Abstract
Polymers based from petrochemical have been widely used in various applications due to their availability and lower cost. Products produced from petrochemicals have excellent properties. However their low biodegradability rate had caused terrible environmental problems. Therefore, polymers based on natural resources such as natural polymers, biopolymers, and synthetic polymers are highly in demand as they can be produced from natural sources which means they are sustainable. Apart of that, due to their nature, they are environmentally friendly and biodegradable. There are many types of biopolymers such as cellulose, poly(lactic acid) (PLA), polyhydroxybutyrate (PHB) and much more. Among these biopolymers, PLA has high potential because it has similar properties to conventional polymers such as polystyrene (PS). This polymer is an aliphatic type polyester which can be polymerized from its’ monomer, lactic acid. Lactic acid (LA) can be obtained from the fermentation process of natural sources such as starch (Auras R, Harte B, Selke S; Macromol Biosci 4(9):835–864, 2004).
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Isocyanate
- Chain extender
- Chemical structure
- Condensation polymerization
- Hydrolytic degradation
- Lactic acid
- Mechanical properties
- Poly(lactic acid)
- Polymerization
- Polyols
- Polyurethane
- Ring-opening polymerization
- Step growth polymerization
- Thermal properties
12.1 Introduction
Polymers based from petrochemical have been widely used in various applications due to their availability and lower cost. Products produced from petrochemicals have excellent properties. However their low biodegradability rate had caused terrible environmental problems. Therefore, polymers based on natural resources such as natural polymers, biopolymers, and synthetic polymers are highly in demand as they can be produced from natural sources which means they are sustainable. Apart of that, due to their nature, they are environmentally friendly and biodegradable. There are many types of biopolymers such as cellulose, poly(lactic acid) (PLA), polyhydroxybutyrate (PHB) and much more. Among these biopolymers, PLA has high potential because it has similar properties to conventional polymers such as polystyrene (PS). This polymer is an aliphatic type polyester which can be polymerized from its’ monomer, lactic acid. Lactic acid (LA) can be obtained from the fermentation process of natural sources such as starch (Auras et al. 2004).
Lactic acid can be polymerized into PLA through two different methods as shown in Fig. 12.1; polycondensation or ring-opening polymerization (ROP) of lactic acid. PLA polymerized by polycondensation method usually produces PLA with low molecular weight (Mw). However, during the polymerization of the polymer through polycondensation method, one molecule of water is generated in each step of polymerization. Formation of water in the polymerization vessel cause shorter polymer chain, thus resulting in the low Mw of the polymer. The shorter polymer chains formed through this reaction usually provides the polymer with a higher polydispersity index (PDI). On the other hand, ROP is a method which has an advantage compared to polycondensation where the higher molecular weight of PLA is prepared. This is usually due to controlled reaction of ROP compared to polycondensation method. In addition, polymers prepared by controlled polymerization method generally produces polymer with low PDI. Therefore, PLA prepared through ROP usually has higher Mw and low PDI, which usually used for further polymerization step such as to produce block copolymers (BCP). Therefore, PLA produced through this method can be potentially used for various applications such as lithography and drug delivery.
Lactide monomer which is obtained from lactic acid usually forms in different structures which consisted of L, D, and meso-lactide forms and called as L-lactide, D-lactide and DL-lactide, respectively (Fig. 12.2). Polymerization of these monomers can produce polymers with different properties due to its different stereochemistry; poly(L-lactic acid) (PLLA), poly(D-lactic acid) (PDLA) and poly(DL-lactic acid) (PDLLA). PLLA type homopolymer is a crystalline type polymer whereas PDLA and PDLLA are amorphous type polymers. Crystallites in polymers influences on the properties of polymers. Therefore PLLA has slightly different properties compared to PDLA and PDLLA.
PLA can be polymerized using the above mentioned steps and this polymer has high mechanical strength, low toxicity and good gas barrier properties. For its biodegradability behavior, PLA has potential and it can be used as environmentally friendly material, especially in packaging materials. However, as mentioned before, PLLA has higher crystallinity and this caused PLLA to be rigid and brittle which limits its usage in packaging application. Therefore, to be used in packaging industry, PLA must have higher elongation with good mechanical strength and this can be achieved through different methods such as blending with other polymers, plasticization of PLA, co-polymerization with different polymers and many more. Among these methods, properties of PLA can be altered through polymerization methods. Through polymerization, the structures of the polymer can be designed and suited according to their desired application.
12.2 Polymerization of PLA
12.2.1 Synthesis of PLA by Condensation Polymerization
PLA can be prepared through two different methods from the lactic acid monomer (Fig. 12.3) which can be produced either by lactic acid or lactide. Polycondensation is performed in bulk by distillation of condensation of water, with or without a catalyst, while vacuum and temperature are progressively increased. This PLA can be used for synthesis of polyurethane. This polymer can be used as it is or coupled with isocyanates, epoxides or peroxide to produce polymers having a range of molecular weights.
12.2.2 Synthesis of PLA by Ring-Opening Polymerization Method (ROP)
The second method is ring opening polymerization of lactide. Ring opening polymerization (ROP) is a type of polymeric reaction where the terminal end of the polymer chain will be activated and act as a reactive center. This reactive site will be used for the addition of monomers which will enhance the polymerization process and form larger polymeric chains. Metals consist of alkoxide are known to be good initiating agent in ROP of lactide and can be used to activate the site as shown in Fig. 12.4. One type of the metal alkoxides is triethylaluminum which has been used for polymerization of PLA where it reacts well with hydroxyl-functionalized polymer (Wang and Hillmyer 2000). PLA produced from this method gave polymer with high molecular weight and low PDI. PLA produced through this method can be used for further polymerization such as for synthesis of block copolymers (BCPs).
12.2.3 Step-Growth Polymerization
In step-growth polymerization method, a polymer is produced when an end chain diol of oligomer reacts with a diisocyanate to provide pre-polymer which is also called as polyurethane (PU) (Fig. 12.5). For an example, ethylene glycol (EG) or different type of diol is added to the isocyanate in the presence of catalyst, a rapid condensation occurs and it gives the pre-polymer. The pre-polymer which has isocyanate at the terminal of the chain, is then can then be reacted with another polyol. In this case, the second polyol function as chain extender and the enhancement of chain extender in the polymer chains substantially give higher performance polymer. The choice of initiator, extender, and molecular weight of the polyol greatly affect its physical state, and the physical properties of the polyurethane. PLA based polyurethane using polycaprolactone diol as chain extender was prepared and the properties suit for environmental friendly packaging material (Ali et al. 2014).
12.2.3.1 Chemical Structure
Polyurethane (PU) is a polymer with urethane linkages in their backbone is shown in Fig. 12.6. It is also a compound having one or more of highly reactive isocyanate group (-N=C=O) that will react with hydrogen atoms that are attached to atoms which having more electronegative than carbon. It is formed through polyaddition polymerization between isocyanates and polyols which will produce different chemical, physical and mechanical properties depending on their types and characteristics. It also belongs to the class of alternating block copolymers where hard and soft segments alternate along the chain. The soft segments are polyether (mainly prepared from the mixture of propylene oxide and ethylene oxide) and polyester (derived from ethylene glycol and adipic acid). For the hard segment, it is produced from residues of diisocyanates and low molecular weight polyols which is also called as chain extender. Polyurethane properties are strongly dependent on the chemical nature of basic components, also the synthesis and processing conditions. As mentioned before, it is prepared mainly from two compounds which are isocyanates and polyols. The addition of other materials such as chain extenders, surfactants and catalysts are required to aid processing the polymer or to change the properties of the polymer.
12.2.3.2 Isocyanate
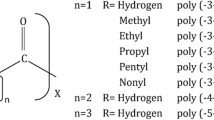
The isocyanate is a compound containing the isocyanate group (-NCO) (Fig. 12.7). It is formed through the reaction with a compound containing alcohol (hydroxyl) groups to produce polyurethane polymers. The interaction between hydroxyl and –NCO determine the properties of the PU, in term of its rigidity, mechanical and also tensile properties (Petrović et al. 2002). It also can react with water to form unstable carbamic acid, which consists of carbon dioxide and amines. The released carbon dioxide if trapped within the polymers results in the formation of cell structure, which known as foams. The formation of polyurethane structure needs two or more isocyanate groups on each molecule. There are many commercially available poly- or di- isocyanate compound usually used in a production of polyurethane, such as methylene diphenyl diisocyanate (MDI), toluene diisocyanate (TDI) and Hexamethylene diisocyanate (HDI). Methylene diphenyl diisocyanate (MDI) and toluene diisocyanate (TDI) is the most commonly used during the process because of their cost and excellent reactivity. Both of them exhibit a different function to the polyurethane. MDI is commonly used to make flexible foams as well as semi-rigid and rigid polyurethane plastic for construction and insulations. It has relatively low human toxicity and the least hazardous isocyanate but is not benign. Examples for MDI available are 4, 4-, 2, 4-, 2, 2- and polymeric MDI as shown in Fig. 12.7.
Meanwhile, for TDI, it is an aromatic diisocyanate which commonly used in the manufacture of flexible foams such as foam cushions for furniture and automotive components. It is usually produced as a mixture of 2,4- and 2,6- TDI isomers. The isocyanates can be adjusted by partially reacting with polyols or adding other materials to reduce volatility, which also will reduce the toxicity to enhance the properties of polyurethane.
12.2.3.3 Polyols
Polyols are compound with multiple hydroxyl functional groups available for organic reactions. The polyols used in polyurethane production are predominantly hydroxyl-polyethers rather than hydroxyl-polyesters. Soft segment of polyurethane itself is derived from polyols either polyester polyols or polyether polyols. Therefore, the degradation rate can be adjusted through a suitable soft segment. For the production of biodegradable packaging, polyester-polyols is an appropriate choice since it is readily biodegradable instead of polyether-polyols which are resistant to biodegradable (Nakajima-Kambe et al. 1999). It is produced by direct esterification in a condensation reaction. Commonly used biodegradable polyesters are polylactic acid (PLA), polycaprolactone (PCL) and polyglycolide (PGA). It is also can be formed by condensation or step-growth polymerization of diols and a dicarboxylic acid, such as diethylene glycol reacting with phthalic acid (Kadkin et al. 2003) (Fig. 12.8).
Polyether polyols are made by reacting epoxides as a high ring stress and polar character of three-membered epoxy rings to allow cleavage easily with the multifunctional initiator in the presence of a catalyst (Fig. 12.9). Some of the examples are polyethylene glycol and polypropylene glycol. It also can be formed by reacting glycol or polyhydric alcohol with propylene oxide or ethylene oxide to yield a branched molecule contains a plurality of ether linkages and a hydroxyl group. The polyether forming reaction is a strong exothermic polymerization reaction.
12.2.3.4 Chain Extender
Chain extender plays an important role in the final mechanical properties of the polymer. It incorporates with low molecular weight diols to enhance the elastomeric properties of the resulting polyurethane in terms of polymer morphology, adhesives, elastomers and microcellular foams. The elastomeric properties are derived from chemical dissimilar of hard and soft segment copolymer, such that the urethane hard segment domains serve as cross-links between the amorphous polyether or soft polyester segments. Most commonly used chain extender is an aliphatic of 1-4 butanediol (Fig. 12.10). Introducing chain extender with different rigidity and bulkiness may provide the change in the degree of phase mixing, hard domain structure and hydrogen bonding (Bae et al. 1999).
12.2.4 Controlled Radical Polymerizations (CRP)
Recent developments in controlled radical polymerization methods, such as atom-transfer radical polymerization (ATRP), nitroxide-mediated living polymerization (NMP) and reversible addition-fragmentation transfer (RAFT) can use for the preparation of functional type polymers. This living polymerization method induces uniform chain length as it offers a proper addition to the polymer chains and it means that the polymer’s chain growth can be controlled and at the same time obtaining low polydisperse polymers.
12.2.4.1 Atom-Transfer Radical Polymerization (ATRP)
Atom-transfer radical polymerization (ATRP) is also a living radical polymerization assisted with transition metal was discovered by Mitsuo Sawamoto (Kato et al. 1995) and by Krzysztof Matyjaszewski (Wang and Matyjaszewski 1995) in 1995. Figure 12.11 showed block copolymers (BCPs) prepared by ATRP is employed by metal-catalyzed controlled radical polymerizations. Controlled radical polymerization produces linear AB diblock copolymer with narrow molecular weight distribution. This AB BCP can be used for the preparation of ABA triblock copolymer while having a low polydispersity (Yuzo Kotani et al. 1996).
12.2.4.2 Reversible Addition-Fragmentation Transfer (RAFT)
Reversible addition-fragmentation transfer (RAFT) can be used to prepare polymers with pre-determined molecular weight and narrow polydispersity (usually <1.2) (Chen et al. 2015; John Chiefari et al. 1998). The polymerization is carried out in the presence of –SH (thiol) compounds which act as RAFT agent. The polymerization using RAFT agent is shown in Fig. 12.12. The transfer agent [S=C(Z)S-R] of the RAFT agent plays an important role in polymerizing monomers. Due to the presence of thiol in the polymer chain, polymerization of a second monomer can be continued to give a block copolymer. Diblock copolymers can be prepared using a macro transfer agent (Zalusky et al. 2002). In Fig. 12.12, PLA macro transfer agent was prepared to polymerize styrene with low polydispersity.
12.3 Characterization
12.3.1 Chemical Structure and Molecular Weight
Chemical structures of the polymer can be confirmed using nuclear magnetic resonance (NMR) and ATR-FTIR. The NMR spectra can be obtained using CDCl3 with a Bruker 400 MHz spectrometer. ATR-FTIR can confirm on the functional group of the polymers. The molecular weight (M n) and polydispersity index (PDI) of each polymer can be determined using size exclusion chromatography (SEC) (PerkinElmer Series 200), calibrated by polystyrene standards.
12.3.2 Thermal Properties
The thermal properties of the polymers can be determined with a TA Instruments DSC-Q100 between −70 and 180 °C and maintained for 5 min to eliminate thermal history. The first scan is carried out to eliminate the thermal history of the polymer. From the DSC, glass transition temperature (Tg), melting temperature (Tm) and crystallization temperature (Tc) can be obtained.
12.3.3 Mechanical Properties
Tensile properties can be measured using a universal testing machine (INSTRON 5583) at 25 °C with a crosshead speed of 10 mm/min in accordance with ASTM D412 specifications. The samples for tensile measurements can be prepared by compression mold in 1-mm thick sheets under a pressure of 10,000 pounds for 5 min at 100 °C.
12.3.4 Polymer Surface Morphology
The field emission scanning electron microscopy (FE-SEM) can be performed to observe the morphology of the tensile-failed samples using a Hitachi S-4800 scanning electron microscope.
12.3.5 Gas Permeability
Gas permeability tests can be performed on a MOCON OX-TRAN 2/21 model in accordance with ASTM D-3985. The gas will penetrate the membrane by a carrier gas (98% N2, 2% H2) at 760 mmHg (test gas, O2).
12.3.6 Hydrolytic Degradation
Hydrolytic degradation tests can be performed to investigate the biodegradability of the polymer. The polymer samples can be placed in 10 mL of PBS (pH = 7.4) at 35 °C, and the hydrolytic degradation should be observed for a month, with measurements taken after 1, 3, 5, 7, and 30 days. The samples can be removed on the specified days and washed thoroughly with distilled water, dried in vacuum, and weighted to determine the weight loss, as shown:
where w o is the initial weight of the samples, and w t is the weight of the samples after degradation.
12.4 Conclusion
Poly(lactic acid) has high potential to be used in an environmental friendly packaging material, and it can be used to substitute well-known commodity polymers. However, its brittleness limits its use and therefore it can be polymerized to improve its properties. Few methods are suggested for the PLA polymerizations, and its characterizations are recommended.
References
Ali FB, Kang DJ, Kim MP, Cho CH, Kim BJ (2014) Synthesis of biodegradable and flexible, polylactic acid-based, thermoplastic polyurethane with high gas barrier properties. Polym Int 63(9):1620–1626
Auras R, Harte B, Selke S (2004) An overview of polylactides as packaging materials. Macromol Biosci 4(9):835–864. https://doi.org/10.1002/mabi.200400043
Bae JY, Chung DJ, An JH, Shin DH (1999) Effect of the structure of chain extenders on the dynamic mechanical behaviour of polyurethane. J Mater Sci 34:2523–2527
Chen CC, Chen CY, Tsay CY, Wang SY, Lin CK (2015) Influence of Fe3O4 nanoparticles on pseudocapacitive behavior of the charge storage process. J Alloys Compd 645:250–258. https://doi.org/10.1016/j.jallcom.2015.04.123
John Chiefari YKBC, Ercole F, Krstina J, Jeffery J, Le TPT, Mayadunne RTA, Meijs GF, Moad CL, Moad G, Rizzardo E, Thang SH (1998) Living free-radical polymerization by reversible addition−fragmentation chain transfer: the RAFT process. Macromolecules 31:5559–5562
Kadkin O, Osajda K, Kaszynski P, Barber TA (2003) Polyester polyols: synthesis and characterization of diethylene glycol terephthalate oligomers. J Polym Sci A Polym Chem 41:1114–1123
Kato M, Kamigaito M, Sawamoto M, Higashimura T (1995) Polymerization of methyl methacrylate with the carbon tetrachloride/dichlorotris- (triphenylphosphine)ruthenium(II)/methylaluminum Bis(2,6-di-tert-butylphenoxide) initiating system: possibility of living radical polymerization. Macromolecules 28(5):1721–1723. https://doi.org/10.1021/ma00109a056
Nakajima-Kambe T, Shigeno-Akutsu Y, Nomura N, Onuma F, Nakahara T (1999) Microbial degradation of polyurethane, polyester polyurethanes and polyether polyurethanes. Appl Microbiol Biotechnol 51(2):134–140
Petrović ZS, Zhang W, Zlatanić A, Lava CC, Ilavský M (2002) Effect of OH/NCO molar ratio on properties of soy-based polyurethane networks. J Polym Environ 10(1–2):5–12. https://doi.org/10.1023/A:1021009821007
Wang Y, Hillmyer MA (2000) Synthesis of polybutadiene-polylactide diblock copolymers using aluminum alkoxide macroinitiators. Kinetics and mechanism. Macromolecules 33(20):7395–7403
Wang J-S, Matyjaszewski K (1995) Controlled/“living” radical polymerization. Atom transfer radical polymerization in the presence of transition-metal complexes. J Am Chem Soc 117(20):5614–5615. https://doi.org/10.1021/ja00125a035
Yuzo Kotani MK, Kamigaito M, Sawamoto M (1996) Living radical polymerization of alkyl methacrylates with ruthenium complex and synthesis of their block copolymers. Macromolecules 29:6979–6982
Zalusky AS, Olayo-Valles R, Wolf JH, Hillmyer MA (2002) Ordered nanoporous polymers from polystyrene-polylactide block copolymers. J Am Chem Soc 124(43):12761–12773. https://doi.org/10.1021/Ja0278584
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Ali, F.B., Ismail, N. (2018). Polymerization Methods and Characterizations for Poly(Lactic Acid) (PLA) Based Polymers. In: Amid, A., Sulaiman, S., Jimat, D., Azmin, N. (eds) Multifaceted Protocol in Biotechnology. Springer, Singapore. https://doi.org/10.1007/978-981-13-2257-0_12
Download citation
DOI: https://doi.org/10.1007/978-981-13-2257-0_12
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-2256-3
Online ISBN: 978-981-13-2257-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)