Abstract
A titanium phosphonate cluster with a formula of [Ti4(μ3-O)(OiPr)5(μ-OiPr)3(PhPO3)3]·thf was synthesized by the reaction of titanium tetraisopropoxide with phenylphosphonic acid in tetrahydrofuran and the following hydrolysis. The titanium cluster phosphonate was mixed with poly(dimethylsiloxane) (PDMS), poly(methylsilsesquioxane), poly(ethoxysilsesquioxane), poly(methyl methacrylate) (PMMA), poly(vinyl alcohol) (PVA), poly(4-vinylphenol), poly(styrene-co-allyl alcohol), or poly(bisphenol A-co-epichlorohydrin) to form a hybrid film. The mechanical strengths and strains of PDMS hybrids were very low. The tensile strengths and elongations of PMMA hybrids increased with the increase in the titanium cluster concentration. The tensile strengths and elongations of PVA hybrids were highest when the titanium cluster concentration was 10 wt%.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Element-block
- Titanium phosphonate cluster
- Organic-inorganic hybrid
- Poly(methyl methacrylate)
- Poly(vinyl alcohol)
1 Introduction
Organic-inorganic hybrids containing element-blocks, a structural unit consisting of various groups of elements, are promising materials by virtue of their strong performance characteristics, such as high mechanical strength, thermal stability, gas permeability, light emission, and electron conductivity [1]. These characteristics appeared by the hybridization of organic polymer with inorganic material. Indeed, organic-inorganic hybrid materials containing polyhedral octasilsesquioxane (POSS) are reported to show strong gas separation [2] and refractive index [3] qualities.
Metal oxide clusters are unique compounds composed of metal-oxygen bonds as a main-chain bearing side chain, such as organic groups or hydrides, halogens. Polysilsesquioxane cages [4] are famous compounds having silicon and oxygen atoms in a main framework. Oxo-titanium clusters [5] composed of titanium and oxygen atoms are also examples of well-studied metal oxide clusters. Recently, organic-inorganic hybrid polymers prepared by using these clusters were found to improve both thermostability and wear resistivity when compared to the corresponding organic polymers [6,7,8,9]. Organic-inorganic hybrid materials are divided into two classes on the basis of bonding type. Class I: the polymers are mixed by weak interactions such as van der Waals force and hydrogen bonding. Class II: the polymers are mixed by strong interactions such as covalent bonding or ionic bonding between organic and inorganic components [10]. For example, Chujo reported that the hybrid materials of polysulfide-bridged POSS with poly(methyl methacrylate) or polystyrene (class I) showed high thermal stability and high refractive indices compared to the calculated values [3]. Schubert reported that poly(methyl methacrylate) cross-linked with Ti4O2(OiPr)6(OMc)6 (OMc = methacrylate) (class II) has high thermal stability [6]. Therefore, organic-inorganic hybrid materials are very interesting because we can expect the improvement of their physical properties to open new application in materials science.
Clusters containing three components are being reported these days. Titanium phosphonate clusters formed by titanium, oxygen, and phosphorus have the potential for high chemical and thermostable properties bearing Ti-O-P bonds. The interactions among organophosphonate groups are easily controlled by changing the organic groups on titanium or phosphorus atoms. Organic-inorganic hybrid materials having titanium phosphonate clusters would be prepared by an alcohol exchange reaction or the sol-gel process [11] due to the alkoxy group on titanium. The synthesis of titanium phosphonate clusters has been reported [12,13,14,15,16,17], however, that of organic-inorganic hybrid materials containing these clusters has not been investigated. One of the problems for preparing hybrid materials using previous clusters is dimethyl sulfoxide as coordinating solvent. Dimethyl sulfoxide has strong coordinating properties compared with tetrahydrofuran (THF) that is one of the common coordinating solvents.
2 Experimental
2.1 Measurements
Nuclear magnetic resonance (NMR) spectra were recorded using a JEOL Resonance JNM-ECP 500 spectrometer (1H at 500.16 MHz, 13C at 125.77 MHz, 31P at 202.46 MHz) at 24 °C. The chemical shifts were reported in ppm with reference to chloroform-d (CDCl3) as an internal standard (for 1H: 7.24 ppm in residual chloroform, 13C: 77.00 ppm) and phosphoric acid (H3PO4) as an external standard (for 31P: 0.00 ppm). Infrared (IR) spectra were recorded on a JASCO FT/IR-6100 FT-IR spectrophotometer using attenuated total reflectance (ATR, ZnSe prism, JASCO ATR PRO 0450-S). Thermogravimetric-differential thermal analysis (TG-DTA) was performed with a NETZSCH JAPAN TG-DTA 2000SE analyzer. The samples were heated to 1000 °C under air flow at a rate of 10 °C/min. Transmittance spectra were recorded on a JASCO V-670 spectrophotometer equipped with an integrating-sphere photometer (JASCO ISN-470 type) in the range of 200–800 nm. Refractive indices were determined using an Otsuka Electronics FE-3000 refractive film thickness monitor. Gel permeation chromatography (GPC) was performed using a high-performance liquid chromatography system (LC-6AD, Shimadzu, Kyoto, Japan) attached to a Polymer Laboratory gel 5 μm Mixed-D column. Tetrahydrofuran was used as the eluent (1 mL min−1). RID-10A was used as the detector. The molecular weight was calculated based on polystyrene standards. Transmittance spectra were recorded using a JASCO V-670 spectrophotometer equipped with an integrating-sphere photometer (JASCO ISN-470 type) in the 200–800 nm wavelength range. The tensile strength was recorded using a Shimadzu Autograph AG-50 kN Xplus at the rate of 2 mm/min. The size of the test samples is shown below: height 25 mm, width 5 mm, thickness 50–100 nm.
2.2 X-Ray Structure Analyses
For structural determination, crystal data were collected using a Bruker AXS SMART APEX CCD X-ray diffractometer equipped with a rotating-anode X-ray generator emitting graphite-monochromatic Mo-Kα radiation (λ = 0.71073 Å) at 77 K. Empirical absorption corrections using equivalent reflections and Lorentzian polarization correction were performed using the SADABS program [18]. All data were collected with SMART and Bruker SAINTPLUS (Version 6.45) software packages. The structures were solved using the SHELXS-97 program [19] and refined against F 2 using SHELEXL-97 [19]. CCDC 1487366 contains the supplementary crystallographic data for [Ti4(μ 3-O)(OiPr)5(μ-OiPr)3(PhPO3)3]·thf. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html, or from the Cambridge Crystallographic Data Center, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223 336 033; or e-mail: deposit@ccdc.cam.ac.uk.
2.3 Materials
The structure and abbreviation of compounds are summarized in Scheme 12.1. All solvents were purified by a standard process [20] and stored over activated molecular sieves. Titanium tetraisopropoxide (Ti(OiPr)4) and PVA (degree of polymerization, 500) and tetraethylammonium hydroxide (Et4NOH 20% aqueous solution) were purchased from Wako Pure Chemical Industries. Et4NOH was concentrated by removal of the water under vacuum. Phenylphosphonic acid (PhPO3H2) was purchased from Tokyo Chemical Industry. Poly(vinyl alcohol) (PVP) (M w = 25,000 g/mol), poly(styrene-co-allyl alcohol) (PSA) (M w = 2200 g/mol, allyl alcohol 40 mol%), poly(bisphenol A-co-epichlorohydrin) (PBE) (M w = 40,000 g/mol), and poly(methyl methacrylate) (PMMA) (M w = 997,000 g/mol) were purchased from Sigma-Aldrich (Tokyo, Japan) and used as received. Trimethoxy(methyl)silane (MTMS), tetraethoxysilane (TEOS), and chloro(trimethyl)silane (TMSCl) were purchased from Tokyo Chemical Industry, Tokyo, Japan, and purified by distillation before use. Octamethylcyclotetrasiloxane was purchased from Shin-Etsu Chemical Co., Tokyo, Japan and purified by distillation before use.
2.4 Synthesis of [Ti4(μ 3-O)(OiPr)5(μ-OiPr)3(PhPO3)3]·thf (TiOPPh)
The reaction was carried out in an argon atmosphere. Ti(OiPr)4 (36 mL, 0.12 mol) was added to PhPO3H2 (9.56 g, 60.5 mmol) and H2O (0.36 mL, 20 mmol) in 48 mL THF at room temperature, after which white precipitation appeared quickly. After several hours of stirring, the mixture became a colorless solution, whereupon stirring was stopped. Colorless black crystals from the solution appeared after several weeks and were then filtrated and dried under reduced pressure. White block crystals were obtained in a yield of 8.25 g (34%).
1H NMR (500 MHz, CDCl3/7.24 ppm): δ = 1.06 (d, J = 5.5 Hz, 12H), 1.37 (d, J = 6.0 Hz, 18H), 1.44 (d, J = 6.0 Hz, 18H), 1.75–1.81 (m, 6H), 3.79–3.82 (m, 6H), 4.69 (sept, J = 6.0 Hz, 3H), 4.94 (br-s, 2H), 5.03 (sept, J = 6.0 Hz, 3H), 7.30–7.38 (m, 9H), 7.88 (dd, J = 7.5 Hz, 3J P-H = 12.0 Hz, 6H).
13C{1H} NMR (126 MHz, CDCl3/77.0 ppm): δ = 24.51, 24.83, 25.36, 68.84, 78.45, 79.14, 79.40, 127.31 (d, 2J P-C = 14.4 Hz), 129.72 (d, 4J P-C = 2.9 Hz), 131.05 (d, 3J P-C = 9.6 Hz), 134.44 (d, 1J P-C = 203.9 Hz) 31P{1H} NMR (202 MHz, CDCl3/ppm): δ = 9.22. Ceramic yield: 40.4% (calcd. for Ti4P3O16 41.7%).
2.5 Preparation of Poly(dimethylsiloxane) (PDMS)
Octamethylcyclotetrasiloxane (30.0 g, 0.10 mol) and Et4NOH (0.60 g, 4 mmol) were placed into a 100 mL four-necked flask equipped with an Allihn condenser and a mechanical stirrer. The mixture was stirred at 100 °C for 3 h at 200 rpm. The viscous liquid was extracted with dichloromethane. The organic layer was washed with brine and dried with anhydrous magnesium sulfate. After filtration, the solution was concentrated to remove vapor compounds under vacuum at 60 °C. PDMS was obtained as a colorless viscous liquid (27.2 g, 91%).
1H NMR (300 MHz, CDCl3/ppm) δ 0.08 (brs, Si-CH 3). 29Si NMR (60 MHz, CDCl3/ppm) δ −21.55. M w = 93,300 g/mol, M w/M n = 1.9.
2.6 Preparation of Poly(methylsilsesquioxane) (PMS)
MTMS (27.2 g, 0.20 mol) and MeOH (13.3 g, 0.42 mol) were placed into a 200 mL four-necked flask equipped with nitrogen inlet and outlet tubes and a mechanical stirrer. The mixture was then cooled in an ice bath for 10 min. Water and hydrochloric acid (molar ratios; H2O/MTMS = 1.0, HCl/MTMS = 0.105) were added. The mixture was stirred in an ice bath for 10 min and then at room temperature for 10 min, followed by heating at 70 °C for 3 h at 150 rpm with a 360 mL/min nitrogen flow. PMS was obtained as colorless liquid (16.3 g).
1H NMR (300 MHz, CDCl3/ppm) δ 0.12 (brs, Si-CH 3), 3.51 (brs, Si-OCH 3). 29Si NMR (60 MHz, CDCl3/ppm) δ −49.35 (T1, content ratio of 6%), −58.87 (T2, that of 50%), −67.17 (T3, that of 44%). M w = 3800 g/mol, M w/M n = 1.8.
2.7 Preparation of Poly(ethoxysilsesquioxane) (PEOS)
TEOS (34.8 g, 0.17 mol) and EtOH (15.9 g, 0.35 mol) were placed into a 300 mL four-necked flask equipped with nitrogen inlet and outlet tubes and a mechanical stirrer. The mixture was then cooled in an ice bath for 10 min. Water and hydrochloric acid (molar ratios; H2O/TEOS = 1.7, HCl/TEOS = 0.105) were added. The mixture was stirred in an ice bath for 10 min and then at room temperature for 10 min, followed by heating at 80 °C for 2 h at 150 rpm with a 360 mL/min nitrogen flow. The resulting product was dissolved into 40 mL THF, then 2 mL TMSCl was added, followed by stirring for 1 day. The mixture was concentrated to remove vapor compounds, and PEOS was obtained (15.7 g).
1H NMR (300 MHz, CDCl3/ppm) δ 0.16 (brs, Si-CH 3), 1.25 (brs, Si-OCH2CH 3), 3.89 (brs, Si-OCH 2CH3). 29Si NMR (60 MHz, CDCl3/ppm) δ 12.76 (M, content ratio of 8%), −95.86 (Q2, that of 12%), −102.78 (Q3, that of 80%). M w = 7200 g/mol, M w/M n = 2.7.
2.8 Preparation of Freestanding Hybrid Films
Polymer solution of THF or toluene was added to TiOPPh and stirred for 3 h at room temperature. The mixture was poured into a 50 mmϕ Teflon petri dish followed by curing at 50 °C for 1 day and then at 120 °C for 1 day.
2.9 Preparation of Hybrid Thin Films
A solution of 0.12 g of PVA in 5 mL of deionized water was added to TiOPPh in 5 mL of THF, and the mixture was stirred at room temperature for overnight. Hybrid thin films were prepared by spin-coating of the solution on silicon wafer at 1000 rpm for 20 s and then heating at 120 °C for 2 min in air.
3 Results and Discussion
3.1 Characterization of TiOPPh
TiOPPh was synthesized by the reaction of Ti(OiPr)4 with PhPO3H2 and water in THF. The formation of this cluster was determined by various NMR spectra, FT-IR spectra, and single-crystal X-ray structural analysis as shown in Fig. 12.1 and Table 12.1. From the single-crystal X-ray structural diffraction, TiOPPh was proved to be built up from four titanium atoms and three RPO3, five terminal OiPr, three bridged OiPr, one μ 3-O, and one THF molecules. The main framework of TiOPPh consisted of three bridged RPO3 between one Ti atom coordinated by THF and one Ti3O containing a μ 3-O atom. Three phosphonates are arranged in one direction, one on top of the other, and are capped by a THF-coordinated Ti atom. This structure is very similar to that of [Ti4(μ 3-O)(OiPr)5(μ-OiPr)3(PhPO3)3]·dmso [10]. In the 1H NMR spectrum, three doublet signals attributable to CH3 in the isopropoxy group were found at 1.06, 1.37, and 1.44 ppm with respective integration ratios of 12H, 18H, and 18H. These signals are assigned to the terminal isopropoxy group on the Ti coordinated by THF, the terminal isopropoxy group on the Ti3O unit, and the bridged isopropoxy group, respectively. CH in the isopropoxy group show signals at 4.69, 4.94, and 5.03 ppm. The signals derived from the phenyl group shown at 7.30–7.38 ppm as a multiplet are assigned to m- and p-CH in the phenyl group with overlapping, and a signal at 7.88 ppm in the double doublet assigned to o-CH in the phenyl group was observed. 13C NMR spectrum shows signals due to CH3 at 24.51 and 24.83 ppm, signals due to THF at 25.36 and 68.84 ppm, and signals due to CH at 78.45, 79.14, and 79.40 ppm. The signals derived from the phenyl group appear in doublet because of the coupling between phosphorus and carbon atoms. Signals were observed at 127.31 ppm assigned to o-CH, at 129.72 ppm assigned to p-CH, at 131.05 ppm assigned to m-CH, and at 134.44 ppm assigned to ipso-CH. The 31P NMR spectrum shows signals at 9.22 ppm in CDCl3; in C6D6, it shows signals at 6.82 ppm with an integrated intensity of 2 and at 7.16 ppm with an integrated intensity of 1.
ORTEP drawing of TiOPPh with thermal ellipsoids at the 50% probability level. Hydrogen atoms are omitted for clarity. Selected interatomic distances (Å) and bond angles (°) are as follows: Ti(1)–O(1) 1.972(6), Ti(1)–O(7) 1.962(4), Ti(1)–O(11) 2.010(5), Ti(1)–O(14) 1.779(5), Ti(4)–O(8) 1.924(6), Ti(4)–O(17) 1.820(7), Ti(4)–O(19) 2.162(6), P(1)–O(1) 1.536(4); O(1)–Ti(1)-O(3) 90.7(2), O(1)–Ti(1)-O(11) 87.5(2), O(7)-Ti(1)-O(14) 175.4(2), O(8)-Ti(4)-O(9) 94.5(2), O(8)-Ti(4)-O(19) 174.3(3), O(1)-P(1)-O(2) 111.4(3), O(1)-P(1)-O(8) 110.9(3)
The FT-IR spectrum of TiOPPh shows the following absorption bands: νC-H in phenyl at 3056 cm−1, νC-H in the isopropoxy group at 2970 and 2928 cm−1, νC-H in THF at 2862 cm−1, νC=C in phenyl at 1439 cm−1, δC-H at 1375 and 1362 cm−1, νC-C at 1156 and 1132 cm−1, νC-O and νP-O at 989–949 cm−1 overlapping with δC=C at 754 and 696 cm−1 assigned to out-of-plane of phenyl, and νTi-O at 614–558 cm−1. The TG-DTA of TiOPPh shows weight losses at 80–250 °C (41%) assigned to the decomposition of the isopropoxy group, THF, and carbons in phenyl, and at 450 °C (8%) and at 800 °C (10%).
3.2 Preparation of Freestanding Hybrid Films
PMMA hybrid films were prepared from toluene solution, and PVA hybrid films were prepared from DMSO and THF solution. A photograph of the hybrid films is shown in Fig. 12.2 The PMMA films changed from colorless to yellow as the concentration of TiOPPh increased. On the other hand, colorless and transparent films were prepared by using PVA. We note that we have tried to prepare hybrid films containing 30 wt% of PMMA and 50 wt% of PVA. Unfortunately, these films were not prepared because they were too rigid to retain the form of freestanding films.
FT-IR spectra are shown in Fig. 12.3. All PMMA hybrid films show νC-H at 3000–2950 cm−1, νC-H at 1723 cm−1, and νC-O-C at 1190 and 1144 cm−1. PMMA-TiOPPh hybrid films show new bands due to νP=O at 1040 cm−1 and νTi-O at around 550 cm−1. However, the band of νP-O could be observed only for the 20 wt% TiOPPh. These bands derived from PMMA were very similar, especially νC=O at 1723 cm−1. These results indicated that the carbonyl groups of PMMA did not coordinate to Ti atom of TiOPPh via an exchange reaction between THF and the carbonyl groups. However, νC-O-C shifted from 1144 cm−1 (PMMA) to 1142 cm−1 (PMMA-TiOPPh hybrid). Therefore, PMMA and TiOPPh can be blended by using weak interaction between methoxy group of PMMA and TiOPPh. On the other hand, all PVA hybrid films showed νO-H at 3300 cm−1, νC-H at 2911 cm−1, and νC-C at 1421 cm−1. The signal intensity due to νP-O at 1000 cm−1 was increased, and that due to νO-H decreased when TiOPPh was increased. From these results, an alcohol-exchange reaction between the hydroxy group in PVA and the isopropoxy group in TiOPPh.
Transmittance spectra are shown in Fig. 12.4 and the data are summarized in Table 12.2. The transparency of PMMA hybrid films significantly decreased in the visible region as the TiOPPh concentration increased. Moreover, these TiOPPh-PMMA hybrid films were yellow. On the other hand, the transparency of the PVA hybrid films was high compared with that of the PVA films. The origin of the yellow color in PMMA films is the aggregation and/or polymerization of TiOPPh caused by water in air. In contrast, TiOPPh in PVA seems to be highly dispersed. Therefore, PVA hybrid films were obtained as colorless film. These ideas are also supported by the results of IR spectroscopy.
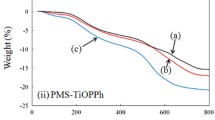
TG-DTA thermograms of hybrid films are shown in Fig. 12.5, and the data are summarized in Table 12.2. The temperatures of 10% weight loss (Td10) were 279 °C and 278 °C for PMMA and PVA, respectively. The values of Td10 for PMMA-TiOPPh hybrid films were about 310 °C and increased 30 °C compared to that of PMMA. TiOPPh improves the thermal stability of PMMA such that the weight loss is 40 wt% at 310 °C. The improvement of thermal stability was as same as other blend or hybrid PMMA materials in previously reported papers which contain polysulfide-bridged POSS [3], zirconia nanocrystals modified with 3-(methacryloxy)propyl-trimethoxysilane [21], and titania modified with 2-hydroxyethyl methacrylate [19]. On the other hand, the Td10 of the PVA-TiOPPh hybrid films decreased as the concentration of TiOPPh increased, such that PVA-30 wt% of the TiOPPh hybrid film was decreased at 45 °C compared to PVA. The decrease in the value of Td10 is attributable to the reaction of the isopropoxy group in TiOPPh with the residual hydroxy group in PVA.
The refractive indices of PVA-hybrid thin films at 633 nm were measured. The refractive index of only PVA was 1.488, and those of PVA-2.5 wt% TiOPPh and PVA-10 wt% TiOPPh were 1.500 and 1.501, respectively. The increase of refractive index supports the formation of hybrids.
3.3 Preparation and Properties of Silicone Polymers/TiOPPh Hybrid
The silicone/TiOPPh hybrids were prepared by mixing silicone polymers with TiOPPh in toluene (Table 12.3). Freestanding PDMS/TiOPPh films were prepared in the concentration of 20–40 wt% TiOPPh, and the obtained films were highly flexible. When the concentration was less than 20 wt%, the hybrids were oily, viscous, and adhesive. On the other hand, when the concentration was more than 40 wt%, the hybrids were obtained as glassy solids. In the IR spectra of the hybrids, no absorption band assigned to ν Si-O-Ti (around 950 cm−1) [22] was observed. The transparency of PDMS hybrid was higher than 89% (at 420 nm) even at a concentration of 40 wt%. This transparency is higher than the PMMA composite (43% at 420 nm, due to the aggregation of TiOPPh in PMMA) [23]. The hybrids using PDMS were, therefore, composed of segregated polymer domains with uniform dispersions of TiOPPh in PDMS. The thermogravimetric traces for PDMS hybrids are shown in Table 12.2. The temperatures at which 10% weight loss occurred (T d10) were 414 °C (PDMS), 281 °C (TiOPPh 20 wt%), and 264 °C (TiOPPh 40 wt%). The decrease of T d10 was very similar to the tendency of PDMS/metal oxide composite that was reported to accelerate the oxidation of methyl groups and the depolymerization with formation of cyclosiloxanes [24]. The char yield of PDMS only and TiOPPh only at 1000 °C in air were 28.2% and 40.4%, respectively. Thus, the thermal decomposition of PDMS mainly occurs by the depolymerization because the char yield would be 81.0% if the thermal decomposition of PDMS was proceed to oxidize all of the methyl groups. The char yields of PDMS/20 wt%, 30 wt%, and 40 wt% TiOPPh were 28.9%, 31.2%, and 37.2%, respectively, where we can expect 30.6% (for 20 wt%), 31.9% (for 30 wt%), and 33.1% (for 40 wt%). The depolymerization of PDMS would be more accelerated when the content of TiOPPh is low, and the oxidation of PDMS would be slightly accelerated at high concentration.
The freestanding PMS/TiOPPh films were prepared in the concentration of 10–40 wt% TiOPPh, but the films were brittle and easily broken. The PMS hybrid containing 40 wt% TiOPPh was yellow and 67% transparent at 420 nm. Other films were colorless with 93% transparent at 420 nm. In the IR spectra of 40 wt%, a new small absorption band was observed at 912 cm−1, which was assigned to νSi-O-Ti. Unfortunately, this band was so weak that this band was not observed when the concentration was less than 40 wt%. The T d10 decreased when the concentration of TiOPPh was increased as 573 °C for PMS, 557 °C for 20 wt% TiOPPh, and 452 °C for 40 wt% TiOPPh. TiOPPh might accelerate the oxidation of methyl group on PMS as with thermal decomposition of PDMS.
Freestanding PEOS hybrid films were barely formed because the hybrids were rigid and brittle. In the IR spectrum of the hybrid containing 40 wt% TiOPPh, a new absorption band assigned to ν Si-O-Ti (at 908 cm−1) was observed. The T d10 of the PEOS hybrid decreased slightly compared to that of only PEOS. TiOPPh maybe act as a catalyst of hydrolytic polycondensation of alkoxysilane such as metal acetylacetonate complexes [25].
3.4 Preparation and Properties of Organic Polymers/TiOPPh Hybrids
The organic polymers/TiOPPh hybrids were prepared by mixing organic polymers with TiOPPh in THF (Table 12.3). When TiOPPh was added to PVP solution, the solution immediately changed from colorless to red, and the coloration increased with the concentration of TiOPPh. This phenomenon was similar to that observed in the synthesis of [Ti2(μ-OPh)2(OPh)6(HOPh)2] by the reaction of Ti(OiPr)4 with phenol [26]. Thus, the alcohol exchange reaction is expected to proceed between PVP and TiOPPh. These freestanding films were rigid. The coating films were prepared on a silicon wafer by spin-coating, which were colored in orange. The intensity of the hydroxyl group at 3200–3500 cm−1 in the FT-IR spectra decreased with the increase in TiOPPh. The T d10 were 242 °C (PVP) and 406 °C (50 wt% concentration), showing that TiOPPh acts as a good cross-linker to PVP matrix.
Freestanding PSA hybrid films were barely formed because of the low molecular weight (2200 g/mol) of PSA. In the IR spectra, the intensity of the hydroxyl group decreased as the concentration of TiOPPh increased. The T d10 is summarized in Table 12.4 as 332 °C (PSA), 241 °C (PSA 20 wt%), and 279 °C (PSA 40 wt%).
Freestanding PBE hybrid films were prepared at concentrations of less than 50 wt% TiOPPh, and the obtained films were orange. In the IR spectra of PBE hybrids, the intensity of ν OH decreased with the increase in TiOPPh content. Thus, TiOPPh was reacted with hydroxyl groups of PBE. Also, the top of the absorption bands assigned to ether groups (νC-O-C) shifted from 1036 cm−1 (PBE) to 1032 cm−1 (PEB hybrids). In the case of Zn crown ether-type complex coordinated from the oxygen atoms to zinc atom, the band of νC-O-C was shifted to decrease 6 cm−1 less than only crown ether ligand [12]. Therefore, the PBE hybrids suggest the formation of chelate by the coordination of the oxygen atom to titanium. The transmittance of freestanding films is summarized in Table 12.4 as 89% (PBE), 55% (10 wt% TiOPPh), and 40% (50 wt% TiOPPh) at 420 nm. The T d10 values were 397 °C (PBE), 350 °C (20 wt% TiOPPh), and 341 °C (50 wt% TiOPPh). The thermal stabilities of the PSA and PBE hybrids were lower than those of PSA and PBE polymers and PVA hybrid as reported before [23].
3.5 Tensile Strengths of Freestanding Films
The measured tensile strengths of freestanding films of PDMS and PVA hybrids and PMMA composites are summarized in Table 12.5. The freestanding PDMS hybrid films show very low tensile strengths: 0.6 MPa (PDMS 20 wt% TiOPPh) and 0.2 MPa (30 wt% TiOPPh). The tensile strengths of PDMS-TEOS hybrid materials containing a titanium cross-linker were reported to be increased by the increase in the titanium content [27]. Moreover, the tensile strength of PDMS materials is known to depend strongly on the cross-linked network [28]. Therefore, the molecular interaction between PDMS and TiOPPh would be weak.
The tensile strengths of PMMA composite films are shown in Fig. 12.3 (i). Stress and strain increased with the increase in the concentration of TiOPPh for 0, 2.5, and 10 wt%. The tensile strength was increased about threefold from only PMMA film by mixing TiOPPh in 10 wt %. This improvement is higher than that in PMMA–montmorillonite composite material, which showed an improvement of ca. 1.1-fold [29]. The strain was also increased with the increase in the TiOPPh concentration, suggesting mixing PMMA with TiOPPh will yield hard and brittle composites.
The tensile strengths of PVA hybrid films are shown in Fig. 12.6 (ii). The strain increased with the similar manner with PMMA hybrids to 10 wt% cluster contain. On the other hand, the strain decreased when the content was increased from 20 to 30 wt %. The Young’s modulus was evaluated for the stress and strain values of the initial stage. The value was increased by the TiOPPh content that suggests the formation of brittle and hard hybrids due to the cross-linking by TiOPPh. Maximum strain and stress were observed when the TiOPPh content was 10 wt % because TiOPPh acts as a good cross-linker to form hard and brittle hybrids.
4 Conclusion
A titanium phosphonate cluster with a formula of [Ti4(μ3-O)(OiPr)5(μ-OiPr)3(PhPO3)3]·thf was synthesized by the reaction of titanium tetraisopropoxide and phenylphosphonic acid in water and tetrahydrofuran. Titanium phosphonate was mixed with PMMA to form a yellow hybrid film at a concentration of less than 30 wt%. The degradation temperature increased about 30 °C when the titanium phosphonate cluster was hybridized with PMMA. On the other hand, titanium phosphonate was mixed with PVA to form a hybrid film. The resulting content was less than 50 wt% and colorless. Isopropyl alcohol was detected after the formation of the hybrid films, which confirms the reaction of PVA with the isopropoxy group in titanium phosphonate cluster. The application of titanium phosphonate clusters for preparing hybrid materials is expected to be useful for developing new organic-inorganic hybrid materials including various reactive positions in a molecule.
Organic-inorganic hybrids containing TiOPPh as a new element-block were prepared by using silicon polymers such as PDMS, PMS, and PEOS and organic polymers such as PMMA, PVA, PVP, PSA, and PBE. The concentration of TiOPPh was increased to 40 wt% to form a transparent film for PDMS, PMS, PVA, and PBE, whereas the concentration was 20 wt% to form such a film for PMMA. The temperature at which the 10 wt% weight loss occurred was increased for the hybrid using PVP because the alcohol exchange reaction between TiOPPh and PVP was expected to form a rigid network, whereas the 10 wt% weight loss temperature decreased with the increase in TiOPPh concentration for PSA, PBE, PDMS, PMS, and PEOS. The PDMS hybrids showed very low tensile strengths and elongations. The tensile strengths and elongations of PMMA hybrids increased with the increase in the TiOPPh concentration. The tensile strengths and elongations of PVA hybrid were highest when the concentration of TiOPPh was 10 wt%. As a result, TiOPPh was found to be a good cross-linker to form hard and brittle hybrids.
References
Chujo Y, Tanaka K (2015) New polymer materials based on element–blocks. Bull Chem Soc Jpn 88:633–643
Kanezashi M, Shioda T, Gunji T, Tsuru T (2012) Gas permeation properties of silica membranes with uniform pore sizes derived from polyhedral oligomeric silsesquioxane. AICHE J 58:1733–1743
Tanaka K, Yamane H, Mitamura K, Watase S, Matsukawa K, Chujo Y (2014) Transformation of sulfur to organic–inorganic hybrids employed by networks and their application for the modulation of refractive indices. J Polym Sci Part A Polym Chem 52:2588–2595
Cordes DB, Lickiss PD, Rataboul F (2010) Recent developments in the chemistry of cubic polyhedral oligosilsesquioxanes. Chem Rev 110:2081–2173
Bradley DC, Mehrotra RC, Rothwell IP, Singh A (2001) Alkoxo and aryloxo derivatives of metals. Academic, San Diego
Schubert U (2004) Organofunctional metal oxide clusters as building blocks for inorganic-organic hybrid materials. J Sol-Gel Sci Techn 31:19–24
Rozes L, Steunou N, Fornasieri G, Sanchez C (2006) Titanium-oxo clusters, versatile nanobuilding blocks for the design of advanced hybrid materials. Monatsh Chem 137:501–528
Gross S (2011) Oxocluster-reinforced organic–inorganic hybrid materials: effect of transition metal oxoclusters on structural and functional properties. J Mater Chem 21:15853–15861
Schubert U (2011) Cluster-based inorganic–organic hybrid materials. Chem Soc Rev 40:575–582
Kickelbick G (2006) Hybrid materials. Wiley VCH, Weinheim
Guerrero G, Mutin PH, Vioux A (2000) Mixed nonhydrolytic/hydrolytic sol−gel routes to novel metal oxide/phosphonate hybrids. Chem Mater 12:1268–1272
Guerrero G, Mehring M, Mutin PH, Dahan F, Vioux A (1999) Syntheses and single-crystal structures of novel soluble phosphonate- and phosphinato-bridged titanium oxo alkoxides. J Chem Soc Dalton Trans:1537–1538
Mehring M, Guerrero G, Dahan F, Mutin PH, Vioux A (2000) Syntheses, characterizations, and single-crystal x-ray structures of soluble titanium alkoxide phosphonates. Inorg Chem 39:3325–3332
Czakler M, Artner C, Schubert U (2013) Influence of the phosphonate ligand on the structure of phosphonate-substituted titanium oxo clusters. Eur J Inorg Chem:5790–5796
Czakler M, Artner C, Schubert U (2014) Acetic acid mediated synthesis of phosphonate-substituted titanium Oxo clusters. Eur J Inorg Chem 2014:2038–2045
Czakler M, Artner C, Schubert U (2015) Titanium oxo/alkoxo clusters with both phosphonate and methacrylate ligands. Monatsh Chem 146:1249–1256
Kalita L, Kalita AC, Murugavel R (2014) Organotitanium phosphates with free P–OH groups: synthesis, spectroscopy and solid state structures. J Organomet Chem 751:555–562
Sheldrick GM (1996) SADABS, program for Siemens area detector absorption correction. University of Göttingen, Germany
Sheldrick GM (1997) SHELXS-97, program for crystal structure solution. University of Göttingen, Germany
Armarego WLF, Chai C (2012) Purification of laboratory chemicals, 7th edn. Elsevier, Oxford
Otsuka T, Chujo Y (2010) Poly(methyl methacrylate) (PMMA)-based hybrid materials with reactive zirconium oxide nanocrystals. Polym J 42:58–65
Julián B, Gervais C, Cordoncillo E, Escribano P, Babonneau F, Sanchez C (2003) Synthesis and characterization of transparent PDMS-metal-oxo based organic–inorganic nanocomposites. Chem Mater 15:3026–3034
Hayami R, Wada K, Sagawa T, Tsukada S, Watase S, Gunji T (2017) Preparation and properties of organic-inorganic hybrid polymer films using [Ti4(μ 3-O)(OiPr)5(μ-OiPr)3(O3PPh)3]·thf. Polym J 49:223–228
Guńko VM, Borysenko MV, Pissis P, Spanoudaki A, Shinyashiki N, Sulim IY, Kulik TV, Palyanytsya BB (2007) Polydimethylsiloxane at the interfaces of fumed silica and zirconia/fumed silica. Appl Surf Sci 253:7143–7156
Adachi K, Hirano T (2008) Good linear relationship between logarithms of Eigen’s water exchange constants for several divalent metal ions and activation energies of corresponding metal-catalyzed alkoxysilane hydrolysis in ethylene-propylene copolymer system. Eur Polym J 44:542–549
Svetich GW, Voge AA (1972) The crystal and molecular structure of sym-trans-di-μ-phenoxyhexaphenoxydiphenolatodititanium (IV). Acta Crystallogr B 28:1760
Glaser RH, Wilkes GL (1988) Structure property behavior of polydimethylsiloxane and poly(tetramethylene oxide) modified TEOS based sol-gel materials V. effect of titaniumisopropoxide incorporation. Polym Bull 19:51–57
Liu M, Sun J, Sun Y, Bock C, Chen Q (2009) Thickness-dependent mechanical properties of polydimethylsiloxane membranes. J Micromech Microeng 19:35028–35031. https://doi.org/10.1088/0960-1317/19/3/035028
Lee DC, Jang LW (1996) Preparation and characterization of PMMA–clay hybrid composite by emulsion polymerization. J Appl Polym Sci 61:1117–1122
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research on Innovative Areas “New Polymeric Materials Based on Element-Blocks (No.2401)” (JSPS KAKENHI Grant Number JP24102008). This work was supported by JSPS KAKENHI Grant Number JP16K17951.
Shinji Ogihara, Ryuta Kitamura, and Ryosuke Matsuzaki are greatly acknowledged for their technical assistance in the tensile strength measurement.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Gunji, T., Tsukada, S. (2019). Metalloxane Cage Compounds as an Element-Block. In: Chujo, Y. (eds) New Polymeric Materials Based on Element-Blocks. Springer, Singapore. https://doi.org/10.1007/978-981-13-2889-3_12
Download citation
DOI: https://doi.org/10.1007/978-981-13-2889-3_12
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-2888-6
Online ISBN: 978-981-13-2889-3
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)