Abstract
Alcoholic liver disease (ALD) is one of the most common forms of chronic liver disease in the world; it is a major cause of chronic illness and mortality associated with alcohol over-consumption. ALD represents a broad spectrum of liver injury, such as hepatocyte cell death, liver inflammation, angiogenesis, and fibrosis leading to cirrhosis and hepatocellular carcinoma. Chronic ethanol consumption results in hepatic lipid accumulation and increases cell stress, which leads to inflammation and liver injury during the progression of ALD. It has been shown that crosstalk between hepatocytes and non-parenchymal cells is significantly important. The identifying factors that communicate stress signals from hepatocytes, and may initiate and perpetuate the inflammatory reaction responsible for liver injury and disease progression from steatohepatitis to cirrhosis may have a tremendous biomedical impact. Furthermore, the elucidation of these molecular mechanisms of crosstalk may allow for the identification of an individualized therapeutic approach in the treatment of patients with different stages of ALD and for the development of biomarkers to diagnose ALD progression. Recently, extracellular vesicles (EVs) have been identified as cell-to-cell communicators, the cellular contents of which contain proteins, lipids, and RNAs from stressed/activated cells and transfer this cellular payload to target cells. In this chapter, we will focus on current reports of EV function, how they are involved in the molecular pathogenesis of ALD, and EV biomarkers using EV composition.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
1.1 Alcoholic Liver Injury
Alcoholic liver disease (ALD) is the common cause of chronic liver disease in the world [1]. ALD represents a wide spectrum of liver injury ranging from alcoholic steatosis, alcoholic steatohepatitis (ASH) , cirrhosis to liver failure and hepatocellular carcinoma [2]. In the process of ALD progression, hepatocyte damage, inflammation, fibrosis, and angiogenesis are key events and are closely interconnected [3], suggesting that multiple-hits involve in the progression of ALD. Current growing evidences show that extracellular vesicles (EVs) releasing from damaged hepatocytes or non-parenchymal cells contribute to the progression of liver diseases through activation of target cells, such as hepatic stellate cells and hepatic macrophages [4, 5]. Furthermore, EV composition, such as proteins and microRNAs (miRNAs), can be used to identify the degree of liver diseases including ALD/ASH [4, 6].
1.2 Extracellular Vesicles (EVs)
EVs are released from various cell types with their cell contents, such as proteins, non-coding RNAs, and lipids, in a highly regulated manner and circulated into the blood with high stability. Circulating EV levels are increased in many diseases due to up-regulation of EV release from damaged and activated cells, thus EVs including EV compositions will be able to use for biomarkers [7]. EVs are mainly categorized as exosomes or microparticles (MPs)/microvesicles. Exosomes are enclosed in the multi-vesicle body (MVB) and released from the cells in the endosomal pathway, whereas MPs are budded from the plasma membrane. Traditionally, their size was defined below 100–150 nm for exosomes and around 200–500 nm for MPs, but small vesicles (~100 nm) were identified as budding form as same as MPs [8]. As larger than nano-size, apoptotic bodies (above 1 μm) and oncosomes (1–10 μm) from cancer cells, which are budded from the plasma membrane, are also categorized in EVs [9]. In the molecular content, some of their composition may be different, CD63 for exosomes and annexin V for MPs, but traditionally identified molecules, such as CD81, CD9, or TSG101, are contained both in exosomes and MPs [8]. Lacking a clear categorization of EV type by size and contents, a new system of nomenclature has been proposed for studies lacking a detailed analysis of EV biogenesis whereby vesicles are grouped into one of two categories, small EVs or large EVs [9]. Notably, EVs are efficiently internalized into target cells and the subsequent transferring of their molecular composition, such as proteins, non-coding RNAs including microRNAs (miRNAs), messenger RNAs (mRNAs), DNA, and lipids, is a key mechanism by which EVs modulate cell signaling in target cells [8, 9], so called cell-to-cell communication. For instance, ligands on EVs bind to their specific receptor on the target cells and release encapsulated miRNAs, which in turn bind to target cell mRNA, thus altering the cell signaling pathway via translational suppression [10].
2 The Mechanism of EV Release and the Role of EVs in ALD/ASH
Circulating EV levels were increased in both animal models of ALD and human ALD patients. The source of the EVs was identified using EV composition, such as asialoglycoprotein receptor 1 (ASGPR1), vanin-1, and miR-122, which would indicate that a portion of the circulating EVs were derived from hepatocytes [4]. Supporting evidence that the liver releases hepatocyte-derived EVs (Hep-EVs) was directly confirmed when large quantities of EVs were found to be released from damaged hepatocytes isolated from alcoholic steatohepatitis (ASH) mice compared to control-diet mice [11]. Non-parenchymal cells also release EVs in ALD that are circulated in the blood. Liver EVs derived from hepatocytes and non-parenchymal cells contribute to the progression of ALD.
2.1 Hepatocyte-Derived Extracellular Vesicles (Hep-EVs)
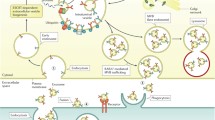
Various pathways, including the activation of caspase and pho-kinase, as well as ER stress are involved in Hep-EV release [4, 11], resulting in Hep-EVs containing damage-associated cellular molecules, such as proteins, ligands, miRNAs, and mtDNAs, used in the activation of target cells (Fig. 6.1).
Hepatocyte-derived EVs are cell-to-cell communicators for the progression of ALD. Hepatocyte-derived EVs (Hep-EVs), exosomes and microparticles, released from damaged hepatocytes activate target cells (hepatic macrophages, monocytes, neutrophils) via cell-to-cell communication. Hep-EVs contain unique molecular cargo, such as proteins and miRNAs, that reflects cellular damage/stress and this cargo modulates the activation of target cells
A significant amount of Hep-EVs, which contained CD40 ligand (CD40L), were released in a caspase-3-dependent-manner from HepG2 cells treated with EtOH and overexpressing cytochrome P450 2E1, which is related to ethanol metabolism [12]. CD40L containing Hep-EVs activated macrophages to the M1 type inflammatory phenotype through the activation of ERK, whereas Hep-EV macrophage activation was attenuated using a CD40L-specific antibody. In a chronic Lieber-DeCarli diet plus single binge ethanol feeding model , wild-type mice receiving a pan-caspase/Rho kinase inhibitor or with a genetic deletion of either CD40 (CD40 −/−) or the caspase-activating tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) receptor (TR−/−) were protected from ethanol induced liver injury due to the attenuation of macrophage infiltration. Macrophage activation with Hep-EVs was also observed in a different experimental model that isolated hepatocytes from an intra-gastric infusion model of ASH, which significantly released Hep-EVs in a caspase 3-dependent-manner and these Hep-EVs were internalized and activated primary hepatic macrophages into the inflammatory M1 type [11, 13]. In the chronic Lieber-DeCarli diet-plus single binge ethanol feeding model, mtDNA-enriched circulating EVs were increased associated with the activation of hepatic ER stress and inflammatory responses, particularly the inflammasome [14]. Hepatocytes were the main source of mtDNA-enriched EVs, since mtDNA-enriched EVs were decreased in a hepatocyte-specific deletion of the protein kinase RNA-like ER kinase (Perk) gene in mice. mtDNA-enriched circulating EV levels, and the degree of neutrophil infiltration in the liver, were attenuated in transcriptional factor C/EBP homologous protein (Chop) KO mice, Jun-amino-terminal kinase 2 (JNK2) KO mice, or caspase-1 inhibitor treated mice, which is an ER stress-related gene, an ER stress-associated protein, or an inflammasome-associated gene, respectively. In an in vivo transfer EV assay, chronic ethanol fed mice injected with mtDNA-enriched circulating EVs induced neutrophilic inflammation through TLR9 activation. In chronic ethanol fed (Lieber-DeCarli diet) mice, which delivers a more mild liver pathology compared to the Lieber-DeCarli plus binge feeding model, circulating EVs (Hep-EVs plus other EVs) from ALD (ALD-EVs) were internalized via an in vivo transfer EV assay into the hepatocytes and Kupffer cells (KCs) of naïve mice which induced an increase of MCP-1 mRNA levels in hepatocytes and elevated inflammatory M1 type KCs and infiltrating monocytes [15]. Heat shock protein 90 (Hsp90) was highly enriched in ALD-EVs, as assessed by mass spectrometry analysis, and contributed to RAW macrophage activation associated with TNFα elevation. Conversely, macrophage activation was suppressed in RAW cells treated with a competitive inhibitor of Hsp90 plus ALD-EVs. These results suggest that Hep-EVs are an Hsp90 carrier and are involved in macrophage activation, since Hsp90 is a key player in macrophage activation as the literature has shown [16].
2.2 Non-parenchymal Cell-Derived Extracellular Vesicles
Hep-EVs from damaged hepatocytes were the major source of EVs in ASH mice (intra-gastric EtOH infusion), but non-parenchymal cells, such as hepatic macrophages, also released EVs associated with liver injury [11]. Indeed, alcohol-exposed monocytes, human primary monocytes, and THP-1 monocytic cells released miR-27a-enriched EVs [17]. miR-27a-enriched EVs stimulated naive monocytes into M2 macrophages associated with the up-regulation of IL-10 and TGF-β followed by increased monocyte phagocytosis. Circulating miR-27a-enriched EVs from AH patients polarize monocytes into an M2 phenotype associated with an elevation of IL-10.
3 EVs as Novel Biomarkers to Monitor Liver Injury in ALD/ASH
Various imaging modalities, such as ultrasound- and MR-based elastography, are increasingly being used for the assessment of liver fibrosis. However, liver biopsy still remains the gold standard in which to determine ALD staging with hepatocellular injury and hepatic inflammation. EVs have a key pathophysiological role in liver injury, as described in the previous sections, and EVs are remarkably stable in the blood during circulation, thus EVs, as well as EV composition, carry the potential to be developed into noninvasive biomarkers.
Growing evidence using animal models and human patients shows that the number of circulating EVs and liver-specific EV composition levels, such as asialoglycoprotein receptor 1 (ASGPR1), miR-122, and miR-192, are increased in various liver diseases including ALD, non-alcoholic steatohepatitis, viral hepatitis, and cirrhosis [4, 6, 18,19,20]. Circulating EV levels and liver specific EV composition levels may not be able to distinguish liver diseases by type, thus we need to identify specific EV composition to confirm ALD diagnosis. We introduce biomarkers, proteins, mtDNAs, and miRNAs in ALD/ASH, but focus exclusively on liver-specific proteins and miRNAs in this section (Table 6.1).
3.1 Ligands and Proteins
CD40L levels on circulating EVs were increased in alcoholic hepatitis patients compared to healthy individual or individuals who consume alcohol [12]. CD40L enriched-EVs were involved in macrophage activation. Using proteomic analysis, many proteins relating to the inflammatory response, cellular development, and cellular movement were enriched in circulating ALD-EVs from chronic ethanol feeding mice compared to circulating control-EVs [15]. One of the identified proteins was Hsp90, which induced macrophage activation, and high Hsp90 expression was validated in circulating ALD-EVs compared to control-EVs. Interestingly, at least ten proteins were only expressed in ALD-EVs and they were related to alcohol metabolism and redox regulation. These proteins are not yet validated in EVs from human alcoholic patients.
3.2 mtDNAs and miRNAs
mtDNAs levels in circulating EVs were higher in chronic-plus single binge ethanol feeding mice compared to pair-feeding mice, chronic ethanol feeding mice, or single binge ethanol feeding mice [14]. Furthermore, mtDNAs levels in circulating EVs were also elevated in chronic excessive alcohol use (EAU) with recent excessive drinking (RD) patients compared to EAU without RD patient or healthy controls. mtDNA-enriched EVs led to neutrophilia and liver injury. For miRNAs, miR-27a levels in circulating EVs were increased in AH patients compared to healthy controls [17]. miR-27a-enriched EVs mediated a polarization from monocytes to M2 type macrophage. Using firefly miRNA multiplex assay , seven miRNAs including miR-30a were significantly up-regulated and two miRNAs were significantly down-regulated in circulating ALD-EVs from chronic ethanol feeding mice compared to control-EVs from pair-feeding mice [19]. miR-30a had an excellent diagnostic value in ALD mice and miR-30a was significantly increased in alcoholic hepatitis patients compared to healthy controls. Using RNA-sequencing approach to assess miRNA composition in Hep-EVs released by hepatocytes isolated from the intra-gastric infusion model of ASH, nine miRNAs were significantly up-regulated and four miRNAs were significantly down-regulated in ASH Hep-EVs compared to control Hep-EVs [11]. miR-29a, miR-340, and let7f were increased in circulating EVs from ASH mice, but not in circulating EVs from bile duct ligation, NASH, and obese, indicating these miRNAs identify ASH. Three miRNAs were also elevated in ALD patients compared to non-alcoholics.
4 Conclusions
We have summarized some of the most recent and original studies investigating the biological function of EVs and their potential as biomarkers specific for ALD/ASH. In particular, many studies have pointed to the biological role of Hep-EVs released by stressed/damaged hepatocytes as key modulators for target cells as cell-to-cell communicator during ALD progression. According to many studies that looked into the biological role of EVs in different liver diseases—fatty liver, NASH, cirrhosis—some of the roles or release mechanisms of EVs are similar in ALD. For instance, mtDNA-enriched Hep-EVs were increased in NASH patients and mediated macrophage activation through TLR9 activation [21]. Damaged hepatocytes released Hep-EVs by lipotoxicity in a caspase3-dependent-manner and activated target cells [22], although EV composition was different in the process of target cell modulation. Since EVs have various biological roles in the progression of other liver diseases [5], we expect to identify other roles for EVs in ALD for future study. In addition, our work with EVs will contribute in the development of specific biomarkers for alcoholic liver injury.
References
Rehm J, Samokhvalov AV, Shield KD. Global burden of alcoholic liver diseases. J Hepatol. 2013;59:160–8. https://doi.org/10.1016/j.jhep.2013.03.007.
Stickel F, Datz C, Hampe J, Bataller R. Pathophysiology and management of alcoholic liver disease: update 2016. Gut Liver. 2017;11:173–88. https://doi.org/10.5009/gnl16477.
Bocca C, Novo E, Miglietta A, Parola M. Angiogenesis and fibrogenesis in chronic liver diseases. Cell Mol Gastroenterol Hepatol. 2015;1:477–88. https://doi.org/10.1016/j.jcmgh.2015.06.011.
Hirsova P, Ibrahim SH, Verma VK, Morton LA, Shah VH, LaRusso NF, Gores GJ, Malhi H. Extracellular vesicles in liver pathobiology: small particles with big impact. Hepatology. 2016;64:2219–33. https://doi.org/10.1002/hep.28814.
Povero D, Feldstein AE. Novel molecular mechanisms in the development of non-alcoholic steatohepatitis. Diabetes Metab J. 2016;40:1–11. https://doi.org/10.4093/dmj.2016.40.1.1.
Szabo G, Momen-Heravi F. Extracellular vesicles in liver disease and potential as biomarkers and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2017;14:455–66. https://doi.org/10.1038/nrgastro.2017.71.
Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–83. https://doi.org/10.1083/jcb.201211138.
Yanez-Mo M, Siljander PR, Andreu Z, Zavec AB, Borras FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. https://doi.org/10.3402/jev.v4.27066.
Maas SLN, Breakefield XO, Weaver AM. Extracellular vesicles: unique intercellular delivery vehicles. Trends Cell Biol. 2017;27:172–88. https://doi.org/10.1016/j.tcb.2016.11.003.
Chen K, Rajewsky N. The evolution of gene regulation by transcription factors and microRNAs. Nat Rev Genet. 2007;8:93–103. https://doi.org/10.1038/nrg1990.
Eguchi A, Lazaro RG, Wang J, Kim J, Povero D, Willliams B, Ho SB, Starkel P, Schnabl B, Ohno-Machado L, et al. Extracellular vesicles released by hepatocytes from gastric infusion model of alcoholic liver disease contain a MicroRNA barcode that can be detected in blood. Hepatology. 2017;65:475–90. https://doi.org/10.1002/hep.28838.
Verma VK, Li H, Wang R, Hirsova P, Mushref M, Liu Y, Cao S, Contreras PC, Malhi H, Kamath PS, et al. Alcohol stimulates macrophage activation through caspase-dependent hepatocyte derived release of CD40L containing extracellular vesicles. J Hepatol. 2016;64:651–60. https://doi.org/10.1016/j.jhep.2015.11.020.
Eguchi A, Kim J, Ohno-Machado L, Tsukamoto H, Feldstein AE. Hepatocytes from mice on intragastric feeding model of alcoholic steatohepatitis release extracellular vesicles with specific microRNA cargo that modulate hepatic stellate cell and macrophage phenotype. Hepatology. 2015;62:266A–7A.
Cai Y, Xu MJ, Koritzinsky EH, Zhou Z, Wang W, Cao H, Yuen PS, Ross RA, Star RA, Liangpunsakul S, et al. Mitochondrial DNA-enriched microparticles promote acute-on-chronic alcoholic neutrophilia and hepatotoxicity. JCI Insight. 2017;2:92634. https://doi.org/10.1172/jci.insight.92634.
Saha B, Momen-Heravi F, Furi I, Kodys K, Catalano D, Gangopadhyay A, Haraszti R, Satishchandran A, Iracheta-Vellve A, Adejumo A, et al. Extracellular vesicles from mice with alcoholic liver disease carry a distinct protein cargo and induce macrophage activation via Hsp90. Hepatology. 2017;67(5):1986–2000. https://doi.org/10.1002/hep.29732.
Ambade A, Catalano D, Lim A, Kopoyan A, Shaffer SA, Mandrekar P. Inhibition of heat shock protein 90 alleviates steatosis and macrophage activation in murine alcoholic liver injury. J Hepatol. 2014;61:903–11. https://doi.org/10.1016/j.jhep.2014.05.024.
Saha B, Momen-Heravi F, Kodys K, Szabo G. MicroRNA cargo of extracellular vesicles from alcohol-exposed monocytes signals naive monocytes to differentiate into M2 macrophages. J Biol Chem. 2016;291:149–59. https://doi.org/10.1074/jbc.M115.694133.
Eguchi A, Wree A, Feldstein AE. Biomarkers of liver cell death. J Hepatol. 2014;60:1063–74. https://doi.org/10.1016/j.jhep.2013.12.026.
Momen-Heravi F, Saha B, Kodys K, Catalano D, Satishchandran A, Szabo G. Increased number of circulating exosomes and their microRNA cargos are potential novel biomarkers in alcoholic hepatitis. J Transl Med. 2015;13:261. https://doi.org/10.1186/s12967-015-0623-9.
Povero D, Eguchi A, Li H, Johnson CD, Papouchado BG, Wree A, Messer K, Feldstein AE. Circulating extracellular vesicles with specific proteome and liver microRNAs are potential biomarkers for liver injury in experimental fatty liver disease. PLoS One. 2014;9:e113651. https://doi.org/10.1371/journal.pone.0113651.
Garcia-Martinez I, Santoro N, Chen Y, Hoque R, Ouyang X, Caprio S, Shlomchik MJ, Coffman RL, Candia A, Mehal WZ. Hepatocyte mitochondrial DNA drives nonalcoholic steatohepatitis by activation of TLR9. J Clin Invest. 2016;126:859–64. https://doi.org/10.1172/JCI83885.
Povero D, Eguchi A, Niesman IR, Andronikou N, de Mollerat du Jeu X, Mulya A, Berk M, Lazic M, Thapaliya S, Parola M, et al. Lipid-induced toxicity stimulates hepatocytes to release angiogenic microparticles that require Vanin-1 for uptake by endothelial cells. Sci Signal. 2013;6:ra88. https://doi.org/10.1126/scisignal.2004512.
Acknowledgments
This work was partially supported by JSPS KAKENHI Grant Number JP16H06872 to AE and JSPS KAKENHI Grant Number JP17K09419 to YT.
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Eguchi, A., Takei, Y. (2019). Extracellular Vesicles in Alcoholic Liver Injury. In: Yoshiji, H., Kaji, K. (eds) Alcoholic/Non-Alcoholic Digestive Diseases. Springer, Singapore. https://doi.org/10.1007/978-981-13-1465-0_6
Download citation
DOI: https://doi.org/10.1007/978-981-13-1465-0_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-1464-3
Online ISBN: 978-981-13-1465-0
eBook Packages: MedicineMedicine (R0)