Abstract
Recently, Alzheimer’s disease (AD) is understood as “diabetes of the brain” or “type 3 diabetes.” Recent clinical trials of anti-amyloid β-protein (Aβ) therapies have not proved to be successful. Thus, glucose-insulin metabolism in the brain is thought to be an alternative therapeutic target. Various types of antidiabetic drugs such as insulin, thiazolidinediones, dipeptidyl peptidase-4 (DPP4) inhibitors, glucagon-like peptide-1 (GLP-1) agonists, biguanides, and others have been reported to be effective on cognitive impairment in animal models and patients with DM or AD. Here, recent reports are reviewed. While we identified apomorphine (APO) as a novel drug that promoted intracellular Aβ degradation and improved memory function in an AD mouse model, more recently, we have revealed that APO treatment improves neuronal insulin resistance and activates insulin-degrading enzyme (IDE), a major Aβ-degrading enzyme. In this context, recovery of impaired insulin signaling in AD neurons may be a promising therapeutic strategy for AD dementia.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Alzheimer’s disease
- Diabetes mellitus
- Insulin
- Thiazolidinediones
- DPP4 inhibitors
- GLP-1 agonists
- Apomorphine
11.1 Introduction
Alzheimer’s disease (AD) is the major cause of dementia in the elderly people, and the therapeutics for AD is the major topic in the world. At present, four drugs are approved to use for AD patients. Among them, three drugs (donepezil, galantamine, rivastigmine) are acetylcholinesterase (AChE) inhibitors, and one is a glutamate antagonist, memantine. All these drugs have effects to slow the progression of dementia but not to improve cognitive function persistently (symptom-modifying drugs). Thus, many pharmaceutical companies and researchers have been investigating to develop novel drugs that completely inhibit disease progression and improve cognitive function (disease-modifying drugs).
To date, one of the most widely known mechanisms of AD pathogenesis has been “amyloid cascade hypothesis.” There are two major pathological hallmarks of AD, neurofibrillary tangles (NFTs) and senile plaques (SPs) (Serrano-Pozo et al. 2011). NFTs consist of hyper-phosphorylated tau protein (p-tau), and SPs consist of amyloid β-protein (Aβ). Remarkably, Aβ has long been thought to play a pivotal role in the pathogenesis of AD. Because, Aβ deposition is one of the earliest phenomena in brain, followed by p-tau formation and cognitive decline (Jack et al. 2010). As shown in Fig. 11.1, Aβ is produced from Aβ protein precursor (APP) by two enzymes, i.e., β-secretase and γ-secretase. Although approximately 90% of Aβ species secreted physiologically is Aβ40, only 10% Aβ species, Aβ42, is more aggregative and forms Aβ oligomers. Aβ oligomers are more neurotoxic than Aβ monomer. Toxicity of Aβ may induce synaptic dysfunction leading to cognitive impairment (Ferreira et al. 2015) and may also accelerate p-tau formation (Hu et al. 2014). In addition, toxic turn Aβ42 form has recently been found (Murakami et al. 2010). Such a pathogenic cascade is named “amyloid cascade hypothesis.” Based on this hypothesis, many therapeutic strategies targeting Aβ have been investigated. As shown in Fig. 11.1, β- and γ-secretase inhibitors that inhibit Aβ generation, anti-Aβ aggregation drugs, and immunological therapies using specific anti-Aβ antibodies or vaccination with Aβ peptides have been developed. Although many of these drugs were effective on AD mouse models, almost all phase III clinical trials for AD patients did not reach the primary end point. Some evidences of amyloid imaging and biomarkers in cerebrospinal fluids have been demonstrated, but cognitive impairment was not improved sufficiently (Doody et al. 2014; Salloway et al. 2014; Siemers et al. 2016). Possible causes for such unsuccessfulness are the following: (i) Aβ-targeting therapy may be effective only in preclinical and prodromal AD; (ii) sporadic AD cases in the elderly may be caused by some mechanisms different from those in AD mouse models produced by the gene engineering; and (iii) “amyloid cascade hypothesis” may not be the true mechanism in dementia of AD. As to the possibility (i), clinical trials of anti-Aβ therapy for the preclinical and prodromal AD patients are still pursued. As to possibilities (ii) and (iii), clinical trials of anti-Aβ therapy for the early-onset familial AD patients with the genes determined are now ongoing. The results of such investigation will provide us the validity of “amyloid cascade hypothesis.” More recently, p-tau is focused on as a new therapeutic target other than Aβ (Boutajangout and Wisniewski 2014). However, it is unclear whether or not only the abnormal proteins accumulating in the brain are the powerful therapeutic targets to improve dementia. It is important to recover the neuronal network system improving generation of energy and metabolism in AD neurons. In this point of view, glucose-insulin metabolism may be an important therapeutic target.
“Amyloid cascade hypothesis” and therapeutic targets. Aβ is generated by β-secretase (β-amyloid clipping enzyme, BACE) and γ-secretase. Aβ42 takes only 10% in secreted Aβ but is highly aggregative and readily forms Aβ oligomers that are toxic for synapse and cause memory impairment. Aβ oligomers also promote hyperphosphorylation of tau protein. To attenuate this process, many inhibitors of β- or γ-secretase, anti-Aβ aggregation drugs, and immunotherapeutics such as anti-Aβ antibodies and Aβ vaccination have been developed. However, to date, almost all clinical trials of these drugs have been unsuccessful
11.2 Association Between Diabetes Mellitus (DM) and AD
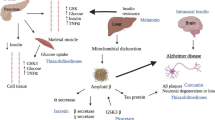
Hypertension and DM have been widely known as the major risk factors for arteriosclerosis resulting in brain and cardiac infarction. Thus, DM has been thought to be one of the strong risk factors for vascular dementia. On the other hand, correlation between DM and AD has been investigated epidemiologically. Some reports found no correlation (Luchsinger et al. 2001; MacKnight et al. 2002; Hassing et al. 2002), but others found positive correlation between them (Leibson et al. 1997; Ott et al. 1999; Peila et al. 2002). Such discrepancies may be due to differences in determination of DM. DM was diagnosed by oral glucose tolerance tests (OGTT) in the reports that showed positive correlation but not in the negative reports. It suggests that subclinical diabetic status may contribute to AD risk. Recently, an epidemiological study in Japanese population (the Hisayama study) has clearly revealed that glucose intolerance may increase the risk of AD as well as VD in the future (Ohara et al. 2011). In the same study, correlation between glucose intolerance and Aβ deposition (Matsuzaki et al. 2010) and DM-like gene expression patterns in the postmortem brain tissues (Hokama et al. 2014) were also demonstrated. In addition, Talbot et al. revealed an increased insulin resistance of neurons in the AD brain (Talbot et al. 2012). Taken together, increased peripheral insulin resistance, i.e., type 2 DM (T2DM), may be linked to increased neuronal insulin resistance in AD. Therefore, AD has recently been named “type 3 DM” or “brain DM” (De la Monte 2014) (Fig. 11.2). In addition, recurrent hypoglycemic attacks (Whitmer et al. 2009) and both increases and decreases in mean blood glucose levels (Crane et al. 2013) may increase the risk for dementia, indicating that marked alteration of blood glucose levels may strongly affect neuronal network function and cognitive function. Moreover, increased insulin resistance in neurons may decrease insulin-degrading enzyme (IDE), also a major Aβ-degrading enzyme (Miners et al. 2011), and may increase dephosphorylated GSK-3β, a major phosphokinase of tau protein (Avila et al. 2010), resulting in enhancing progression of the AD pathology. In this context, brain insulin resistance would be a new target in therapeutic approach for dementia in AD patients.
AD pathogenesis associating with DM. DM is well known to accelerate arteriosclerosis and ischemic changes in the brain, leading to vascular dementia (VD) (left: vascular factors). While, DM may cause hyperinsulinemia, increase brain insulin resistance and oxidative stress, and decrease insulin-degrading enzyme (IDE), accelerating AD-related pathology (Aβ deposition and NFT formation). It is also known that vascular lesion due to ischemia may enhance the progression of AD pathology
Recently increasing reports suggest that exercise may contribute to prevention of dementia (Barnes 2015). Also, the National Institutes of Health in the USA recommends control of T2DM, exercise habits, and healthy foods for prevention of dementia. Such recommendations may be similar to the prevention of DM. Such facts may imply a common basis of AD and DM.
11.3 Insulin Therapy
If insulin resistance is increased in neurons, insulin signaling may not work sufficiently. Insulin signaling may play a major role in signal transduction in cells, regulating cell cycle proteins (Yang and Guan 2007). Thus, first simple therapeutic strategy may be supply of insulin in AD brain. Recent reports demonstrating efficacy of insulin administration on cognitive function in rodents and human are listed in Table 11.1. In an AD model, 3xTg-AD mice (APPKM670/671NL/PS1M146V/TauP301L), high-fat diet (HFD), which increases peripheral insulin resistance, may accelerate Aβ deposition in brain and memory impairment; such phenomena may be improved by insulin injection (Vandal et al. 2014). Moreover, in these HFD-treated 3xTg-AD mice, Aβ deposition is observed in the pancreas, indicating a pathogenic self-amplifying loop between AD and T2DM (Vandal et al. 2015). More recently, many reports have demonstrated that nasal administration of insulin improved memory function, reduced Aβ deposition, increased brain-derived neurotrophic factor (BDNF) and its receptor protein tropomyosin receptor kinase B (TrkB), improved hippocampal afterhyperpolarization (AHP), etc., in APP/presenilin-1 (PS1) double transgenic mice, anesthetic mice, Aβ-injected rats, aged rats, rats with brain trauma, streptozotocin (STZ)-treated rats, and senescence-accelerated mice (SAMP8) (Mao et al. 2016; Zhang et al. 2016; Farzampour et al. 2016; Haas et al. 2016; Maimaiti et al. 2016; Brabazon et al. 2017; Rajasekar et al. 2017; Kamei et al. 2017) (see Table 11.1).
While, clinical trials of nasal insulin administration to human preceded the investigation using the animal models. Craft and colleagues have demonstrated that nasal administration of insulin improves memory function in MCI and mild AD patients (Craft et al. 2012) and that such effects may be different among sex (Claxton et al. 2013) and apolipoprotein E gene alleles (Claxton et al. 2015). Insulin administered via nasal pathway did not cause systemic hypoglycemia (Craft et al. 2012; Claxton et al. 2013, 2015) and may thus seem a promising method to develop new drugs to improve the hippocampal function.
11.4 Thiazolidinediones (Glitazones)
Thiazolidinediones (glitazones) are peroxisome proliferator-activated receptor γ (PPARγ) agonists, which reduce insulin resistance of the liver and muscle. There is a possibility that these drugs improve insulin resistance of neurons in the AD brain, since neuronal insulin resistance may be increased in AD (Talbot et al. 2012). Recent reports demonstrating efficacy of thiazolidinediones (glitazones) on cognitive function in rodents and human are listed in Table 11.2. At present, there are two major glitazones, rosiglitazone (Ros) and pioglitazone (Pio). Disease models consist of some different types. First, transgenic mice with mutant APP, mutant APP + presenilin-1 (PS1) double, and mutant APP+PS1+tau triple genes were used as an early-onset familial AD models. Second, HFD- and high-fructose-diet (HFuD)-fed rats are models of T2DM, because those diets are well known to induce peripheral insulin resistance. Third, STZ-injected mouse is a model of type 1 DM (T1DM), because STZ causes selective damages in pancreatic β cells resulting in peripheral insulin deficiency. At last, congenital DM rats or mice (db/db mice) were also used. All these DM-associated mice or rats were exactly not the models of AD. However, based on the concept that AD may be “brain diabetes,” drugs that improve cognitive function in these DM-associated animal models may become promising candidates for AD.
As shown in Table 11.2, Ros treatment improved memory function in HFD rats (Pathan et al. 2008), APP-Tg mice (Escribano et al. 2010), 3xTg-AD mice (Yu et al. 2015), DM rats (Ma et al. 2015), and db/db mice (Wang et al. 2016). Remarkably, Ros treatment removed the amyloid plaques and decreased p-tau in the hippocampus of APP-Tg mice (Escribano et al. 2010). Also, Pio treatment improved memory function of HFuD rats (Yin et al. 2013), STZ mice (Liu et al. 2013), 3xTg-AD mice (Yu et al. 2015), APP/PS1 mice (Toba et al. 2016), and db/db mice (Wang et al. 2016). In addition, Pio treatment prevented the β-amyloidogenic process such as Aβ overproduction and decreased Aβ degradation induced by insulin resistance in HFuD rats (Luo et al. 2011), strengthened antioxidant defense system in HFuD rats (Yin et al. 2013), reduced brain β-amyloid clipping enzyme 1 (BACE1) in STZ mice (Liu et al. 2013). Both Ros and Pio treatments attenuated hyperphosphorylation of tau and neuroinflammation in 3xTg-AD mice (Yu et al. 2015) and promoted Aβ clearance across the blood-brain barrier (BBB) and enhanced hippocampal long-term potentiation (LTP) in db/db mice (Wang et al. 2016). In the human studies, there have been the reports indicating dementia protective efficacy of both Ros and Pio in patients with MCI/AD (Watson et al. 2005), with AD and T2DM (Sato et al. 2011), with T2DM (Heneka et al. 2015), and with DM (Chou et al. 2017), whereas some other reports indicated the negative data as to the efficacy of Ros and Pio (Miller et al. 2011; Harrington et al. 2011; Seaquist et al. 2013; Hildreth et al. 2015; Galimberti and Scarpini 2017). Further large-size clinical trials are necessary to determine their effects.
11.5 DPP4 Inhibitors
Dipeptidyl peptidase-4 (DPP4) degrades incretin hormones, which stimulate secretion of insulin from the pancreas and decrease blood glucose levels. Incretin hormones contain glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). Thus, DPP4 inhibitors enhance incretin hormone activity followed by increase in levels of plasma insulin. DPP4 inhibitors would therefore increase insulin stimulation in the AD brain. Recent reports demonstrating efficacy of DPP4 inhibitors (gliptins) on cognitive function in rodents and human are listed in Table 11.3. Currently, there are some well-known gliptins such as sitagliptin (Sita), saxagliptin (Saxa), vildagliptin (Vilda), alogliptin (Alo), and linagliptin (Lina). As well as PPAR-γ agonists, many reports indicate that these gliptins may improve memory function and mitochondrial function and inhibit Aβ deposition, p-tau deposition, and neuroinflammation (D’Amico et al. 2010; Kosaraju et al. 2013a, b, 2016; Sakr 2013; Pipatpiboon et al. 2013; Sripetchwandee et al. 2014; El-Sahar et al. 2015; Gault et al. 2015; Tsai et al. 2015; Pintata et al. 2016; Qin et al. 2016) (Table 11.3). The fact that such drugs may be effective for AD mouse models as well as for cognitive deficit in mice with T1DM (STZ) and T2DM (HFD), indicates a common mechanism in cognitive impairment in AD and DM. In human studies, although there have not been clinical trials for MCI or AD patients, DPP4 inhibitors may be beneficial to protect against cognitive impairment in patients with T2DM (Tasci et al. 2013; Rizzo et al. 2014) and may also be effective on patients with AD (Isik et al. 2017). Thus, further clinical trials of DPP4 inhibitors for AD patients are necessary.
11.6 GLP-1 Agonists
As mentioned above, GLP-1 is one of incretin hormones that stimulate insulin secretion. As well as DPP4 inhibitors, GLP-1 agonists and GIP are included in the incretin-related drugs. Recent reports demonstrating efficacy of GLP-1 agonists (glutides) on cognitive function in rodents and human are listed in Table 11.4. To date, liraglutide (Lira), lixisenatide (Lixi), exenatide (Exen), and exendin-4 (Ex-4) have been investigated using animal models. As shown in Table 11.4, many reports demonstrated that these GLP-1 agonists improved memory function and hippocampal LTP, inhibited Aβ deposition and microglial activation, and decreased insulin resistance and tau phosphorylation in HFD mice, STZ mice, APP/PS1 mice, intraventricular Aβ-injected mice, and 3xTg-AD mice (Table 11.4). Interestingly, Ex-4 treatment recovered permeability of BBB and blood-CSF barrier (BCSFB) damaged by DM, indicating a novel efficacy of GLP-1 agonists other than stimulation of insulin secretion (Zanotto et al. 2017). Remarkably, there have been much evidence for the efficacy of Lira treatment, and Lira seems to be a promising drug in the AD therapeutics. However, only a few reports have shown negative results (Egefjord et al. 2012) and a limited effect in patients with mood disorder (Mansur et al. 2017). Currently, further clinical trials for AD patients are still under investigation.
11.7 Other Antidiabetic Drugs
Recent reports demonstrating efficacy of other antidiabetic drugs on cognitive function in rodents and human are listed in Table 11.5. Sulfonylureas, biguanides, α-glucosidase inhibitors (α-GIs), and sodium-glucose cotransporter-2 (SGLT-2) inhibitors are also known as antidiabetic drugs. Sulfonylureas stimulate insulin secretion from β-cells in the pancreas. Glibenclamide, a sulfonylurea drug, improved memory function in the rats intracerebroventicularly injected with Aβ peptide (Baraka and ElGhotny 2010) and in the rats with traumatic brain injury (TBI) (Patel et al. 2010). Also, inhibition of the Sur1-Trpm4 channel by glibenclamide reduces neuroinflammation and ameliorates cognitive impairments in rat and human with subarachnoid hemorrhage (SAH) (Tosun et al. 2013). Although glibenclamide may have protective effects on cognitive function, there have been no studies using AD mouse models. In human studies, there is a report that indicates no association between sulfonylurea and risk of AD (Imfeld et al. 2012), while sulfonylurea would reduce the risk for dementia in T2DM patients (Cheng et al. 2014). Since hypoglycemic attacks may increase the risk for dementia (Whitmer et al. 2009), evaluation of the efficacy of sulfonylurea should be carefully investigated.
Biguanides inhibit glycogenesis in the liver and uptake of glucose from the intestine and improve insulin resistance. A well-known biguanide metformin was reported to attenuate tau phosphorylation in db/db mice (Li et al. 2012) and to have protective effects on cognitive function in combination in HFD mice (Asadbegi et al. 2016; Allard et al. 2016). In human, it is suggested that metformin treatment reduced the risk of cognitive decline in DM patients (Ng et al. 2014; Herath et al. 2016). Also, a meta-analysis suggests that metformin and thiazolidinediones may reduce the incidence rate of dementia with the relative risks, 0.79 and 0.75, respectively (Ye et al. 2016). Efficacy of biguanides for AD patients should be evaluated in the future studies.
α-GIs inhibit postprandial hyperglycemia and would thus inhibit glucotoxicity in the brain. Although there have been no reports of investigation about efficacy on rodents or patients with AD, chronic acarbose treatment may have a protective effect on behavioral impairment (Tong et al. 2015) and alleviated memory impairment (Yan et al. 2015) in SAMP8 mice. The efficacy of α-GIs for the cognitive impairment in DM and AD remains to be elucidated.
SGLT-2 inhibitors are newcomers in antidiabetic drugs. These drugs inhibit reuptake of glucose in the kidney and lower the blood glucose level. Since the term of usage of SGLT-2 inhibitors is not long, there have been few reports studying about its efficacy on cognitive impairment. A recent report demonstrated that empagliflozin treatment ameliorates cardiovascular injury and cognitive dysfunction in db/db mice (Lin et al. 2014). At present, many SGLT-2 inhibitors are used for control of blood glucose levels. Thus, further investigation about its efficacy should be continued.
11.8 Apomorphine (APO)
Lastly, we describe about our recent finding of novel efficacy of apomorphine (APO) for AD. Although APO is well known to be a dopamine agonist for patients with Parkinson’s disease (PD), we have recently found efficacy of APO for cognitive improvement in AD and have also found APO to be effective on brain diabetes.
In the beginning of this century, based on many studies using AD mouse models produced by mutant APP and PS1 genes, anti-Aβ therapies such as Aβ vaccination and anti-Aβ antibodies were thought to be a promising therapeutic strategy for AD. However, it is well known that many clinical trials targeting Aβ in AD patients have failed. While, our previous studies first revealed that oxidative stress-related apoptosis stimulation induced intracellular Aβ42 deposition in contrast to reduction of extracellular Aβ secretion in primary neuronal cultures (Ohyagi et al. 2000). Subsequently, we found intracellular accumulation of Aβ42 to promote the p53 mRNA expression resulting in neuronal apoptosis (Ohyagi et al. 2005). In addition, intracellular Aβ42 was reported to promote apoptosis via various pathways (Ohyagi 2008). Therefore, we did search for novel drugs that may promote intracellular Aβ42 degradation. Using SH-SY5Y cells, we established an assay system for intracellular Aβ degradation and found that treatment with APO, which has been suggested to protect neurons from oxidative stress in PD mouse models and from brain infarction in a gerbil stroke model (Mandel et al. 2004; Castri et al. 2006), accelerated Aβ42 degradation through activating insulin-degrading enzyme (IDE) and proteasome system (Himeno et al. 2011). Furthermore, APO therapy improved memory function and the AD pathology in 3xTg-AD mice (Himeno et al. 2011) (Fig. 11.3).
Efficacy of APO on 3xTg-AD mice (Himeno et al. 2011; Nakamura et al. 2017). (a) Morris water maze (MWM) of the representative 3xTg-AD mice treated with APO. 6-month-old and 12-month-old mice were subcutaneously injected with 5 m/kg APO once a week for 1 month (total five times). After memorizing the platform location, track of 60 s free swimming was analyzed. Both 7- and 13-month-old mice exhibited improvement of spatial memory posttreatment compared to pretreatment. (b) Immunohistochemistry of hippocampus CA1 in 7-month-old mice. Both Aβ42 and p-tau levels were lower in APO-treated mice compared to untreated mice. Bars = 100 μm
Further investigation has revealed that APO treatment may enhance intracellular antioxidative stress system protecting cells from apoptosis (Ma et al. 2011). In addition, DNA microarray analysis has revealed that APO treatment may effect on regulation of cell cycle, which is a quite different characteristic from other kind of dopamine agonists, and upregulates molecules relating to insulin signaling (unpublished data). Taken together, we hypothesized that APO treatment may upregulate IDE through activating insulin signaling. In our recent report (Nakamura et al. 2017), western blotting and immunostaining revealed that IDE was upregulated and two types of serine-phosphorylated insulin receptor substrate-1 (pS616 and pS636+639 IRS-1) were downregulated in APO-treated 3xTg-AD mice brain. Figure 11.4 shows immunostaining data of hippocampus (CA1) in 13-month-old mice in that report (Nakamura et al. 2017). IDE was increased in 3xTg-AD mice compared to non-Tg mice and was further increased by APO treatment, while Aβ was decreased by APO treatment (Fig. 11.4a). In the same 13-month-old mice, pS616 and pS636+639 IRS-1 were increased in 3xTg-AD mice compared to non-Tg mice and were decreased by APO treatment (Fig. 11.4b). All the alterations were statistically significant (Fig. 11.4a, b, right panels), indicating that APO treatment may decrease insulin resistance of neurons (decreases in pS616 and pS636+639 IRS-1) and may enhance insulin signaling associating with IDE upregulation.
Quantitative analysis of immunohistochemistry of hippocampus CA1 (Nakamura et al. 2017). (a) IDE and Aβ. IDE is increased in 3xTg-AD compared to non-Tg mice. APO treatment further increased IDE level. In contrast, Aβ is decreased by APO treatment. Inset shows a solitary neuron. (b) pS616 and pS636+639 IRS-1. Both types of IRS-1 are increased in 3xTg-AD mice compared to non-Tg mice and are decreased by APO treatment. *P < 0.05, **P < 0.01, ***P < 0.001. Bars = 100 μm
Since APO is currently used as a subcutaneous injection drug for PD patients, we checked its effects on five AD patients without DM and have observed slight improvement of memory function (unpublished data). Also, APO treatment may reduce Aβ burden in the brains of PD patients (Yarnall et al. 2016). Thus, APO may be effective on “brain diabetes” as well as PPAR-γ agonists, DPP4 inhibitors, and GLP-1 agonists. In Fig. 11.5, our hypothesis of molecular pathogenesis in AD brain and therapeutic targets of APO therapy are presented. In AD neurons, increased insulin resistance decreases insulin signaling, leading to decreases in IDE levels and increases in GSK-3β, which may accelerate accumulation of both Aβ and p-tau, respectively. Increased Aβ oligomers may inhibit insulin signaling, which may result in a vicious cycle. APO may activate insulin signaling and IDE and may inhibit GSK-3β, thereby inhibiting AD pathology. In this context, APO may become a novel drug for AD targeting glucose-insulin metabolism in neurons. To evaluate the significance of APO treatment, APO effects should be checked in comparison with other DM drugs in the future.
Hypothetic scheme of pathogenesis related to insulin metabolism and therapeutic targets of APO in AD brain (Nakamura et al. 2017). Increased insulin resistance attenuates insulin signaling. Decreased insulin signaling downregulates IDE and upregulates GSK-3β, which increases Aβ oligomers and tau phosphorylation/neurofibrillary tangle, respectively. Increased Aβ42 oligomers attenuate insulin receptor stimulation, thereby fostering vicious cycle. APO treatment may improve insulin resistance, upregulate IDE, and attenuate GSK-3β activity
References
Allard JS, Perez EJ, Fukui K, Carpenter P, Ingram DK, de Cabo R (2016) Prolonged metformin treatment leads to reduced transcription of Nrf2 and neurotrophic factors without cognitive impairment in older C57BL/6J mice. Behav Brain Res 301:1–9
Asadbegi M, Yaghmaei P, Salehi I, Ebrahim-Habibi A, Komaki A (2016) Neuroprotective effects of metformin against Aβ-mediated inhibition of long-term potentiation in rats fed a high-fat diet. Brain Res Bull 121:178–185
Avila J, Wandosell F, Hemandez F (2010) Role of glycogen synthase kinase-3 in Alzheimer’s disease pathogenesis and glycogen synthase kinase-3 inhibitors. Expert Rev Neurother 10:703–710
Baraka A, ElGhotny S (2010) Study of the effect of inhibiting galanin in Alzheimer’s disease induced in rats. Eur J Pharmacol 641:123–127
Barnes JN (2015) Exercise, cognitive function, and aging. Adv Physiol Educ 39:55–62
Bomfim TR, Forny-Germano L, Sathler LB, Brito-Morelia J, Houzel JC, Decker H, Silverman MA, Kazi H, Melo HM, McClean PL, Holscher C, Arnold SE, Talbot K, Klein WL, Munoz DP, Ferreira ST, De Felice FG (2012) An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer’s disease-associated Aβ oligomers. J Clin Invest 122:1339–1353
Boutajangout A, Wisniewski T (2014) Tau based therapeutic approaches for Alzheimer’s disease. Gerontology 60:381–385
Brabazon F, Wilson CM, Jaiswal S, Reed J, Frey WH (2017) Nd, Byrnes KR. Intranasal insulin treatment of an experimental model of moderate traumatic brain injury. J Cereb Blood Flow Metab 37:3203–3218
Castri P, Busceti C, Battaglia G, Girardi F, Cavallari M, Orzi F, Fornai F (2006) Protection by apomorphine in two independent models of acute inhibition of oxidative metabolism in rodents. Clin Exp Hypertens 28:387–394
Chen S, Sun J, Zhao G, Guo A, Chen Y, Fu R, Deng Y (2017) Liraglutide improves water maze learning and memory performance while reduces hyperphosphorylation of tau and neurofilaments in APP/PS1/tau triple transgenic mice. Neurochem Res 42:2326–2335
Cheng C, Lin CH, Tsai YW, Tsai CJ, Chou PH, Lan TH (2014) Type 2 diabetes and antibiotic medications in relation to dementia diagnosis. J Gerontol A Biol Sci Med Sci 69:1299–1305
Chou PS, Ho BL, Yang YH (2017) Effects of pioglitazone on the incidence of dementia in patients with diabetes. J Diabetes Complicat 31:1053–1057
Claxton A, Baker LD, Wilkinson CW, Trittschuh EH, Chapman D, Watson GS, Cholerton B, Plymate SR, Arbuckle M, Craft S (2013) Sex and Apo E genotype differences in treatment response to two doses of intranasal insulin in adults with mild cognitive impairment or Alzheimer’s disease. J Alzheimers Dis 35:789–797
Claxton A, Baker LD, Hanson A, Trittschuh EH, Cholerton B, Morgan A, Callaghan M, Arbuckle M, Behl C, Craft S (2015) Long-acting intranasal insulin detemir improves cognition for adults with mild cognitive impairment or early-stage Alzheimer’s disease dementia. J Alzheimers Dis 44:897–906
Craft S, Baker LD, Montine TJ, Minoshima S, Watson GS, Claxton A, Arbuckle M, Callaghan M, Tsai E, Plymate SR, Green PS, Leverenz J, Cross D, Gerton B (2012) Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol 69:29–38
Crane PK, Walker R, Hubbard RA, Li G, Nathan DM, Zheng H, Haneuse S, Craft S, Montine TJ, Kahn SE, McCormick W, McCurry SM, Bowen JD, Larson EB (2013) Glucose levels and risk of dementia. N Engl J Med 369:540–548
D’Amico M, Di Filippo C, Marfella R, Abbatecola AM, Ferraraccio F, Rossi F, Paolisso G (2010) Long-term inhibition of dipeptidyl peptidase-4 in Alzheimer’s prone mice. Exp Gerontol 45:202–207
De la Monte SM (2014) Type 3 diabetes is sporadic Alzheimer’s disease: mini-review. Eur Neuropsychopharmacol 24:1954–1960
Doody RS, Thomas RG, Farlow M, Iwatsubo T, Vellas B, Joffe S, Kieburtz K, Raman R, Sun X, Aisen PS, Siemers E, Liu-Seifert H, Mohs R (2014) Alzheimer’s disease cooperative study steering committee: Solanezumab study group. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer’s disease. N Engl J Med 370:311–321
Egefjord L, Gejl M, Moller A, Braendgaard H, Gottrup H, Antropova O, Moller N, Poulsen HE, Gjedde A, Brock B, Rungby J (2012) Effects of liraglutide on neurodegeneration, blood flow and cognition in Alzheimer’s disease – protocol for a controlled, randomized double-blinded trial. Dan Med J 59:A4519
El-Sahar AE, Safer MM, Zaki HF, Attia AS, Ahi-Shoka AA (2015) Sitagliptin attenuates transient cerebral ischemia/reperfusion injury in diabetic rats: implication of the oxidative-inflammatory-apoptotic pathway. Life Sci 126:81–86
Escribano L, Simon AM, Gimeno E, Cuadrado-Tejedor M, Lopez de Maturana R, Garcia-Osta A, Rcobaraza A, Perez-Mediavilla A, Del Rio J, Frechilla D (2010) Rosiglitazone rescues memory impairment in Alzheimer’s transgenic mice: mechanisms involving a reduced amyloid and tau pathology. Neuropsychopharmacology 35:1593–1604
Faivre E, Hölscher C (2013) D-Ala2GIP facilitated synaptic plasticity and reduces plaque load in aged wild type mice and in an Alzheimer’s disease mouse model. J Alzheimers Dis 35:267–283
Farzampour S, Majdi A, Sadigh-Eteghad S (2016) Intranasal insulin treatment improves memory and learning in a rat amyloid-β model of Alzheimer’s disease. Physiol Int 103:344–353
Ferreira ST, Lourenco MV, Olivia MM, De Felice FG (2015) Soluble amyloid-β oligomers as synaptotoxins leading to cognitive impairment in Alzheimer’s disease. Front Cell Neurosci 9:191
Galimberti D, Scarpini E (2017) Pioglitazone for the treatment of Alzheimer’s disease. Expert Opin Investig Drugs 26:97–101
Gault VA, Porter WD, Flatt PR, Hölscher C (2010) Actions of exendin-4 therapy on cognitive function and hippocampal synaptic plasticity in mice fed a high-fat diet. Int J Obe (Lond) 34:1341–1344
Gault VA, Lennox R, Flatt PR (2015) Sitagliptin, a dipeptidyl peptidase-4 inhibitor, improves recognition memory, oxidative stress and hippocampal neurogenesis and upregulates key genes involved in cognitive decline. Diabetes Obes Metab 17:403–413
Gumuslu E, Mutlu O, Celikyurt IK, Ulak G, Akar F, Erden F, Ertan M (2016) Exenatide enhances cognitive performance and upregulates neurotrophic factor gene expression levels in diabetic mice. Fundam Clin Pharmacol 30:376–384
Haas CB, Kalinine E, Zimmer ER, Hansel G, Brochier AW, Oses JP, Portela LV, Muller AP (2016) Brain insulin administration triggers distinct cognitive and neurotrophic responses in young and aged rats. Mol Neurobiol 53:5807–5817
Hansen HH, Barkholt P, Fabricius K, Jelsing J, Terwel D, Pyke C, Knudsen LB, Vrang N (2016) The GLP-1 receptor agonist liraglutide reduces pathology-specific tau phosphorylation and improves motor function in a transgenic hTauP301L mouse model of tauopathy. Brain Res 1634:158–170
Harrington C, Sawchak S, Chiang C, Davies J, Donovan C, Saunders AM, Irizarry M, Jeter B, Zvartau-Hind M, van Dyck CH, Gold M (2011) Rosiglitazone does not improve cognition or global function when used as adjunctive therapy to AChE inhibitors in mild-to-moderate Alzheimer’s disease: two phase 3 studies. Curr Alzheimer Res 8:592–606
Hassing LB, Johansson B, Nilsson SE, Berg S, Pedersen NL, Gatz M, McClearn G (2002) Diabetes mellitus is a risk factor for vascular dementia, but not for Alzheimer’s disease: a population-based study of the oldest old. Int Psychogeriatr 14:239–248
Heneka MT, Fink A, Doblhammer G (2015) Effect of pioglitazone medication on the incidence of dementia. Ann Neurol 78:284–294
Herath PM, Cherbuin N, Eramudugolla R, Anstey KJ (2016) The effect of diabetes medication on cognitive function: evidence from the PATH through life study. Biomed Res Int 2016:7208429
Hildreth KL, Van Pelt RE, Moreau KL, Grigsby J, Hoth KF, Pelak V, Anderson CA, Parnes B, Kittelson J, Wolfe P, Nakamura T, Linnebur SA, Trujillo JM, Aquilante CL, Schwartz RS (2015) Effects of pioglitazone or exercise in older adults with mild cognitive impairment and insulin resistance: a pilot study. Dement Geriatr Cogn Dis Extra 5:51–63
Himeno E, Ohyagi Y, Ma L, Nakamura N, Miyoshi K, Sakae N, Motomura K, Soejima N, Yamasaki R, Hashimoto T, Tabira T, LaFerla FM, Kira J (2011) Apomorphine treatment in Alzheimer mice promoting amyloid-β degradation. Ann Neurol 69:248–256
Hokama M, Oka S, Leon J, Ninomiya T, Honda H, Sasaki K, Iwaki T, Ohara T, Sasaki T, LaFerla FM, Kiyohara Y, Nakabeppu Y (2014) Altered expression of diabetes-related genes in Alzheimer’s disease brains: the Hisayama study. Cereb Cortex 24:2476–2488
Hu X, Li X, Zhao M, Gottesdiener A, Luo W, Paul S (2014) Tau pathogensis is promoted by Aβ1-42 but not Aβ1-40. Mol Neurodegener 9:52
Imfeld P, Bodmer M, Jick SS, Meier CR (2012) Metformin, other antidiabetic drugs, and risk of Alzheimer’s disease: a population-based case-control study. J Am Geriatr Soc 60:916–921
Isik AT, Soysal P, Yay A, Usarel C (2017) The effects of sitagliptin, a DPP-4 inhibitor, on cognitive functions in elderly diabetic patients with or without Alzheimer’s disease. Diabetes Res Clin Pract 123:192–198
Jack CR Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen RC, Trojanowski JQ (2010) Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol 9:119–128
Kamei N, Tanaka M, Choi H, Okada N, Ikeda T, Itokazu R, Takeda-Morishima M (2017) Effect of an enhanced nose-to-brain delivery of insulin on mild and progressive memory loss in the senescence-accelerated mouse. Mol Pharm 14:916–927
Kosaraju J, Gali CC, Khatwal RB, Dubala A, Chinni S, Holsinger RM, Madhunapantula VS, Mathureddy Nataraj SK, Basavan D (2013a) Saxagliptin: a dipeptidyl peptidase-4 inhibitor ameliorates streptozotocin induced Alzheimer’s disease. Neuropharmacology 72:291–300
Kosaraju J, Murthy V, Khatwal RB, Dubala A, Chinni S, Muthureddy Nataraj SK, Basavan D (2013b) Vildagliptin: an anti-diabetes agent ameliorates cognitive deficits and pathology observed in streptozotocin-induced Alzheimer’s disease. J Pharm Pharmacol 65:1773–1784
Kosaraju J, Holsinger RM, Guo L, Tam KY (2016) Linagliptin, a dipeptidyl peptidase-4 inhibitor, mitigates cognitive deficits and pathology in the 3xTg-AD mouse model of Alzheimer’s disease. Mol Neurobiol (Epub ahead of print)
Leibson CL, Rocca WA, Hanson VA, Cha R, Kokmen E, O’Brien PC, Palumbo PJ (1997) Risk of dementia among persons with diabetes mellitus: a population-based cohort study. Am J Epidemiol 145:301–308
Lennox R, Flatt PR, Gault VA (2014a) Lixisenatide improves recognition memory and exerts neuroprotective actions in high-fat fed mice. Peptides 61:38–47
Lennox R, Porter DW, Flatt PR, Hölscher C, Irwin N, Gault VA (2014b) Comparison of the independent and combined effects of sub-chronic therapy with metformin and a stable GLP-1 receptor agonist on cognitive function, hippocampal synaptic plasticity and metabolic control in high-fat fed mice. Neuropharmacology 86:22–30
Li J, Deng J, Sheng W, Zuo Z (2012) Metformin attenuates Alzheimer’s disease-like neuropathology in obese, leptin-resistant mice. Pharmacol Biochem Behav 101:564–574
Lin B, Koibuchi N, Hasegawa Y, Sueta D, Toyama K, Uekawa K, Ma M, Nakagawa T, Kusaka H, Kim-Mitsuyama S (2014) Glycemic control with empagliflozin, a novel selective SGLT2 inhibitor, ameliorates cardiovascular injury and cognitive dysfunction in obese and type 2 diabetic mice. Cardiovasc Diabetol 13:148
Liu LP, Yan TH, Jiang LY, Hu W, Hu M, Wang C, Zhang Q, Long Y, Wang JQ, Li YQ, Hu M, Hong H (2013) Pioglitazone ameliorates memory deficits in streptozotocin-induced diabetic mice by reducing brain β-amyloid through PPARγ activation. Acta Pharmacol Sin 34:455–463
Long-Smith CM, Manning S, McClean PL, Coakley MF, O’Halloran DJ, Hölscher C, O’Neill C (2013) NeuroMolecular Med 15:102–114
Luchsinger JA, Tang MX, Stern Y, Shea S, Mayeux R (2001) Diabetes mellitus and risk of Alzheimer’s disease and dementia with stroke in a multiethnic cohort. Am J Epidemiol 154:635–641
Luo D, Hou X, Hou L, Wang M, Xu S, Dong C, Liu X (2011) Effect of pioglitazone on altered expression of Ab metabolism-associated molecules in the brain of fructose-drinking rats, a rodent model of insulin resistance. Eur J Pharmacol 664:14–19
Ma L, Ohyagi Y, Nakamura N, Iinuma KM, Miyoshi K, Himeno E, Soejima N, Yanagihara YT, Sakae N, Yamasaki R, Kira J (2011) Activation of glutathione peroxidese and inhibition of p53-related apoptosis by apomorphine. J Alzheimers Dis 27:225–237
Ma T, Du X, Pick JE, Sui G, Brownlee M, Klann E (2012) Glucagon-like peptide-1 cleavage product GLP-1 (9-36) amide rescues synaptic plasticity and memory deficits in Alzheimer’s disease model mice. J Neurosci 32:13701–13708
Ma L, Shao Z, Wang R, Zhao Z, Dong W, Zhang J, Zhang X, Sheng S, Ji Z, Zhang J (2015) Rosiglitazone improves learning and memory ability in rats with type 2 diabetes through the insulin signaling pathway. Am J Med Sci 350:121–128
MacKnight C, Rockwood K, Awalt E, McDowell I (2002) Diabetes mellitus and the risk of dementia, Alzheimer’s disease and vascular cognitive impairment in the Canadian study of health and aging. Dement Geriatr Cogn Disord 14:77–83
Maimaiti S, Anderson KL, DeMoll C, Brewer LD, Rauh BA, Gant JC, Blalock EM, Porter NM, Thibault O (2016) Intranasal insulin improves age-related cognitive deficits and reverses electrophysiological correlates of brain aging. J Gerontol A Biol Sci Med Sci 71:30–39
Mandel S, Maor G, Youdim MB (2004) Iron and α-synuclein in the substantia nigra of MPTP-treated mice: effect of neuroprotective drugs R-apomorphine and green tea polyphenol (−)-epigallocatechin-3-gallate. J Mol Neurosci 24:401–416
Mansur RB, Ahmed J, Cha DS, Woldeyohannes HO, Subramaniapillai M, Lovshin J, Lee JG, Lee JH, Brietzke E, Reininghaus EZ, Sim K, Vinberg M, Rasgon N, Hajek T, Mclntyre RS (2017) Liraglutide promotes improvements in objective measures of cognitive dysfunction in individuals with mood disorders: a pilot, open-label study. J Affect Disord 207:114–120
Mao YF, Guo Z, Zheng T, Jiang Y, Yan Y, Yin X, Chen Y, Zhang B (2016) Intranasal insulin alleviates cognitive deficits and amyloid pathology in young adult APPswe/PS1dE9 mice. Aging Cell 15:893–902
Matsuzaki T, Sasaki K, Tanizaki Y, Hata J, Fujimi K, Matsui Y, Sekita A, Suzuki SO, Kanba S, Kiyohara Y, Iwaki T (2010) Insulin resistance is associated with the pathology of Alzheimer disease: the Hisayama study. Neurology 75:764–770
McClean PL, Hölscher C (2014) Liraglutide can reverse memory impairment, synaptic loss and reduce plaque load in aged APP/PS1 mice, a model of Alzheimer’s disease. Neuropharmacology 76(Pt A):57–67
McClean PL, Parthsarathy V, Faivre E, Hölscher C (2011) The diabetes drug liraglutide prevents degenerative processes in a model of Alzheimer’s disease. J Neurosci 31:6587–6594
Miller BW, Willett KC, Desilets AR (2011) Rosiglitazone and pioglitazone for the treatment of Alzheimer’s disease. Ann Pharmacother 45:1416–1424
Miners JS, Barua N, Kehoe PG, Gill S, Love S (2011) Aβ-degrading enzymes: potential for treatment of Alzheimer disease. J Neuropathol Exp Neurol 70:944–959
Murakami K, Horikoshi-Sakurababa Y, Murata N, Noda Y, Masuda Y, Kinoshita N, Hatsuta H, Murayama S, Shirasawa T, Shimizu T, Irie K (2010) Monoclonal antibody against the turn of the 42-reidue amyloid β-protein at positions 22 and 23. ACS Chem Neurosci 1:747–756
Nakamura N, Ohyagi Y, Imamura T, Yanagihara YT, Iinuma KM, Soejima N, Murai H, Yamasaki R, Kira J (2017) Apomorphine therapy for neuronal insulin resistance in a mouse model of Alzheimer’s disease. J Alzheimers Dis 58:1151–1161
Ng TP, Feng L, Yap KB, Lee TS, Tan CH, Winblad B (2014) Long-term metformin usage and cognitive function among older adults with diabetes. J Alzheimers Dis 41:61–68
Ohara T, Doi Y, Ninomiya T, Hirakawa Y, Hata J, Iwaki T, Kanba S, Kiyohara Y (2011) Glucose tolerance status and risk of dementia in the community: the Hisayama study. Neurology 77:1126–1134
Ohyagi Y (2008) Intracelluler amyloid β-protein as a therapeutic target for treating Alzheimer’s disease. Curr Alzheimer Res 5:555–561
Ohyagi Y, Yamada T, Nishioka K, Clarke NJ, Tomlinson AJ, Naylor S, Nakabeppu Y, Kira J, Younkin SG (2000) Selective increase in cellular Aβ42 is related to apoptosis but not necrosis. Neuroreport 11:167–171
Ohyagi Y, Asahara H, Chui DH, Tsuruta Y, Sakae N, Miyoshi K, Yamada T, Kikuchi H, Taniwaki T, Murai H, Ikezoe K, Furuya H, Kawarabayashi T, Shoji M, Checler F, Iwaki T, Makifuchi T, Takeda K, Kira J, Tabira T (2005) Intracellular Aβ42 activates p53 promoter: a pathway to neurodegeneration in Alzheimer’s disease. FASEB J 19:255–257
Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM (1999) Diabetes mellitus and the risk of dementia: the Rotterdam study. Neurology 53:1937–1942
Palleria C, Leo A, Andreozzi F, Citraro R, Iannone M, Spiga R, Sesti G, Constanti A, De Sarro G, Arturi F, Russo E (2017) Liraglutide prevents cognitive decline in a rat model of streptozotocin-induced diabetes independently from its peripheral metabolic effects. Behav Brain Res 321:157–169
Patel AD, Gerzanich V, Genz Z, Simard JM (2010) Glibenclamide reduces hippocampal injury and preserves rapid spatial learning in a model of traumatic brain injury. J Neuropathol Exp Neurol 69:1177–1190
Pathan AR, Gaikwad AB, Viswanad B, Ramarao P (2008) Rosiglitazone attenuates the cognitive deficits induced by high fat diet feeding in rats. Eur J Pharmacol 589:176–179
Peila R, Rodriguez BL, Launer LJ (2002) Honolulu-Asia aging study. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: the Honolulu-Asia aging study. Diabetes 51:1256–1262
Pintata H, Tanajak P, Pratchayasakul W, Sa-Nguanmoo P, Chunchai T, Satjaritanun P, Leelarphat L, Chattipatorn N, Chattipakorn SC (2016) Energy restriction combined with dipeptidyl peptidase-4 inhibitor exerts neuroprotection in obese male rats. Br J Nutr 17:1–9. (Epub ahead of print)
Pipatpiboon N, Pintana H, Pratchayasakul W, Chattipakorn N, Chattipakorn SC (2013) DPP4-inhibitor improves neural insulin receptor function, brain mitochondrial function and cognitive function in rats with insulin resistance induced by high-fat diet consumption. Eur J Neurosci 37:839–849
Porter DW, Kerr BD, Flatt PR, Hölscher C, Gault VA (2010) Four weeks administration of Liraglutide improves memory and learning as well as glycemic control in mice with high fat dietary-induced obesity and insulin resistance. Diabetes Obes Metab 12:891–899
Porter DW, Irwin N, Flatt PR, Hölscher C, Gault GA (2011) Prolonged GIP receptor activation improves cognitive function, hippocampal synaptic plasticity and glucose homeostasis in high-fat fed mice. Eur J Pharmacol 650:688–693
Qi L, Ke L, Liu X, Liao L, Ke S, Liu X, Wang Y, Lin X, Zhou Y, Wu L, Chen Z, Liu L (2016) Subcutaneous administration of liraglutide ameliorates learning and memory impairment by modulating tau hyperphosphorylation via the glycogen synthase kinase-3β pathway in an amyloid β protein induced Alzheimer disease mouse model. Eur J Phramacol 783:23–32
Qin L, Chong T, Rodriguez R, Pugazhenthi S (2016) Glucagon-like peptide-1-mediated modulation of inflammatory pathways in the diabetic brain: relevance to Alzheimer’s disease. Curr Alzheimer Res 13:1346–1355
Rajasekar N, Nath C, Hanif K, Shukla R (2017) Intranasal insulin improves cerebral blood flow, Nrf-2 expression and BDNF in STZ (ICV)-induced memory impaired rats. Life Sci 173:1–10
Rizzo MR, Barbieri M, Boccardi V, Angellotti E, Marfella R, Paolisso G (2014) Dipeptidyl peptidase-4 inhibitors have protective effect on cognitive impairment in aged diabetic patients with mild cognitive impairment. J Gerontol A Biol Sci Med Sci 69:1122–1131
Sakr HF (2013) Effect of sitagliptin on the working memory and reference memory in type 2 diabetic Sprague-Dawley rats: possible role of adiponectin receptor 1. J Phyasiol Pharmacol 64:613–623
Salloway S, Sperling R, Fox NC, Blennow K, Klunk W, Raskind M, Sabbagh M, Honig LS, Porsteinsson AP, Ferris S, Reichert M, Ketter N, Nejadnik B, Guenzler V, Miloslavsky M, Wang D, Lu Y, Lull J, Tudor IC, Liu E, Grundman M, Yuen E, Black R, Brashear HR, Bapineuzumab 301 and 302 Clinical Trial Investigators (2014) Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. N Engl J Med 370:322–333
Sato T, Hanyu H, Hirao K, Kanetaka H, Sakurai H, Iwamoto T (2011) Efficacy of PPAR-γ agonist pioglitazone in mild Alzheimer disease. Neurobiol Aging 32:1626–1633
Seaquist ER, Miller ME, Fonseca V, Ismail-Beigi F, Launer LJ, Punthakee Z, Sood A (2013) Effect of thiazolidinediones and insulin on cognitive outcomes in ACCORD-MIND. J Diabetes Complicat 27:485–491
Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT (2011) Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med 1:a006189
Siemers ER, Sundell KL, Carlson C, Case M, Sethuraman G, Liu-Seifert H, Dowsett SA, Pontecorvo MJ, Dean RA, Demattos R (2016) Phase 3 solanezumab trials: secondary outcomes in mild Alzheimer’s disease patients. Alzheimers Dement 12:110–120
Sripetchwandee J, Pipatpiboon N, Pratchayasakul W, Chattipakorn N, Chattipakorn SC (2014) DPP-4 inhibitor and PPARγ agonist restore the loss of CA1 dendritic spines in obese insulin-resistant rats. Arch Med Res 45:547–552
Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A, Fuino RL, Kawaguchi KR, Samoyedny AJ, Wilson RS, Arvanitakis Z, Schneider JA, Wolf BA, Bennett DA, Trojanowski JQ, Arnold SE (2012) Demonstrated brain insulin resistance in Alzheimer’s disease patients associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest 122:1316–1338
Tasci I, Naharci MI, Bozoglu E, Safer U, Aydogdu A, Yilmaz BF, Yilmaz G, Doruk H (2013) Cognitive and functional influences of vildagliptin, a DPP-4 inhibitor, added to ongoing metformin therapy in elderly with type 2 diabetes. Endocr Metab Immune Disord Drug Targets 13:256–263
Toba J, Nikkuni M, Ishizeki M, Yoshii A, Watamura N, Inoue T, Ohshima T (2016) PPARγ agonist pioglitazone improves cerebellar dysfunction at pre-Aβ deposition stage in APPswe/PS1dE9 Alzheimer’s disease model mice. Biochem Biophys Res Commun 473:1039–1044
Tong JJ, Chen GH, Wang F, Li XW, Cao L, Sui X, Tao F, Yan WW, Wei ZJ (2015) Chronic acarbose treatment alleviates age-related behavioral and biochemical changes in SAMP8 mice. Behav Brain Res 284:138–152
Tosun C, Kurland DB, Mehta R, Castellani RJ, deJong JL, Kwon MS, Woo SK, Gerzanich V, Simard JM (2013) Inhibition of the Sur1-Trpm4 channel reduces neuroinflammation and cognitive impairment in subarachnoid hemorrhage. Stroke 44:3522–3528
Tsai TH, Sun CK, Su CH, Sung PH, Chua S, Zhen YY, Leu S, Chang HW, Yang JL, Yip HK (2015) Sitagliptin attenuated brain damage and cognitive impairment in mice with chronic cerebral hypo-perfusion through suppressing oxidative stress and inflammatory reaction. J Hypertens 33:1001–1013
Vandal M, White PJ, Tremblay C, St-Amour I, Chevrier G, Emond V, Lefrrancois D, Virgili J, Planel E, Giguere Y, Marette A, Calon F (2014) Insulin reverses the high-fat diet-induced increase in brain Aβ and improves memory in an animal model of Alzheimer disease. Diabetes 63:4291–4301
Vandal M, White PJ, Chevrier G, Tremblay C, St-Amour I, Planel E, Marette A, Calon F (2015) Age-dependent impairment of glucose tolerance in the 3xTg-AD mouse model of Alzheimer’s disease. FASEB J 29:4273–4284
Wang H, Chen F, Zhong KL, Tang SS, Hu M, Long Y, Miao MX, Liao JM, Sun HB, Hong H (2016) PPARγ agonists regulate bidirectional transport of amyloid-β across the blood-brain barrier and hippocampus plasticity in db/db mice. Br J Pharmacol 173:372–385
Watson GS, Cholerton BA, Reger MA, Baker LD, Plymate SR, Asthana S, Fishel MA, Kulstad JJ, Green PS, Cook DG, Kahn SE, Keeling ML, Craft S (2005) Preserved cognition in patients with early Alzheimer disease and amnestic mild cognitive impairment during treatment with rosiglitazone: a preliminary study. Am J Geriatr Psychiatry 13:950–958
Whitmer RA, Karter AJ, Yaffe K, Quesenberry CP Jr, Selby JV (2009) Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA 301:1565–1572
Yan WW, Chen GH, Wang F, Tong JJ, Tao F (2015) Long-term acarbose administration alleviating the impairment of spatial learning and memory in the SAMP8 mice was associated with alleviated reduction of insulin system and acetylated H4K8. Brain Res 1603:22–31
Yang Q, Guan KL (2007) Expanding mTOR signaling. Cell Res 17:666–681
Yarnall AJ, Lashley T, Ling H, Lees AJ, Coleman SY, O’Sullivan SS, Compta Y, Revesz T, Burn DJ (2016) Apomorphine: a potential modifier of amyloid deposition in Parkinson’s disease? Mov Disord 31:668–675
Ye F, Luo YJ, Xiao J, Yu NW, Yi G (2016) Impact of insulin sensitizers on the incidence of dementia: a meta-analysis. Dement Geriatr Cogn Disord 41:251–260
Yin QQ, Pei JJ, Xu S, Luo DZ, Dong SQ, Sun MH, You L, Sun ZJ, Liu XP (2013) Pioglitazone improves cognitive function via increasing insulin sensitivity and strengthening antioxidant defence system in fructose-drinking insulin resistance rats. PLoS One 8:e59313
Yu Y, Li X, Blanchard J, Li Y, Iqbal K, Liu F, Gong CX (2015) Insulin sensitizers improve learning and attenuate tau hyperphosphorylation and neuroinflammation in 3xTg-AD mice. J Neural Transm (Vienna) 122:593–606
Zanotto C, Simao F, Gasparin MS, Biasibetti R, Tortorelli LS, Nardin P, Goncalves CA (2017) Exendin-4 reverses biochemical and functional alterations in the blood-brain and blood-CSF barriers in diabetic rats. Mol Neurobiol 54:2154–2166
Zhang Y, Dai CL, Chen Y, Iqbal K, Liu F, Gong CX (2016) Intranasal insulin prevents anesthesia-induced spatial learning and memory deficit in mice. Sci Rep 6:21186
Acknowledgments
I appreciate to Dr. Frank M. LaFerla for providing 3xTg-AD mice. This work was supported by a Grant-in-Aid for Scientific Research (C) from Japan Society for the Promotion of Science (Y.O., 22590936, 26461274), by Japan Science and Technology Agency, and by Kakihara Science and Technology Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Ohyagi, Y., Miyoshi, K., Nakamura, N. (2019). Therapeutic Strategies for Alzheimer’s Disease in the View of Diabetes Mellitus. In: Nakabeppu, Y., Ninomiya, T. (eds) Diabetes Mellitus. Advances in Experimental Medicine and Biology, vol 1128. Springer, Singapore. https://doi.org/10.1007/978-981-13-3540-2_11
Download citation
DOI: https://doi.org/10.1007/978-981-13-3540-2_11
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-3539-6
Online ISBN: 978-981-13-3540-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)