Abstract
Periostin is a protein having two characteristics—an extracellular matrix (ECM) protein important for maintaining tissue or organ structures and in generating fibrosis by binding to other ECM proteins and a matricellular protein transducing intracellular signaling by binding to several integrins on the cell surface. IL-4 and IL-13, signature type 2 cytokines abundantly expressed in the inflamed sites of asthma, induce periostin in several tissue-resident cells—fibroblasts, airway epithelial cells, and endothelial cells—and then periostin contributes to generating thick basement membranes, a typical histological feature in asthma patients. Periostin would play a role in accelerating airway allergic inflammation in asthma by acting as a matricellular protein. Serum periostin has characteristics as a biomarker, reflecting both type 2 inflammation and remodeling/fibrosis in asthma patients. These characteristics are useful for stratifying asthma patients and can predict the ICS resistance or efficacy of molecularly targeted drugs such as IL-4/IL-13 antagonists for asthma patients.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Periostin was originally identified in 1993 as osteoblast-specific factor 2, a molecule highly expressed in the mouse osteoblastic cell line MC3T3-E1 [1]. It was then renamed periostin in 1999 because it is preferentially expressed in the periosteum and in periodontal ligaments [2].
Periostin has two characteristics: an extracellular matrix (ECM) protein and a matricellular protein [3, 4]. Periostin has a cysteine-rich EMILIN (EMI) domain at the N terminus and four fasciclin (FAS) 1 domains in the middle. These domains are important for binding to other ECM proteins such as collagen 1, fibronectin (EMI domain), and tenascin-C (FAS1 domain). Such functions of periostin as an ECM protein are important for maintaining tissue or organ structures in physiological conditions and in generating fibrosis in pathological conditions. Additionally, periostin binds to several integrins on the cell surface, transducing intracellular signaling via FAK, PI3-kinase, Akt, ERK, NF-κB, STAT3, and so on [3]. That is why periostin is classified as a matricellular protein defined as an ECM protein binding to its receptor on cell surfaces and functioning in cell activation rather than in maintenance of tissue structure. The aspect of periostin as a matricellular protein is important in the onset of inflammatory conditions, including asthma. It has been shown that periostin is expressed in various pathological conditions or diseases—allergic diseases, pulmonary fibrosis, scleroderma, renal diseases, ocular diseases, and malignancies—and plays a role in accelerating those by acting as both an ECM protein and a matricellular protein [4,5,6,7,8,9].
In this chapter, we focus on the expression and functions of periostin in asthma and then explain the usefulness of periostin as a biomarker for asthma.
2 Expression of Periostin in Asthma
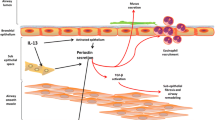
It is known that various triggers—TGF-β, angiotensin II, connective tissue growth factor 2, bone morphogenetic protein 2, mechanical stress, and stimuli from malignancies—induce periostin expression [10,11,12] (Fig. 7.1). We found for the first time that IL-4 and IL-13, signature type 2 cytokines, induce periostin [13, 14]. In our hands, both airway epithelial cells and lung fibroblasts express periostin at the mRNA level; lung fibroblasts, but not airway epithelial cells, secrete periostin protein following stimulation by IL-4 or IL-13. That is why periostin can be used as a surrogate biomarker for IL-4 or IL-13, as we will mention below. It was then reported that periostin is secreted from the basal side, but not from the apical side of airway epithelial cells stimulated by IL-13, using an air/liquid interface method in which one side of the airway epithelial cells faces air, whereas the other side faces liquid medium [15]. Moreover, it was reported that microvascular endothelial cells can secrete periostin by stimulation of IL-4 or IL-13 [16]. Thus, at least three kinds of tissue-resident cells in the lung—fibroblasts, epithelial cells, and endothelial cells—can produce periostin in response to IL-4 or IL-13.
Under normal conditions, periostin is only very weakly deposited in part of the bronchiolar basement membrane [17]. In contrast, periostin is robustly deposited on the thickened basement membrane in asthma patients, consistent with the notion that IL-4 and IL-13, triggers to induce periostin, are abundantly expressed in the bronchial tissues of these patients [14] (Fig. 7.2). Periostin is co-localized with collagens I, III, and V, fibronectin, and tenascin-C—all of them are ECM components of thickened basement membrane in asthma. This suggests that by binding to these ECM proteins, periostin thickens the basement membrane, a typical histological feature of asthma. In asthma patients, periostin in inflamed sites is likely to move easily to blood [18,19,20] and sputum [21, 22], although the precise mechanism of the movement or secretion remains elusive. That is also why periostin has an advantage as a biomarker for asthma.
High expression of periostin in asthma patients [14]. Periostin expression in bronchial tissues from a normal subject (a) and an asthma patient (b)
3 Functions of Periostin in Asthma
Several trials to clarify the significance of periostin in the pathogenesis of asthma have been carried out using asthma model mice; however, the results are controversial and the conclusion remains uncertain. It was initially reported that when periostin-deficient mice were challenged with ovalbumin or Aspergillus, airway hyperresponsiveness (AHR), type 2 inflammation, and mucus production were also enhanced in these mice [23, 24]. These results suggested that periostin protects against allergic inflammation in these model mice. In contrast, Bentley et al. reported that all of these features—AHR, type 2 inflammation, and mucus production—were impaired in house dust mite (HDM)-challenged periostin-deficient mice and by administration of neutralizing anti-periostin antibodies [25]. These results suggested that, in contrast to the initial studies, periostin accelerates allergic inflammation in these model mice. They showed the importance of periostin in dendritic cells (DCs) in this context as adoptive transfer of HDM-treated bone marrow-derived DCs from wild-type mice into periostin-deficient mice restored HDM-induced asthma-like phenotypes. The reason for this contradiction is not yet known.
Kanemitsu et al. examined the importance of periostin deposition in asthma patients [26]. They analyzed the correlation between periostin expression in some biopsy samples that they took from asthma patients more than 20 years ago and recent changes of pulmonary function in those same patients. They found that deposition of periostin in the bronchial subepithelium in the samples was strongly inversely correlated with decline of ΔFEV1. These results support the notion that periostin plays a role in accelerating airway allergic inflammation in asthma patients.
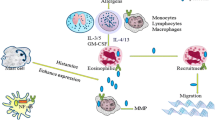
We have examined the importance of periostin as a matricellular protein in the process of inflammation using in vitro systems. We have shown that particularly the involvement of periostin in the epithelial/mesenchymal interaction would be important for the pathogenesis of allergic diseases, including asthma (Fig. 7.3). In the three-dimensional organotypic co-culture system mimicking the epithelial/mesenchymal interaction in skin tissues, we showed that periostin derived from fibroblasts stimulated by IL-13 acts on keratinocytes by itself activating NF-κB inducing production of pro-inflammatory cytokines including TSLP [27]. Using the same system, we also showed that periostin acts on fibroblasts together with IL-1α activating NF-κB inducing IL-6 [28]. Moreover, we found that cooperative actions of periostin and either TNF-α or IL-1α activate NF-κB in lung fibroblasts, followed by production of MCP1/3, CXCL1/2, and IL-1β, pro-inflammatory cytokines important for recruiting neutrophils and macrophages [29]. These results point to the capability of periostin to activate NF-κB in tissue-resident cells such as epithelial cells and fibroblasts by itself or by cross talk with other pro-inflammatory mediators. Regarding periostin’s actions on immune cells, it has been reported that it can act on eosinophils, inducing adhesion, superoxide generation, and TGF-β production [30, 31]. Thus, it is assumed that periostin plays various roles as a pro-inflammatory mediator acting on both tissue-resident cells and immune cells.
Epithelial/mesenchymal interaction via periostin in the pathogenesis of allergic diseases [4]. (a) IL-4/IL-13 produced by TH2 cells activated by exposure to allergens induces periostin production in fibroblasts. Periostin acts on keratinocytes, activating NF-κB followed by production of pro-inflammatory cytokines including TSLP, which acts on dendritic cells (DCs), accelerating type 2 inflammation. Thus, IL-4/IL-13, periostin, and TSLP generate a vicious cycle in the pathogenesis of skin allergic diseases. (b) IL-1α and periostin produced by keratinocytes and fibroblasts, respectively, cooperate to act on fibroblasts activating NF-κB. Activated fibroblasts produce IL-6 accelerating proliferation of keratinocytes
4 Periostin as a Biomarker for Asthma
4.1 A Biomarker for Type 2 (Th2-High) Asthma
It is now recognized that asthma is not a single disease but a syndrome [32]. We have empirically classified asthma patients based on clinical features such as age of onset (pediatric vs. adult), IgE dependency (atopic vs. nonatopic), and responsiveness to inhaled corticosteroids (ICSs, steroid-responsive vs. steroid-resistant). These classifications are based on phenotypes. In contrast, the significance of classifications based on molecular mechanisms of diseases, called endotypes, has recently emerged. Classifying asthma by type 2 vs. non-type 2 (or Th2-high vs. Th2-low) is an example of using endotypes [32]. This concept, “stratification of asthma patients,” is the basis for applying molecularly targeted drugs for asthma, as we will discuss later.
The proportion of type 2 asthma defined by high expression of IL-5 and IL-13, signature type 2 cytokines, is estimated to be 50–70% of total asthma patients [33, 34]. Asano and his colleagues have recently estimated the proportion of type 2 in severe asthma patients to be ~80% in a Japanese population [35]. Fahy and his colleagues searched for biomarkers for type 2 asthma, finding that periostin is highly expressed in bronchial tissues of type 2 asthma patients together with chloride channel regulator 1 (CLCA1) and serpin peptidase inhibitor, clade B, member 2 (SERPINB2) [33]. They then found that serum periostin is high in type 2 asthma correlated with airway eosinophilia, compared to the fraction of exhaled nitric oxide (FeNO), peripheral blood eosinophils, YKL-40, and IgE levels [34]. These results demonstrate that periostin has emerged as a novel biomarker for type 2 asthma (Fig. 7.4).
Our collaborators have intensively examined the characteristics of asthma patients correlated with serum periostin levels, using periostin ELISA kits with high sensitivity that we developed compared to other kits [36]. It has turned out that periostin is associated with eosinophil dominance [18,19,20, 37], late onset [18, 19], aspirin intolerance [19, 20], chronic sinusitis/olfactory dysfunction [18,19,20], and high FeNO [36,37,38]. These characteristics are known to be correlated with type 2 inflammation, which is consistent with the concept that periostin reflects type 2 inflammation. Moreover, these characteristics are known to be correlated with remodeling or fibrosis leading to resistance to treatments for asthma as we will mention next.
4.2 A Biomarker for ICS Resistance
We and our collaborators have shown that periostin is a component of the thickened basement membranes of asthma and is correlated with poor long-term prognosis [14, 26], suggesting that periostin has another characteristic as a biomarker for asthma reflecting remodeling or fibrosis. Matsumoto and her colleagues have demonstrated that periostin is associated with resistance to ICSs, the first-line drugs for asthma patients, which would be explained by this characteristic (Fig. 7.4). In the KiHAC study, when they divided asthma patients into rapid decliners and non-rapid decliners, defined by patients with treatments by ICS showing a decline in FEV1 of more than or less than 30 mL/year, respectively, serum periostin was higher in rapid decliners than in non-rapid decliners. This suggests that serum periostin is associated with hyporesponsiveness to ICSs in asthma [18]. When they clustered these patients based on their peripheral eosinophil and neutrophil numbers, cluster 3, which was characterized by high eosinophils and low neutrophil numbers and late onset, showed that the difference in decline of FEV1 between the periostin-high and periostin-low groups was more significant compared to the overall patients. This suggests that in this cluster, serum periostin is more associated with poor responsiveness to ICSs [39]. The association of serum periostin with poor responsiveness to ICSs was also observed in other studies [19, 36, 37]. Kato et al. showed more direct evidence for this association; when they tapered ICS treatment, asthma patients with high periostin showed a higher risk for instability than those with low periostin [40]. Introducing ICS to asthma patients promptly decreased FeNO levels, whereas it sustained high serum periostin levels. This finding suggests that ICS improves superficial inflammation consistent with decreased FeNO secreted from epithelial cells, whereas ICS does not improve the inflammation of deep layers consistent with sustained serum periostin [41]. Such limited efficacy of ICSs for asthma patients may lead to resistance to ICSs in periostin-high asthma patients showing high remodeling or fibrosis.
4.3 A Biomarker for Predicting the Efficacy of Molecularly Targeted Drugs for Type 2 Asthma
Currently, many drugs for type 2 asthma targeting IgE, IL-4/IL-13(receptor), IL-5(receptor), TSLP, CCR3, CCR4, CCL11, and CRTH2 are being developed. Some of them, two kinds of anti-IL-5 antibodies—mepolizumab and reslizumab—are already available at the start of 2018 [42]. Since periostin is a surrogate biomarker for type 2 asthma, particularly a downstream molecule of IL-4 and IL-13, several trials to apply periostin to a biomarker to predict efficacy of asthma drugs targeting IL-4 and IL-13 have been performed.
The first trial was carried out in the phase IIb study for lebrikizumab, an anti-IL-13 antibody, developed by Roche/Genentech [43]. They demonstrated that when they set the cutoff value of serum periostin at 50 ng/mL, the high periostin group showed good responsiveness to lebrikizumab, whereas the low periostin group showed poor responsiveness to it, demonstrating that serum periostin is a very useful biomarker to predict the efficacy of lebrikizumab. However, in the phase III study, lebrikizumab did not show enough efficacy for asthma patients, and development was ended [44]. In the phase IIb study of tralokinumab, another anti-IL-13 antibody, developed by AstraZeneca/MedImmune, both periostin and DPP-4, another type 2 biomarker, showed good ability to discriminate between good and poor responders to it as well as to lebrikizumab [45]. It is now in phase III study. Sanofi/Regeneron has developed dupilumab, an anti-IL-4 receptor α chain antibody that inhibits both IL-4 and IL-13 signals, as the first molecularly targeted drug for atopic dermatitis [46]. They have also developed dupilumab as an anti-asthma drug. In the phase IIb study, they used the blood eosinophil number as a biomarker to stratify patients; however, although the high eosinophil group tended to respond better than the low eosinophil group, dupilumab showed statistically significant efficacy in both groups [47]. It is also now in phase III.
We have examined the ability of serum periostin for this purpose instead of blood eosinophil number using the same samples as a post hoc study, finding that serum periostin showed a good ability to discriminate between good and poor responders as defined by improved lung functions (unpublished data, presented at the ERS Congress, 2016). Taken together, serum periostin has the potential to be a useful biomarker to predict the efficacy of IL-4/IL-13 antagonists (Fig. 7.4).
The usefulness of periostin as a biomarker to predict of efficacy of molecularly targeted drugs for type 2 asthma was examined for omalizumab, an anti-IgE antibody, provided by Novartis/Genentech. At this point, two studies have shown its usefulness [48, 49]. To our knowledge, these are the only studies so far to examine the usefulness of serum periostin as a biomarker to predict efficacy of molecularly targeted drugs for type 2 asthma except IL-4/IL-13 antagonists. These results point to the possibility that serum periostin is useful to predict efficacy of more molecularly targeted drugs for type 2 asthma other than IL-4/IL-13 antagonists.
5 Conclusion
It is strongly suggested that periostin acts as a pro-inflammatory mediator in asthma, although it has not been conclusively shown. Moreover, the usefulness of periostin as a biomarker for asthma—reflection of type 2 inflammation, resistance to ICS treatment, and prediction efficacy of several anti-asthma drugs targeting type 2 asthma—has been demonstrated. However, there still remain unresolved issues in this field—the pathological role of periostin in asthma, the usefulness of periostin as a biomarker to predict efficacy of molecularly targeted drugs for asthma, and the development of periostin detection systems more suitable for treating asthma patients. We need to begin to address these questions now.
References
Takeshita S, Kikuno R, Tezuka K, Amann E. Osteoblast-specific factor 2: cloning of a putative bone adhesion protein with homology with the insect protein fasciclin I. Biochem J. 1993;294:271–8.
Horiuchi K, Amizuka N, Takeshita S, Takamatsu H, Katsuura M, Ozawa H, et al. Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor β. J Bone Miner Res. 1999;14:1239–49.
Izuhara K, Arima K, Ohta S, Suzuki S, Inamitsu M, Yamamoto K. Periostin in allergic inflammation. Allergol Int. 2014;63:143–51.
Izuhara K, Nunomura S, Nanri Y, Ogawa M, Ono J, Mitamura Y, et al. Periostin in inflammation and allergy. Cell Mol Life Sci. 2017;74:4293–303.
O’Dwyer DN, Moore BB. The role of periostin in lung fibrosis and airway remodeling. Cell Mol Life Sci. 2017;74:4305–14.
Murota H, Lingli Y, Katayama I. Periostin in the pathogenesis of skin diseases. Cell Mol Life Sci. 2017;74:4321–8.
Prakoura N, Chatziantoniou C. Periostin in kidney diseases. Cell Mol Life Sci. 2017;74:4315–20.
Yoshida S, Nakama T, Ishikawa K, Nakao S, Sonoda KH, Ishibashi T. Periostin in vitreoretinal diseases. Cell Mol Life Sci. 2017;74:4329–37.
Cui D, Huang Z, Liu Y, Ouyang G. The multifaceted role of periostin in priming the tumor microenvironments for tumor progression. Cell Mol Life Sci. 2017;74:4287–91.
Conway SJ, Izuhara K, Kudo Y, Litvin J, Markwald R, Ouyang G, et al. The role of periostin in tissue remodeling across health and disease. Cell Mol Life Sci. 2014;71:1279–88.
Nishida M, Sato Y, Uemura A, Narita Y, Tozaki-Saitoh H, Nakaya M, et al. P2Y6 receptor-Gα12/13 signalling in cardiomyocytes triggers pressure overload-induced cardiac fibrosis. EMBO J. 2008;27:3104–15.
Liu AY, Zheng H, Ouyang G. Periostin, a multifunctional matricellular protein in inflammatory and tumor microenvironments. Matrix Biol. 2014;37:150–6.
Yuyama N, Davies DE, Akaiwa M, Matsui K, Hamasaki Y, Suminami Y, et al. Analysis of novel disease-related genes in bronchial asthma. Cytokine. 2002;19:287–96.
Takayama G, Arima K, Kanaji T, Toda S, Tanaka H, Shoji S, et al. Periostin: a novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J Allergy Clin Immunol. 2006;118:98–104.
Sidhu SS, Yuan S, Innes AL, Kerr S, Woodruff PG, Hou L, et al. Roles of epithelial cell-derived periostin in TGF-β activation, collagen production, and collagen gel elasticity in asthma. Proc Natl Acad Sci U S A. 2010;107:14170–5.
Shoda T, Futamura K, Kobayashi F, Saito H, Matsumoto K, Matsuda A. Cell type-dependent effects of corticosteroid on periostin production by primary human tissue cells. Allergy. 2013;68:1467–70.
Okamoto M, Hoshino T, Kitasato Y, Sakazaki Y, Kawayama T, Fujimoto K, et al. Periostin, a matrix protein, is a novel biomarker for idiopathic interstitial pneumonias. Eur Respir J. 2011;37:1119–27.
Kanemitsu Y, Matsumoto H, Izuhara K, Tohda Y, Kita H, Horiguchi T, et al. Increased periostin associates with greater airflow limitation in patients receiving inhaled corticosteroids. J Allergy Clin Immunol. 2013;132:305–12.
Matsusaka M, Kabata H, Fukunaga K, Suzuki Y, Masaki K, Mochimaru T, et al. Phenotype of asthma related with high serum periostin levels. Allergol Int. 2015;64:175–80.
Kim MA, Izuhara K, Ohta S, Ono J, Yoon MK, Ban GY, et al. Association of serum periostin with aspirin-exacerbated respiratory disease. Ann Allergy Asthma Immunol. 2014;113:314–20.
Bobolea I, Barranco P, Del Pozo V, Romero D, Sanz V, Lopez-Carrasco V, et al. Sputum periostin in patients with different severe asthma phenotypes. Allergy. 2015;70:540–6.
Simpson JL, Yang IA, Upham JW, Reynolds PN, Hodge S, James AL, et al. Periostin levels and eosinophilic inflammation in poorly-controlled asthma. BMC Pulm Med. 2016;16:67.
Sehra S, Yao W, Nguyen ET, Ahyi AN, Tuana FM, Ahlfeld SK, et al. Periostin regulates goblet cell metaplasia in a model of allergic airway inflammation. J Immunol. 2011;186:4959–66.
Gordon ED, Sidhu SS, Wang ZE, Woodruff PG, Yuan S, Solon MC, et al. A protective role for periostin and TGF-β in IgE-mediated allergy and airway hyperresponsiveness. Clin Exp Allergy. 2012;42:144–55.
Bentley JK, Chen Q, Hong JY, Popova AP, Lei J, Moore BB, et al. Periostin is required for maximal airways inflammation and hyperresponsiveness in mice. J Allergy Clin Immunol. 2014;134:1433–42.
Kanemitsu Y, Ito I, Niimi A, Izuhara K, Ohta S, Ono J, et al. Osteopontin and periostin are associated with a 20-year decline of pulmonary function in patients with asthma. Am J Respir Crit Care Med. 2014;190:472–4.
Masuoka M, Shiraishi H, Ohta S, Suzuki S, Arima K, Aoki S, et al. Periostin promotes chronic allergic inflammation in response to Th2 cytokines. J Clin Invest. 2012;122:2590–600.
Taniguchi K, Arima K, Masuoka M, Ohta S, Shiraishi H, Ontsuka K, et al. Periostin controls keratinocyte proliferation and differentiation by interacting with the paracrine IL-1α/IL-6 loop. J Invest Dermatol. 2014;134:1295–304.
Uchida M, Shiraishi H, Ohta S, Arima K, Taniguchi K, Suzuki S, et al. Periostin, a matricellular protein, plays a role in the induction of chemokines in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2012;46:677–86.
Blanchard C, Mingler MK, McBride M, Putnam PE, Collins MH, Chang G, et al. Periostin facilitates eosinophil tissue infiltration in allergic lung and esophageal responses. Mucosal Immunol. 2008;1:289–96.
Noguchi T, Nakagome K, Kobayashi T, Uchida Y, Soma T, Nakamoto H, et al. Periostin upregulates the effector functions of eosinophils. J Allergy Clin Immunol. 2016;138:1449–52.
Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18:716–25.
Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009;180:388–95.
Jia G, Erickson RW, Choy DF, Mosesova S, Wu LC, Solberg OD, et al. Periostin is a systemic biomarker of eosinophilic airway inflammation in asthmatic patients. J Allergy Clin Immunol. 2012;130:647–54.
Matsusaka M, Fukunaga K, Kabata H, Izuhara K, Asano K, Betsuyaku T. Subphenotypes of type 2 severe asthma in adults. J Allergy Clin Immunol Pract. 2018;6:274–6.
James A, Janson C, Malinovschi A, Holweg C, Alving K, Ono J, et al. Serum periostin relates to type-2 inflammation and lung function in asthma: data from the large population-based cohort Swedish GA(2)LEN. Allergy. 2017;72:1753–60.
Hinks TS, Brown T, Lau LC, Rupani H, Barber C, Elliott S, et al. Multidimensional endotyping in patients with severe asthma reveals inflammatory heterogeneity in matrix metalloproteinases and chitinase 3-like protein 1. J Allergy Clin Immunol. 2016;138:61–75.
Nagasaki T, Matsumoto H, Kanemitsu Y, Izuhara K, Tohda Y, Horiguchi T, et al. Using exhaled nitric oxide and serum periostin as a composite marker to identify severe/steroid-insensitive asthma. Am J Respir Crit Care Med. 2014;190:1449–52.
Nagasaki T, Matsumoto H, Kanemitsu Y, Izuhara K, Tohda Y, Kita H, et al. Integrating longitudinal information on pulmonary function and inflammation using asthma phenotypes. J Allergy Clin Immunol. 2014;133:1474–7.
Kato G, Takahashi K, Izuhara K, Komiya K, Kimura S, Hayashi S. Markers that can reflect asthmatic activity before and after reduction of inhaled corticosteroids: a pilot study. Biomark Insights. 2013;8:97–105.
Matsumoto H. Serum periostin: a novel biomarker for asthma management. Allergol Int. 2014;63:153–60.
Izuhara K, Matsumoto H, Ohta S, Ono J, Arima K, Ogawa M. Recent developments regarding periostin in bronchial asthma. Allergol Int. 2015;64(Suppl):S3–S10.
Corren J, Lemanske RF, Hanania NA, Korenblat PE, Parsey MV, Arron JR, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011;365:1088–98.
Hanania NA, Korenblat P, Chapman KR, Bateman ED, Kopecky P, Paggiaro P, et al. Efficacy and safety of lebrikizumab in patients with uncontrolled asthma (LAVOLTA I and LAVOLTA II): replicate, phase 3, randomised, double-blind, placebo-controlled trials. Lancet Respir Med. 2016;4:781–96.
Brightling CE, Chanez P, Leigh R, O’Byrne PM, Korn S, She D, et al. Efficacy and safety of tralokinumab in patients with severe uncontrolled asthma: a randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Respir Med. 2015;3:692–701.
Simpson EL, Bieber T, Guttman-Yassky E, Beck LA, Blauvelt A, Cork MJ, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;376:1090–1.
Wenzel S, Castro M, Corren J, Maspero J, Wang L, Zhang B, et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting β2 agonist: a randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet. 2016;388:31–44.
Hanania NA, Wenzel S, Rosen K, Hsieh HJ, Mosesova S, Choy DF, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med. 2013;187:804–11.
Tajiri T, Matsumoto H, Gon Y, Ito R, Hashimoto S, Izuhara K, et al. Utility of serum periostin and free IgE levels in evaluating responsiveness to omalizumab in patients with severe asthma. Allergy. 2016;71:1472–9.
Acknowledgments
We thank Dr. Dovie R. Wylie for the critical review of this manuscript. We also thank the following colleagues and collaborators for contributing to the present work: Go Takayama, Masaru Uchida, Miho Masuoka, Hiroshi Shiraishi, Kanako Ontsuka, Kazuto Taniguchi, Yasutaka Mitamura, Tomohito Yoshihara, Kazuhiko Arima, Shoichi Suzuki, Shoichiro Ohta, Go Kato, Koichiro Takahashi, Shin-ichiro Hayashi (Saga Medical School), Noriko Yuyama (Genox Research, Inc.), Akihiro Ishida, Nobuo Ohta (Yamagata University), Hiroshi Fujishima (Tsurumi University), Naoko Okada, Kenji Matsumoto (National Research Institute for Child Health and Development Laboratory), Yoshihiro Kanemitsu, Tadao Nagasaki, Tomoko Tajiri, Hisako Matsumoto (Kyoto University), Masako Matsuzaka, Koichi Fukunaga (Keio University), Koichiro Asano (Tokai University), Yorihisa Kotobuki, Ichiro Katayama (Osaka University), Kenzen Kou, Yukie Yamaguchi, Michiko Aihara (Yokohama City University), Timothy Hinks, Peter Howarth (Southampton University Hospital), Mi-Ae Kim, Hae-Sim Park (Ajou University), Anna James, Sven-Erik Dahlen (Karolinska Institutet), Ayami Kamei, Yoshinori Azuma (Shino-Test Co.), Simon J. Conway (Indiana University), Masaki Okamoto, Tomoaki Hoshino, and Kiminori Fujimoto (Kurume University).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Izuhara, K., Nunomura, S., Ono, J., Takai, M., Nanri, Y. (2019). Periostin as a Biomarker for Type 2 Asthma. In: Yokoyama, A. (eds) Advances in Asthma. Respiratory Disease Series: Diagnostic Tools and Disease Managements. Springer, Singapore. https://doi.org/10.1007/978-981-13-2790-2_7
Download citation
DOI: https://doi.org/10.1007/978-981-13-2790-2_7
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-2789-6
Online ISBN: 978-981-13-2790-2
eBook Packages: MedicineMedicine (R0)