their barrier function breaks down. Together they form soft supramolecular assemblies which can change thickness, morphology, and macroscopic phase properties globally or locally. Therefore, in the presence of antimicrobial peptides, Soft Membranes Adapt and Respond, also Transiently, a concept that makes up the SMART model for antimicrobial or other membrane-active peptides (Bechinger 2015). The model thereby extends on the molecular shape concept by taking into account that the lipids have the capacity to respond to the membrane-disruptive properties of the peptides which themselves adjust their properties when associating with lipid membranes. However, once a critical concentration is reached, phase transitions of the membrane are observed locally or globally. Due to lateral diffusion, weak peptide-peptide interactions, transient lipid phase separation, etc., the local peptide concentrations can vary which can explain how stochastic fluctuations of peptide density result in the transient pore events observed in, for example, electrophysiological recordings (Christensen et al. 1988; Duclohier et al. 1989; Cruciani et al. 1991; Watanabe and Kawano 2016). Transient openings also occur during membrane crossing when the peptide density equilibrates between the outer and the inner leaflet of the bilayer (Matsuzaki et al. 1995a, b; Tamba et al. 2010; Wheaten et al. 2013). The different supramolecular morphologies of the SMART model can be nicely represented by phase diagrams where regions corresponding to bilayer, wormholes, tubular structures, bicelle, micelle, or hexagonal phases are represented as a function of the peptide-to-lipid ratio, the detailed membrane composition, temperature, hydration, salt, pH, and other environmental factors (Bechinger and Lohner 2006; Bechinger 2011). Notably, when bacteria are exposed to AMPs, the peptides diffuse to the cell, across the cell wall and other cellular barriers, before they interact with the cellular membranes. Therefore, the peptide local concentrations vary over time, and intermediate states have been observed for magainin 2 where membranes temporarily lyse and recover (Hall et al. 2014).
At low peptide concentrations, the bilayer structure is maintained; however, transient openings may form stochastically due to the lateral diffusion of peptides and lipids concomitant with density alterations. Notably, whereas in most models the membranes mechanically break or water-filled pores form, it seems also possible that the physicochemical properties change in such a manner to allow diffusion of ions through a less densely packed lipid phase or along phase boundaries which form when the peptides are responsible for the lateral phase separation of membrane constituents (Cruzeiro-Hansson and Mouritsen 1988; Jean-Francois et al. 2008; Gallaher et al. 2010; Aisenbrey and Bechinger 2014). Furthermore, it has been pointed out that membrane morphological changes, including the thinning of the lipid bilayer, which has been shown to occur in the presence of magainin 2 (Ludtke et al. 1995), are associated with changes in membrane capacity and concomitantly electrical currents (Heimburg 2012; Laub et al. 2012).
At higher peptide concentrations, the system enters non-bilayer phases (at least locally) which come along with more stable openings (Gregory et al. 2008) and macroscopic phase transitions of the membranes (Fig. 4.5g–i) (Bechinger 2009). The threshold concentrations that have been identified in some biophysical studies thus represent boundaries in phase diagrams (Bechinger and Lohner 2006; Bechinger 2011). The loss of bilayer integrity has been proposed by the “carpet model” (Shai 1999), whereas stochastic and transient openings occur when peptides orient along the membrane surface at low peptide-to-lipid ratios (Christensen et al. 1988; Duclohier et al. 1989; Cruciani et al. 1991).
It should be noted that the peptide concentration at the membrane surface may be different by orders of magnitude from those in bulk solution because the cationic peptides are attracted to the surface of negatively charged membranes (Wenk and Seelig 1998; Wieprecht et al. 1999a, b). Thereby, the apparent partitioning coefficients are also much increased, which provides one explanation why these AMPs kill bacteria which expose a highly negative surface charge. In contrast, the eukaryotic host cells are neutral in charge at their outside monolayer (Matsuzaki et al. 1991; Wenk and Seelig 1998; Bechinger 2004; Klocek and Seelig 2008; Lohner 2009). Electrostatic interactions also play a role when AMPs cause the lateral phase separation of lipids (Mason et al. 2006; Voievoda 2014), when the peptides arrange in mesophase structures along the membrane surface (Aisenbrey and Bechinger 2014), or when peripheral membrane proteins are repelled from the bilayer surface due to a more positive surface charge in the presence of cationic AMPs (Wenzel et al. 2014).
Notably the molecular shape concept and the SMART model have allowed the design of novel peptide and non-peptidic mimetics of AMPs with high antimicrobial efficiency that are cationic or amphipathic and partition into the interface without filling the hydrophobic volume to the same extent such as short peptides (Oyston et al. 2009; Schweizer 2009; Hadley and Hancock 2010; Kindrachuk and Napper 2010; Liu et al. 2010; Mangoni and Shai 2011; Chou et al. 2016; Ahn et al. 2017), foldamers (Porter et al. 2002; Patch and Barron 2003; Kuroda and DeGrado 2005; Violette et al. 2006; Makovitzki et al. 2008; Scott et al. 2008; Rotem and Mor 2009; Palermo and Kuroda 2010), polymers (Rank et al. 2017), and small organic molecules (Ghosh et al. 2014).
12 Dimers of Antimicrobial Peptides
In the context of the SMART model, an interesting question arises how the oligomerization of peptides along the membrane surface would affect their activity (Bechinger 1999). Figure 4.6a, b shows possible mechanisms how the membrane can adjust to two tightly packed side-by-side helices. On the one hand, one could imagine a situation where the lipids of the peptide-bearing monolayer compensate for the packing deficiency underneath in-plane oriented helices. Because they are excluded from the peptide-peptide interface, they have to move in exclusively from the opposite helical side affecting the lipids that are neighboring the peptide even more significantly (Fig. 4.6a). On the other hand, the opposite leaflet of the bilayer could be used to cover the hydrophobic face of the peptide dimer (Fig. 4.6b). In yet another situation, a mesophase structure of many helices may form along the bilayer surface (Aisenbrey and Bechinger 2014). In this case the peptides are separated by one or few lipids only (Fig. 4.1c). Notably, previously the membrane-disruptive properties of magainin 2 have been shown to extend over a radius of 5 nm (Chen et al. 2003; Mecke et al. 2005) which involves many lipids of an estimated diameter of about 0.8 nm. Within a mesophase supramolecular arrangement, the close proximity of helices thus results in a concerted destabilization of the membrane (Fig. 4.1c).
The figure shows how amphipathic helix dimers potentially interact with membranes. (a) The disruptive properties of two side-by-side helices should be more pronounced and/or (b) result in the approach of the opposite monolayer to interact with the hydrophobic face of the peptides. (c) Membrane openings form by bilayer disruptions. (d) The two helices of heterodimers can exhibit very different properties and affect the membrane similar to a monomer. Helices are schematized as cylinders and circles, where the combination of different colors is indicative of a heterodimer
Only a few dimers have been identified in nature and have been investigated by biophysical methods. Distinctin is composed of two different polypeptide chains that are linked near their carboxy-terminus by a cystine bond. In solution, the peptide forms a compact four-helix dimer of heterodimers (Raimondo et al. 2005). When interacting with membranes, the solution structure unfolds. Whereas the 25-residue chain 2 partitions into the membrane in a manner similar to magainin with a stable alignment parallel to the membrane surface, chain 1 encompassing 22 residues associates more loosely with the lipid bilayer (Fig. 4.6d) (Resende et al. 2009; Verardi et al. 2011). The slightly increased antimicrobial activity of the dimer when compared to the monomers (Dalla Serra et al. 2008) is thought to be due to the better resistance to proteolysis (Raimondo et al. 2005).
More recently a natural homodimer of twice 24 residues has been described and its structure investigated in solution by NMR spectroscopy. Interestingly doubling of some NMR cross peaks are indicative of slight asymmetries of the fold (Verly et al. 2017). The global structure shows a tightly packed coiled coil where the homodimer is stabilized by hydrophobic interactions encapsulating a hydrophilic cluster made up from the two chains (Verly et al. 2017). The dimer is considerably more active than the monomers (Verly et al. 2017) suggesting that the homodimer is more membrane-disruptive than two monomers (illustrated in Fig. 4.6c). Additional investigations of the homotarsinin membrane interactions are ongoing.
Notably a cystine-linked magainin 2 dimer was also found to exhibit enhanced membrane permeabilization and antimicrobial activities (Dempsey et al. 2003). The higher efficiency was correlated to an enhanced association with negatively charged bilayers and a reduction in the concentration dependence in the dimer when compared to the monomer. On the one hand, the formation of magainin oligomers had been suggested early on; FRET measurements in the presence of membranes did not yield evidence for dimer or higher oligomer formation (Clark et al. 2011). On the other hand, NMR structural investigations of 5 mM magainin in the presence of 0.5 mM DLPC showed a dimer arrangement of the peptide (Wakamatsu et al. 2002). In this densely packed peptide-lipid supramolecular aggregate, aromatic interactions involve mostly the F5Y and F16W sites, which had been introduced artificially for better assignment, and these amino acids are involved in the dimer interface (Wakamatsu et al. 2002). In combination with magainin monomers, the dimer stabilizes the pore formation in egg-PG membranes (Hara et al. 2001). Notably whereas a dimer linked through a carboxy-terminal lysine extension was considerably more active than the monomer, the amino-terminal linkage through glutamic acid has no effect (Lorenzon et al. 2016).
Furthermore, the activities of homodimers of magainin 2 or PGLa carrying GGC extensions and of a heterodimeric hybrid made of the two peptides were tested (Nishida et al. 2007). Whereas the antimicrobial activities of the heterodimer were about the same as those of an equimolar mixture of monomers, the calcein release activity was somewhat stronger for the dimers. However, both the hybrid and the mixture are much more potent that the individual peptides alone. In a recent extension of this previous study, the (PGLa-GGC)2 and (magainin-GGC)2 homodimers as well as the magainin-GGC/PGLa-GGC heterodimer showed increased calcein release activities from POPE/POPG 3/1 mole/mole liposomes when compared to unmodified peptides in mixtures (Leber et al. 2018). In POPC/cholesterol 3/1 mole/mole mixtures, only the PGLa-homodimer and the PGLa-magainin heterodimer but not unlinked peptides showed significant release activities at all (Leber et al. 2018). These findings are in line with increased membrane-perturbing properties of larger peptide aggregates (illustrated in Fig. 4.6a–c) (Dempsey et al. 2003; Lorenzon et al. 2016; Verly et al. 2017).
13 Synergistic Enhancement of the Activities of Antimicrobial Peptides
Synergistic enhancement of antimicrobial activities has been described (McCafferty et al. 1999; Acar 2000) and includes mixtures of peptides with conventional antibiotics (Chou et al. 2016; Bolosov et al. 2017; Kim et al. 2017; Payne et al. 2017; Rank et al. 2017; Sakoulas et al. 2017). Furthermore, combinations of different peptides from the dermaseptin or the bacteriocin family (Mor et al. 1994; McCafferty et al. 1999), magainin 2 and PGLa (Vaz Gomes et al. 1993), or magainin and the cyclic β-sheet tachyplesin I sequence have been shown to interact in a synergistic manner (Kobayashi et al. 2001). Here we will shortly summarize findings made with magainin 2 and PGLa which have recently been reviewed in more detail (Marquette and Bechinger 2018). This mixture is of particular interest because synergism has been observed in antimicrobial assays but also when model membranes are tested (Westerhoff et al. 1995; Matsuzaki et al. 1998; Leber et al. 2018), suggesting that the mode of action should reveal itself when studying the membrane interactions. In cellular assays, it is also possible that one component helps to path the way of the second component to its active site. In this context it is noteworthy that synergism in calcein release experiments was more pronounced for membranes with high negative intrinsic curvature such as POPE/POPG 3/1 mole/mole (Leber et al. 2018). Furthermore, these peptides naturally occur as a mixture in the skin of Xenopus laevis frogs, suggesting that our conventional approach to separate such components to analyze each one individually should be reconsidered.
Early on Matsuzaki et al. suggested that the formation of magainin pores is slow, but once formed, they are more stable than those of PGLa (Matsuzaki et al. 1998). It was also suggested that synergistic vesicle leakage results from optimizing both pore size and distribution among the liposomes present in the suspension (Patel et al. 2014). The openings should be large enough and at the same time abundant in order for all the entrapped dye being released. Such more general concepts are nicely complemented by experiments aiming to reveal the mechanisms of synergism at a molecular, even at an atomistic level.
Solid-state NMR investigations show that in equimolar mixtures and in bilayers whose lipid composition resembles that of membranes that occur in nature, PGLa and magainin exhibit an alignment parallel to the membrane surface (Salnikov and Bechinger 2011; Strandberg et al. 2013; Glattard et al. 2016; Salnikov et al. 2016a). Thus, the helix topology does not change much when compared to investigations of magainin or PGLa individually (Bechinger 2011; Salnikov and Bechinger 2011). It should be mentioned that the PGLa behavior is somewhat different in fully saturated membranes (Salnikov and Bechinger 2011; Strandberg et al. 2013; Harmouche and Bechinger 2018). However, here we focus on studies that were obtained with more biological lipid compositions, carrying an unsaturation. In dye release experiments, mutating the carboxyterminal residues of magainin showed some effect in reducing the synergistic activity, whereas modifying F5W was neutral (Matsuzaki et al. 1998). Thus, when replacing the negative E19 and the carboxy-terminus of magainin 2, synergism is abolished (Zerweck et al. 2017). Notably, amidation of the magainin 2 carboxyterminus has been shown to increase the activity of the peptide (Cuervo et al. 1988), but within experimental error, the combination with PGLa exhibits a similar degree of synergism (Marquette et al. 2015; Glattard et al. 2016).
When the PGLa sequence was modified, the positively charged K15 and K19 sites had a favorable synergistic effect (Zerweck et al. 2017). Furthermore, the G7, G11, and L18 positions of PGLa are important for the synergistic enhancement of activities between the two peptides (Zerweck et al. 2017). Cross-linking experiments with PGLa and magainin 2 both carrying a GGC extensions indicate that in egg-PC/PG 1/1 mole/mole lipid membranes, parallel dimers preferentially form (Hara et al. 2001).
From fluorescence binding experiments, energies were derived that suggest favorable interactions when magainin and PGLa are added to egg-PG membranes (Matsuzaki et al. 1998). However, FRET experiments do not reveal strong, long-lasting contacts between the peptides when associated with POPE/POPG 3/1 or POPC/POPS 3/1 membranes (Marquette et al. 2015). When investigated by a combination of ITC and dynamic light scattering, an additional exothermic contribution in the presence of both peptides was attributed to the agglutination of the liposomes (Marquette and Bechinger 2018) similar to studies with other amphipathic helical peptides (Marquette et al. 2010; Vermeer et al. 2016). Such processes involving the lipids and/or changes in the supramolecular assembly of peptides and lipids also contribute to the total interaction energies (Bechinger 1996; Harmouche and Bechinger 2018). Considering such supramolecular changes, it should also be mentioned that a reduced bilayer repeat distance has been observed when both peptides are present but not with PGLa alone (Grage et al. 2016).
14 Conclusions
During three decades of research, we have moved from a textbook view where AMPs form pores in the shape of transmembrane helical bundles to a more complex vision where lipids play an essential role (Bechinger et al. 1991a, b; Pouny et al. 1992). Thus, in order to understand the mechanism of action not only of AMPs alone but also of their synergism, an in-depth understanding of the interactions within the supramolecular lipid-peptide architectures is required (Bechinger and Lohner 2006; Imura et al. 2008; Kim et al. 2009; Aisenbrey and Bechinger 2014). The molecular shape and SMART models (Bechinger 2009; 2015) provide valuable concepts from where to further explore these activities, where new technological approaches reveal how structure, topology, dynamics, and interactions evolve in a spatiotemporal manner.
Abbreviations
- Aib:
-
α-Aminobutyric acid
- AMP:
-
Antimicrobial peptide
- ATR FTIR:
-
Attenuated total reflection Fourier transform infrared
- CD:
-
Circular dichroism
- CL:
-
Cardiolipin
- CMC:
-
Critical micelle concentration
- DLPC:
-
1,2-Lauroyl-sn-glycero-3-phosphocholine
- DMPC:
-
1,2-Dimyristoyl-sn-glycero-3-zhosphocholine
- DMPG:
-
1,2-Dimyristoyl-sn-glycero-3-phospho-(1′-rac-glycerol)
- DOPC:
-
1,2-Dioleoyl-sn-glycero-3-phosphocholine
- DOPG:
-
1,2-Dioleoyl-sn-glycero-3-phospho-(1′-rac-glycerol)
- DPC:
-
Dodecylphosphocholine
- GUV:
-
Giant unilamellar vesicle
- ITC:
-
Isothermal titration calorimetry
- LUV:
-
Large unilamellar vesicle
- MD:
-
Molecular dynamics
- MIC:
-
Minimal inhibitory concentration
- NMR:
-
Nuclear magnetic resonance
- PC:
-
Phosphatidylcholine
- PE:
-
Phosphatidylethanolamine
- PG:
-
Phosphatidylglycerol
- POPC:
-
1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine
- POPE:
-
1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine
- POPG:
-
1-Palmitoyl-2-oleoyl-sn-glycero-3-phospho-(1′-rac-glycerol)
- POPS:
-
1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphoserine
- SMART:
-
Soft Membranes Adapt and Respond, also Transiently
References
Acar JF (2000) Antibiotic synergy and antagonism. Med Clin N Am 84:1391–1406
Agerberth B, Gunne H, Odeberg J, Kogner P, Boman HG, Gudmundsson GH (1995) FALL-39, a putative human peptide antibiotic, is cysteine-free and expressed in bone marrow and testis. Proc Natl Acad Sci U S A 92:195–199
Ahn M, Gunasekaran P, Rajasekaran G, Kim EY, Lee SJ, Bang G, Cho K, Hyun JK, Lee HJ, Jeon YH, Kim NH, Ryu EK, Shin SY, Bang JK (2017) Pyrazole derived ultra-short antimicrobial peptidomimetics with potent anti-biofilm activity. Eur J Med Chem 125:551–564
Aisenbrey C, Bechinger B (2004) Tilt and rotational pitch angles of membrane-inserted polypeptides from combined 15N and 2H solid-state NMR spectroscopy. Biochemistry 43:10502–10512
Aisenbrey C, Bechinger B (2014) Molecular packing of amphipathic peptides on the surface of lipid membranes. Langmuir 30:10374–10383
Amos ST, Vermeer LS, Ferguson PM, Kozlowska J, Davy M, Bui TT, Drake AF, Lorenz CD, Mason AJ (2016) Antimicrobial peptide potency is facilitated by greater conformational flexibility when binding to gram-negative bacterial inner membranes. Sci Rep 6:37639
Arnusch CJ, Albada HB, van Vaardegem M, Liskamp RMJ, Sahl HG, Shadkchan Y, Osherov N, Shai Y (2012) Trivalent ultrashort lipopeptides are potent pH dependent antifungal agents. J Med Chem 55:1296–1302
Avitabile C, D’Andrea LD, Romanelli A (2014) Circular dichroism studies on the interactions of antimicrobial peptides with bacterial cells. Sci Rep 4:4293
Bak M, Bywater RP, Hohwy M, Thomsen JK, Adelhorst K, Jakobsen HJ, Sorensen OW, Nielsen NC (2001) Conformation of alamethicin in oriented phospholipid bilayers determined by N-15 solid-state nuclear magnetic resonance. Biophys J 81:1684–1698
Barns KJ, Weisshaar JC (2013) Real-time attack of LL-37 on single Bacillus subtilis cells. Biochim Biophys Acta 1828:1511–1520
Barns KJ, Weisshaar JC (2016) Single-cell, time-resolved study of the effects of the antimicrobial peptide alamethicin on Bacillus subtilis. Biochim Biophys Acta 1858:725–732
Batenburg AM, van Esch JH, De Kruijff B (1988) Melittin-induced changes of the macroscopic structure of phosphatidylethanolamines. Biochemistry 27:2324–2331
Bechinger B (1996) Towards membrane protein design: pH-sensitive topology of histidine-containing polypeptides. J Mol Biol 263:768–775
Bechinger B (1997) Structure and functions of channel-forming polypeptides: magainins, cecropins, melittin and alamethicin. J Membr Biol 156:197–211
Bechinger B (1999) The structure, dynamics and orientation of antimicrobial peptides in membranes by multidimensional solid-state NMR spectroscopy. Biochim Biophys Acta 1462:157–183
Bechinger B (2004) Membrane-lytic peptides. Crit Rev Plant Sci 23:271–292
Bechinger B (2005) Detergent-like properties of magainin antibiotic peptides: a 31P solid-state NMR study. Biochim Biophys Acta 1712:101–108
Bechinger B (2009) Rationalizing the membrane interactions of cationic amphipathic antimicrobial peptides by their molecular shape. Cur Opin Colloid Interface Sci Surfactant 14:349–355
Bechinger B (2011) Insights into the mechanisms of action of host defence peptides from biophysical and structural investigations. J Pept Sci 17:306–314
Bechinger B (2015) The SMART model: soft membranes adapt and respond, also transiently, to external stimuli. J Pept Sci 21:346–355
Bechinger B, Gorr SU (2017) Antimicrobial peptides: mechanisms of action and resistance. J Dent Res 96:254–260
Bechinger B, Lohner K (2006) Detergent-like action of linear cationic membrane-active antibiotic peptides. Biochim Biophys Acta 1758:1529–1539
Bechinger B, Salnikov ES (2012) The membrane interactions of antimicrobial peptides revealed by solid-state NMR spectroscopy. Chem Phys Lipids 165:282–301
Bechinger B, Sizun C (2003) Alignment and structural analysis of membrane polypeptides by 15N and 31P solid-state NMR spectroscopy. Concepts Magn Reson 18A:130–145
Bechinger B, Shon K, Eck H, Zasloff M, Opella SJ (1990) NMR studies of magainin peptide antibiotics in membranes. Biol Chem Hoppe Seyler 371:758–758
Bechinger B, Kim Y, Chirlian LE, Gesell J, Neumann JM, Montal M, Tomich J, Zasloff M, Opella SJ (1991a) Orientations of amphipathic helical peptides in membrane bilayers determined by solid- state NMR spectroscopy. J Biomol NMR 1:167–173
Bechinger B, Zasloff M, Opella SJ (1991b) Solid state NMR of magainin and PGLa peptide antibiotics in bilayers. J Cell Biochem Suppl 15G:84
Bechinger B, Zasloff M, Opella SJ (1992) Structure and interactions of magainin antibiotic peptides in lipid bilayers: a solid-state NMR investigation. BiophysJ 62:12–14
Bechinger B, Zasloff M, Opella SJ (1993) Structure and orientation of the antibiotic peptide magainin in membranes by solid-state NMR spectroscopy. Protein Sci 2:2077–2084
Bechinger B, Zasloff M, Opella SJ (1998) Structure and dynamics of the antibiotic peptide PGLa in membranes by multidimensional solution and solid-state NMR spectroscopy. Biophys J 74:981–987
Bechinger B, Ruysschaert JM, Goormaghtigh E (1999) Membrane helix orientation from linear dichroism of infrared attenuated Total reflection spectra. Biophys J 76:552–563
Bechinger B, Resende JM, Aisenbrey C (2011) The structural and topological analysis of membrane-associated polypeptides by oriented solid-state NMR spectroscopy: established concepts and novel developments. Biophys Chem 153:115–125
Bolen EJ, Holloway PW (1990) Quenching of tryptophan fluorescence by brominated phospholipid. Biochemistry 29:9638–9643
Bolosov IA, Kalashnikov AA, Panteleev PV, Ovchinnikova TV (2017) Analysis of synergistic effects of antimicrobial peptide arenicin-1 and conventional antibiotics. Bull Exp Biol Med 162:765–768
Boman HG (1995) Peptide antibiotics and their role in innate immunity. Annu Rev Immunol 13:61–92
Bortolus M, Dalzini A, Toniolo C, Hahm KS, Maniero AL (2014) Interaction of hydrophobic and amphipathic antimicrobial peptides with lipid bicelles. J Pept Sci 20:517–525
Brown LR (1979) Use of fully deuterated micelles for conformational studies of membrane proteins by high resolution 1H nuclear magnetic resonance. Biochim Biophys Acta 557:135–148
Bruch MD, Dhingra MM, Gierasch LM (1991) Side chain-backbone hydrogen bonding contributes to helix stability in peptides derived from an alpha-helical region of carboxypeptidase A. Proteins Struct Funct Genet 10:130–139
Cao P, Yang Y, Uche FI, Hart SR, Li WW, Yuan C (2018) Coupling plant-derived cyclotides to metal surfaces: an antibacterial and antibiofilm study. Int J Mol Sci 19:E793
Caputo GA, London E (2003) Using a novel dual fluorescence quenching assay for measurement of tryptophan depth within lipid bilayers to determine hydrophobic alpha-helix locations within membranes. Biochemistry 42:3265–3274
Cardoso MH, de Almeida KC, Candido ES, Murad AM, Dias SC, Franco OL (2017) Comparative NanoUPLC-MS(E) analysis between magainin I-susceptible and -resistant Escherichia coli strains. Sci Rep 7:4197
Carrillo C, Teruel JA, Aranda FJ, Ortiz A (2003) Molecular mechanism of membrane permeabilization by the peptide antibiotic surfactin. Biochim Biophys Acta 1611:91–97
Chang S, Sievert DM, Hageman JC, Boulton ML, Tenover FC, Downes FP, Shah S, Rudrik JT, Pupp GR, Brown WJ, Cardo D, Fridkin SK, Staphylococcus V-R (2003) Infection with vancomycin-resistant Staphylococcus aureus containing the vanA resistance gene. N Engl J Med 348:1342–1347
Chen FY, Lee MT, Huang HW (2003) Evidence for membrane thinning effect as the mechanism for peptide-induced pore formation. Biophys J 84:3751–3758
Cheng JTJ, Hale JD, Elliot M, Hancock REW, Straus SK (2009) Effect of membrane composition on antimicrobial peptides aurein 2.2 and 2.3 from Australian Southern Bell Frogs. Biophys J 96:552–565
Cheng JTJ, Hale JD, Elliott M, Hancock REW, Straus SK (2011) The importance of bacterial membrane composition in the structure and function of aurein 2.2 and selected variants. Biochim Biophys Acta 1808:622–633
Choi H, Rangarajan N, Weisshaar JC (2016) Lights, camera, action! Antimicrobial peptide mechanisms imaged in space and time. Trends Microbiol 24:111–122
Choi H, Yang Z, Weisshaar JC (2017) Oxidative stress induced in E. coli by the human antimicrobial peptide LL-37. PLoS Pathog 13:e1006481
Chou S, Shao C, Wang J, Shan A, Xu L, Dong N, Li Z (2016) Short, multiple-stranded beta-hairpin peptides have antimicrobial potency with high selectivity and salt resistance. Acta Biomater 30:78–93
Christensen B, Fink J, Merrifield RB, Mauzerall D (1988) Channel-forming properties of cecropins and related model compounds incorporated into planar lipid membranes. Proc Natl Acad Sci USA 85:5072–5076
Clark KS, Svetlovics J, McKeown AN, Huskins L, Almeida PF (2011) What determines the activity of antimicrobial and cytolytic peptides in model membranes. Biochemistry 50:7919–7932
Cruciani RA, Barker JL, Zasloff M, Chen HC, Colamonici O (1991) Antibiotic magainins exert cytolytic activity transformed cell lines through channel formation. Proc Natl Acad Sci U S A 88:3792–3796
Cruzeiro-Hansson L, Mouritsen OG (1988) Passive ion permeability of lipid membranes modelled via lipid-domain interfacial area. Biochim Biophys Acta 944:63–72
Cuervo JH, Rodriguez B, Houghten RA (1988) The magainins: sequence factors relevant to increased antimicrobial activity and decreased hemolytic activity. Pept Res 1:81–86
Dalla Serra M, Cirioni O, Vitale RM, Renzone G, Coraiola M, Giacometti A, Potrich C, Baroni E, Guella G, Sanseverino M, De LS, Scalise G, Amodeo P, Scaloni A (2008) Structural features of distinctin affecting peptide biological and biochemical properties. Biochemistry 47:7888–7899
Das N, Dai J, Hung I, Rajagopalan MR, Zhou HX, Cross TA (2015) Structure of CrgA, a cell division structural and regulatory protein from Mycobacterium tuberculosis, in lipid bilayers. Proc Natl Acad Sci U S A 112:E119–E126
del Rio Martinez JM, Zaitseva E, Petersen S, Baaken G, Behrends JC (2015) Automated formation of lipid membrane microarrays for ionic single-molecule sensing with protein nanopores. Small 11:119–125
Dempsey CE, Ueno S, Avison MB (2003) Enhanced membrane permeabilization and antibacterial activity of a disulfide-dimerized magainin analogue. Biochemistry 42:402–409
Diamond G, Beckloff N, Weinberg A, Kisich KO (2009) The roles of antimicrobial peptides in innate host defense. Curr Pharm Des 15:2377–2392
Duclohier H, Molle G, Spach G (1989) Antimicrobial peptide magainin I from xenopus skin forms anion-permeable channels in planar lipid bilayers. Biophys J 56:1017–1021
Eddy MT, Su Y, Silvers R, Andreas L, Clark L, Wagner G, Pintacuda G, Emsley L, Griffin RG (2015) Lipid bilayer-bound conformation of an integral membrane beta barrel protein by multidimensional MAS NMR. J Biomol NMR 61:299–310
Farrotti A, Bocchinfuso G, Palleschi A, Rosato N, Salnikov ES, Voievoda N, Bechinger B, Stella L (2015) Molecular dynamics methods to predict peptide location in membranes: LAH4 as a stringent test case. Biochim Biophys Acta 1848:581–592
Gallaher J, Wodzinska K, Heimburg T, Bier M (2010) Ion-channel-like behavior in lipid bilayer membranes at the melting transition. Phys Rev E Stat Nonlinear Soft Matter Phys 81:061925
Gazit E, Miller IR, Biggin PC, Sansom MSP, Shai Y (1996) Structure and orientation of the mammalian antibacterial peptide cecropin P1 within phospholipid membranes. J Mol Biol 258:860–870
Georgescu J, Munhoz VHO, Bechinger B (2010) NMR structures of the histidine-rich peptide LAH4 in micellar environments: membrane insertion, pH-dependent mode of antimicrobial action and DNA transfection. Biophys J 99:2507–2515
Ghosh C, Haldar J (2015) Membrane-active small molecules: designs inspired by antimicrobial peptides. ChemMedChem 10:1606–1624
Ghosh C, Manjunath GB, Akkapeddi P, Yarlagadda V, Hoque J, Uppu DSSM, Konai MM, Haldar J (2014) Small molecular antibacterial peptoid mimics: the simpler the better! J Med Chem 57:1428–1436
Ghosh C, Harmouche N, Bechinger B, Haldar J (2018) Aryl-alkyl-lysines interact with anionic lipid components of bacterial cell envelope eliciting anti-inflammatory and anti-biofilm properties. ACS Omega 3:9182–9190
Giovannini MG, Poulter L, Gibson BW, Williams DH (1987) Biosynthesis and degradation of peptides derived from Xenopus laevis prohormones. Biochem J 243:113–120
Glattard E, Salnikov ES, Aisenbrey C, Bechinger B (2016) Investigations of the synergistic enhancement of antimicrobial activity in mixtures of magainin 2 and PGLa. Biophys Chem 210:35–44
Gogonea V (2015) Structural insights into high density lipoprotein: old models and new facts. Front Pharmacol 6:318
Gopinath T, Veglia G (2015) Multiple acquisition of magic angle spinning solid-state NMR experiments using one receiver: application to microcrystalline and membrane protein preparations. J Magn Reson 253:143–153
Gopinath T, Mote KR, Veglia G (2015) Simultaneous acquisition of 2D and 3D solid-state NMR experiments for sequential assignment of oriented membrane protein samples. J Biomol NMR 62:53–61
Grage SL, Afonin S, Kara S, Buth G, Ulrich AS (2016) Membrane thinning and thickening induced by membrane-active amphipathic peptides. Front Cell Dev Biol 4:65
Grau A, Gomez Fernandez JC, Peypoux F, Ortiz A (1999) A study on the interactions of surfactin with phospholipid vesicles. Biochim Biophys Acta 1418:307–319
Gregory SM, Cavenaugh A, Journigan V, Pokorny A, Almeida PFF (2008) A quantitative model for the all-or-none permeabilization of phospholipid vesicles by the antimicrobial peptide cecropin A. Biophys J 94:1667–1680
Hadley EB, Hancock RE (2010) Strategies for the discovery and advancement of novel cationic antimicrobial peptides. Curr Top Med Chem 10:1872–1881
Hall K, Lee TH, Mechler AI, Swann MJ, Aguilar MI (2014) Real-time measurement of membrane conformational states induced by antimicrobial peptides: balance between recovery and lysis. Sci Rep 4:5479
Hallock KJ, Lee DK, Omnaas J, Mosberg HI, Ramamoorthy A (2002) Membrane composition determines pardaxin’s mechanism of lipid bilayer disruption. Biophys J 83:1004–1013
Hara T, Kodama H, Kondo M, Wakamatsu K, Takeda A, Tachi T, Matsuzaki K (2001) Effects of peptide dimerization on pore formation: antiparallel disulfide-dimerized magainin 2 analogue. Biopolymers 58:437–446
Harmouche N, Bechinger B (2018) Lipid-mediated interactions between the amphipathic antimicrobial peptides magainin 2 and PGLa in phospholipid bilayers. Biophys J 115:1033-1044
Harpole TJ, Delemotte L (2018) Conformational landscapes of membrane proteins delineated by enhanced sampling molecular dynamics simulations. Biochim Biophys Acta 1860:909–926
Harzer U, Bechinger B (2000) The alignment of lysine-anchored membrane peptides under conditions of hydrophobic mismatch: a CD, 15 N and 31 P solid-state NMR spectroscopy investigation. Biochemistry 39:13106–13114
Hasan M, Karal MAS, Levadnyy V, Yamazaki M (2018) Mechanism of initial stage of pore formation induced by antimicrobial peptide magainin 2. Langmuir 34:3349–3362
Hayden RM, Goldberg GK, Ferguson BM, Schoeneck MW, Libardo MD, Mayeux SE, Shrestha A, Bogardus KA, Hammer J, Pryshchep S, Lehman HK, McCormick ML, Blazyk J, Angeles-Boza AM, Fu R, Cotten ML (2015) Complementary effects of host defense peptides piscidin 1 and piscidin 3 on DNA and lipid membranes: biophysical insights into contrasting biological activities. J Phys Chem B 119:15235–15246
He K, Ludtke SJ, Heller WT, Huang HW (1996) Mechanism of alamethicin insertion into lipid bilayers. Biophys J 71:2669–2679
Heerklotz H, Seelig J (2001) Detergent-like action of the antibiotic peptide surfactin on lipid membranes. Biophys J 81:1547–1554
Heerklotz H, Seelig J (2007) Leakage and lysis of lipid membranes induced by the lipopeptide surfactin. Eur Biophys J 36:305–314
Heerklotz H, Wieprecht T, Seelig J (2004) Membrane perturbation by the lipopeptide surfactin and detergents as studied by deuterium. J Phys Chem B 108:4909–4915
Heimburg T (2012) The capacitance and electromechanical coupling of lipid membranes close to transitions: the effect of electrostriction. Biophys J 103:918–929
Henderson JM, Waring AJ, Separovic F, Lee KYC (2016) Antimicrobial peptides share a common interaction driven by membrane line tension reduction. Biophys J 111:2176–2189
Holzl MA, Hofer J, Steinberger P, Pfistershammer K, Zlabinger GJ (2008) Host antimicrobial proteins as endogenous immunomodulators. Immunol Lett 119:4–11
Hong M, Su Y (2011) Structure and dynamics of cationic membrane peptides and proteins: insights from solid-state NMR. Protein Sci 20:641–655
Imura Y, Choda N, Matsuzaki K (2008) Magainin 2 in action: distinct modes of membrane permeabilization in living bacterial and mammalian cells. Biophys J 95:5757–5765
Ines M, Dhouha G (2015) Lipopeptide surfactants: production, recovery and pore forming capacity. Peptides 71:100–112
Islam MZ, Alam JM, Tamba Y, Karal MAS, Yamazaki M (2014) The single GUV method for revealing the functions of antimicrobial, pore-forming toxin, and cell-penetrating peptides or proteins. Phys Chem Chem Phys 16:15752–15767
Israelachvili JN, Marcelja S, Horn RG (1980) Physical principles of membrane organization. Q Rev Biophys 13:121–200
Itkin A, Salnikov ES, Aisenbrey C, Raya J, Raussens V, Ruysschaert JM, Bechinger B (2017) Evidence for heterogeneous conformations of the gamma cleavage site within the amyloid precursor proteins transmembrane domain. ACS Omega 2:6525–6534
Jaipuria G, Leonov A, Giller K, Vasa SK, Jaremko L, Jaremko M, Linser R, Becker S, Zweckstetter M (2017) Cholesterol-mediated allosteric regulation of the mitochondrial translocator protein structure. Nat Commun 8:14893
Jean-Francois F, Castano S, Desbat B, Odaert B, Roux M, Metz-Boutigue MH, Dufourc EJ (2008) Aggregation of cateslytin beta-sheets on negatively charged lipids promotes rigid membrane domains. A new mode of action for antimicrobial peptides? Biochemistry 47:6394–6402
Jenssen H, Hamill P, Hancock RE (2006) Peptide antimicrobial agents. Clin Microbiol Rev 19:491–511
Kandaswamy K, Liew TH, Wang CY, Huston-Warren E, Meyer-Hoffert U, Hultenby K, Schroder JM, Caparon MG, Normark S, Henriques-Normark B, Hultgren SJ, Kline KA (2013) Focal targeting by human beta-defensin 2 disrupts localized virulence factor assembly sites in Enterococcus faecalis. Proc Natl Acad Sci U S A 110:20230–20235
Karal MA, Alam JM, Takahashi T, Levadny V, Yamazaki M (2015) Stretch-activated pore of the antimicrobial peptide, magainin 2. Langmuir 31:3391–3401
Killian JA, de Planque MRR, van der Wel PCA, Salemink I, De Kruijff B, Greathouse DV, Koeppe RE (1998) Modulation of membrane structure and function by hydrophobic mismatch between proteins and lipids. Pure Appl Chem 70:75–82
Kim C, Spano J, Park EK, Wi S (2009) Evidence of pores and thinned lipid bilayers induced in oriented lipid membranes interacting with the antimicrobial peptides, magainin-2 and aurein-3.3. Biochim Biophys Acta 1788:1482–1496
Kim EY, Rajasekaran G, Shin SY (2017) LL-37-derived short antimicrobial peptide KR-12-a5 and its d-amino acid substituted analogs with cell selectivity, anti-biofilm activity, synergistic effect with conventional antibiotics, and anti-inflammatory activity. Eur J Med Chem 136:428–441
Kindrachuk J, Napper S (2010) Structure-activity relationships of multifunctional host defence peptides. Mini-Rev Med Chem 10:596–614
Kiss G, Michl H (1962) Über das Giftsekret der Gelbbauchunke Bombina variegata L. Toxicon (Oxford) 1:33–39
Klocek G, Seelig J (2008) Melittin interaction with sulfated cell surface sugars. Biochemistry 47:2841–2849
Kmiecik S, Gront D, Kolinski M, Wieteska L, Dawid AE, Kolinski A (2016) Coarse-grained protein models and their applications. Chem Rev 116:7898–7936
Kobayashi S, Hirakura Y, Matsuzaki K (2001) Bacteria-selective synergism between the antimicrobial peptides alpha-helical magainin 2 and cyclic beta-sheet tachyplesin I: toward cocktail therapy. Biochemistry 40:14330–14335
Kollmitzer B, Heftberger P, Rappolt M, Pabst G (2013) Monolayer spontaneous curvature of raft-forming membrane lipids. Soft Matter 9:10877–10884
Kozlowska J, Vermeer LS, Rogers GB, Rehnnuma N, Amos SB, Koller G, McArthur M, Bruce KD, Mason AJ (2014) Combined systems approaches reveal highly plastic responses to antimicrobial peptide challenge in Escherichia coli. PLoS Pathog 10:e1004104
Kuroda K, DeGrado WF (2005) Amphiphilic polymethacrylate derivatives as antimicrobial agents. J Am Chem Soc 127:4128–4129
Ladokhin AS, Wimley WC, White SH (1995) Leakage of membrane vesicle contents – determination of mechanisms using fluorescence requenching. Biophys J 69:1964–1971
Laub KR, Witschas K, Blicher A, Madsen SB, Luckhoff A, Heimburg T (2012) Comparing ion conductance recordings of synthetic lipid bilayers with cell membranes containing TRP channels. Biochim Biophys Acta 1818:1123–1134
Laurencin M, Simon M, Fleury Y, Baudy-Floc’h M, Bondon A, Legrand B (2018) Selectivity modulation and structure of alpha/aza-beta(3) cyclic antimicrobial peptides. Chemistry 24:6191–6201
Lear JD, Wasserman ZR, DeGrado WF (1988) Synthetic amphiphilic peptide models for protein ion channels. Science 240:1177–1181
Leber R, Pachler M, Kabelka I, Svoboda I, Enkoller D, Vácha R, Lohner K, Pabst G (2018) Synergism of antimicrobial frog peptides couples to membrane intrinsic curvature strain. Biophys J 114:1945–1954
Leitgeb B, Szekeres A, Manczinger L, Vagvolgyi C, Kredics L (2007) The history of alamethicin: a review of the most extensively studied peptaibol. Chem Biodivers 4:1027–1051
Liu SP, Zhou L, Lakshminarayanan R, Beuerman RW (2010) Multivalent antimicrobial peptides as therapeutics: design principles and structural diversities. Int J Pept Res Ther 16:199–213
Lohner K (2009) New strategies for novel antibiotics: peptides targeting bacterial cell membranes. Gen Physiol Biophys 28:105–116
Lorenzon EN, Santos-Filho NA, Ramos MA, Bauab TM, Camargo IL, Cilli EM (2016) C-terminal lysine-linked magainin 2 with increased activity against multidrug-resistant bacteria. Protein Pept Lett 23:738–747
Loudet C, Khemtemourian L, Aussenac F, Gineste S, Achard MF, Dufourc EJ (2005) Bicelle membranes and their use for hydrophobic peptide studies by circular dichroism and solid state NMR. Biochim Biophys Acta 1724:315–323
Ludtke SJ, He K, Wu Y, Huang HW (1994) Cooperative membrane insertion of magainin correlated with its cytolytic activity. Biochim Biophys Acta 1190:181–184
Ludtke S, He K, Huang H (1995) Membrane thinning caused by magainin 2. Biochemistry 34:16764–16769
Ludtke SJ, He K, Heller WT, Harroun TA, Yang L, Huang HW (1996) Membrane pores induced by magainin. Biochemistry 35:13723–13728
Maget-Dana R, Peypoux F (1994) Iturins, a special class of pore-forming lipopeptides: biological and physicochemical properties. Toxicology 87:151–174
Maget-Dana R, Ptak M (1995) Interactions of surfactin with membrane models. Biophys J 68:1937–1943
Makovitzki A, Baram J, Shai Y (2008) Antimicrobial lipopolypeptides composed of palmitoyl Di- and tricationic peptides: in vitro and in vivo activities, self-assembly to nanostructures, and a plausible mode of action. Biochemistry 47:10630–10636
Mangoni ML, Shai Y (2011) Short native antimicrobial peptides and engineered ultrashort lipopeptides: similarities and differences in cell specificities and modes of action. Cell Mol Life Sci 68:2267–2280
Marquette A, Bechinger B (2018) Biophysical investigations elucidating the mechanisms of action of antimicrobial peptides and their synergism. Biomol Ther 8:E18
Marquette A, Mason AJ, Bechinger B (2008) Aggregation and membrane permeabilizing properties of designed histidine-containing cationic linear peptide antibiotics. J Pept Sci 14:488–495
Marquette A, Lorber B, Bechinger B (2010) Reversible liposome association induced by LAH4: a peptide with potent antimicrobial and nucleic acid transfection activities. Biophys J 98:2544–2553
Marquette A, Salnikov E, Glattard E, Aisenbrey C, Bechinger B (2015) Magainin 2-PGLa interactions in membranes – two peptides that exhibit synergistic enhancement of antimicrobial activity. Curr Top Med Chem 16:65–75
Mason AJ, Martinez A, Glaubitz C, Danos O, Kichler A, Bechinger B (2006) The antibiotic and DNA-transfecting peptide LAH4 selectively associates with, and disorders, anionic lipids in mixed membranes. FASEB J 20:320–322
Mason AJ, Moussaoui W, Abdelrhaman T, Boukhari A, Bertani P, Marquette A, Shooshtarizaheh P, Moulay G, Boehm N, Guerold B, Sawers RJH, Kichler A, Metz-Boutigue MH, Candolfi E, Prevost G, Bechinger B (2009) Structural determinants of antimicrobial and antiplasmodial activity and selectivity in histidine rich amphipathic cationic peptides. J Biol Chem 284:119–133
Matos PM, Franquelim HG, Castanho MA, Santos NC (2010) Quantitative assessment of peptide-lipid interactions. Ubiquitous fluorescence methodologies. Biochim Biophys Acta 1798:1999–2012
Matsuzaki K (1998) Magainins as paradigm for the mode of action of pore forming polypeptides. Biochim Biophys Acta 1376:391–400
Matsuzaki K, Harada M, Funakoshi S, Fujii N, Miyajima K (1991) Physicochemical determinants for the interactions of magainins 1 and 2 with acidic lipid bilayers. Biochim Biophys Acta 1063:162–170
Matsuzaki K, Murase O, Tokuda H, Funakoshi S, Fujii N, Miyajima K (1994) Orientational and aggregational states of magainin 2 in phospholipid bilayers. Biochemistry 33:3342–3349
Matsuzaki K, Murase O, Fujii N, Miyajima K (1995a) Translocation of a channel-forming antimicrobial peptide, magainin2, across lipid bilayers by forming a pore. Biochemistry 34:6521–6526
Matsuzaki K, Murase O, Miyajima K (1995b) Kinetics of pore formation by an antimicrobial peptide, magainin 2, in phospholipid bilayers. Biochemistry 34:12553–12559
Matsuzaki K, Murase O, Fujii N, Miyajima K (1996) An antimicrobial peptide, magainin 2, induced flip-flop of phospholipid coupled with pore formation and peptide translocation. Biochemistry 35:11361–11368
Matsuzaki K, Mitani Y, Akada K, Murase O, Yoneyama S, Zasloff M, Miyajima K (1998) Mechanism of synergism between antimicrobial peptides magainin 2 and PGLa. Biochemistry 37:15144–15153
McCafferty DG, Cudic P, Yu MK, Behenna DC, Kruger R (1999) Synergy and duality in peptide antibiotic mechanisms. Curr Opin Chem Biol 3:672–680
Mecke A, Lee DK, Ramamoorthy A, Orr BG, Banaszak Holl MM (2005) Membrane thinning due to antimicrobial peptide binding: an atomic force microscopy study of MSI-78 in lipid bilayers. Biophys J 89:4043–4050
Medintz IL, Hildebrandt N (2013) Förster resonance energy transfer: from theory to applications. Wiley-VCH, Weinheim
Michalek M, Aisenbrey C, Bechinger B (2014) Investigation of membrane penetration depth and interactions of the amino-terminal domain of huntingtin: refined analysis by tryptophan fluorescence measurement. Eur Biophys J 43:347–360
Miles AJ, Wallace BA (2006) Synchrotron radiation circular dichroism spectroscopy of proteins and applications in structural and functional genomics. Chem Soc Rev 35:39–51
Miles AJ, Wallace BA (2016) Circular dichroism spectroscopy of membrane proteins. Chem Soc Rev 45:4859–4872
Milov AD, Samoilova RI, Tsvetkov YD, De Zotti M, Formaggio F, Toniolo C, Handgraaf JW, Raap J (2009) Structure of self-aggregated alamethicin in ePC membranes detected by pulsed electron-electron double resonance and electron spin Echo envelope modulation spectroscopies. Biophys J 96:3197–3209
Montal M, Mueller P (1972) Formation of bimolecular membranes from lipid monolayers and a study of their electrical properties. Proc Natl Acad Sci U S A 69:3561–3566
Mor A, Hani K, Nicolas P (1994) The vertebrate peptide antibiotics dermaseptins have overlapping structural features but target specific microorganisms. J Biol Chem 269:31635–31641
Naito A, Matsumori N, Ramamoorthy A (2018) Dynamic membrane interactions of antibacterial and antifungal biomolecules, and amyloid peptides, revealed by solid-state NMR spectroscopy. Biochim Biophys Acta 1862:307–323
Nishida M, Imura Y, Yamamoto M, Kobayashi S, Yano Y, Matsuzaki K (2007) Interaction of a magainin-PGLa hybrid peptide with membranes: insight into the mechanism of synergism. Biochemistry 46:14284–14290
North CL, Barranger-Mathys M, Cafiso DS (1995) Membrane orientation of the N-terminal segment of alamethicin determined by solid-state 15 N NMR. Biophys J 69:2392–2397
Otzen DE (2017) Biosurfactants and surfactants interacting with membranes and proteins: same but different? Biochim Biophys Acta 1859:639–649
Oyston PC, Fox MA, Richards SJ, Clark GC (2009) Novel peptide therapeutics for treatment of infections. J Med Microbiol 58:977–987
Palermo EF, Kuroda K (2010) Structural determinants of antimicrobial activity in polymers which mimic host defense peptides. Appl Microbiol Biotechnol 87:1605–1615
Patch JA, Barron AE (2003) Helical peptoid mimics of magainin-2 amide. J Am Chem Soc 125:12092–12093
Patel H, Tscheka C, Edwards K, Karlsson G, Heerklotz H (2011) All-or-none membrane permeabilization by fengycin-type lipopeptides from Bacillus subtilis QST713. Biochim Biophys Acta 1808:2000–2008
Patel H, Huynh Q, Barlehner D, Heerklotz H (2014) Additive and synergistic membrane permeabilization by antimicrobial (lipo)peptides and detergents. Biophys J 106:2115–2125
Paterson DJ, Tassieri M, Reboud J, Wilson R, Cooper JM (2017) Lipid topology and electrostatic interactions underpin lytic activity of linear cationic antimicrobial peptides in membranes. Proc Natl Acad Sci U S A 114:E8324–E8332
Payne JE, Dubois AV, Ingram RJ, Weldon S, Taggart CC, Elborn JS, Tunney MM (2017) Activity of innate antimicrobial peptides and ivacaftor against clinical cystic fibrosis respiratory pathogens. Int J Antimicrob Agents 50:427–435
Perrone B, Miles AJ, Salnikov ES, Wallace B, Bechinger B (2014) Lipid- interactions of the LAH4, a peptide with antimicrobial and nucleic transfection activities. Eur Biophys J 43:499–507
Pino-Angeles A, Leveritt JM 3rd, Lazaridis T (2016) Pore structure and synergy in antimicrobial peptides of the magainin family. PLoS Comput Biol 12:e1004570
Pirtskhalava M, Gabrielian A, Cruz P, Griggs HL, Squires RB, Hurt DE, Grigolava M, Chubinidze M, Gogoladze G, Vishnepolsky B, Alekseyev V, Rosenthal A, Tartakovsky M (2016) DBAASP v.2: an enhanced database of structure and antimicrobial/cytotoxic activity of natural and synthetic peptides. Nucleic Acids Res 44:D1104–D1112
Porter EA, Weisblum B, Gellman SH (2002) Mimicry of host-defense peptides by unnatural oligomers: antimicrobial beta-peptides. J Am Chem Soc 124:7324–7330
Pouny Y, Rapaport D, Mor A, Nicolas P, Shai Y (1992) Interaction of antimicrobial dermaseptin and its fluorescently labeled analogues with phospholipid membranes. Biochemistry 31:12416–12423
Raimondo D, Andreotti G, Saint N, Amodeo P, Renzone G, Sanseverino M, Zocchi I, Molle G, Motta A, Scaloni A (2005) A folding-dependent mechanism of antimicrobial peptide resistance to degradation unveiled by solution structure of distinctin. Proc Natl Acad Sci U S A 102:6309–6314
Ramamoorthy A, Thennarasu S, Lee DK, Tan A, Maloy L (2006) Solid-state NMR investigation of the membrane-disrupting mechanism of antimicrobial peptides MSI-78 and MSI-594 derived from magainin 2 and melittin. Biophys J 91:206–216
Rangarajan N, Bakshi S, Weisshaar JC (2013) Localized permeabilization of E. coli membranes by the antimicrobial peptide cecropin A. Biochemistry 52:6584–6594
Rank LA, Walsh NM, Liu R, Lim FY, Bok JW, Huang M, Keller NP, Gellman SH, Hull CM (2017) A cationic polymer that shows high antifungal activity against diverse human pathogens. Antimicrob Agents Chemother 61:e00204–e00217
Rautenbach M, Troskie AM, Vosloo JA (2016a) Antifungal peptides: to be or not to be membrane active. Biochimie 130:132–145
Rautenbach M, Troskie AM, Vosloo JA, Dathe ME (2016b) Antifungal membranolytic activity of the tyrocidines against filamentous plant fungi. Biochimie 130:122–131
Reijmar K, Edwards K, Andersson K, Agmo Hernandez V (2016) Characterizing and controlling the loading and release of cationic amphiphilic peptides onto and from PEG-stabilized lipodisks. Langmuir 32:12091–12099
Resende JM, Moraes CM, Munhoz VHDO, Aisenbrey C, Verly RM, Bertani P, Cesar A, Pilo-Veloso D, Bechinger B (2009) Membrane structure and conformational changes of the antibiotic heterodimeric peptide distinctin by solid-state NMR spectroscopy. Proc Natl Acad Sci U S A 106:16639–16644
Resende JM, Verly RM, Aisenbrey C, Amary C, Bertani P, Pilo-Veloso D, Bechinger B (2014) Membrane interactions of phylloseptin-1, −2, and −3 peptides by oriented solid-state NMR spectroscopy. Biophys J 107:901–911
Rollins-Smith LA, Doersam JK, Longcore JE, Taylor SK, Shamblin JC, Carey C, Zasloff MA (2002) Antimicrobial peptide defenses against pathogens associated with global amphibian declines. Dev Comp Immunol 26:63–72
Rotem S, Mor A (2009) Antimicrobial peptide mimics for improved therapeutic properties. Biochim Biophys Acta 1788:1582–1592
Roversi D, Luca V, Aureli S, Park Y, Mangoni ML, Stella L (2014) How many AMP molecules kill a bacterium? Spectroscopic determination of PMAP-23 binding to E. Coli. ACS Chem Biol 9:2003–2007
Russ WP, Engelman DM (2000) The GxxxG motif: a framework for transmembrane helix-helix association. J Mol Biol 296:911–919
Sakoulas G, Kumaraswamy M, Kousha A, Nizet V (2017) Interaction of antibiotics with innate host defense factors against Salmonella enterica serotype Newport. mSphere 2:e00410–e00417
Salnikov E, Bechinger B (2011) Lipid-controlled peptide topology and interactions in bilayers: structural insights into the synergistic enhancement of the antimicrobial activities of PGLa and magainin 2. Biophys J 100:1473–1480
Salnikov ES, Friedrich H, Li X, Bertani P, Reissmann S, Hertweck C, O’Neil JD, Raap J, Bechinger B (2009a) Structure and alignment of the membrane-associated peptaibols ampullosporin A and alamethicin by oriented 15 N and 31 P solid-state NMR spectroscopy. Biophys J 96:86–100
Salnikov ES, Mason AJ, Bechinger B (2009b) Membrane order perturbation in the presence of antimicrobial peptides by 2H solid-state NMR spectroscopy. Biochimie 91:734–743
Salnikov E, Aisenbrey C, Vidovic V, Bechinger B (2010) Solid-state NMR approaches to measure topological equilibria and dynamics of membrane polypeptides. Biochim Biophys Acta 1798:258–265
Salnikov E, Aisenbrey C, Balandin SV, Zhmak MN, Ovchinnikova AY, Bechinger B (2011) Structure and alignment of the membrane-associated antimicrobial peptide arenicin by oriented solid-state NMR spectroscopy. Biochemistry 50:3784–3795
Salnikov ES, Aisenbrey C, Aussenac F, Ouari O, Sarrouj H, Reiter C, Tordo P, Engelke F, Bechinger B (2016a) Membrane topologies of the PGLa antimicrobial peptide and a transmembrane anchor sequence by Dynamic Nuclear Polarization/solid-state NMR spectroscopy. Sci Rep 6:20895
Salnikov ES, Raya J, De Zotti M, Zaitseva E, Peggion C, Ballano G, Toniolo C, Raap J, Bechinger B (2016b) Alamethicin supramolecular organization in lipid membranes from 19F solid- state NMR. Biophys J 111:2450–2459
Salnikov ES, Anantharamaiah GM, Bechinger B (2018) Supramolecular organization of apolipoprotein A-I – derived peptides within disc-like arrangements. Biophys J 115:467–477 in press
Sani MA, Separovic F (2018) Antimicrobial peptide structures: from model membranes to live cells. Chemistry 24:286–291
Sansom MSP (1991) The biophysics of peptide models of ion channels. Prog Biophysmol Biol 55:139–235
Sansom MS (1993) Alamethicin and related peptaibols – model ion channels. Eur Biophys J 22:105–124
Scherer PG, Seelig J (1989) Electric charge effects on phospholipid headgroups. Phosphatidylcholine in mixtures with cationic and anionic amphiphiles. Biochemistry 28:7720–7727
Schweizer F (2009) Cationic amphiphilic peptides with cancer-selective toxicity. Eur J Pharmacol 625:190–194
Scott RW, DeGrado WF, Tew GN (2008) De novo designed synthetic mimics of antimicrobial peptides. Curr Opin Biotechnol 19:620–627
Seelig J (2004) Thermodynamics of lipid-peptide interactions. Biochim Biophys Acta 1666:40–50
Shai Y (1999) Mechanism of the binding, insertion, and destabilization of phospholipid bilayer membranes by alpha-helical antimicrobial and cell non-selective lytic peptides. Biochim Biophys Acta 1462:55–70
Smart M, Rajagopal A, Liu WK, Ha BY (2017) Opposing effects of cationic antimicrobial peptides and divalent cations on bacterial lipopolysaccharides. Phys Rev E 96:042405
Sreerama N, Woody RW (2000) Estimation of protein secondary structure from circular dichroism spectra: comparison of CONTIN, SELCON, and CDSSTR methods with an expanded reference set. Anal Biochem 287:252–260
Steinstraesser L, Kraneburg U, Jacobsen F, Al-Benna S (2010) Host defense peptides and their antimicrobial-immunomodulatory duality. Immunobiology 216:322–333
Strandberg E, Tiltak D, Ehni S, Wadhwani P, Ulrich AS (2012) Lipid shape is a key factor for membrane interactions of amphipathic helical peptides. Biochim Biophys Acta 1818:1764–1776
Strandberg E, Zerweck J, Wadhwani P, Ulrich AS (2013) Synergistic insertion of antimicrobial magainin-family peptides in membranes depends on the lipid spontaneous curvature. Biophys J 104:L09–L11
Sychev SV, Sukhanov SV, Panteleev PV, Shenkarev ZO, Ovchinnikova TV (2018) Marine antimicrobial peptide arenicin adopts a monomeric twisted beta-hairpin structure and forms low conductivity pores in zwitterionic lipid bilayers. Pept Science 110:e23093
Tamba Y, Yamazaki M (2005) Single giant unilamellar vesicle method reveals effect of antimicrobial peptide magainin 2 on membrane permeability. Biochemistry 44:15823–15833
Tamba Y, Yamazaki M (2009) Magainin 2-induced pore formation in the lipid membranes depends on its concentration in the membrane interface. J Phys Chem B 113:4846–4852
Tamba Y, Ariyama H, Levadny V, Yamazaki M (2010) Kinetic pathway of antimicrobial peptide magainin 2-induced pore formation in lipid membranes. J Phys Chem B 114:12018–12026
Tieleman DP, Hess B, Sansom MS (2002) Analysis and evaluation of channel models: simulations of alamethicin. Biophys J 83:2393–2407
Tremouilhac P, Strandberg E, Wadhwani P, Ulrich AS (2006a) Conditions affecting the re-alignment of the antimicrobial peptide PGLa in membranes as monitored by solid state 2H-NMR. Biochim Biophys Acta 1758:1330–1342
Tremouilhac P, Strandberg E, Wadhwani P, Ulrich AS (2006b) Synergistic transmembrane alignment of the antimicrobial heterodimer PGLa/magainin. J Biol Chem 281:32089–32094
Tsutsumi LS, Elmore JM, Dang UT, Wallace MJ, Marreddy R, Lee RB, Tan GT, Hurdle JG, Lee RE, Sun D (2018) Solid-phase synthesis and antibacterial activity of cyclohexapeptide wollamide B analogs. ACS Comb Sci 20:172–185
Ulmschneider JP, Smith JC, Ulmschneider MB, Ulrich AS, Strandberg E (2012) Reorientation and dimerization of the membrane-bound antimicrobial peptide PGLa from microsecond all-atom MD simulations. Biophys J 103:472–482
Usachev KS, Kolosova OA, Klochkova EA, Yulmetov AR, Aganov AV, Klochkov VV (2017) Oligomerization of the antimicrobial peptide protegrin-5 in a membrane-mimicking environment. Structural studies by high-resolution NMR spectroscopy. Eur Biophys J 46:293–300
Vacha R, Frenkel D (2014) Simulations suggest possible novel membrane pore structure. Langmuir 30:1304–1310
Vaz Gomes A, de Waal A, Berden JA, Westerhoff HV (1993) Electric potentiation, cooperativity, and synergism of magainin peptides in protein-free liposomes. Biochemistry 32:5365–5372
Verardi R, Traaseth NJ, Shi L, Porcelli F, Monfregola L, De Luca S, Amodeo P, Veglia G, Scaloni A (2011) Probing membrane topology of the antimicrobial peptide distinctin by solid-state NMR spectroscopy in zwitterionic and charged lipid bilayers. Biochim Biophys Acta 1808:34–40
Verly RM, Resende JM, Junior EFC, de Magalhães MTC, Guimarães CFCR, Munhoz VHO, Bemquerer MP, Almeida FCL, Santoro MM, Piló-Veloso D, Bechinger B (2017) Structure and membrane interactions of the homodimeric antibiotic peptide homotarsinin. Sci Rep 7:40854
Vermeer LS, Marquette A, Schoup M, Fenard D, Galy A, Bechinger B (2016) Simultaneous analysis of secondary structure and light scattering from circular dichroism titrations: application to vectofusin-1. Sci Rep 6:39450
Violette A, Fournel S, Lamour K, Chaloin O, Frisch B, Briand JP, Monteil H, Guichard G (2006) Mimicking helical antibacterial peptides with nonpeptidic folding oligomers. Chem Biol 13:531–538
Visscher KM, Medeiros-Silva J, Mance D, Rodrigues J, Daniels M, Bonvin A, Baldus M, Weingarth M (2017) Supramolecular organization and functional implications of K+ channel clusters in membranes. Angew Chem Int Ed Eng 56:13222–13227
Vogt TCB, Bechinger B (1999) The interactions of histidine-containing amphipathic helical peptide antibiotics with lipid bilayers: the effects of charges and pH. J Biol Chem 274:29115–29121
Voievoda N (2014) Biophysical investigations of the membrane and nucleic acids interactions of the transfection peptide LAH4-L1. PhD thesis, University of Strasbourg PhD
Voievoda N, Schulthess T, Bechinger B, Seelig J (2015) Thermodynamic and biophysical analysis of the membrane-association of a histidine-rich peptide with efficient antimicrobial and transfection activities. J Phys Chem B 119:9678–9687
Wakamatsu K, Takeda A, Tachi T, Matsuzaki K (2002) Dimer structure of magainin 2 bound to phospholipid vesicles. Biopolymers 64:314–327
Wallace BA, Moa B (1984) Circular dichroism analyses of membrane proteins: an examination of differential light scattering and absorption flattening effects in large membrane vesicles and membrane sheets. Anal Biochem 142:317–328
Wang G, Li X, Wang Z (2016) APD3: the antimicrobial peptide database as a tool for research and education. Nucleic Acids Res 44:D1087–D1093
Watanabe H, Kawano R (2016) Channel current analysis for pore-forming properties of an antimicrobial peptide, magainin 1, using the droplet contact method. Anal Sci 32:57–60
Wenk M, Seelig J (1998) Magainin 2 amide interaction with lipid membranes: calorimetric detection of peptide binding and pore formation. Biochemistry 37:3909–3916
Wenzel M, Chiriac AI, Otto A, Zweytick D, May C, Schumacher C, Gust R, Albada HB, Penkova M, Kramer U, Erdmann R, Metzler-Nolte N, Straus SK, Bremer E, Becher D, Brotz-Oesterhelt H, Sahl HG, Bandow JE (2014) Small cationic antimicrobial peptides delocalize peripheral membrane proteins. Proc Natl Acad Sci USA 111:E1409–E1418
Westerhoff HV, Zasloff M, Rosner JL, Hendler RW, de Waal A, Vaz G, Jongsma PM, Riethorst A, Juretic D (1995) Functional synergism of the magainins PGLa and magainin-2 in Escherichia coli, tumor cells and liposomes. Eur J Biochem 228:257–264
Wheaten SA, Ablan FD, Spaller BL, Trieu JM, Almeida PF (2013) Translocation of cationic amphipathic peptides across the membranes of pure phospholipid giant vesicles. J Am Chem Soc 135:16517–16525
Wieprecht T, Apostolov O, Beyermann M, Seelig J (1999a) Thermodynamics of the alpha-helix-coil transition of amphipathic peptides in a membrane environment: implications for the peptide-membrane binding equilibrium. J Mol Biol 294:785–794
Wieprecht T, Beyermann M, Seelig J (1999b) Binding of antibacterial magainin peptides to electrically neutral membranes: thermodynamics and structure. Biochemistry 38:10377–10378
Wieprecht T, Apostolov O, Beyermann M, Seelig J (2000a) Membrane binding and pore formation of the antibacterial peptide PGLa: thermodynamic and mechanistic aspects. Biochemistry 39:442–452
Wieprecht T, Apostolov O, Seelig J (2000b) Binding of the antibacterial peptide magainin 2 amide to small and large unilamellar vesicles. Biophys Chem 85:187–198
Wieprecht T, Beyermann M, Seelig J (2002) Thermodynamics of the coil-alpha-helix transition of amphipathic peptides in a membrane environment: the role of vesicle curvature. Biophys Chem 96:191–201
Wolf J, Aisenbrey C, Harmouche N, Raya J, Bertani P, Voievoda N, Süss R, Bechinger B (2017) pH-dependent membrane interactions of the histidine-rich cell penetrating peptide LAH4-L1. Biophys J 113:1290–1300
Wu Y, Huang HW, Olah GA (1990) Method of oriented circular dichroism. Biophys J 57:797–806
Wu YS, Ngai SC, Goh BH, Chan KG, Lee LH, Chuah LH (2017) Anticancer activities of surfactin and potential application of nanotechnology assisted surfactin delivery. Front Pharmacol 8:761
Yang D, Zou R, Zhu Y, Liu B, Yao D, Jiang J, Wu J, Tian H (2014) Magainin II modified polydiacetylene micelles for cancer therapy. Nanoscale 6:14772–14783
Yang Z, Choi H, Weisshaar JC (2018) Melittin-induced permeabilization, re-sealing, and re-permeabilization of E. coli membranes. Biophys J 114:368–379
Yuksel E, Karakecili A (2014) Antibacterial activity on electrospun poly(lactide-co-glycolide) based membranes via magainin II grafting. Mater Sci Eng C Mater Biol Appl 45:510–518
Zanin LM, Dos Santos Alvares D, Juliano MA, Pazin WM, Ito AS, Ruggiero Neto J (2013) Interaction of a synthetic antimicrobial peptide with model membrane by fluorescence spectroscopy. Eur Biophys J 42:819–831
Zasloff M (1987) Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc Natl Acad Sci U S A 84:5449–5453
Zasloff M (2002) Antimicrobial peptides of multicellular organisms. Nature 415:389–395
Zerweck J, Strandberg E, Kukharenko O, Reichert J, Burck J, Wadhwani P, Ulrich AS (2017) Molecular mechanism of synergy between the antimicrobial peptides PGLa and magainin 2. Sci Rep 7:13153
Zhao H, Shao D, Jiang C, Shi J, Li Q, Huang Q, Rajoka MSR, Yang H, Jin M (2017) Biological activity of lipopeptides from Bacillus. Appl Microbiol Biotechnol 101:5951–5960
Zhao P, Xue Y, Gao W, Li J, Zu X, Fu D, Bai X, Zuo Y, Hu Z, Zhang F (2018) Bacillaceae-derived peptide antibiotics since 2000. Peptides 101:10–16
Acknowledgments
We gratefully acknowledge the discussion with and contributions from many coworkers and colleagues from our own team and from outside. We are grateful to Ekaterina Zaitseva for her feedback on electrophysiology and Roland Stote for much helping to refine the text on molecular dynamics. Over the years, the Agence Nationale de la Recherche (projects TRANSPEP 07-PCV-0018, ProLipIn 10-BLAN-731, membraneDNP 12-BSV5-0012, MemPepSyn 14-CE34-0001-01, InMembrane 15-CE11-0017-01, Biosupramol 17-CE18-0033-3, and the LabEx Chemistry of Complex Systems 10-LABX-0026_CSC), the IRTG Soft Matter Science (Freiburg, Strasbourg), the Marie-Curie Research and Training Network 33439 of the European Commission BIOCONTROL, the University of Strasbourg, the CNRS, the Région Alsace, the RTRA International Center of Frontier Research in Chemistry, and the French Foundation for Medical Research (FRM) have provided financial support. BB thanks the Institut Universitaire de France for providing additional time to be dedicated to research.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Aisenbrey, C., Marquette, A., Bechinger, B. (2019). The Mechanisms of Action of Cationic Antimicrobial Peptides Refined by Novel Concepts from Biophysical Investigations. In: Matsuzaki, K. (eds) Antimicrobial Peptides. Advances in Experimental Medicine and Biology, vol 1117. Springer, Singapore. https://doi.org/10.1007/978-981-13-3588-4_4
Download citation
DOI: https://doi.org/10.1007/978-981-13-3588-4_4
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-3587-7
Online ISBN: 978-981-13-3588-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)