Abstract
The technological ability to capture electrophysiological activity of populations of cortical neurons through chronic implantable devices has led to significant advancements in the field of brain-computer interfaces. Recent progress in the field has been driven by developments in integrated microelectronics, wireless communications, materials science, and computational neuroscience. Here, we review major device development landmarks in the arena of neural interfaces from FDA-approved clinical systems to prototype head-mounted and fully implantable wireless systems for multi-channel neural recording. Additionally, we provide an outlook toward next-generation, highly miniaturized technologies for minimally invasive, vastly parallel neural interfaces for naturalistic, closed-loop neuroprostheses.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Brain-computer interfaces
- Wireless neural interfaces
- Spatially distributed neural sensors
- Neuroprostheses
9.1 Overview: Where We Are Now
This volume contains many illustrative examples of the early development and specific applications, across a spectrum of contemporary techniques, whereby direct electronic access to brain circuits, primarily from/to the neocortex, offers opportunities for basic brain science, emergence of medical devices for assisting and/or correcting neurological deficits, as well as discovering potential device-based therapies as an alternative to pharmaceuticals. The emergence of electronic neural interfaces, in particular, reflects outcomes of worldwide multidisciplinary work over the past two decades whereby device engineering, neuroscience, and clinical needs have met at the intersection of fundamental and applied brain science. Here, we consider progress—and challenges ahead—for the development of brain-computer interfaces for neural prostheses. We narrow the focus to cortical brain-computer interfaces (BCI) in offering a subjective view of future assistive technologies, particularly those applicable to severe neurological injury to the motor system such as in case of paralysis from neuromotor diseases, stroke, or spinal cord injury. To gain a broader perspective, the reader is referred elsewhere for reviews of neural interfaces including those which engage deeper brain structures such as deep brain stimulation (DBS) which has reached medical device maturity in widespread treatment of, e.g., Parkinsonian disorder [33]. The DBS technology is one example of neuromodulation application of electrical stimulation; many other approved electrical stimulation-based medical devices are also in widespread clinical use, such as spinal cord treatment for chronic pain.
When compared to maturity of the DBS-like neuromodulation technologies, and leaving questions about their therapeutic efficacy to clinicians, cortical BCIs are still at relative infancy, limited at this writing to perhaps up to a dozen human pilot trials worldwide. The fundamental challenges are, in our opinion, considerably more difficult than devices which deploy electrical stimulation from a handful of electrodes near/at the anatomical central or peripheral nervous system target. In particular, for BCIs, one must be able to record cortical circuit activity at sufficient level of detail and then decode typically many parallel channels of electrophysiological activity (action potentials, field potentials, etc.). The decoding, in turn, for essentially reconstructing cortical network activity in a predictive context requires sophisticated computational models and tools to decipher, e.g., a subject’s intention to move a limb.
One set of illustrative examples of progress is from research where intracortical microelectrode arrays (MEA) are used to record population dynamics from (<1 cm2 area) cortical patches at single neuron-level and subsequent application of stochastic state-space dynamical models to decode, e.g., movement planning intention of a subject. The genesis and progress have been well documented in a number of review articles and book chapters. Suffice it to say here that from early discoveries in non-human primates [9, 21, 30, 51], the adaptation of these methods has enabled tetraplegic subjects to control communication devices and electromechanical devices such as robotic arms and hands [3, 13, 22, 23, 46, 52]. The cortical locations where such recordings are made have typically involved primary and/or premotor and parietal areas where, e.g., direction and velocity tuning is distinctly encoded in subsets of neurons. While specific tuning properties of neurons can be helpful across a population which is part of an interconnected network with manifolds of internal state representation (e.g., motor and visual cortices), new decoding techniques are emerging using, e.g., deep learning methods, where presence of such tuning properties is not necessary for successful decoding.
While it follows from biophysical fundamentals that intracortical probes with their inherently single-neuron space-time resolution offer the most direct means to communicate with neuronal populations and their associate networks, they are by no means the only, and not always necessary, requirement for building electronic neural interfaces for prosthetic and other assistive use. As shown elsewhere in this volume, whether scalp-based (EEG) electrode arrays [36], epidural or subdural electrocorticography (ECoG) [34, 50], peripheral nerves, or elsewhere, there are multiple means to acquire meaningful control signals for assistive use. Broadly, for any assistive medical technology, the overall cost-to-benefit calculations must weigh in many factors: engineering and technological complexity, required medical procedures (such as surgery), safety and reliability—and above all, the level of required performance of the neural interface from the user’s point of view.
This chapter examines electrophysiological BCIs only. While the brain is an electrical (electrochemical) biological machine with remarkable level of performance (e.g., per Watt of metabolic power) and lends itself readily to electrophysiology, active research is being pursued to search for neural interfaces relying on recording/stimulation modalities which range from optical to acoustic (ultrasound), to magnetic to chemical modalities, and combinations thereof. Time will tell if these alternative biophysical agents/modalities can compete or augment the direct electrical/electronic approaches.
We summarize in Sect. 2 some recent progress where innovation of wireless neural interfaces can now be envisioned to enable mobile BCIs, for untethered movement of subjects. In Sect. 3, we peek into the early ambitious efforts to develop very large-scale neural recording and microstimulation systems where scaling the electrophysiological access to, say, thousands of cortical points requires entirely new ways of approaching the problem, from developing new types of neural probes to re-examining systems level neuroengineering concepts.
9.2 Examples of State-of-the-Art Electronic Brain Interfaces
From a systems level perspective, a brain-computer interface may be broadly partitioned into (i) the physical neural probe (electrodes), (ii) an electronic custom integrated circuit (IC) core for signal acquistion, (iii) the (generally noisy) signal conditioning and telemetry, and (iv) the computing modules with dedicated back-end hardware embedding computational algorithms specific to the neural decoding task. The last decade has seen significant advances in each of these areas, even if a challenging road still lies ahead to meet the overarching goal of developing versatile BCI systems that can adaptively engage with the underlying non-stationary neural circuits in a closed-loop fashion, while compatible with long-term chronic implantation of neural probes in a (future) mobile subject without eliciting significant immune response.
The fundamental challenges of chronic intracortical or deep-brain neural probe design are manyfold. First, it is arguably desirable that the spatial scale and density of microscale probes reach close to single neuron resolution (say, ~20–100 μm electrode proximity for a layer V pyramidal neural cell body). Second, the probe material in direct electrical contact with tissue must enable high signal to noise ratio (SNR) recordings and high-charge-density biphasic microstimulation (the latter being critical for eventual fully closed-loop BCIs) while maintaining designed form factor (rigidity, etc.) and microscale surface characteristics that hopefully limit unavoidable immunologic response. There have been multiple recent reports of successful refinement of electrode microfabrication methodologies to yield, for example, high-density surface ECoG electrodes [26, 49], as well as penetrating 3-D probes [40] approaching an electrode pitch of tens of microns for high-density neural sampling. In contrast to traditional epi and intracortical approaches, Oxley et al. [37] have described a minimally invasive intravascular approach for the placement of a “stentrode” in cortical veins to access neural information at high spatial resolution (where the latter approach is currently being commercialized by Synchron med). Meanwhile, material science research has provided several promising candidates for the integration of next-generation BCI probes in addition to established work horses of Pt and IrOx, from silicon-carbide [15, 16], carbon nanotubes [12], nanoelectronic threads [32] to hybrids of conductive polymers like poly(3,4-ethylenedioxythiophene) (PEDOT) [10, 11, 27]. Success of optical techniques such as optogenetics has led to the development of hybrid probes integrating, for example, optical stimulation and electronic recording onto the same intracortical platform [22, 28, 45], where the latter target closed-loop hybrid optoelectronic BCIs in rodents and non-human primates. Successful transitioning of these novel experimental probe technologies for a chronic implantable BCI is the next major challenge and goal.
The electronic core of a BCI system invariably includes signal amplifiers, conditioning (filters) and dedicated analog-to-digital neural signal conversion. A number of groups worldwide have been developing custom IC solutions for low-power and low-noise neural signal acquisition, particularly focusing on channel scalability (from a handful to >100) and ease of physical integration with the typically monolithic multi-element neural probe. While it is not possible to review these developments exhaustively here, we highlight one recent work, namely, the “Neuropixel” probe system where up to 1356 recording channels were microfabricated within one silicon shank with integrated CMOS signal preconditioning [25, 39]. Systems such as these which allow high-resolution, sub-acute (up to a few months) of robust, wired access to the brain are instrumental in driving neuroscientific knowledge forward, yet are limited for translational chronic use by the complex percutaneous connectors in terms of full subcutaneous implantation. In general, adding a wireless telecommunication capability to neural probes defines one crucial need to extend the utility of these methods to freely moving animals and clinical applications.
9.2.1 BCI Going Wireless: General Considerations
In designing wireless neural interfaces, it is important to consider the requirements and constraints in the full ecosystem context. Relevant questions to ask up front might include, e.g., how a given intracortical MEA or subdural ECoG array may be interfaced with the electronic payload, the latter now located within the hermetically sealed envelope of the wireless device system. A key question is where within or on the body will the electronics package locate. We start with the design where the system electronics are configured as a head-mounted compact package whereby percutaneous/transcranial connectorization from, e.g., an MEA, is required. One rationale for this configuration as a starting choice on the wireless path is that silicon-based commercial intracortical MEAs are now widely available with a variety of channel counts compatible with small-animal (rodent) as well as non-human and human primate models, including reasonable chronic robustness demonstrated in clinical trials.
Given that contemporary intracortical (or subdual ECoG) sensor arrays typically sample on the order of 100 nodes (single neurons for MEAs, summated field potentials for ECoGs), it is remarkable how such pronounced under-sampling of neuronal population can be successful in enabling subjects to control, e.g., a robotic hand/arm with multiple degrees of freedom of motion. The good news is that this limited amount of neural data sets not unreasonable requirements for wireless telemetry. As also noted elsewhere in this volume, much credit to BCI development must be assigned to (scalable) sophisticated statistical models of neuronal dynamics which have produced powerful algorithms for effective decoding of, e.g., patient’s movement intention, from this under-sampled pool of “noisy” brain signals, for real-time assistive device use. Since the “noise” typically hides valuable neural information, it is important that the signal fidelity of a wireless link be maintained (use of data compression techniques must thus be approached judiciously). The wireless neural population data provides direct inputs to the neuro-computational decoding models. There are numerous approaches to neural decoding; one representative example is state-space dynamical modeling, which distils multidimensional neural data (spikes and field potentials) to lower dimensional cortical state representation, suitable for interpretation both by visualization (graphics algorithms), as well as for prosthetic use through forwarding the outputs to assistive electronic devices (e.g., direct cortically operated laptops). The role of machine learning applied to neural decoding and encoding is a rapidly evolving new area of computational neuroscience, with very promising preliminary results suggestive of significant performance and efficiency benefits for real-time BCIs. We bypass the field of neural decoding/encoding in this article and refer the reader to the rich literature, which by now exists for this topic.
From a generic system level view, a fully wired BCI instrumentation collects the raw neural (multi-channel) data via multi-wire cables into electronic neural signal processors (composed of combinations of analog and digital electronics). The digitized data is fed to computers where even today’s desktop machines have enough on-board processing power to carry out much of neural decoding to run a simple set of assistive devices. Figure 9.1 shows a block diagram of the basic electronic ecosystem which is more or less common to most (non-human and human) primate researchers in the field.
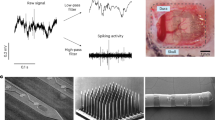
The next level of significance in advancing this type of electronic brain interface neurotechnology (even if still in early days) is to “untether the patient” by engineering direct wireless access to neural probes such as depicted in Fig. 9.2. While for external body wearable biosensors (whether EEG, EKG, etc.), the transition to a wireless system for enhancing subject’s mobility is already possible by adapting any number of wireless electronics developed in the past decade for consumer and industrial use, the situation for implanted wireless BCIs poses a number of challenges. These include the following which must be designed and ultimately integrated seamlessly into one viable medical grade implant device system:
Block diagram and representative device view of a head-mounted wireless neural recording system [55]. Integrated microelectronics are placed within a compact package powered by a replaceable Li-ion battery
-
Very low-power and high-fidelity analog and digital (custom) application specific integrated circuits (ASICs) for broadband (spikes, field potentials) neural signal acquisition and electrical microstimulation.
-
Very low-power radio frequency transmitter capable of high data rate transmission (~100 MBits/s and beyond), custom designed as an integrated circuit (“RF ASIC”).
-
Connectorization of the multi-element neural probe front-end (such as one or more MEAs) to and integration with the on-board ASICs.
-
Packaging the electronics within a compact housing which is hermetically viable for long-term chronic use.
-
Designing external RF receivers which capture the wirelessly emitted, digitally-encoded signals for inputs to computational devices for decoding.
-
Strategy for powering the implant electronics (internal battery or RF inductive coupling by external coils).
These engineering challenges are further bound by the first priority in any medical device candidate—safety—which overrides even the main functional claim of the device, here the neurotechnological performance. In the wireless device system which we use here as an example (from our laboratories), safety has multiple components such as (and revisited further below):
-
Electrical safety: the electrical energy required by the active ASICs in the implant must have zero accidental probability of discharging into the body/brain tissue.
-
Mechanical safety: if the implant unit locates, for example, in the subject’s head, the packaging of the electronics must be correspondingly impact-proof.
-
Chemical safety: the hermetic sealing of the electronics package must ensure that there is no leakage of toxic or other chemically harmful materials into tissue.
-
Thermal safety: ensure that maximum temperature, e.g., in the subcutaneous vicinity of the implant during operation (or recharging batteries), does not exceed ΔT < 2.0 °C and that corresponding temperature rise in the cortical space are kept at ΔT < 0.1 °C.
-
Electromagnetic (EM) safety: RF (or similar wireless) signal and power transmission of the device are secure, and are not susceptible to external EM interference by ambient RF traffic and vice versa.
Most of these requirements follow the footsteps of established protocols, such as those for pacemakers or cochlear implants for human use, thereby mirroring established regulatory guidelines determined by FDA in the USA. We note, however, that at the frontiers of neurotechnology such as discussed in this volume, the device systems involve considerably complex electronics and require orders of magnitude larger wireless data rates so that early discussions by the technology developers with regulatory agencies is very important.
A number of neurotechnology research groups have developed compact, externally head-mounted wireless neural recording systems as the first step, whereby this approach has allowed leveraging commercially available microelectronics for the development of proof-of-concept wireless BCI systems. Among some of the earliest innovation in this arena, a set of contributions came from the Shenoy group at Stanford. This team has built a series of incrementally sophisticated “Hermes” series of wireless neural recording platforms [12, 19, 30, 38] beginning from a two-channel Hermes-B [41] to a 96-channel battery-operated Hermes-E [17]. In the laboratories of the authors of this chapter, we have similarly developed an external battery-operated, 96-channel broadband neural recording system, featuring custom ICs for neural signal capture and conditioning as well as a dedicated RF IC for 3.5 GHz short-range (~4 m), very-low power wireless transmission with a net power consumption of 61.2 mW [55]. The latter is now a licensed technology which is commercially available, allowing animal researchers to conduct studies on freely moving animals, for example in the context of foraging and naturalistic locomotion.
In addition to the systems level achievements highlighted above, new research is particularly focused at pushing the limits of microelectronic technology to attain improved performance for wireless neural recording with ever-shrinking power budgets. This research extends from subsystems utilizing commercial technologies such as UWB radios, WiFi, and Zigbee to highly optimized low-power integrated approaches. For example, a 4096 channel multiplexed ECoG recording chip with 64-channel amplifiers and 5.12 Mbps data rata, transmitted at 7.8 GHz using UWB radio has been described by Ando et al. [2] with a net power consumption of 1400 mW. Schwarz et al. [42] similarly describe a 128-channel head-mounted system working with microwire array implants, but now integrating the capability for on-board neural signal processing and bidirectional communications with a power consumption of 264 mW, or ~2 mW per channel. Several other groups have described multi-channel ASICs with sub-milliwatt power consumptions per channel while representing various trade-offs between signal compression and power budgets [5, 8]. Finally, multi-modal sensing and stimulation, for example, integrating dopamine sensing and electrical stimulation alongside electrical neural recording [7, 22], and approaches incorporating optogenetic approaches, are increasingly building a niche in the BCI component level landscape which is anticipated to grow significantly in future.
9.2.2 Fully Implanted Wireless Devices—A Case Example
The development of head-mounted and other outside-body wireless neural recording systems has provided a new BCI tool for neuroscience research by enabling more complex and naturalistic animal experiments. However, the need for a percutaneous connection still poses a limitation for BCI use of these systems, in particular, limiting their translation into the human/clinical BCI domain. In considering the bridge from a head-mounted to a fully implanted wireless neural interface device system, we reiterate key challenges which have to be addressed, typically through extensive animal testing and performance validation:
-
Implantation of the system’s electronic components within the body in a hermetically encapsulated unit, and choice for the anatomical location of this payload relative to the actual neural probes, where the electronic package has a form factor compatible with subcutaneous placement, for example, in the epicranial space.
-
ASIC design and optimization for ultra-low power, consistent with thermal safety guidelines defined by FDA for active brain implants (maximum of 0.5 °C increment in tissue temperature). Extensive thermal simulations and metrology of implants in animal models are typically required.
-
Implant power management (rechargeable batteries or continuous inductive coupling) and transcutaneous wireless communication, mitigating the impact of tissue RF absorption, and keeping within the specific absorption rate (SAR) limits prescribed by IEEE Std C95.1–2005.
The basic microelectronic building blocks of a fully implantable neural interface system are nearly identical to those described in previous sections; the caveat is that now the spatial scale and performance metrics and requirements (such as those listed above) for a fully implantable device are far more stringent. As the case example for this chapter, the authors’ labs have developed a 100-channel hermetically-sealed, fully implantable broadband wireless neural recording system utilizing a 100-channel neural amplifier ASIC, 12-bit SAR ADCs, and a custom RF transmitter IC. The components mirror those developed for the head-mounted system described previously, but now focus on a system architecture with a titanium (Ti)-based hermetic enclosure. The Ti-enclosure has been equipped with a sapphire window to provide full electromagnetic transparency for wireless charging and telecommunications. Further, a custom planar interconnect interface has been built to feed the 100 microwires from the intracortical MEA to the active electronics via multi-channel custom high-density hermetic feedthroughs as shown in Fig. 9.3 [6]. In keeping with the need for further electronic integration, there have been several recent efforts to develop system on chip electronics (SoC), where a single chip solution provides neural recording in conjunction with wireless charging, compressive sensing or spike detection, electrical stimulation and bidirectional telecommunications [4, 31, 36]. These advancements are important elements for the further development of fully-implantable systems, but the most critical current bottle-neck for these devices is the availability of scalable hermetic packaging solutions. Hermetic sealing approaches being further studied include ceramic, metallic, or thin-film materials, among others. In addition to the materials choice, a major engineering challenge is to microfabricate the large number of electrical “feedthroughs” without compromising the hermeticity of the implant.
9.3 New Horizons: Large-Scale Neural Interfaces to 10,000 Nodes and Beyond
There has been significant progress in enhancing and maintaining neural access through dense-electrode arrays and biocompatible surface modifications as described in previous sections, yet the scalability of practical neural interfaces to orders of magnitude higher numbers of recording and/or stimulating nodes (thousands and tens of thousands) remains a persistent challenge. As we move from the realm of highly controlled, experimental BCIs to more naturalistic, deployable systems, neural task complexity (NTC) is expected to grow. To gauge the expected number of microelectrode sites with increasing NTC, Gao et al. [18] have postulated that neural decoding would be critically dependent on access to significantly higher numbers of neurons at high densities, ideally from an anatomically diverse neural population. Figure 9.4 represents the outcome from a high-dimensionality neural state-space dynamical theoretical model. Current monolithic multi-electrode constructs are, in our view, inherently incompatible with large-scale implementations of BCIs due to a variety of anatomical and fabrication constraints. For example, traditional hermetic sealing approaches require bulky Ti or ceramic cases, which add significant volume overhead to device size. The intricacy of interconnect design, particularly in the case of a multi-areal implant, is an added complication. While the short tethers between intracortical arrays and the wireless electronics described in the previous section represent a significant improvement over percutaneous tethers, they are still relying on the traditional monolithic intra- or epicortical electrode arrays. Looking forward, one needs to consider paradigm shifts in approaching future BCI-compatible neural interface designs to mitigate these issues in the design of vastly scalable systems. One such approach is to split individual recording and/or neurostimulation electrodes into separate autonomous microscale active electronic chiplets, each with its own internal electronics and (wireless) means to communicate with a central information processing hub. The idea takes advantage of the remarkable progress made by silicon microelectronics in the past 20 years which has pushed component and transistor sizes deep into the sub-100 nm regime. For neural and BCI applications, having access to such CMOS technologies is very attractive provided that commensurate expertise is available for custom design of “mixed-signal” CMOS chips (analog-digital-RF).
A dimensionality frontier in motor cortical data. Allowed possibilities of dimensionality D and neural task complexity, NTC, exhibit three distinct regimes: (i) the number of recorded neurons M but not NTC restricts dimensionality, (ii) NTC but not M restricts D, and (iii) D is far less than both M and NT C, reflecting an unexplained circuit constraint beyond smoothness and task simplicity. (Courtesy of [18])
Among contemporary work is the concept of free-floating individual “neural dust” sensors in conjunction with a sub-cranial interrogator which was proposed by a number of neuroengineering researchers, most notably Seo et al. [43] (we show concrete examples from our own work below to illustrate a related approach for wireless microsensors and stimulators). The proposed system by Seo et al. [43] involves ultrasonic power and telemetry to circumvent the challenges of efficient electromagnetic energy coupling at millimeter-scale and has been demonstrated at the level of a single 1 mm3 recording node in the peripheral nervous system [44]. While the concept is presented as an ultra-miniaturizable sensor system, the authors identify several fabrication and materials challenges requiring further advances in CMOS die post-processing completion of microscale implantable microdevices. Elsewhere, a purely electromagnetic-based distributed neural sensor has been demonstrated by Yeon et al. [53], where a three-coil resonant near field inductively coupled system is used to improve the efficiency of wireless power transfer to a millimeter-sized free-floating wireless implantable neural recording system (FF-WINeR). The approach to assembling the FF-WINeR sensor node by manual techniques is also described by Yeon et al. [54] including integration of microwire electrodes, ASICs with through-silicon vias (TSVs), discrete microcoils, and surface mount components. Finally, hermetic sealing with polymeric film deposition (parylene in this case) was applied to form a single “push-pin” recording node measuring 1.05 × 1.05 × 0.3 mm3. Here too, the authors outline a number of challenges ahead in scaling to many devices given the number of heterogeneous materials and the complexity plus fabrication requirements comprising each sensor node.
With these snapshots of recent early work, we support the view that spatially distributed sensor systems, once developed as full-fledged medical devices can contribute to future breakthroughs in the field of BCIs while providing new tools for brain science. There are, however, several caveats for the development of a truly scalable distributed sensor system. First, it is imperative that it be possible to pursue the fabrication of sensors in a uniform, high throughput batch process. Second, sophisticated and ultra-miniaturized sensor node electronics are needed to support semi-autonomous operation including critical functions such as memory and networking. Third, the full neural interface system needs to be defined in the context of a highly-efficient multi-node telecom network. This includes the design and implementation of a compact wearable telecommunications hub that would drive and tune the performance specifications of individual sensor nodes in an adaptive fashion, depending on the specific neural interface application. Fourth, the next frontier in BCI development beyond neural recording for decoding must include neural encoding implemented as patterned cortical microstimulation by a spatially distributed ensemble. This capability needs to be integrated into the individual nodes to form an independent, bidirectional neural interfacing element. Fifth, for chronic implants, it is critical to develop the means for (thin film) hermetic seals which provide a chemically impermeable envelope for the microdevices over decades in the body environment. Finally, the implementation of any neural interface system for use in a human subject requires a real-time neural encoding/decoding using a wearable neurocomputational processor with wireless telemetry to supporting computational platforms, including cloud computing. The concept image of Fig. 9.5 shows schematically the general approach (with some specifics to the approach adopted in the authors’ laboratories).
Designing a system to meet the above-mentioned caveats is a challenging proposition on multiple levels. Recent work in our laboratories has focused on developing a spatially distributed implantable wireless “cortical communication” system toward these aspirations, which is built around 500 × 500 μm individually addressable custom microelectronic “neurograins” chiplets (see Fig. 9.6, which also compares several other recent microdevices). Each chiplet integrates RF-energy harvesting, broadband neural recording or cortical microstimulation. Ensembles of neurograins operate as a synchronized, networked bidirectional RF telecommunication network, scalable in principle across populations of nodes up to tens of thousands. We have chosen to utilize near-field electromagnetic coupling at ~1 GHz to mitigate RF tissue absorption for SAR compliance and designed a single RF-channel simultaneous power-data link for network level simplicity. An external RF telecommunications hub, implemented as a wearable module, wirelessly manages implant performance, and is envisioned also to integrate real-time data processing for neural decoding [19, 20]. Further, we have addressed the microscale packaging challenge by utilizing a batch-process stacked multi-layer thin-film deposition process which is able to yield packaged individual devices that are <0.01 mm3 in physical volume. This thin film hermetic sealing technique using atomic layer epitaxy, has been demonstrated to provide implant impermeability in accelerated lifetime tests exceeding 10 years [24]. These features have been validated in the context of ex vivo rodent brain slices as well as acute in vivo experiments [29], and now mature for transition to a specific application context.
The above-described system from our labs is one example of an approach for a completely untethered neural interface system that is minimally invasive from the perspective of implant volume burden. Alternative approaches involve flexible, floating epicortical probes with very high channel counts [48] versus integration of genetic approaches to modify neural responsiveness (optogenetics, etc.) toward the realization of next-generation highly efficient, integrated neural interface systems.
9.4 Summary: Outlook for High-Performance BCIs
In this chapter, we have described approaches to high data rate neural interfaces via examples of technical solutions to intracortical/intracranial recording systems. While early clinical trials are under way, and mainly use well-established, but bulky cabled systems, there is a need and opportunity to invest resources to pursue innovative approaches across the entire ecosystem from micro- and optoelectronic probe arrays to chipscale integration of tailored on-chip signal processing and storage functions, to name two areas.
The microminiaturization approaches reviewed here offer a particular opportunity to enhance the functionality of the wireless interfaces by adding neuromodulation/stimulation capabilities. Among such functions is the implementation of patterned electrical (e.g., [14, 47]) or/and optical microstimulation for enabling truly bidirectional communication opportunities with the brain. Efforts to develop such multi-node targeted microstimulation tools are being pursued in several academic and commercial laboratories and are likely to reach primates in the near term. These efforts must link closely with neural decoding/encoding models using combination of theoretical neuroscience and machine learning tools.
We re-emphasize the importance of close synergy between closed-loop sense/stimulate-based algorithms which have recently made progress in deep brain stimulation [1]; however, the challenges encountered herein, for bidirectional interfaces comprising hundreds of channels (nodes) and beyond require that large amounts of real-time data, recorded from the nervous system, must be processed and decoded (e.g., [38, 52]). Among the many challenges to this aspiration are the inherent variability and statistical entropy-driven fluctuations in neural circuits, the latter requiring approaches which can adapt to such “non-stationarities.”
References
Afshar P, Khambhati A, Stanslaski S, Carlson D, Jensen R, Dani S, …, Denison T (2013) A translational platform for prototyping closed-loop neuromodulation systems. Front Neural Circuits 6:117
Ando H, Takizawa K, Yoshida T, Matsushita K, Hirata M, Suzuki T (2016) Wireless multichannel neural recording with a 128-mbps UWB transmitter for an implantable brain-machine interfaces. IEEE Trans Biomed Circuits Syst 10(6):1068–1078
Aflalo T, Kellis S, Klaes C, Lee B, Shi Y, Pejsa K, …, Liu C (2015) Decoding motor imagery from the posterior parietal cortex of a tetraplegic human. Science 348(6237):906–910
Biederman W, Yeager DJ, Narevsky N, Leverett J, Neely R, Carmena JM, …, Rabaey JM (2015) A 4.78 mm 2 fully-integrated neuromodulation SoC combining 64 acquisition channels with digital compression and simultaneous dual stimulation. IEEE J Solid State Circuits 50(4):1038–1047
Bonfanti A, Ceravolo M, Zambra G, Gusmeroli R, Borghi T, Spinelli AS, Lacaita AL (2010) A multi-channel low-power IC for neural spike recording with data compression and narrowband 400-MHz MC-FSK wireless transmission. In: 2010 Proceedings of ESSCIRC, September. IEEE, pp 330–333
Borton DA, Yin M, Aceros J, Nurmikko A (2013) An implantable wireless neural interface for recording cortical circuit dynamics in moving primates. J Neural Eng 10(2):026010
Bozorgzadeh B, Schuweiler DR, Bobak MJ, Garris PA, Mohseni P (2016) Neurochemostat: a neural interface SoC with integrated chemometrics for closed-loop regulation of brain dopamine. IEEE Trans Biomed Circuits Syst 10(3):654–667
Brenna S, Padovan F, Neviani A, Bevilacqua A, Bonfanti A, Lacaita AL (2016) A 64-channel 965-$\mu\text {W} $ neural recording SoC with UWB wireless transmission in 130-nm CMOS. IEEE Trans Circuits Syst Express Briefs 63(6):528–532
Carmena JM, Lebedev MA, Crist RE, O’Doherty JE, Santucci DM, Dimitrov DF, …, Nicolelis MA (2003) Learning to control a brain–machine interface for reaching and grasping by primates. PLoS Biol 1(2):e42
Castagnola E, Maiolo L, Maggiolini E, Minotti A, Marrani M, Maita F, …, Fadiga L (2015a) PEDOT-CNT-coated low-impedance, ultra-flexible, and brain-conformable micro-ECoG arrays. IEEE Trans Neural Syst Rehabil Eng 23(3):342–350
Castagnola V, Descamps E, Lecestre A, Dahan L, Remaud J, Nowak LG, Bergaud C (2015b) Parylene-based flexible neural probes with PEDOT coated surface for brain stimulation and recording. Biosens Bioelectron 67:450–457
Chen G, Dodson B, Hedges DM, Steffensen SC, Harb JN, Puleo C, …, Davis RC (2018) Fabrication of high aspect ratio millimeter-tall free-standing carbon nanotube-based microelectrode arrays. ACS Biomater Sci Eng 4(5):1900–1907
Collinger JL, Wodlinger B, Downey JE, Wang W, Tyler-Kabara EC, Weber DJ, …, Schwartz AB (2013) High-performance neuroprosthetic control by an individual with tetraplegia. Lancet 381(9866):557–564
Dadarlat MC, O’doherty JE, Sabes PN (2015) A learning-based approach to artificial sensory feedback leads to optimal integration. Nat Neurosci 18(1):138
Deku F, Cohen Y, Joshi-Imre A, Kanneganti A, Gardner TJ, Cogan SF (2018) Amorphous silicon carbide ultramicroelectrode arrays for neural stimulation and recording. J Neural Eng 15(1):016007
Diaz-Botia CA, Luna LE, Neely RM, Chamanzar M, Carraro C, Carmena JM, …, Maharbiz MM (2017) A silicon carbide array for electrocorticography and peripheral nerve recording. J Neural Eng 14(5):056006
Gao H, Walker RM, Nuyujukian P, Makinwa KA, Shenoy KV, Murmann B, Meng TH (2012) HermesE: a 96-channel full data rate direct neural Interface in 0.13μm CMOS. IEEE J Solid State Circuits 47(4):1043–1055
Gao P, Trautmann E, Byron MY, Santhanam G, Ryu S, Shenoy K, Ganguli S (2017) A theory of multineuronal dimensionality, dynamics and measurement. bioRxiv 1:214262
Heelan C, Komar J, Vargas-Irwin CE, Simeral JD, Nurmikko AV (2015) A mobile embedded platform for high performance neural signal computation and communication. In: 2015 IEEE biomedical circuits and systems conference (BioCAS), October. IEEE, pp 1–4
Heelan C, Nurmikko AV, Truccolo W (2018) FPGA implementation of deep-learning recurrent neural networks with sub-millisecond real-time latency for BCI-decoding of large-scale neural sensors (104 nodes). In: 2018 40th annual international conference of the ieee engineering in medicine and biology society (EMBC), July. IEEE, pp 1070–1073
Hochberg LR, Serruya MD, Friehs GM, Mukand JA, Saleh M, Caplan AH, …, Donoghue JP (2006) Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature 442(7099):164
Hochberg LR, Bacher D, Jarosiewicz B, Masse NY, Simeral JD, Vogel J, …, Donoghue JP (2012) Reach and grasp by people with tetraplegia using a neurally controlled robotic arm. Nature 485(7398):372
Jarosiewicz B, Sarma AA, Bacher D, Masse NY, Simeral JD, Sorice B, …, Cash SS (2015) Virtual typing by people with tetraplegia using a self-calibrating intracortical brain-computer interface. Sci Trans Med 7(313):313ra179
Jeong J, Laiwalla F, Lee J, Ritasalo R, Pudas M, Larson L, …, Nurmikko A (2019) Conformal hermetic sealing of wireless microelectronic implantable Chiplets by multilayered atomic layer deposition (ALD). Adv Funct Mater 29(5):1806440
Jun JJ, Steinmetz NA, Siegle JH, Denman DJ, Bauza M, Barbarits B et al (2017) Fully integrated silicon probes for high-density recording of neural activity. Nature 551(7679):232
Khodagholy D, Gelinas JN, Thesen T, Doyle W, Devinsky O, Malliaras GG, Buzsáki G (2015) NeuroGrid: recording action potentials from the surface of the brain. Nat Neurosci 18(2):310
Kozai TD, Catt K, Du Z, Na K, Srivannavit O, Razi-ul MH, …, Cui XT (2016) Chronic in vivo evaluation of PEDOT/CNT for stable neural recordings. IEEE Trans Biomed Eng 63(1):111–119
Kwon KY, Lee HM, Ghovanloo M, Weber A, Li W (2015) Design, fabrication, and packaging of an integrated, wirelessly-powered optrode array for optogenetics application. Front Syst Neurosci 9:69
Lee J, Mok E, Huang J, Cui L, Lee AH, Leung VW, Mercier P, Shellhammer S, Larson L, Asbeck A, Song YK, Nurmikko A, Laiwalla F (2019) An implantable wireless network of distributed microscale sensors for neural applications. IEEE EMBS conference on neural engineering 2019
Leuthardt EC, Schalk G, Wolpaw JR, Ojemann JG, Moran DW (2004) A brain–computer interface using electrocorticographic signals in humans. J Neural Eng 1(2):63
Liu X, Zhang M, Xiong T, Richardson AG, Lucas TH, Chin PS, …, Van der Spiegel J (2016) A fully integrated wireless compressed sensing neural signal acquisition system for chronic recording and brain machine interface. IEEE Trans Biomed Circuits Syst 10(4):874–883
Luan L, Wei X, Zhao Z, Siegel JJ, Potnis O, Tuppen CA, …, Dunn AK (2017) Ultraflexible nanoelectronic probes form reliable, glial scar–free neural integration. Sci Adv 3(2):e1601966
McDermott H (2016) Neurobionics: treatments for disorders of the central nervous system. In: Neurobionics: the biomedical engineering of neural prostheses. Wiley, Hoboken, pp 213–230
Mestais CS, Charvet G, Sauter-Starace F, Foerster M, Ratel D, Benabid AL (2015) WIMAGINE: wireless 64-channel ECoG recording implant for long term clinical applications. IEEE Trans Neural Syst Rehabil Eng 23(1):10–21
Mirbozorgi SA, Bahrami H, Sawan M, Rusch LA, Gosselin B (2016) A single-chip full-duplex high speed transceiver for multi-site stimulating and recording neural implants. IEEE Trans Biomed Circuits Syst 10(3):643–653
Müller-Putz GR, Schwarz A, Pereira J, Ofner P (2016) From classic motor imagery to complex movement intention decoding: the noninvasive Graz-BCI approach. In: Progress in brain research, vol 228. Elsevier, pp 39–70
Oxley TJ, Opie NL, John SE, Rind GS, Ronayne SM, Wheeler TL, …, Steward C (2016) Minimally invasive endovascular stent-electrode array for high-fidelity, chronic recordings of cortical neural activity. Nat Biotechnol 34(3):320
Park YS, Hochberg LR, Eskandar EN, Cash SS, Truccolo W (2013) Early detection of human epileptic seizures based on intracortical local field potentials. In: 2013 6th international IEEE/EMBS conference on neural engineering (NER), November. IEEE, pp 323–326
Raducanu BC, Yazicioglu RF, Lopez CM, Ballini M, Putzeys J, Wang S et al (2016) Time multiplexed active neural probe with 678 parallel recording sites. In: 2016 46th European solid-state device research conference (ESSDERC). IEEE, pp 385–388
Rios G, Lubenov EV, Chi D, Roukes ML, Siapas AG (2016) Nanofabricated neural probes for dense 3-D recordings of brain activity. Nano Lett 16(11):6857–6862
Santhanam G, Linderman MD, Gilja V, Afshar A, Ryu SI, Meng TH, Shenoy KV (2007) HermesB: a continuous neural recording system for freely behaving primates. IEEE Trans Biomed Eng 54(11):2037–2050
Schwarz DA, Lebedev MA, Hanson TL, Dimitrov DF, Lehew G, Meloy J, …, Ramakrishnan A (2014) Chronic, wireless recordings of large-scale brain activity in freely moving rhesus monkeys. Nat Methods 11(6):670
Seo D, Carmena JM, Rabaey JM, Alon E, Maharbiz MM (2013) Neural dust: an ultrasonic, low power solution for chronic brain-machine interfaces. arXiv preprint arXiv:1307.2196
Seo D, Neely RM, Shen K, Singhal U, Alon E, Rabaey JM, …, Maharbiz MM (2016) Wireless recording in the peripheral nervous system with ultrasonic neural dust. Neuron 91(3):529–539
Seymour EÇ, Freedman DS, Gökkavas M, Özbay E, Sahin M, Ünlü MS (2014) Improved selectivity from a wavelength addressable device for wireless stimulation of neural tissue. Front Neuroeng 7:5
Simeral JD, Kim SP, Black MJ, Donoghue JP, Hochberg LR (2011) Neural control of cursor trajectory and click by a human with tetraplegia 1000 days after implant of an intracortical microelectrode array. J Neural Eng 8(2):025027
Tabot GA, Dammann JF, Berg JA, Tenore FV, Boback JL, Vogelstein RJ, Bensmaia SJ (2013) Restoring the sense of touch with a prosthetic hand through a brain interface. Proc Natl Acad Sci 110(45):18279–18284
Tsai D, Sawyer D, Bradd A, Yuste R, Shepard KL (2017) A very large-scale microelectrode array for cellular-resolution electrophysiology. Nat Commun 8(1):1802
Viventi J, Kim DH, Vigeland L, Frechette ES, Blanco JA, Kim YS, …, Wulsin DF (2011) Flexible, foldable, actively multiplexed, high-density electrode array for mapping brain activity in vivo. Nat Neurosci 14(12):1599
Wang W, Collinger JL, Degenhart AD, Tyler-Kabara EC, Schwartz AB, Moran DW, …, Kelly JW (2013) An electrocorticographic brain interface in an individual with tetraplegia. PLoS One 8(2):e55344
Wessberg J, Stambaugh CR, Kralik JD, Beck PD, Laubach M, Chapin JK, …, Nicolelis MA (2000) Real-time prediction of hand trajectory by ensembles of cortical neurons in primates. Nature 408(6810):361
Wodlinger B, Downey JE, Tyler-Kabara EC, Schwartz AB, Boninger ML, Collinger JL (2014) Ten-dimensional anthropomorphic arm control in a human brain− machine interface: difficulties, solutions, and limitations. J Neural Eng 12(1):016011
Yeon P, Mirbozorgi S, Ash B, Eckhardt H, Ghovanloo M (2016) Fabrication and microassembly of a mm-sized floating probe for a distributed wireless neural interface. Micromachines 7(9):154
Yeon P, Gonzalez JL, Zia M, Rajan SK, May GS, Bakir MS, Ghovanloo M (2017) Microfabrication, assembly, and hermetic packaging of mm-sized free-floating neural probes. In 2017 IEEE biomedical circuits and systems conference (BioCAS), October. IEEE, pp 1–4
Yin M, Borton DA, Komar J, Agha N, Lu Y, Li H, …, Larson L (2014) Wireless neurosensor for full-spectrum electrophysiology recordings during free behavior. Neuron 84(6):1170–1182
Acknowledgments
The authors are very grateful to many members, past and present, in their laboratory. These include Jihun Lee, Joonsoo Jeong, David Borton, Ming Yin, Y.-K. Song, William R. Patterson III, Naubahar Agha, and Chris Heelan, among others. At Brown University, the authors are part of a synergistic brain science and neurotechnology effort with many colleagues including John Donoghue, Leigh Hochberg, David Rosler, John Simeral, and Carlos Vargas-Irwin whom we thank for their continuing input and expertise. Special thanks are also extended to Krishna Shenoy and his colleagues at Stanford for providing early leadership in the field.
All animal procedures referred in this chapter were conducted according to protocols approved by Institutional Animal Care and Use Committee (IACUC) at each institution. Research in the authors’ laboratory was supported by US National Institutes of Health, Defense Advanced Projects Agency and the National Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Laiwalla, F., Nurmikko, A. (2019). Future of Neural Interfaces. In: Zheng, X. (eds) Neural Interface: Frontiers and Applications. Advances in Experimental Medicine and Biology, vol 1101. Springer, Singapore. https://doi.org/10.1007/978-981-13-2050-7_9
Download citation
DOI: https://doi.org/10.1007/978-981-13-2050-7_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-2049-1
Online ISBN: 978-981-13-2050-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)