Abstract
Polyaniline-vanadium pentoxide (PANI-V2O5) nanocomposites have been synthesized via hydrothermal autoclave polymerization approach in acidic environment. The synthesis has not only stabilized the bulk morphology but has also improved the electronic conductivity due to the presence of doped polyaniline (PANI). This property is an important criterion for any material to be used as an electrode. The synthesized material is characterized by using FTIR, XRD and four-probe method, and the particle size is revealed by optical microscopy. It has been observed that the band gap value of nanocomposites has increased. This is attributed to increase in the concentration of metal oxides in nanocomposites. The results acquired are encouraging and presents themselves as a base for future advancements in energy storage applications.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Nanocomposites (NCPs) offer extensive possibilities as hybrid conducting materials. These are formed by combining both the organic and inorganic entities and have exhibited improved characteristics in comparison with that shown by the conventional materials used in solitude [1,2,3,4]. An important development in synthesis of nanocomposites has been advent of conducting polymers. These have been comprehensively examined by several researchers. Polyaniline (PANI) has presented itself as one such compound with several characteristics suitable for wide range of applications such as electronics, biosciences and pharmaceutics [5].
The primary reason behind extensive amount of research carried out on polyaniline is its molecular structure and more specifically, its conducting ability. PANI has intrinsic redox states which is key to its unique properties. For evaluating NCPs as electrode materials, the properties that are needed to be evaluated are, “Specific capacity (m Ah g−1),” “Charge/discharge cycle” and “Degree of capacity change upon cycling at different demands” [6,7,8].
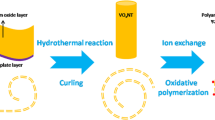
The aim of this research is to synthesize polyaniline-vanadium pentoxide nanocomposites and to explore their morphology and characteristics. PANI-vanadium pentoxide NCPs have been prepared using hydrothermal autoclave polymerization approach in acidic environment.
2 Material and Experimental Method
The chemicals used for synthesis of nanocomposites (NCPs) were procured from “Sigma Aldrich” and used directly in as received form. These chemicals are “AR”-graded. It means that these are analytical reagents with high degree of purity.
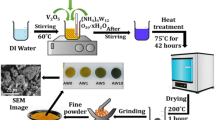
2.1 Synthesis of V2O5 Particles
As per reported stoichiometry, a mixture of Fe3O4·9H2O and V2O5 were added to base solution of NaOH and mechanically stirred for half an hour at 500 rpm [9]. The mix is then transferred into the autoclave for hydrothermal synthesis of the powder. The sample was heated for 8 h by ensuring temperature of 180 °C. The resultant product was taken together and cleaned up with distilled water. Drying was carried out using IR lamp with the temperature around 60 °C.
2.2 Synthesis of Polyaniline-Vanadium Pentoxide Nanocomposites (NCPs)
The synthesis was carried out by vigorous stirring along with addition of appropriate amount of V2O5 particles and aniline (1.25M) for 20 min. Then, ammonium peroxydisulfate was added to the mix and kept undisturbed for half an hour until dark green color was obtained in the presence of HCl (1M). The polymerization process took place for 24 h. The temperature of synthesis was 30 °C. The shady olive color products were filtered and washed by ethanol several times and dried at 60 °C by IR lamp. Pure PANI with a doping of HCl has also been synthesized by following the same procedure.
3 Results and Discussion
In order to verify the applicability of the synthesized nanocomposites, characterization of the powdered samples has been carried out using X-ray diffraction (XRD) done through Bruker D8 Advance XRD. Particle size is determined by optical microscope. For the identification of functional groups in the nanocomposites, Fourier transform infrared (FTIR) images were obtained using Burker Germany model vertex 70 FTIR Spectrometer. The most important characteristics for any material to be used as an electrode, i.e., electrical conductivity has been determined using a standard four-probe apparatus.
XRD spectra (See Figs. 1 and 2) of the synthesized NCPs observed sharp Bragg peaks signifying excellent morphology of vanadium ferrites. The results endorse hexagonal structure. The crystalline morphology of magnetic particles seems to be well-preserved during synthesis. This fact supports that there is no variation in peak position between V2Fe3O9 and the NCPs observed. Moreover, wide peak in the range of 2θ = 20–35° verifies the presence of PANI polymer [9, 10]. It has been observed that the peak falls with aggregation of hexaferrite in the polymer.
FTIR spectrum (See Fig. 3) image of the sample has been generated in the frequency band of 400–4000 cm−1 to reveal the nature of chemical bonds along with the functional groups in the NCPs. The absorption peaks around 1485.19 cm−1 is due to the presence of benzene ring. The sudden peaks and valleys around the band at 1535 cm−1 is due to symmetric–nonsymmetric stretching in C–C bond. FTIR images of V2O5 nanosized particles have shown three characteristic vibration modes: V=O vibrations at 960.55 cm−1, the symmetric stretch of V–O–V around 516 cm−1 and the asymmetric stretch of V–O–V at 802 cm−1. As clearly seen, the bands appearing between 914 and 1095 cm−1 are attributed to vanadium-oxide stretching modes. Bands between 800 and 914 cm−1 represents the bridged V–O–V stretching [11, 12].
Electrical conductivity (at room temperature) of the NCPs has been measured by the four-probe method. The process involved compression of composite powders in the form of pellets having thickness of 0.71 mm diameter of 8.0 mm under constant pressure. Values of electrical conductivity (σdc) reduce with the accumulation of vanadium particles in the NCPs [13, 14]. Their extreme σdc value is found to be 1.4 × 10−3 S cm−1 which supports the excellent conductivity characteristics of the synthesized NCPs.
During synthesis, acidic environment is maintained throughout. Mechanical stirring helped in reducing the acid attack of the iron-oxide coat on NCPs, thereby preventing it from getting suspended.
The size of the particle was analyzed by optical microscopy. The image was captured at 10× magnification. The clusters of the particles have radius size in microns which reveals that the individual particles are of nanosize. For instance, in Fig. 4, the greenish part has nine particles and covers only 1.24 × 10−3% of the total area of the image, and thus it is evident that the particles synthesized are of nanodimensions.
4 Conclusion
Polyaniline-vanadium pentoxide NCPs have been successfully synthesized by hydrothermal approach. The synthesized material exhibits desired morphology and material characteristics required for conducting materials. The electric conductivity of NCPs obtained is admirable and enhances as the content of vanadium-hexaferrite increases in NCPs. Peaks obtained by FTIR reveals that synthesized NCPs possess excellent conductivity due to elevated oxidation intensity and doping degree.
Thus, the hydrothermal approach of polymerization is a reliable process, and at the same time, it is found to be convenient for processing, eco-friendly and appropriate with reference to desired outcome for bulk fabrication of NCPs.
References
G.R. Pedro, Hybrid organic-inorganic materials-in search of synergic activity, Adv. Mater. (2001) 163–174.
P.T. Nguyen, U. Rammelt & W. Plieth, Electrochemical impedance spectroscopy for characterization of coatings with intrinsically conducting polymers, Macromol. Symp. 187 (2002) 929–938.
G.A. Lamzoudi, F. Pillier, H. Nguyen, Thi Le & C. Deslouis, Oxide/Polypyrrole composite films for corrosion protection of iron, J. Electrochem. Soc. 149 (2002) 560–566.
P.H.C. Camargo , K.G. Satyanarayana & F. Wypych, Nanocomposites: synthesis, structure, properties and new application opportunities. Materials Research, 12(1) (2009) 1–39.
H. Ahmad, Magnetic polyaniline composites: recent developments in preparation, properties and applications, J. Colloid Sci. Biotechnol. 2 (2013) 155–170.
Y. Wang, Microwave absorbing materials based on polyaniline composites: a review, Int. J. Mater. Res. 105 (2014) 3–12.
M.X. Wan & J.H. Fan, Synthesis and ferromagnetic properties of composites of a water-soluble polyaniline copolymer containing iron oxide, J. Polym. Sci. Part A: Polym. Chem. 36 (1998) 2749–2755.
J. Zhao, Y. Xie, C. Yu, Z. Le, R. Zhong, Y. Qin, J. Pan & F. Liu, Preparation and characterization of the graphene-carbon nanotube/CoFe2O4/polyaniline composite with reticular branch structures, Mater. Chem. Phys. 123 (2013) 395–402.
M. Drofenika, I. Bana, D. Makovec, A. Znidarsicc, Z. Jaglicic, D. Hanzel & D. Lisjak, The hydrothermal synthesis of super-paramagnetic barium hexaferrite particles, Mater. Chem. Phys. 127 (2011) 415–419.
S. Ejiri, T. Sasaki & Y. Hirose, Residual Stress Analysis of Textured Materials by X-Ray Diffraction Method, Materials Science Forum. 706–709 (2012) 1673–1678.
M. Qiu, Y. Zhang, & B.J. Wen, Facile synthesis of polyaniline nanostructures with effective electromagnetic interference shielding performance, Journal of Materials Science: Materials in Electronics 29.12 (2018),10437–10444.
D. Mishraa, S. Ananda, R.K. Pandab, R.P. Dasa, Studies on characterization, microstructures and magnetic properties of nano-size barium hexa-ferrite prepared through a hydrothermal precipitation—calcination route, Mater. Chem. Phys. 86 (2004)132–136.
S.H. Deng, Y. Wang & X.Yang, The study of electrochemical synthesis, properties and composite mechanism of PANI/PVA and PANI/PVA/Ag composite films. Pigment and Resin Technology. 47 (2018) 133–14.
N. Odzak, D. Kistler, R.Behra & L. Sigg, Dissolution of metal and metal oxide nanoparticles in aqueous media, Environmental pollution, 191C (2014) 132–138.
Acknowledgements
The authors are thankful to SVKM’s NMIMS (Deemed-to-be University) for providing research facilities and financial assistance.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Shivhare, S., Loharkar, P.K., Vyas, S., Bagal, V., Sharma, M. (2019). Synthesis of Polyaniline-Vanadium Pentoxide Nanocomposites: A High-Performance Conducting Material for Energy Storage. In: Vasudevan, H., Kottur, V., Raina, A. (eds) Proceedings of International Conference on Intelligent Manufacturing and Automation. Lecture Notes in Mechanical Engineering. Springer, Singapore. https://doi.org/10.1007/978-981-13-2490-1_31
Download citation
DOI: https://doi.org/10.1007/978-981-13-2490-1_31
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-2489-5
Online ISBN: 978-981-13-2490-1
eBook Packages: EngineeringEngineering (R0)