Abstract
Multiple organ dysfunction syndrome (MODS) is a life-threatening condition with high morbidity and mortality. Mitochondria are multifunctional organelles, whose failure triggers multiple organ dysfunction and is directly associated with patient’s vicious outcome. Physiologically, mitochondria undergo continuous fission, fusion, biogenesis, and mitophagy (selective mitochondrial autophagy) to maintain homeostasis, whose disruption may heavily impact the mitochondrial quality and result in damaged cell and organ functions under pathological conditions such as severe trauma, shock, and sepsis. Mitochondrial quality imbalance is a key step in MODS process, and rebalancing the mitochondrial quality may be a promising approach in the treatment of MODS following severe trauma, shock, and sepsis.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
4.1 Introduction
As medical techniques greatly develop, some diseases have been effectively controlled or got better care; however, severe trauma, shock, sepsis, severe pancreatitis, etc. which induced multiple organ dysfunction syndrome (MODS) have not been controlled well yet. It is reported that approximately 50% of patients in ICU can develop MODS, and their mortality is up to 30–100%. Patients with two organ dysfunction have more than 70% mortality rate [1]. However, such high mortality has not changed much since the 1980s.
Several mechanisms have been put forward to explain the process of MODS, such as immunosuppression and hypometabolism, ischemia and hypoxia induced by hypoperfusion, uncontrolled inflammation-induced extensive tissue damage, and so on. Some measures have been widely applied, such as anti-infection therapy, blood purification and immune adsorption, microcirculation modulation and hemodynamic support, etc., which made some contributions to the improvement of the outcome to these critical diseases. However, studies toward further understanding of MODS are still necessary. This review will focus on the mitochondrial quality imbalance and its role in MODS following severe trauma, shock, and sepsis; this might help to provide the better prevention and management of MODS.
4.2 Role of Mitochondrial Quality Imbalance in Diseases
Mitochondria are power houses of cells and responsible for aerobic respiration. Mitochondria not only produce ATP through oxidative phosphorylation but also participate in the regulation of metabolism, calcium homeostasis [2], oxidative signaling [3], steroid synthesis [4], etc. In pathologic conditions, mitochondria also induce oxidative injury and calcium overload, initiate cell apoptosis, etc. Mitochondria undergo continuous fission, fusion, biogenesis, and mitophagy to maintain the good quality and function of mitochondrial population. More and more studies revealed that mitochondrial quality imbalance plays important role in various pathological conditions.
4.2.1 Role of Mitochondria Dysfunction in Various Diseases
Mitochondrial dysfunction is involved in a wide range of clinical diseases, ranging from heart and brain diseases and diabetes to acute illnesses such as sepsis, traumatic injuries, and poisoning. As mitochondria are the center of cellular function and energy production, it is not surprising that these disease processes result in bioenergetic dysfunction [5]. Oxidative injury is considered as another major source of cell damage in various organ systems. Oxidative stress initiates diverse pathological shifts, such as mitochondrial DNA (mtDNA) mutation and apoptosis [6], and eventually boosts cell injuries. Since ROS generates in mitochondria, mitochondria are easily attacked by ROS. ROS-induced mitochondrial DNA (mtDNA) mutation and depletion would result in more downstream mitochondrial stresses [6].
Oxidative stress is one of the important pathological factors of chronic diseases including cardiovascular diseases (i.e., atherosclerosis, hypertension, cardiomyopathy, and congestive heart failure), neurodegeneration diseases (i.e., Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis), asthma and chronic obstructive pulmonary disease (COPD), and renal failure. In acute pathologies such as trauma, stroke, and acute myocardial infarction, oxidative stress is also an important inducing factor of secondary injury of organs and tissues.
Besides, mitochondria store calcium and sustain calcium hemostasis, which are the important cytosolic buffer for calcium. In pathological conditions, mitochondrial permeability transition pore (mPTP) and mitochondrial calcium uniporter (MCU) complex might mediate excessive calcium inflow toward mitochondria and provoke calcium overload, which induces apoptosis afterward [7,8,9]. Intensive long-lasting excitatory stimuli eventually cause a persistent mitochondrial calcium accumulation and cytotoxicity; this is a mechanism that has been implicated in the pathogenesis of neurological disorders such as Alzheimer’s and Huntington’s disease and schizophrenia [10]. Mitochondria-derived cell injury can finally initiate intrinsic apoptosis, which has been widely studied in many cell types (as reviewed by Wu et al.) [11], including immune cells, cardiomyocytes, hepatocytes, hemotocytes, etc. Damaged mitochondria can be fixed or cleared to avoid the final detrimental effect. This process relies on mitochondrial quality control. However, the underlying mechanism for mitochondrial quality control is not fully understood yet.
4.2.2 Mitochondrial Quality Balance Is a Dynamic Process to Regulate Mitochondrial Function
Continuous fission, fusion, mitophagy, and biogenesis sustain mitochondrial quality balance, including adequate mitochondrial mass and function, which is indispensable for normal cell function. In mitochondria fission, parental mitochondrion divides into at least two mitochondria to achieve cell mitogen or meet energy demand; in mitochondria fusion, mitochondria merge to achieve self-renewal and promote mitochondrial function; in mitophagy, damaged mitochondria are segregated and degraded by selective autophagy process to diminish detrimental effects; in mitochondria biogenesis, mitochondrial functional or structural proteins are synthesized to replenish mitochondrial pool.
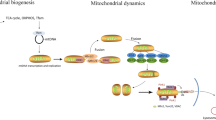
Basic studies indicated that mitochondrial characteristics vary from one kind of cell to another. Mitochondria account for ~40% of cardiomyocytes cell volume while 10–20% in vascular smooth muscle cells or vascular endothelia cells; cardiomyocyte completes rejuvenation in 2–3 days while vascular endothelial cells or intestinal epithelial cells in 2–3 h. Despite such heterogeneity, mitochondria share almost common fission, fusion, mitophagy, and biogenesis mechanisms (Fig. 4.1).
4.2.2.1 Mitochondrial Fission
Mitochondrial fission is essential for stabilizing mitochondrial genome, regulating energy production, and modulating oxidative signaling [12,13,14,15]. The mechanism of mitochondrial fission is complex and has attracted much attention recently. Dynamin-related protein (DRP1) is recruited from cytoplasm to bind receptors (e.g., FIS1, MFF, Mid49/51) on mitochondria outer membrane [16]. Then, DRP1 oligomerizes and hydrolizes GTP to mediate constriction of the fission site [17, 18]. DRP1 possesses an N-terminal GTPase domain thought to provide mechanical force, a dynamin-like middle domain, and a GTPase effector domain (GED) located in the C-terminal region domain. DRP1 is predicted to exist as a T-shaped dimer or tetramer that contains a head (containing GTPase domain), leg (VD), and stalk (middle and GED domains). GTP induces the rearrangement of the head and stalk, which generates a force ultimately resulting in membrane constriction [19].
Phosphorylation, ubiquitination, S-nitrosylation, SUMOylation, and O-GlcNAcylation of DRP1 regulate mitochondrial fission. The phosphorylation of DRP1 is the most studied. Generally, the phosphorylation of DRP1 Ser616 activates DRP1, while DRP1 Ser637/600/716/656 phosphorylation inhibits it [19]. CaMKII [20], calcineurin [21, 22], PINK1 [23], Parkin [24], RhoA/ROCK pathway [25, 26], ERK [27, 28], cyclins [29], and Cdk5 [30] are proven to be responsible for the phosphorylation or dephosphorylation of DRP1. March5 (also known as MITOL) may ubiquitinate DRP1 or MiD49 on the MOM and reduces mitochondrial fragmentation [31,32,33]. S-nitrosylation of DRP1 at Cys-644 may enhance GTPase activity and DRP1 oligomerization and results in excessive mitochondrial fission in neurons and neuronal damage [34]. In addition, increased DRP1 SUMOylation by MAPL overexpression may upregulate mitochondrial fission [35], and decreased DRP1 SUMOylation (i.e., deSUMOylation) by SENP5 may rescue the mitochondrial fission [36]; O-GlcNAcylation at Thr-585 and Thr-586 may reduce the mitochondrial fragmentation [37].
4.2.2.2 Mitochondrial Fusion
The most important function of mitochondrial fusion is to facilitate the heterogeneous mitochondria to mix and exchange content. Mitochondrial fusion participates in the quality control of mtDNA [12] and energy metabolism regulation, e.g., ameliorating oxidative stress, cell differentiation, stress adaption and steroidogenesis, and so on [38,39,40].
Since mitochondria are double-membrane bound organelles, the complete fusion calls for merging of both the outer mitochondrial membrane (OMM) and inner mitochondrial membrane (IMM), either process possesses different mechanisms, respectively. MFN1 and MFN2 form homo-oligomers or hetero-oligomers (i.e., MFN1/MFN1, MFN2/MFN2, or MFN1/MFN2) to mediate OMM fusion [41]. OPA1 is responsible for IMM fusion and cristae morphology formation [42, 43]. Mitofusin harbors a GTPase domain close to the N-terminus that is involved in the GTP hydrolysis. Two hydrophobic heptad repeat (HR) domains are localized in the middle (HR1) and C-terminal (HR2) regions and provide the basis for most coiled-coil interactions. The HR2 domain was shown to be of great importance for mitochondrial fusion activity; formation of HR2 dimers promotes the generation of a mitochondrial tether before mitochondrial fusion. OPA1 contains an N-terminal mitochondrial localization sequence, responsible for import of the protein into the mitochondrial inner membrane. This region also has three putative cleavage sites for the mitochondrial processing peptidase. Other structural motifs include a transmembrane domain that anchors OPA1 in the mitochondrial inner membrane; the first coiled-coil domain, involved in protein-protein interactions; a GTPase domain crucial for protein activity; the middle domain, which participates in tetramerization and higher-order assembly of OPA1; and a second coiled-coil domain in the C-terminus that mediates the interaction between OPA1 and MFN1/2 [44], but the exact mechanism in which OPA1 drives IMM fusion is far from being elucidated. Expression level of MFNs or OPA1 influences mitochondrial fusion. Mitochondrial fusion is also regulated by ubiquitination, deacetylation of MFNs, or protease-dependent cleavage of OPA1 by OMA1, YME1L. MFN1/MFN2 are generally regulated at transcriptional and posttranscriptional levels. Upregulation of MFN1/MFN2 may result in the increased fusion [40, 45, 46], and downregulation of MFN1 or MFN2 may result in the decreased fusion [47, 48]. Deacetylation of MFN1 by HDAC6 may increase the fusion in fasting mice skeletal cells so as to produce more energy [49]. Ubiquitination of MFNs by USP30 or Parkin may result in the reduced fusion, which contributes to selectively isolate damaged mitochondria [50, 51]. Parra et al. found OPA1 upregulation induced by insulin in cardiomyocytes resulted in the increased mitochondrial fusion, along with the increase of the intracellular ATP levels and oxygen consumption [52]. Exercise pretreatment is protective in nervous system ischemia/reperfusion injury via upregulation of OPA1 and mitochondrial fusion [53]. Besides, protease-dependent cleavage seems to conduct the functional shift of OPA1. OPA1 could be processed into long form (L-OPA1) and short form (S-OPA1) possessing different functions, respectively. Mitochondrial fusion regulation is thought to depend on the coordination of L- and S-OPA1 [54]. Two key players for OPA1 proteolysis are the IMM peptidase OMA1 and the i-AAA protease YME1L. Loss function study of YME1L suggests that it is responsible for constitutive processing of OPA1 [54, 55], while OMA1 regulates stress-induced OPA1 cleavage [56]. But the role of OMA1 and YME1L in mitochondrial morphology remains controversial. Anand et al. found that long OPA1 forms were sufficient to mediate mitochondrial fusion in these cells and expression of short OPA1 forms promoted mitochondrial fragmentation, demonstrating a dispensable role of OPA1 processing in mitochondrial fusion and a stimulatory role of S-OPA1 [57]. Detailed studies into mitochondrial fusion mechanism are eagerly needed to facilitate the adequate manipulation of mitochondrial dynamics, which may further contribute to mitochondrial function management.
4.2.2.3 Mitophagy
Mitophagy is a selective autophagic process conserved in eukaryotes and plays an essential role in mitochondrial quality and quantity control. It is involved in myogenic differentiation, cardiomyocyte mitochondrial plasticity and metabolic transitioning of perinatal hearts [58], and metabolism regulation in the liver [59] and β cells [60]. Mitophagy can be classified into ubiquitin-dependent and receptor-dependent pathways. A ubiquitin/PINK1/Parkin-dependent mitophagy pathway was unraveled and was extensively characterized, in short: (1) damaged mitochondria are isolated by fission mechanisms and PINK1 is preserved on OMM; (2) ubiquitin chain is connected to OMM proteins (e.g., MFNs, DRP1, FIS1) of damaged mitochondria by the E3 ubiquitin ligase Parkin, which is activated by PINK1; (3) adaptors (p62/SQSTM1, optineurin/OPTN, NBR1, etc.) associate ubiquitin with membrane protein LC3 through their LC3-interacting region (LIR); and (4) damaged mitochondria are encapsulated by autophagosome and degraded in autophagolysosome. Besides, the consistent mitochondrial outer membrane receptors FUNDC1, NIX/BNIP3L, BNIP3, and Bcl2L13 mediate receptor-dependent mitophagy; they interact with LC3 directly through LIR and promote encapsulation and latter processes [61, 62].
PINK1 serves as the sensor for the mitochondrial polarization state. Mitochondrial depolarization inactivates its import and proteasomal degradation, leading to PINK1 accumulation on the OMM, then PINK1 phosphorylates MFN2 (at serine 442, threonine 111, etc.), and phosphorylated MFN2 can act as a receptor to recruit Parkin, an E3 ubiquitin ligase. PINK1 also phosphorylates ubiquitin at serine 65 and the ubiquitin-like domain of Parkin at serine 65, which recruit more Parkin to OMM and activate its E3 ligase activity. As for adaptors, p62 and NBR1 were found to be dispensable, and primary but redundant autophagy receptor functions were defined for OPTN and NDP52. USP8 deubiquitylation of auto-ubiquitylated Parkin is required for its localization to depolarized mitochondria and thereby for efficient activation of mitophagy. On the other hand, the ubiquitin-specific proteases USP30, which localizes at the OMM via a transmembrane domain, and USP15, which can fractionally localize to mitochondria, remove Parkin-ligated ubiquitin from OMM proteins and reduce mitophagy. In addition, mitophagy receptors upon expression, constitutively localize at the OMM via transmembrane domains, are transcriptionally regulated, and engagement of mitophagy receptor activity is controlled through the phosphorylation status of their LIR. Phosphorylation of serine residues 17 and 24 flanking the Bnip3 LIR specifically promotes binding to LC3B and GATE-16, but to date it remains undetermined which kinases and phosphatases are responsible for controlling the phosphorylation state of the Bnip3. In response to hypoxia or mitochondrial uncoupling, PGAM5 dephosphorylates CK2-phosphorylated serine 13 of FUNDC1 to activate LC3 binding. In addition, ULK1 phosphorylates serine 17 of the FUNDC1 LIR motif, resulting in increased LC3 binding [63].
4.2.2.4 Mitochondrial Biogenesis
Mitochondrial biogenesis includes synthesis of mtDNA-encoded protein, synthesis and import of nuclear-encoded proteins, assembly of the dual genetic origin-derived proteins and mtDNA replication, and finally formation of new organelle structures [64]. Nuclear-encoded mitochondrial proteins are synthesized in cytoplasm and are then imported into mitochondria. mtDNA-encoded proteins are synthesized within the organelle itself.
The regulation of mitochondrial biogenesis includes a set of nuclear transcription factors. The nuclear transcription factor, NRF1, governs the expression of nuclear OXPHOS genes as well as the expression of nuclear-encoded factors involved in mitochondrial transcription, protein import, and protein assembly. NRF2 binding sites have been also identified in several other mitochondrial genes including the OXPHOS subunits, mitochondrial protein import machinery, and mitochondrial translation factors. Additional transcription factors such as the estrogen-related receptor α (ERRα), cAMP response element-binding protein (CREB), and Yin Yang 1 (YY1) are also involved. A higher level of regulation is achieved by the family of coactivators of the peroxisome proliferator-activated receptors (PPARs). The best studied member of this family is the PPAR coactivator 1α (PGC-1α), which is termed the master regulator of mitochondrial biogenesis. PGC-1α expression is upregulated by PPARs, CREB, and YY1/mTOR and is downregulated by RIP140, 160MYB, DNMT3b, p53, etc. Besides, PGC-1α can be phosphorylated by p38, MAPK, and AMPK and inhibited by Akt. Activation of Sirt1 and the deacetylation of PGC-1α can also activate PGC-1α [65].
Mitochondrial biogenesis and mitochondrial mass can be modulated through several stimuli and cellular pathways, including but not limited to hormones such as thyroid hormone [66] and estrogen [67], inflammatory signaling [68], as well as calcium signaling [69]. As a result, mitochondrial biogenesis plays vital role in cell differentiation [70,71,72], immune response [73,74,75], and inflammation response in the liver, kidney, heart, and lung [76,77,78,79].
4.2.3 Role of Mitochondrial Quality Imbalance in Various Diseases
Diversified stimuli disrupt such dynamics by hindering any of the above four processes and results in mitochondrial quality imbalance, which induces functional impairment or structural damage in mitochondria and strongly insults organ function [80].
Mitochondrial quality imbalance is largely seen in neurodegeneration [81, 82]. Downregulation of MFN2 may cause mitochondrial dysfunction, alter calcium homeostasis, and enhance Bax translocation to mitochondria, resulting in delayed neuronal death in in vitro and in vivo models of excitotoxicity [48]. Defect in mitophagy may result in damaged mitochondria accumulation and neurodegeneration [83]. Loss of PINK1 function is associated with early-onset recessive Parkinson’s disease, in vitro studies showed that cells lacking Pink1 had lower DRP1 and MFN2 expression, and mitochondrial morphology was fragmented [84]. Impaired mitochondrial biogenesis contributed to mitochondrial dysfunction in Alzheimer’s disease [85]. TNF-α may activate NF-κB signaling and increase OPA1 expression, while IL-6 may upregulate fission inducer FIS1 and downregulate MFN2, both signal axes contributed to islet cell apoptosis and type 2 diabetes [86]. Disturbances in mitochondrial biogenesis and PGC-1α levels are involved in type 2 diabetes, neurodegenerative disease, and many age-related pathologies [87, 88].
Mitochondrial quality imbalance also participates in infection and inflammatory diseases. S. mansoni infection may change hepatocyte mitochondrial morphology and affect mitochondrial function, in which mitochondrial biogenesis and fission were also upregulated [89]. The toxic bile salt glycochenodeoxycholate-induced mitochondrial fragmentation was associated with an increase in ROS levels and hepatic cell death [90]. Induction of mitochondrial fission by cathepsin E in lung epithelial contributed to increased caspase activation/apoptosis, and lung epithelial-targeted transgenic cathepsin E mice developed emphysema similarly [91].
Mitochondrial quality balance is also studied in the cardiovascular system. Rat cardiac arrest model showed excessive autophagy and mitophagy, along with increased apoptosis in cardiomyocytes [92]. Genetic ablation of both MFN1 and MFN2 in the adult murine heart resulted in mitochondrial fragmentation, impairment in mitochondrial respiration, and a severe lethal cardiomyopathy after 7–8 weeks [93, 94]. Upregulation of miR-140 and downregulation of MFN1 were found in right ventricles of pulmonary arterial hypertension rats, which correlated with increased right ventricular systolic pressure and hypertrophy [95].
4.3 Role of Mitochondrial Quality Imbalance in MODS Following Severe Trauma, Shock, and Sepsis
4.3.1 Role of Mitochondrial Dysfunction in MODS Following Severe Trauma, Shock, and Sepsis
Studies revealed pivotal role of mitochondria dysfunction in MODS, and mitochondrial status is directly associated with patient outcome [96]. Decreased mitochondrial complex I activity was associated with the degree of nitric oxide (NO) production in the skeletal muscle of patients admitted to intensive care unit with septic shock [97]. Respiratory protein subunits and transcripts were depleted in critically ill patients [98]. In rat sepsis models induced by cecal ligation and puncture (CLP), Karlsson et al. found a mismatch between reduced oxygen delivery and increased oxygen demand which impaired mitochondria and vital organs including the brain and liver [99]. Besides, adenosine diphosphate (ADP)-stimulated respiratory rates of cardiac fibers were reduced in septic mice due to reduced Ser-58 phosphorylation of cytochrome c oxidase subunit IV-1, resulted in cardiac dysfunction [100]. LPS reduced ATP content in HepG2 cell and primary human hepatocytes, partly by modulating complex II respiration [101]. LPS significantly induced heart oxidative stress and abnormal oxidative phosphorylation, which further impair cardiac contractile and bioenergetic function [25]. In addition, Herminghaus A et al. investigated the varying degrees of sepsis on hepatic mitochondrial function and related varied respiratory control ratio (RCR) to the severity of sepsis [102]. Joseph L et al. found the increased cardiac oxidative stress and decreased systolic function were accompanied with mitochondrial calcium overload and depolarization of the mitochondrial inner membrane potential in a mouse model of endotoxemia [103]. Besides, studies also showed increased renal tubular cell [104] and endothelial cell [105] apoptosis by LPS stimulus. In addition, in experimental models of hemorrhagic shock and resuscitation in murine and porcine, mitochondrial injury was observed, as well as cell injury and organ dysfunction [106]. These studies remind us of the fact that mitochondrial respiration is impaired and decreased mitochondria function is closely related to organ function in sepsis and other critical illness.
4.3.2 Possible Role of Mitochondrial Quality Imbalance in MODS Following Severe Trauma, Shock, and Sepsis
As above discussed, mitochondrial quality imbalance has been studied in chronic pathologies, such as neurodegeneration, diabetes, and inflammatory diseases. However, mitochondria are highly dynamic organelles, and acute stresses including trauma, shock- and sepsis-induced ischemia, hypoxia, endotoxemia, and cytokines could induce rapid changes in mitochondrial morphology and function in multiple organs (Fig. 4.2).
Schematics for mitochondrial quality balance maintenance. Biogenesis: PGC-1α is the master of mitochondrial biogenesis, which activates NRF and TFAM in mitochondrial plasma to mediate mitochondrial protein synthesis. Fission: DRP1 is recruited from cytoplasm to bind receptors (e.g., MFF, FIS1) on mitochondrial outer membrane and hydrolyze GTP to drive mitochondrial fission. Fusion: MFN1 and MFN2 on mitochondrial outer membrane form homo-oligomers or hetero-oligomers and hydrolyze GTP to drive adjacent mitochondrial outer membrane fusion; OPA1 is responsible for inner membrane fusion and cristae morphology. Mitophagy: PINK1 is preserved on mitochondrial outer membrane and activates Parkin by phosphorylation, and then Parkin ubiquitin OMM proteins (e.g., MFN1, MFN2) and adaptors (e.g., OPTN, NDP52, p62, NBR1, etc.) are recruited, and then adaptors are connected to LC3 on autophagosome membrane; mitophagy receptors (e.g., FUNDC1, BNIP3, BNIP3L/NIX, etc.) on mitochondrial outer membrane interact with LC3 directly to mediate mitophagy
Dysregulated fission and fusion are widely reported. In a traumatic rat model, the number of heart mitochondria was increased, while smaller-sized mitochondria were extensively observed. Translocation of DRP1 and phosphorylation of its Ser-616 were significantly increased. Inhibition of mitochondrial fission with melatonin pretreatment significantly reduced cardiac function impairment [107]. Rat sepsis model induced by CLP showed significant decrease of MFN2 mRNA and increase of DRP1 mRNA, while there was MFN2 mRNA decrease in the endotoxemia model. Both models showed mitochondrial fragmentation in the heart [108]. Besides, serum from burn rats significantly increased mitochondrial fission in murine myoblast C2C12 cells, consistent with the decreased mitochondrial membrane potential and increased cell apoptosis. Treatment with IL-6 antibody prevented mitochondrial fragmentation and cell death, suggesting cytokine-induced mitochondrial fission plays an important role in second-hit tissue damage [109]. These studies suggest that abnormal fission-fusion balance may contribute to the progression of trauma-, burn-, and sepsis-induced organ function.
Mitochondrial biogenesis is also impaired in these acute pathologies. In LPS-treated hepatocytes [76], the septic heart [78], sepsis- or I/R-induced kidney injury [77, 110], etc., mitochondria mass was reduced, accompanied with deceased ATP production, decreased mitochondrial membrane potential, and increased oxidative injury and apoptosis. Decreased mitochondrial biogenesis and mitophagy caused by inhibition of SIRT1, PINK1, and Parkin are associated with higher risk of lung injury in sepsis [111]. Suppression of mitochondrial biogenesis through Toll-like receptor 4-dependent MAPK kinase increased endotoxin-induced acute kidney injury [112].
The behavior of mitophagy is complex. A sublethal dose of E. coli lipopolysaccharide (LPS) was injected to mouse; mitochondrial function was decreased temporarily and gets fully recovered later, but Parkin-deficient mice exhibited impaired recovery of cardiac contractility and constant degradation of mitochondrial metabolic functions, suggesting a protective role of mitophagy [113]. Ischemia/reperfusion injury is common in liver resection, hemorrhagic shock, and resuscitation. Due to nutrition and ATP depletion, calcium overload, and oxidative injury during ischemia, autophagy-related protein BECN1 and ATG7 are degraded, causing defective autophagy and mitochondrial clearance; more ROS production and CytC release were induced during fluid reperfusion and resulted in a severer liver injury, suggesting a harmful role of inadequate mitophagy [114]. By enhancing mitophagy, ischemic hepatocytes were protected from apoptosis [115], VSMC was prevented from LDL-induced injury [116], and lung epithelial cells were prevented from hypoxic injury [117]. However, the cross talk between autophagy and apoptosis makes the situation complex. A mouse model of cardiac myocyte ischemia/reperfusion injury showed increased autophagic flux, inhibiting autophagy attenuated I/R-induced increase in oxidative stress, along with decrease in myocardial infarction size, suggesting that autophagy mediated myocardial injury during I/R [118]. However, whether mitophagy is a double-edged sword remains to be investigated.
4.3.3 Cross Talk of Fission, Fusion, Mitophagy, and Biogenesis Regulates Organ Function
Mitochondrial fission and fusion, mitophagy, and biogenesis are the dynamic process for maintaining the mitochondrial function. After fission, healthy mitochondria participate in normal function maintaining of cell, while slightly injured mitochondria can be restored with the help of fusion, severely damaged mitochondria with decreased membrane potential are degraded by mitophagy, and mitochondrial biogenesis counteracts the mitochondrial mass loss caused by mitochondrial clearance [119].
Studies showed that mitochondrial fission protein DRP1 may regulate mitochondrial fusion and mitophagy. The differential binding of MFN2 and DRP1 regulates mitochondrial fusion. DRP1 may act as a regulatory factor for both mitochondrial fusion and fission [120]. Studies found ablating DRP1 in myocytes not only interrupted the mitochondrial fission but also provoking mitophagy by regulating parkin [121], indicating a contrary role of fission and mitophagy.
The cross talk between fusion and mitophagy was also discovered. PINK1 may phosphorylate MFN2 at Thr-111 and Ser-442 and promote its Parkin-mediated ubiquitination and mitophagy [122]; ablation of either DRP1 or mitofusins (Mfn) in cardiomyocytes showed abnormal mitochondrial morphology; MFN null cells showed increased damaged mitochondria, while DRP1 null cells showed increased loss of mitochondria [123].
Mitochondrial biogenesis and mitophagy may cooperate to realize mitochondrial modification. Skeletal myoblasts may specifically shift from a highly glycolytic state to relying predominantly on oxidative phosphorylation (OXPHOS) upon differentiation; this phenomenon requires both mitochondrial clearance and biogenesis. During early myogenic differentiation, autophagy is robustly upregulated, and this coincides with mitophagy. Mitochondria are then repopulated via PPARGC1A/PGC-1alpha-mediated biogenesis; inhibiting autophagy may result in reduced mitochondrial biogenesis [58].
We may safely draw the conclusion that the regulation of mitochondrial fission, fusion, biogenesis, and mitophagy network has important influences on cell status and organ function. MODS is a consequence of various factors action, including infection, endotoxemia, hypoxia or ischemia, cytokines, etc. These factors may contribute to the impairment of one of the four processes (i.e., fission, fusion, biogenesis, and mitophagy). Multifactor-induced imbalance of mitochondrial quality may expand cell injury and participates in the occurrence of MODS (Fig. 4.3).
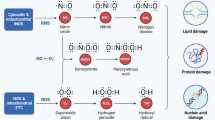
Schematic for mitochondrial quality imbalance in MODS Mitochondria in normal cells undergo continuous fission, fusion, biogenesis, and mitophagy to maintain mitochondrial quality balance. Under stress conditions (e.g., severe trauma, shock, sepsis, MODS), the mitochondrial dynamics, biogenesis, and clearance are dysregulated and appear to be quality imbalance. Imbalanced mitochondrial quality may appear as mitochondrial fragmentation, decreased mitochondrial membrane potential and calcium overload, increased ROS and decreased ATP generation, etc. Restoring mitochondrial quality balance might be a therapeutic target to manage MODS. The blue inner mitochondrial membrane indicates a healthy status, the orange indicates unhealthy, and the red indicates damaged.
4.4 Restoring Mitochondrial Quality Balance Preserves Cellular and Organ Function
Some treatments have been applied to restore mitochondrial function such as vitamin C, vitamin E, β-carotene, coenzyme Q and resveratrol, and so on [124, 125]. Some novel antioxidants such as MitoQ, SS-31, MitoGSH, and MitoTEMPO have been developed and used to improve mitochondrial function. MitoQ, a mitochondria-targeted antioxidant, which is designed to protect against oxidative damage within mitochondria, has been proven to be beneficial for ischemic renal damage [126], intestinal inflammation [127], and sepsis [128]. SS-31 has gained benefits in neurodegeneration [129, 130] and hypoxic renal tubular cell injury [131] by decreasing free radical production and oxidative damage. MitoGSH may directly restore GSH levels and preserve mitochondrial redox buffering and signaling capacity [132]. Modifying mitochondrial respiration by manipulating substrate or electron transport chain enzyme activity are the other ways to improve mitochondrial function. A newly developed synthetic antimicrobial peptide 19-2.5 (Pep2.5) was found to prevent mitochondrial dysfunction in murine cardiomyocytes stimulated with serum from septic shock patients by enhancing the mitochondrial respiratory function and increasing cellular ATP levels [133]. In addition, other efforts aiming at decreasing calcium overload and apoptosis have also been made.
Large amount of studies observed the beneficial effect of modification of mitochondrial dynamics. Research showed inhibition of ROCK pathway with Fasudil significantly reduced the mitochondrial fragmentation in endotoxemic cardiomyocytes and improved the cardiac function [25]. Inhibition of the excessive mitochondrial fission by blocking DRP1 with P110 at the onset of reperfusion significantly raised the long-term benefits of acute myocardial infarction [134]. Inhibition of mitochondrial fission with mdivi-1, the inhibitor of DRP1, significantly reduced Aβ-induced microglial apoptosis, exerting neuroprotective effect [135]. Mdivi-1 inhibition of mitochondrial fission may help to attenuate TBI-induced cell death through maintaining normal mitochondrial morphology and inhibiting activation of apoptosis [136]. In cardiomyocyte cell model of ischemia, overexpression of mitofusin proteins, MFN1 or MFN2, was found to be mitochondrial and cytoprotective [137].
Regulating mitochondrial biogenesis and PGC-1α expression was also found beneficial in some diseases including muscular, neurodegenerative disorders and renal and cardiac dysfunction [76, 110, 112, 138,139,140,141]. For example, overexpression of PINK1 and parkin could increase mitophagy of VSMC and decrease oxidized LDL-induced apoptosis [116].
4.5 Perspective
Mitochondrial quality imbalance might play a critical role in the occurrence and development of MODS. Severe trauma, shock, hypoxia, infection, and overproduction of inflammatory mediators and cytokines are the most common damage factors for mitochondrial quality and organ function. These factors can regulate or interrupt the balance of mitochondrial quality by regulating mitochondrial fission-, fusion-, mitophagy-, and biogenesis-related proteins such as DRP1, MFN1/MFN2, FIS1, OPA1, PGC1α, etc. [142,143,144].
p53 has been extensively studied in cancer and apoptosis; recent studies found p53 made a great contribution to directly modulating mitochondrial fission and fusion [145]. By attenuating the impairment of mitochondrial fission/fusion, biogenesis, and mitophagy, melatonin was found to be effective in restoring mitochondrial function in liver fibrosis induced by chronic carbon tetrachloride exposure [146]. Overexpression of heme oxygenase-1 (HO-1) may inhibit fission; promote fusion, biogenesis, and basal mitophagy; and thus play a protective role in heart oxidative injury [147]. Phospholipids in mitochondria are associated with dynamin-related GTPase (i.e., MFN1/MFN2, OPA1, DRP1), which regulate not only mitochondria fission and fusion but also mitophagy [148]. These studies suggested that manipulating mitochondrial fission and fusion, biogenesis, and mitophagy simultaneously seems to be more effective in correcting mitochondrial quality imbalance in some complicated situations.
Endoplasmic reticulum (ER) and mitochondria contact were considered a transport platform for calcium, the lipid between mitochondria and ER. Recent study found mitochondrial fission occurred at the mito-ER contact site. This contact may regulate mitochondrial fission, fusion, and mitophagy [149]. Studies are called to examine the exact role of such contact, which may inspire new therapeutic measures for mitochondrial imbalance and organ function protection.
References
Ulvik A, Kvale R, Wentzel-Larsen T, Flaatten H. Multiple organ failure after trauma affects even long-term survival and functional status. Crit Care. 2007;11(5):R95.
Santulli G, Xie W, Reiken SR, Marks AR. Mitochondrial calcium overload is a key determinant in heart failure. Proc Natl Acad Sci U S A. 2015;112(36):11389–94.
Li X, Fang P, Mai J, Choi ET, Wang H, Yang XF. Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers. J Hematol Oncol. 2013;6:19.
Rossier MF. T channels and steroid biosynthesis: in search of a link with mitochondria. Cell Calcium. 2006;40(2):155–64.
Jang DH, Greenwood JC, Spyres MB, Eckmann DM. Measurement of mitochondrial respiration and motility in acute care: sepsis, trauma, and poisoning. J Intensive Care Med. 2017;32(1):86–94.
Scheibye-Knudsen M, Fang EF, Croteau DL, Wilson DM 3rd, Bohr VA. Protecting the mitochondrial powerhouse. Trends Cell Biol. 2015;25(3):158–70.
Marchi S, Pinton P. The mitochondrial calcium uniporter complex: molecular components, structure and physiopathological implications. J Physiol. 2014;592(5):829–39.
Vaseva AV, Marchenko ND, Ji K, Tsirka SE, Holzmann S, Moll UM. p53 opens the mitochondrial permeability transition pore to trigger necrosis. Cell. 2012;149(7):1536–48.
Volgyi K, Juhasz G, Kovacs Z, Penke B. Dysfunction of endoplasmic reticulum (ER) and mitochondria (MT) in Alzheimer’s disease: the role of the ER-MT cross-talk. Curr Alzheimer Res. 2015;12(7):655–72.
Schapira AH, Olanow CW, Greenamyre JT, Bezard E. Slowing of neurodegeneration in Parkinson’s disease and Huntington’s disease: future therapeutic perspectives. Lancet. 2014;384(9942):545–55.
Wu CC, Bratton SB. Regulation of the intrinsic apoptosis pathway by reactive oxygen species. Antioxid Redox Signal. 2013;19(6):546–58.
Busch KB, Kowald A, Spelbrink JN. Quality matters: how does mitochondrial network dynamics and quality control impact on mtDNA integrity? Philos Trans R Soc Lond Ser B Biol Sci. 2014;369(1646):20130442.
Wang L, Ishihara T, Ibayashi Y, Tatsushima K, Setoyama D, Hanada Y, Takeichi Y, Sakamoto S, Yokota S, Mihara K, Kang D, Ishihara N, Takayanagi R, Nomura M. Disruption of mitochondrial fission in the liver protects mice from diet-induced obesity and metabolic deterioration. Diabetologia. 2015;58(10):2371–80.
Hernandez-Alvarez MI, Paz JC, Sebastian D, Munoz JP, Liesa M, Segales J, Palacin M, Zorzano A. Glucocorticoid modulation of mitochondrial function in hepatoma cells requires the mitochondrial fission protein DRP1. Antioxid Redox Signal. 2013;19(4):366–78.
Roth D, Krammer PH, Gulow K. Dynamin related protein 1-dependent mitochondrial fission regulates oxidative signalling in T cells. FEBS Lett. 2014;588(9):1749–54.
Richter V, Palmer CS, Osellame LD, Singh AP, Elgass K, Stroud DA, Sesaki H, Kvansakul M, Ryan MT. Structural and functional analysis of MiD51, a dynamin receptor required for mitochondrial fission. J Cell Biol. 2014;204(4):477–86.
Hatch AL, Gurel PS, Higgs HN. Novel roles for actin in mitochondrial fission. J Cell Sci. 2014;127(Pt 21):4549–60.
Korobova F, Gauvin TJ, Higgs HN. A role for myosin II in mammalian mitochondrial fission. Curr Biol. 2014;24(4):409–14.
Otera H, Ishihara N, Mihara K. New insights into the function and regulation of mitochondrial fission. Biochim Biophys Acta. 2013;1833(5):1256–68.
Godoy JA, Arrazola MS, Ordenes D, Silva-Alvarez C, Braidy N, Inestrosa NC. Wnt-5a ligand modulates mitochondrial fission-fusion in rat hippocampal neurons. J Biol Chem. 2014;289(52):36179–93.
Liang N, Wang P, Wang S, Li S, Li Y, Wang J, Wang M. Role of mitochondrial calcium uniporter in regulating mitochondrial fission in the cerebral cortexes of living rats. J Neural Transm (Vienna). 2014;121(6):593–600.
Pennanen C, Parra V, Lopez-Crisosto C, Morales PE, Del Campo A, Gutierrez T, Rivera-Mejias P, Kuzmicic J, Chiong M, Zorzano A, Rothermel BA, Lavandero S. Mitochondrial fission is required for cardiomyocyte hypertrophy mediated by a Ca2+-calcineurin signaling pathway. J Cell Sci. 2014;127(Pt 12):2659–71.
Chen SD, Lin TK, Yang DI, Lee SY, Shaw FZ, Liou CW, Chuang YC. Roles of PTEN-induced putative kinase 1 and dynamin-related protein 1 in transient global ischemia-induced hippocampal neuronal injury. Biochem Biophys Res Commun. 2015;460(2):397–403.
Buhlman L, Damiano M, Bertolin G, Ferrando-Miguel R, Lombes A, Brice A, Corti O. Functional interplay between Parkin and DRP1 in mitochondrial fission and clearance. Biochim Biophys Acta. 2014;1843(9):2012–26.
Preau S, Delguste F, Yu Y, Remy-Jouet I, Richard V, Saulnier F, Boulanger E, Neviere R. Endotoxemia engages the RhoA kinase pathway to impair cardiac function by altering cytoskeleton, mitochondrial fission, and autophagy. Antioxid Redox Signal. 2016;24(10):529–42.
Wang W, Wang Y, Long J, Wang J, Haudek SB, Overbeek P, Chang BH, Schumacker PT, Danesh FR. Mitochondrial fission triggered by hyperglycemia is mediated by ROCK1 activation in podocytes and endothelial cells. Cell Metab. 2012;15(2):186–200.
Prieto J, Leon M, Ponsoda X, Sendra R, Bort R, Ferrer-Lorente R, Raya A, Lopez-Garcia C, Torres J. Early ERK1/2 activation promotes DRP1-dependent mitochondrial fission necessary for cell reprogramming. Nat Commun. 2016;7:11124.
Kim B, Park J, Chang KT, Lee DS. Peroxiredoxin 5 prevents amyloid-beta oligomer-induced neuronal cell death by inhibiting ERK-DRP1-mediated mitochondrial fragmentation. Free Radic Biol Med. 2016;90:184–94.
Taguchi N, Ishihara N, Jofuku A, Oka T, Mihara K. Mitotic phosphorylation of dynamin-related GTPase DRP1 participates in mitochondrial fission. J Biol Chem. 2007;282(15):11521–9.
Jahani-Asl A, Huang E, Irrcher I, Rashidian J, Ishihara N, Lagace DC, Slack RS, Park DS. CDK5 phosphorylates DRP1 and drives mitochondrial defects in NMDA-induced neuronal death. Hum Mol Genet. 2015;24(16):4573–83.
Nakamura N, Kimura Y, Tokuda M, Honda S, Hirose S. MARCH-V is a novel mitofusin 2- and DRP1-binding protein able to change mitochondrial morphology. EMBO Rep. 2006;7(10):1019–22.
Yonashiro R, Ishido S, Kyo S, Fukuda T, Goto E, Matsuki Y, Ohmura-Hoshino M, Sada K, Hotta H, Yamamura H, Inatome R, Yanagi S. A novel mitochondrial ubiquitin ligase plays a critical role in mitochondrial dynamics. EMBO J. 2006;25(15):3618–26.
Xu S, Cherok E, Das S, Li S, Roelofs BA, Ge SX, Polster BM, Boyman L, Lederer WJ, Wang C, Karbowski M. Mitochondrial E3 ubiquitin ligase MARCH5 controls mitochondrial fission and cell sensitivity to stress-induced apoptosis through regulation of MiD49 protein. Mol Biol Cell. 2016;27(2):349–59.
Cho DH, Nakamura T, Fang J, Cieplak P, Godzik A, Gu Z, Lipton SA. S-nitrosylation of DRP1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science. 2009;324(5923):102–5.
Braschi E, Zunino R, Mcbride HM. MAPL is a new mitochondrial SUMO E3 ligase that regulates mitochondrial fission. EMBO Rep. 2009;10(7):748–54.
Zunino R, Schauss A, Rippstein P, Andrade-Navarro M, Mcbride HM. The SUMO protease SENP5 is required to maintain mitochondrial morphology and function. J Cell Sci. 2007;120(Pt 7):1178–88.
Gawlowski T, Suarez J, Scott B, Torres-Gonzalez M, Wang H, Schwappacher R, Han X, Yates JR 3rd, Hoshijima M, Dillmann W. Modulation of dynamin-related protein 1 (DRP1) function by increased O-linked-beta-N-acetylglucosamine modification (O-GlcNAc) in cardiac myocytes. J Biol Chem. 2012;287(35):30024–34.
Yim N, Ryu SW, Han EC, Yoon J, Choi K, Choi C. Mutant ubiquitin UBB+1 induces mitochondrial fusion by destabilizing mitochondrial fission-specific proteins and confers resistance to oxidative stress-induced cell death in astrocytic cells. PLoS One. 2014;9(6):e99937.
Kasahara A, Cipolat S, Chen Y, Dorn GW 2nd, Scorrano L. Mitochondrial fusion directs cardiomyocyte differentiation via calcineurin and Notch signaling. Science. 2013;342(6159):734–7.
Ballweg K, Mutze K, Konigshoff M, Eickelberg O, Meiners S. Cigarette smoke extract affects mitochondrial function in alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2014;307(11):L895–907.
Hoppins S, Nunnari J. The molecular mechanism of mitochondrial fusion. Biochim Biophys Acta. 2009;1793(1):20–6.
Olichon A, Baricault L, Gas N, Guillou E, Valette A, Belenguer P, Lenaers G. Loss of OPA1 perturbates the mitochondrial inner membrane structure and integrity, leading to cytochrome c release and apoptosis. J Biol Chem. 2003;278(10):7743–6.
Merkwirth C, Dargazanli S, Tatsuta T, Geimer S, Lower B, Wunderlich FT, Von Kleist-Retzow JC, Waisman A, Westermann B, Langer T. Prohibitins control cell proliferation and apoptosis by regulating OPA1-dependent cristae morphogenesis in mitochondria. Genes Dev. 2008;22(4):476–88.
Ranieri M, Brajkovic S, Riboldi G, Ronchi D, Rizzo F, Bresolin N, Corti S, Comi GP. Mitochondrial fusion proteins and human diseases. Neurol Res Int. 2013;2013:293893.
Duarte A, Castillo AF, Podesta EJ, Poderoso C. Mitochondrial fusion and ERK activity regulate steroidogenic acute regulatory protein localization in mitochondria. PLoS One. 2014;9(6):e100387.
Hickey FB, Corcoran JB, Griffin B, Bhreathnach U, Mortiboys H, Reid HM, Andrews D, Byrne S, Furlong F, Martin F, Godson C, Murphy M. IHG-1 increases mitochondrial fusion and bioenergetic function. Diabetes. 2014;63(12):4314–25.
Silvander JSG, Kvarnstrom SM, Kumari-Ilieva A, Shrestha A, Alam CM, Toivola DM. Keratins regulate beta-cell mitochondrial morphology, motility, and homeostasis. FASEB J. 2017;31:4578.
Martorell-Riera A, Segarra-Mondejar M, Munoz JP, Ginet V, Olloquequi J, Perez-Clausell J, Palacin M, Reina M, Puyal J, Zorzano A, Soriano FX. MFN2 downregulation in excitotoxicity causes mitochondrial dysfunction and delayed neuronal death. EMBO J. 2014;33(20):2388–407.
Lee JY, Kapur M, Li M, Choi MC, Choi S, Kim HJ, Kim I, Lee E, Taylor JP, Yao TP. MFN1 deacetylation activates adaptive mitochondrial fusion and protects metabolically challenged mitochondria. J Cell Sci. 2014;127(Pt 22):4954–63.
Glauser L, Sonnay S, Stafa K, Moore DJ. Parkin promotes the ubiquitination and degradation of the mitochondrial fusion factor mitofusin 1. J Neurochem. 2011;118(4):636–45.
Yue W, Chen Z, Liu H, Yan C, Chen M, Feng D, Yan C, Wu H, Du L, Wang Y, Liu J, Huang X, Xia L, Liu L, Wang X, Jin H, Wang J, Song Z, Hao X, Chen Q. A small natural molecule promotes mitochondrial fusion through inhibition of the deubiquitinase USP30. Cell Res. 2014;24(4):482–96.
Parra V, Verdejo HE, Iglewski M, Del Campo A, Troncoso R, Jones D, Zhu Y, Kuzmicic J, Pennanen C, Lopez-Crisosto C, Jana F, Ferreira J, Noguera E, Chiong M, Bernlohr DA, Klip A, Hill JA, Rothermel BA, Abel ED, Zorzano A, Lavandero S. Insulin stimulates mitochondrial fusion and function in cardiomyocytes via the Akt-mTOR-NFkappaB-Opa-1 signaling pathway. Diabetes. 2014;63(1):75–88.
Zhang L, He Z, Zhang Q, Wu Y, Yang X, Niu W, Hu Y, Jia J. Exercise pretreatment promotes mitochondrial dynamic protein OPA1 expression after cerebral ischemia in rats. Int J Mol Sci. 2014;15(3):4453–63.
Song Z, Chen H, Fiket M, Alexander C, Chan DC. OPA1 processing controls mitochondrial fusion and is regulated by mRNA splicing, membrane potential, and Yme1L. J Cell Biol. 2007;178(5):749–55.
Griparic L, Kanazawa T, Van Der Bliek AM. Regulation of the mitochondrial dynamin-like protein OPA1 by proteolytic cleavage. J Cell Biol. 2007;178(5):757–64.
Quiros PM, Ramsay AJ, Sala D, Fernandez-Vizarra E, Rodriguez F, Peinado JR, Fernandez-Garcia MS, Vega JA, Enriquez JA, Zorzano A, Lopez-Otin C. Loss of mitochondrial protease OMA1 alters processing of the GTPase OPA1 and causes obesity and defective thermogenesis in mice. EMBO J. 2012;31(9):2117–33.
Anand R, Wai T, Baker MJ, Kladt N, Schauss AC, Rugarli E, Langer T. The i-AAA protease YME1L and OMA1 cleave OPA1 to balance mitochondrial fusion and fission. J Cell Biol. 2014;204(6):919–29.
Sin J, Andres AM, Taylor DJ, Weston T, Hiraumi Y, Stotland A, Kim BJ, Huang C, Doran KS, Gottlieb RA. Mitophagy is required for mitochondrial biogenesis and myogenic differentiation of C2C12 myoblasts. Autophagy. 2016;12(2):369–80.
Glick D, Zhang W, Beaton M, Marsboom G, Gruber M, Simon MC, Hart J, Dorn GW 2nd, Brady MJ, Macleod KF. BNip3 regulates mitochondrial function and lipid metabolism in the liver. Mol Cell Biol. 2012;32(13):2570–84.
Soleimanpour SA, Gupta A, Bakay M, Ferrari AM, Groff DN, Fadista J, Spruce LA, Kushner JA, Groop L, Seeholzer SH, Kaufman BA, Hakonarson H, Stoffers DA. The diabetes susceptibility gene Clec16a regulates mitophagy. Cell. 2014;157(7):1577–90.
Tan T, Zimmermann M, Reichert AS. Controlling quality and amount of mitochondria by mitophagy: insights into the role of ubiquitination and deubiquitination. Biol Chem. 2016;397(7):637–47.
Yamaguchi O, Murakawa T, Nishida K, Otsu K. Receptor-mediated mitophagy. J Mol Cell Cardiol. 2016;95:50–6.
Hamacher-Brady A, Brady NR. Mitophagy programs: mechanisms and physiological implications of mitochondrial targeting by autophagy. Cell Mol Life Sci. 2016;73(4):775–95.
Scarpulla RC. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim Biophys Acta. 2011;1813(7):1269–78.
Wenz T. Regulation of mitochondrial biogenesis and PGC-1alpha under cellular stress. Mitochondrion. 2013;13(2):134–42.
Fernandez-Vizarra E, Enriquez JA, Perez-Martos A, Montoya J, Fernandez-Silva P. Mitochondrial gene expression is regulated at multiple levels and differentially in the heart and liver by thyroid hormones. Curr Genet. 2008;54(1):13–22.
Chen JQ, Cammarata PR, Baines CP, Yager JD. Regulation of mitochondrial respiratory chain biogenesis by estrogens/estrogen receptors and physiological, pathological and pharmacological implications. Biochim Biophys Acta. 2009;1793(10):1540–70.
Park SJ, Ahmad F, Philp A, Baar K, Williams T, Luo H, Ke H, Rehmann H, Taussig R, Brown AL, Kim MK, Beaven MA, Burgin AB, Manganiello V, Chung JH. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell. 2012;148(3):421–33.
Le Pennec S, Mirebeau-Prunier D, Boutet-Bouzamondo N, Jacques C, Guillotin D, Lauret E, Houlgatte R, Malthiery Y, Savagner F. Nitric oxide and calcium participate in the fine regulation of mitochondrial biogenesis in follicular thyroid carcinoma cells. J Biol Chem. 2011;286(20):18229–39.
Santandreu FM, Oliver J, Roca P. Improvement of mitochondrial energy and oxidative balance during intestinal differentiation. Mitochondrion. 2011;11(1):89–96.
Rogers RP, Rogina B. Increased mitochondrial biogenesis preserves intestinal stem cell homeostasis and contributes to longevity in Indy mutant flies. Aging (Albany NY). 2014;6(4):335–50.
D’Errico I, Salvatore L, Murzilli S, Lo Sasso G, Latorre D, Martelli N, Egorova AV, Polishuck R, Madeyski-Bengtson K, Lelliott C, Vidal-Puig AJ, Seibel P, Villani G, Moschetta A. Peroxisome proliferator-activated receptor-gamma coactivator 1-alpha (PGC1alpha) is a metabolic regulator of intestinal epithelial cell fate. Proc Natl Acad Sci U S A. 2011;108(16):6603–8.
Llimona F, De Lima TM, Moretti AI, Theobaldo M, Jukemura J, Velasco IT, Machado MC, Souza HP. PGC-1alpha expression is increased in leukocytes in experimental acute pancreatitis. Inflammation. 2014;37(4):1231–9.
Mills EL, Kelly B, O’Neill LAJ. Mitochondria are the powerhouses of immunity. Nat Immunol. 2017;18(5):488–98.
Yang CS, Kim JJ, Lee HM, Jin HS, Lee SH, Park JH, Kim SJ, Kim JM, Han YM, Lee MS, Kweon GR, Shong M, Jo EK. The AMPK-PPARGC1A pathway is required for antimicrobial host defense through activation of autophagy. Autophagy. 2014;10(5):785–802.
Xing W, Yang L, Peng Y, Wang Q, Gao M, Yang M, Xiao X. Ginsenoside Rg3 attenuates sepsis-induced injury and mitochondrial dysfunction in liver via AMPK-mediated autophagy flux. Biosci Rep. 2017;37(4):BSR20170934.
Parikh SM, Yang Y, He L, Tang C, Zhan M, Dong Z. Mitochondrial function and disturbances in the septic kidney. Semin Nephrol. 2015;35(1):108–19.
Alvarez S, Vico T, Vanasco V. Cardiac dysfunction, mitochondrial architecture, energy production, and inflammatory pathways: interrelated aspects in endotoxemia and sepsis. Int J Biochem Cell Biol. 2016;81(Pt B):307–14.
Suliman HB, Kraft BD, Bartz RR, Chen L, Welty-Wolf KE, Piantadosi CA. Mitochondrial quality control in alveolar epithelial cells damaged by S. aureus pneumonia in mice. Am J Physiol Lung Cell Mol Physiol. 2017;31:L699.
Wu H, Wei H, Sehgal SA, Liu L, Chen Q. Mitophagy receptors sense stress signals and couple mitochondrial dynamic machinery for mitochondrial quality control. Free Radic Biol Med. 2016;100:199–209.
Valero T. Mitochondrial biogenesis: pharmacological approaches. Curr Pharm Des. 2014;20(35):5507–9.
Rub C, Wilkening A, Voos W. Mitochondrial quality control by the Pink1/Parkin system. Cell Tissue Res. 2017;367(1):111–23.
Bondi H, Zilocchi M, Mare MG, D’Agostino G, Giovannardi S, Ambrosio S, Fasano M, Alberio T. Dopamine induces mitochondrial depolarization without activating PINK1-mediated mitophagy. J Neurochem. 2016;136:1219.
Rojas-Charry L, Cookson MR, Nino A, Arboleda H, Arboleda G. Downregulation of Pink1 influences mitochondrial fusion-fission machinery and sensitizes to neurotoxins in dopaminergic cells. Neurotoxicology. 2014;44:140–8.
Sheng B, Wang X, Su B, Lee HG, Casadesus G, Perry G, Zhu X. Impaired mitochondrial biogenesis contributes to mitochondrial dysfunction in Alzheimer’s disease. J Neurochem. 2012;120(3):419–29.
Baltrusch S. Mitochondrial network regulation and its potential interference with inflammatory signals in pancreatic beta cells. Diabetologia. 2016;59(4):683–7.
Joseph AM, Joanisse DR, Baillot RG, Hood DA. Mitochondrial dysregulation in the pathogenesis of diabetes: potential for mitochondrial biogenesis-mediated interventions. Exp Diabetes Res. 2012;2012:642038.
Mcgill JK, Beal MF. PGC-1alpha, a new therapeutic target in Huntington’s disease? Cell. 2006;127(3):465–8.
Chen TT, Wu LS, Hsu PW, Pang CY, Lee KM, Cheng PC, Peng SY. Mitochondrial dynamics in the mouse liver infected by Schistosoma mansoni. Acta Trop. 2015;148:13–23.
Yu T, Wang L, Lee H, O’Brien DK, Bronk SF, Gores GJ, Yoon Y. Decreasing mitochondrial fission prevents cholestatic liver injury. J Biol Chem. 2014;289(49):34074–88.
Zhang X, Shan P, Homer R, Zhang Y, Petrache I, Mannam P, Lee PJ. Cathepsin E promotes pulmonary emphysema via mitochondrial fission. Am J Pathol. 2014;184(10):2730–41.
Lu J, Shen Y, Liu LJ, Qian HY, Zhu CL. Combining epinephrine and esmolol attenuates excessive autophagy and mitophagy in rat cardiomyocytes after cardiac arrest. J Cardiovasc Pharmacol. 2015;66(5):449–56.
Chen Y, Liu Y, Dorn GW 2nd. Mitochondrial fusion is essential for organelle function and cardiac homeostasis. Circ Res. 2011;109(12):1327–31.
Papanicolaou KN, Kikuchi R, Ngoh GA, Coughlan KA, Dominguez I, Stanley WC, Walsh K. Mitofusins 1 and 2 are essential for postnatal metabolic remodeling in heart. Circ Res. 2012;111(8):1012–26.
Joshi SR, Dhagia V, Gairhe S, Edwards JG, Mcmurtry IF, Gupte SA. MicroRNA-140 is elevated and mitofusin-1 is downregulated in the right ventricle of the Sugen5416/hypoxia/normoxia model of pulmonary arterial hypertension. Am J Physiol Heart Circ Physiol. 2016;311(3):H689–98.
Singer M. The role of mitochondrial dysfunction in sepsis-induced multi-organ failure. Virulence. 2014;5(1):66–72.
Brealey D, Brand M, Hargreaves I, Heales S, Land J, Smolenski R, Davies NA, Cooper CE, Singer M. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet. 2002;360(9328):219–23.
Carre JE, Orban JC, Re L, Felsmann K, Iffert W, Bauer M, Suliman HB, Piantadosi CA, Mayhew TM, Breen P, Stotz M, Singer M. Survival in critical illness is associated with early activation of mitochondrial biogenesis. Am J Respir Crit Care Med. 2010;182(6):745–51.
Karlsson M, Hara N, Morata S, Sjovall F, Kilbaugh T, Hansson MJ, Uchino H, Elmer E. Diverse and tissue-specific mitochondrial respiratory response in a mouse model of sepsis-induced multiple organ failure. Shock. 2016;45(4):404–10.
Neviere R, Delguste F, Durand A, Inamo J, Boulanger E, Preau S. Abnormal mitochondrial cAMP/PKA signaling is involved in sepsis-induced mitochondrial and myocardial dysfunction. Int J Mol Sci. 2016;17(12):E2075.
Jeger V, Brandt S, Porta F, Jakob SM, Takala J, Djafarzadeh S. Dose response of endotoxin on hepatocyte and muscle mitochondrial respiration in vitro. Biomed Res Int. 2015;2015:353074.
Herminghaus A, Barthel F, Heinen A, Beck C, Vollmer C, Bauer I, Weidinger A, Kozlov AV, Picker O. Severity of polymicrobial sepsis modulates mitochondrial function in rat liver. Mitochondrion. 2015;24:122–8.
Joseph LC, Kokkinaki D, Valenti MC, Kim GJ, Barca E, Tomar D, Hoffman NE, Subramanyam P, Colecraft HM, Hirano M, Ratner AJ, Madesh M, Drosatos K, Morrow JP. Inhibition of NADPH oxidase 2 (NOX2) prevents sepsis-induced cardiomyopathy by improving calcium handling and mitochondrial function. JCI Insight. 2017;2(17):94248.
Stoyanoff TR, Todaro JS, Aguirre MV, Zimmermann MC, Brandan NC. Amelioration of lipopolysaccharide-induced acute kidney injury by erythropoietin: involvement of mitochondria-regulated apoptosis. Toxicology. 2014;318:13–21.
Yi L, Huang X, Guo F, Zhou Z, Chang M, Tang J, Huan J. Lipopolysaccharide induces human pulmonary micro-vascular endothelial apoptosis via the YAP signaling pathway. Front Cell Infect Microbiol. 2016;6:133.
Kautza B, Gomez H, Escobar D, Corey C, Ataya B, Luciano J, Botero AM, Gordon L, Brumfield J, Martinez S, Holder A, Ogundele O, Pinsky M, Shiva S, Zuckerbraun BS. Inhaled, nebulized sodium nitrite protects in murine and porcine experimental models of hemorrhagic shock and resuscitation by limiting mitochondrial injury. Nitric Oxide. 2015;51:7–18.
Ding M, Ning J, Feng N, Li Z, Liu Z, Wang Y, Wang Y, Li X, Huo C, Jia X, Xu R, Fu F, Wang X, Pei J. Dynamin-related protein 1-mediated mitochondrial fission contributes to post-traumatic cardiac dysfunction in rats and the protective effect of melatonin. J Pineal Res. 2017;64:e12447.
Gonzalez AS, Elguero ME, Finocchietto P, Holod S, Romorini L, Miriuka SG, Peralta JG, Poderoso JJ, Carreras MC. Abnormal mitochondrial fusion-fission balance contributes to the progression of experimental sepsis. Free Radic Res. 2014;48(7):769–83.
Sehat A, Huebinger RM, Carlson DL, Zang QS, Wolf SE, Song J. Burn serum stimulates myoblast cell death associated with IL-6-induced mitochondrial fragmentation. Shock. 2017;48(2):236–42.
Jesinkey SR, Funk JA, Stallons LJ, Wills LP, Megyesi JK, Beeson CC, Schnellmann RG. Formoterol restores mitochondrial and renal function after ischemia-reperfusion injury. J Am Soc Nephrol. 2014;25(6):1157–62.
Mannam P, Shinn AS, Srivastava A, Neamu RF, Walker WE, Bohanon M, Merkel J, Kang MJ, Dela Cruz CS, Ahasic AM, Pisani MA, Trentalange M, West AP, Shadel GS, Elias JA, Lee PJ. MKK3 regulates mitochondrial biogenesis and mitophagy in sepsis-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2014;306(7):L604–19.
Smith JA, Stallons LJ, Collier JB, Chavin KD, Schnellmann RG. Suppression of mitochondrial biogenesis through toll-like receptor 4-dependent mitogen-activated protein kinase kinase/extracellular signal-regulated kinase signaling in endotoxin-induced acute kidney injury. J Pharmacol Exp Ther. 2015;352(2):346–57.
Piquereau J, Godin R, Deschenes S, Bessi VL, Mofarrahi M, Hussain SN, Burelle Y. Protective role of PARK2/Parkin in sepsis-induced cardiac contractile and mitochondrial dysfunction. Autophagy. 2013;9(11):1837–51.
Go KL, Lee S, Zendejas I, Behrns KE, Kim JS. Mitochondrial dysfunction and autophagy in hepatic ischemia/reperfusion injury. Biomed Res Int. 2015;2015:183469.
Wang K. Autophagy and apoptosis in liver injury. Cell Cycle. 2015;14(11):1631–42.
Swiader A, Nahapetyan H, Faccini J, D’Angelo R, Mucher E, Elbaz M, Boya P, Vindis C. Mitophagy acts as a safeguard mechanism against human vascular smooth muscle cell apoptosis induced by atherogenic lipids. Oncotarget. 2016;7(20):28821–35.
Liang X, Wei SQ, Lee SJ, Fung JK, Zhang M, Tanaka A, Choi AM, Jin Y. p62 sequestosome 1/light chain 3b complex confers cytoprotection on lung epithelial cells after hyperoxia. Am J Respir Cell Mol Biol. 2013;48(4):489–96.
Gao L, Jiang T, Guo J, Liu Y, Cui G, Gu L, Su L, Zhang Y. Inhibition of autophagy contributes to ischemic postconditioning-induced neuroprotection against focal cerebral ischemia in rats. PLoS One. 2012;7(9):e46092.
Yang JY, Yang WY. Bit-by-bit autophagic removal of parkin-labelled mitochondria. Nat Commun. 2013;4:2428.
Huang P, Galloway CA, Yoon Y. Control of mitochondrial morphology through differential interactions of mitochondrial fusion and fission proteins. PLoS One. 2011;6(5):e20655.
Song M, Gong G, Burelle Y, Gustafsson AB, Kitsis RN, Matkovich SJ, Dorn GW 2nd. Interdependence of Parkin-mediated mitophagy and mitochondrial fission in adult mouse hearts. Circ Res. 2015;117(4):346–51.
Chen Y, Dorn GW 2nd. PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science. 2013;340(6131):471–5.
Song M, Mihara K, Chen Y, Scorrano L, Dorn GW 2nd. Mitochondrial fission and fusion factors reciprocally orchestrate mitophagic culling in mouse hearts and cultured fibroblasts. Cell Metab. 2015;21(2):273–85.
Zhang B, Xu L, Zhuo N, Shen J. Resveratrol protects against mitochondrial dysfunction through autophagy activation in human nucleus pulposus cells. Biochem Biophys Res Commun. 2017;493(1):373–81.
Muthulakshmi S, Saravanan R. Protective effects of azelaic acid against high-fat diet-induced oxidative stress in liver, kidney and heart of C57BL/6J mice. Mol Cell Biochem. 2013;377(1-2):23–33.
Dietl A, Maack C. Targeting mitochondrial calcium handling and reactive oxygen species in heart failure. Curr Heart Fail Rep. 2017;14(4):338–49.
Formentini L, Santacatterina F, Nunez De Arenas C, Stamatakis K, Lopez-Martinez D, Logan A, Fresno M, Smits R, Murphy MP, Cuezva JM. Mitochondrial ROS production protects the intestine from inflammation through functional M2 macrophage polarization. Cell Rep. 2017;19(6):1202–13.
Prauchner CA. Oxidative stress in sepsis: pathophysiological implications justifying antioxidant co-therapy. Burns. 2017;43(3):471–85.
Reddy PH, Manczak M, Kandimalla R. Mitochondria-targeted small molecule SS31: a potential candidate for the treatment of Alzheimer’s disease. Hum Mol Genet. 2017;26(8):1483–96.
Yin X, Manczak M, Reddy PH. Mitochondria-targeted molecules MitoQ and SS31 reduce mutant huntingtin-induced mitochondrial toxicity and synaptic damage in Huntington’s disease. Hum Mol Genet. 2016;25(9):1739–53.
Zhao WY, Han S, Zhang L, Zhu YH, Wang LM, Zeng L. Mitochondria-targeted antioxidant peptide SS31 prevents hypoxia/reoxygenation-induced apoptosis by down-regulating p66Shc in renal tubular epithelial cells. Cell Physiol Biochem. 2013;32(3):591–600.
Mailloux RJ. Application of mitochondria-targeted pharmaceuticals for the treatment of heart disease. Curr Pharm Des. 2016;22(31):4763–79.
Martin L, Peters C, Heinbockel L, Moellmann J, Martincuks A, Brandenburg K, Lehrke M, Muller-Newen G, Marx G, Schuerholz T. The synthetic antimicrobial peptide 19-2.5 attenuates mitochondrial dysfunction in cardiomyocytes stimulated with human sepsis serum. Innate Immun. 2016;22(8):612–9.
Disatnik MH, Ferreira JC, Campos JC, Gomes KS, Dourado PM, Qi X, Mochly-Rosen D. Acute inhibition of excessive mitochondrial fission after myocardial infarction prevents long-term cardiac dysfunction. J Am Heart Assoc. 2013;2(5):e000461.
Xie N, Wang C, Lian Y, Wu C, Zhang H, Zhang Q. Inhibition of mitochondrial fission attenuates Abeta-induced microglia apoptosis. Neuroscience. 2014;256:36–42.
Wu Q, Xia SX, Li QQ, Gao Y, Shen X, Ma L, Zhang MY, Wang T, Li YS, Wang ZF, Luo CL, Tao LY. Mitochondrial division inhibitor 1 (Mdivi-1) offers neuroprotection through diminishing cell death and improving functional outcome in a mouse model of traumatic brain injury. Brain Res. 2016;1630:134–43.
Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation. 2010;121(18):2012–22.
Dillon LM, Hida A, Garcia S, Prolla TA, Moraes CT. Long-term bezafibrate treatment improves skin and spleen phenotypes of the mtDNA mutator mouse. PLoS One. 2012;7(9):e44335.
Dillon LM, Williams SL, Hida A, Peacock JD, Prolla TA, Lincoln J, Moraes CT. Increased mitochondrial biogenesis in muscle improves aging phenotypes in the mtDNA mutator mouse. Hum Mol Genet. 2012;21(10):2288–97.
Mudo G, Makela J, Di Liberto V, Tselykh TV, Olivieri M, Piepponen P, Eriksson O, Malkia A, Bonomo A, Kairisalo M, Aguirre JA, Korhonen L, Belluardo N, Lindholm D. Transgenic expression and activation of PGC-1alpha protect dopaminergic neurons in the MPTP mouse model of Parkinson’s disease. Cell Mol Life Sci. 2012;69(7):1153–65.
Mccreath G, Scullion MM, Lowes DA, Webster NR, Galley HF. Pharmacological activation of endogenous protective pathways against oxidative stress under conditions of sepsis. Br J Anaesth. 2016;116(1):131–9.
Rozova EV, Mankovskaya IN, Mironova GD. Structural and dynamic changes in mitochondria of rat myocardium under acute hypoxic hypoxia: role of mitochondrial ATP-dependent potassium channel. Biochemistry (Mosc). 2015;80(8):994–1000.
Sanderson TH, Raghunayakula S, Kumar R. Neuronal hypoxia disrupts mitochondrial fusion. Neuroscience. 2015;301:71–8.
Anusree SS, Nisha VM, Priyanka A, Raghu KG. Insulin resistance by TNF-alpha is associated with mitochondrial dysfunction in 3T3-L1 adipocytes and is ameliorated by punicic acid, a PPARgamma agonist. Mol Cell Endocrinol. 2015;413:120–8.
Wang DB, Kinoshita C, Kinoshita Y, Morrison RS. p53 and mitochondrial function in neurons. Biochim Biophys Acta. 2014;1842(8):1186–97.
Kang JW, Hong JM, Lee SM. Melatonin enhances mitophagy and mitochondrial biogenesis in rats with carbon tetrachloride-induced liver fibrosis. J Pineal Res. 2016;60(4):383–93.
Hull TD, Boddu R, Guo L, Tisher CC, Traylor AM, Patel B, Joseph R, Prabhu SD, Suliman HB, Piantadosi CA, Agarwal A, George JF. Heme oxygenase-1 regulates mitochondrial quality control in the heart. JCI Insight. 2016;1(2):e85817.
Zhang Q, Tamura Y, Roy M, Adachi Y, Iijima M, Sesaki H. Biosynthesis and roles of phospholipids in mitochondrial fusion, division and mitophagy. Cell Mol Life Sci. 2014;71(19):3767–78.
Wu W, Lin C, Wu K, Jiang L, Wang X, Li W, Zhuang H, Zhang X, Chen H, Li S, Yang Y, Lu Y, Wang J, Zhu R, Zhang L, Sui S, Tan N, Zhao B, Zhang J, Li L, Feng D. FUNDC1 regulates mitochondrial dynamics at the ER-mitochondrial contact site under hypoxic conditions. EMBO J. 2016;35(13):1368–84.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Kuang, L., Liu, L., Li, T. (2019). The Role of Mitochondrial Quality Imbalance in Multiple Organ Dysfunction Syndrome Following Severe Trauma, Shock, and Sepsis. In: Fu, X., Liu, L. (eds) Severe Trauma and Sepsis. Springer, Singapore. https://doi.org/10.1007/978-981-13-3353-8_4
Download citation
DOI: https://doi.org/10.1007/978-981-13-3353-8_4
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-3352-1
Online ISBN: 978-981-13-3353-8
eBook Packages: MedicineMedicine (R0)