Abstract
In this chapter, the review of the newest reports on plant-microbe interactions in plant tolerance to arsenic (As) is presented in two aspects. One is the bacteria effect on regulation of As availability in growth environment of the plant, and the second is direct influence of them on plant organism tolerance to As. The role of As oxidization or reduction mechanisms which were developed by microbes colonizing soil or water in plant tolerance to As is discussed. The meaning of rhizospheric bacteria contribution to bioavailability of elements such as phosphorus, iron, silicon or As, by mineral solubilization, as well as the significance of the bacteria siderophores in plant As tolerance is also explained. As and Fe released from iron(III) arsenate by symbiotic bacteria of As-hyperaccumulator fern, Pteris vittata, are not omitted. The role of As-resistant representatives of plant growth-promoting bacteria (PGPB) group in the reduction of As uptake by plants from contaminated soil is also described. Considering novel aspects of plant-microbe interactions under As stress, the content of this chapter refines previous knowledge about plant physiology in terms of As tolerance and in the field of As-resistant plant-microbe model application in environment remediation.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
9.1 Introduction
In the environment, besides herbivores, plants are exposed to different biotic factors, both beneficial and pathogenic such as insects, nematodes, fungi, bacteria, archaea or viruses (Coats and Rumpho 2014; Müller et al. 2016; Busby et al. 2017). They interact with plant affecting its metabolism and development (Martin et al. 2016; Martin et al. 2017). There are long-term symbiotic interactions, bringing bidirectional benefits (mutualism), exploiting one side of the relation (parasitism) or mutualistic and detrimental for both sides of interrelationship at the same time (Martin et al. 2017). Since plants colonized terrestrial environment, they have evolved a variety of interrelationships with actively selected microorganisms, what seemed to be the most beneficial way to provide poorly soluble nutrients and protect from toxins in environment deficient in water, main solvent and transporter, comparing to previous habitat (Bulgarelli et al. 2013; Zgadzaj et al. 2016; Martin et al. 2017). The common pathway of symbiotic signalling is shared by all plants of endosymbiotic interrelationship with fungi and bacteria, including Actinobacteria, from rhizosphere (Martin et al. 2017). Chitin-based signalling molecules secreted by symbiotic fungi and rhizobia, which are detected by receptor-like kinases, activate the signalling pathway of plant host, and this mechanism is common regardless of plant species, genus or family (Martin et al. 2017). That process, although similar to an infection, leads to bidirectional mutualistic benefits and also modifications of hormone activity and such organs as root development towards colonization by determined microorganisms (Martin et al. 2017). Establishing mutualistic interrelationships, such as mycorrhizae or bacteria-host plant relation, can be crucial in case of exposition to different environmental biotic and abiotic stress factors, enhancing plant resistance to metals and metalloids by sequestering them into roots and by protecting these other organs from translocation of uptaken toxic ions or molecules (Garg et al. 2015), on the other hand. It is worth to emphasize, that recently microbial symbionts interacting with plants were distinguished into two groups and are investigated as endophytes or rhizospheric microorganisms.
9.2 Microbial-Mediated Arsenic Transformations in Plant Symbionts
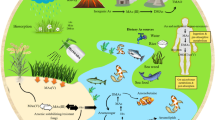
Amongst potentially harmful substances, arsenic (As) contamination is one of the major environmental problems affecting plants (Zhao et al. 2009; Gupta and Khan 2015; Latowski and Kowalczyk 2016). Its uptake by the plants causes damage to natural plant ecosystems and crops as well, ceasing by this continuity of agriculture production. Arsenic causes a variety of damages to nucleic acids, proteins, membranes and other cell compounds (Gupta and Khan 2015). In environment, there are different speciations of this element existing, including organic and inorganic compounds, and it is commonly known that those inorganic, such as arsenite and arsenate, are more toxic to living organisms, including plants (Gupta and Khan 2015; Latowski and Kowalczyk 2016). The toxic effect also depends on valency state of As in given compound (III state of oxidation in arsenite or V state of oxidation in arsenate). Arsenic toxicity effects to plants are mainly due to As(III) because As(V) is immediately reduced to As(III) by arsenate reductase when absorbed into the cell (Hu et al. 2013; Jia et al. 2014). On the other hand, in the external environment, which is rhizosphere, arsenite is oxidized to arsenate either by arsenite-oxidizing microbes (Hu et al. 2013). It was confirmed by several studies that As processing is strongly influenced by microbial activity in addition to chemical transformations in soils or water (Xu et al. 2007; Wang et al. 2011a, b; Hu et al. 2013; Jia et al. 2014; Han et al. 2017b). On the other hand, little is known about interactions between As-resistant plant endosymbionts and root and rhizosphere microbes, particularly on As oxidation and reduction in terms of plant uptake, acquisition, transformation and tolerance to As.
Arsenic transport into plant cells is facilitated, as it is recognized and bound by phosphate or silicon transporters. So far, there are no results explaining what are interactive effects of As and Si to plants (Hu et al. 2013; Schneider et al. 2013); however it was reported that presence of silicon facilitates forming iron plaque on root surface of rice and other wetland plants, thus building barrier for As uptake or space for transformations of As (oxidation/reduction reactions) (Awasthi et al. 2017). In plant roots, arsenate is quickly reduced to arsenite and can outflow back to the soil (Xu et al. 2007; Hu et al. 2013) where under aerobic conditions, it is oxidized rapidly again to arsenate by arsenite-oxidizing microbes. It is worth to notice that As(V) is much stronger bound with soil particles such as ferrihydrite, aluminosilicates or aluminium hydroxides than As(III), and thus oxidation of As(III) to Asin(V) is one of the most significant factors decreasing As bioavailability to plants. It is also known that iron plaque can bind As(V) even four times easier than As(III) (Blute et al. 2004). Studies on rice (Oryza sativa), grown on submerged areas deficient in oxygen, revealed that As uptake by roots is strongly dependent on microbial oxidization of As(III) to As(V) in combination with root radial oxygen loss (ROL) and availability of organic matter, such as addition of extra rice straw (Jia et al. 2014). Rice cultivars with higher ROL demonstrated lower As uptake than those with lower ROL. The enhancement of this rhizospheric effect on the abundance of the As(III) oxidase gene (aroA-like) was greater than on the As(V) reductase gene (arsC) and As(V) respiratory reductase gene (arrA). The direct effect was As(III) oxidation and sequestration in the rhizosphere, protecting the plant from the uptake of As(III), which intoxicate plant much easier than As(V). These rhizospheric processes, together with the addition of rice straw and growth stage dependently, influenced the rhizospheric population of bacteria, which therefore was dominated by α,- β- and γ-Proteobacteria. Mentioned proteobacteria inhabiting rhizosphere are both As(III)-oxidizing, with arsC and arrA gene representatives including Enterobacteriales, Rhizobiales, Sphingomonadales, Burkholderiales and Xanthomonadales, and also As(V)-reducing with aroA-like sequences, such as Phyllobacteriaceae, Bradyrhizobiaceae, Methylobacteriaceae, Rhizobiaceae, Bulkholderiaceae, Comamonadaceae and also seven unidentified mainly rhizospheric clusters (Jia et al. 2014). Recent studies on As hyperaccumulator fern, Pteris vittata, demonstrated that about 50% of the tissue microbiota consists of mentioned above α-, β- and γ-Proteobacteria species, but the half is dominated by Bacteroidetes and Firmicutes (Han et al. 2017a). The important role of the plant organism microbiota was proved e.g. by experiments performed by Mathews et al. in 2010, when under aerobic conditions after 24 h of incubation of 0.1 mM As(III) solution with unsterilized plant samples, considerable amount of the arsenite was oxidized to As(V), while oxidization was not observed in control without plants or with plants sterilized (Mathews et al. 2010).
9.3 Element Availability and Role of Siderophores
Plants and microorganisms involved in biogeochemical element cycles have to deal with reduced access to sufficient nutrients bound in forms of insoluble minerals (Sharma and Sohn 2009; Han et al. 2017b). It is commonly known that mycorrhizal interaction of fungi with plant brings to plant increase of water and nutrient uptake, which is significant concerning such macroelements like P or N, especially in low-fertility soils (Bais et al. 2006; Schneider et al. 2013; Garg et al. 2015).
In terrestrial environments P and As(V), which are chemically P homologue, often bind with Fe. Another factor limiting element availability to plants is pH; the more alkaline, the less mobile are metals and metalloids. P. vittata fern, a model plant organism which Han with the team broadly researches currently (Chen et al. 2016; Han et al. 2016a; Han et al. 2016b; Han et al. 2017a; Han et al. 2017b), as a hyperaccumulator of As had to evolve mechanisms to mobilize nutrients, and As as well, from insoluble minerals in rhizosphere soils and also translocation and detoxification pathways (Chen et al. 2016; Han et al. 2016b; Han et al. 2017b). Obtaining nutrients such as P or Fe by plants is possible, e.g. by excretion to rhizosphere substances such as organic acids, decreasing pH of the microenvironment and thus increasing the concentration of soluble P compounds (Han et al. 2017b; Liu et al. 2017a). Besides plant exudates, also microorganisms play a significant role in mineral solubilization (Han et al. 2017b). In soil different elements, including Fe and As, coexist. Under nutrient deficiency bacteria developed an efficient pathway of their harvest by producing siderophores, low-molecular-mass molecules enhancing Fe uptake (Liu et al. 2015; Han et al. 2017b; Liu et al. 2017a; Liu et al. 2017b) divided by chemical structure into three groups: catecholates, hydroxamates and carboxylates (Liu et al. 2016). The ability of elements such as phosphorus and metals or metalloid uptake and transformation is species- and genotype-specific, and this concerns microorganisms (including bacteria and fungi) and plants as well (Garg and Aggarwal 2012; Garg and Bhandari 2012). Siderophores besides other ions such as Cd2+, Cu2+, Ni2+, Pb2+, Zn2+, Mn3+, Co3+, Al3+, Th4+, U4+ and Pu4+ (Ahmed and Holmström 2014) release Fe3+, As5+ and P5+ from minerals, thus making them available and facilitating their uptake also to plant roots (Azeem et al. 2014; Han et al. 2016a). However, microbial activity within rhizome space and on the root surface can also determine species of As in iron plaque coating roots, i.e. in the direct vicinity of the plant tissues. Acidovorax and Hydrogenophaga genera colonizing iron plaque were reported to be involved in oxidation of As(III) bound within, decreasing uptake, hence total As concentration in the rice roots (from 30 mg kg−1 to about 1 mg kg−1) straw (from 8 mg kg−1 to 1 mg kg−1) and grain (from 23 mg kg−1, to 10 mg kg−1) (Hu et al. 2015). Ghosh with the team (Ghosh et al. 2011; Ghosh et al. 2015) proved that release of ions from iron(III) arsenate (FeAsO4) is observed, when As-resistant bacteria producing siderophores and isolated from P. vittata rhizosphere are present in the direct environment. What is more, some strains, like Pseudomonas PG12 isolated from studied fern and producing catecholate type of siderophores, can thus enhance biomass growth and are more efficient than fungal siderophores. Research performed by Liu et al. (2016) indicated that Pseudomonas PG012 siderophore was more effective in promoting FeAsO4 dissolution and Fe and As plant uptake, than fungal-siderophore desferrioxamine B (DFOB). Assays performed on P. vittata with DFOB demonstrated that DFOB treatment caused uptake and accumulation of mainly As(V) in roots inhibiting its reduction and transport to other organs, whereas bacterial PG12 siderophore treatment resulted in more efficient uptake of As(V) from soil and then its reduction to As(III) and translocation to rhizome and fronds (Liu et al. 2016). Accumulation of As(III) is typical for this fern (Wang et al. 2011a) and beneficial for potential phytoremediation, as above-ground organs are easy to remove from the ground surface. What is interesting, in pot experiments performed on pigeon pea (Cajanus cajan) and pea (Pisum sativum) under As stress (30 or 60 mg kg−1 dry soil), in which arbuscular mycorrhiza with Funneliformis mosseae was investigated in terms of protective for plant role towards As toxicity, demonstrated two important aspects of plant-microbial interactions. Besides beneficial effects of inoculation with mycorrhizal F. mosseae enhancing As tolerance of tested plants, the important role played rhizospheric bacteria Sinorhizobium fredii AR-4 (inoculated to pigeon pea) and Rhizobium leguminosarum bv. viciae strain PRH-1 (inoculated to pea). Those endophytic bacterial strains are reported to fix nitrogen and nodulate legumes (Mora et al. 2014). Pretreatment of sterilized seeds with mentioned strains facilitated fertilization of plants by fixing nitrogen from rhizosphere under stress conditions (As contamination). Interaction of inoculates with germinating seeds and forming organs resulted in increase of P, N and K uptake and, on the other hand, decrease of As uptake, thus diminishing its deleterious effect towards seedlings, as concentration of As after 75 days after sowing was up to about 22% lower in leaves and roots of As-treated plants comparing to uninoculated ones (Garg et al. 2015).
9.4 Plant Growth-Promoting Bacteria Under Arsenic Stress
It is commonly known that community of microbes inhabiting rhizosphere influence coexisting plants. There is a variety of phenomena and processes ranging from biochemical to ecological level, which indirectly or directly cause-effect to plant organisms. Recently research focus on beneficial aspects of them, as so far microorganisms were supposed to be mainly pathogens.
One of the protective roles of microorganisms colonizing rhizosphere is just occupying an environmental niche, potential habitat for pathogens. A consequence is competing and limiting indispensable nutrients, such as discussed P or Fe, for pathogen growth. Direct important benefit for plant organism is mitigation or even elimination of additional stress factor, what can be crucial to survive and develop in the environment with overlapping endangerments exposure. Rhizospheric bacteria can limit pathogen reproduction by synthesizing signal components, lytic enzymes, antibiotics or other toxins for potential pathogens and alter plant defence or induce mechanisms of resistance (Bais et al. 2006; Coats and Rumpho 2014). Several research proved that metal(loid)-resistant microorganisms colonizing rhizosphere and/or becoming plant endosymbionts, besides chemical transformation of molecules or ions thus being “alive targets”, can promote growth of plant (plant growth-promoting bacteria, PGPB) (Wang et al. 2011a, b; Liu et al. 2015; Han et al. 2016b; Liu et al. 2017b). Experimental studies on poplar (Populus deltoides), rice (O. sativa) and ferns P. vittata or Vigna radiata demonstrated that presence of symbiotic bacteria in rhizosphere or within plant tissues not only induces plant growth-promoting effect but also conduces As transformation and detoxification (Mathews et al. 2010; Liu et al. 2015; Han et al. 2016a; Singh et al. 2016; Batool et al. 2017; Liu et al. 2017b; Das and Sarkar 2018). Such parameters as germination percentage, biomass growth, chlorophyll, carotenoid and soluble protein or sugar content, indole-3-acetic acid (IAA) synthesis, the activities of ACC deaminase and oxidative stress enzymes like superoxide dismutase (SOD), catalase (CAT), peroxidase (POD) and the malondialdehyde (MDA) concentrations were controlled to determine As stress of plant organisms treated or untreated with different microbial isolates (Mathews et al. 2010; Zhu et al. 2014; Hu et al. 2015; Liu et al. 2015; Han et al. 2016a; Singh et al. 2016; Batool et al. 2017; Liu et al. 2017b).
Inoculation of poplar cuttings pot cultures with isolate Agrobacterium radiobacter D14 strain isolated from P. vittata rhizosphere contaminated with As resulted in an increase of soluble sugar content in leaves of plants from As-treated cultures in comparison with control assays without As although both types of plants were treated with tested bacterium strain. What is interesting, without inoculation with the D14 strain, the sugar content increased only at a low concentration of As (150 mg kg−1soil), whereas the activity of D14 in inoculated assays resulted in an increase of sugar content also at a high concentration of As (300 mg kg−1). Arsenic contamination caused a decrease of chlorophyll content, but in the presence of microorganisms, chlorophyll contents were higher compared with samples uninoculated (34). Both experiments on rice and poplar plants (Wang et al. 2011b; Pandey et al. 2013) demonstrated increased activity of investigated enzymes, except for POD in poplar tissues. POD activity in poplar was decreased along with the increasing concentration of As, despite inoculation with D14 (Wang et al. 2011b). In experiments on rice (O. sativa), inoculation of plants with As-resistant AsSP9 strain increased amylase and protease activity (from 0.001 mg/g d.w./min up to 0.002 for amylase and 0.002 to 0.0025 mg/g d.w./min for protease, respectively) (Pandey et al. 2013). In lower concentration of As, i.e. 150 mg kg−1 without D14 inoculation, SOD activity was increased in response to stress factor, but the higher the concentration of As in the environment, the higher the enzyme activity inhibition observed. However, inoculation with bacteria diminished the effect of As toxicity and facilitates SOD activity even under high concentration of As, i.e. 300 mg kg−1, whereas without bacteria under such condition, SOD was strongly inhibited (Wang et al. 2011b). What is important, the increased activity of enzymes was correlated with the increased percentage of germination and relative root elongation in presence of As. Moreover, biomass amount, i.e. dry mass weight and plant height in presence of endosymbiotic bacteria, was significantly increasing and was comparable with respective parameters measured in control plants grown without As. The most putative explanation of this mechanism is that the effective availability of the As is decreased by bacterial immobilization or exclusion which in a consequence reduces the inhibitory effect of this stress factor. What is more, this mechanism was observed only in the case of bacterial strains which are plant symbionts and As resistant, results obtained from experiments on non-symbiotic As-resistant species or strains did not demonstrate beneficial effect to investigated parameters of treated plants (Pandey et al. 2013).
Production of IAA by bacteria, causes auxin stimulated root cell division, which is an additional factor promoting the growth of a plant (Lehmann et al. 2010). It was reported that isolates of P. multifida and P. vittata are capable of synthesizing up to 36.5 mg L−1 and 18.5 mg L−1 of IAA, respectively (Zhu et al. 2014). Soil symbiont of chickpea (Cicer arietinum L.), Acinetobacter sp. nbri05 strain, produced IAA on level up to 60.93 μg mg−1 of fresh cell weight. These results allow concluding that additional content in a toxic environment of a compound stimulating root elongation facilitates plant development and on the other hand can therefore enhance and accelerate As uptake (Srivastava and Singh 2014), which may be useful in terms of application in phytoremediation.
The level of lipid peroxidation, which indicates stress factor exposure, is usually estimated by malondialdehyde (MDA) concentration which is the product of peroxidized polyunsaturated fatty acids of the lipid membrane (Kong et al. 2016). MDA concentration was measured in experiments performed on chickpea, pea, pigeon pea, poplar and rice treated with different concentrations of As(III) and As(V) and inoculated with microbial symbiotic organisms, bacteria and fungi as well (Wang et al. 2011b; Pandey et al. 2013; Srivastava and Singh 2014; Garg et al. 2015). The decrease of measured MDA content was observed in all cases if As-treated plants were inoculated. Each concentration of MDA determined in above-ground parts of plant, i.e. shoot for chickpea, pea and pigeon pea or leaves for poplar as well as in roots of rice, chickpea, pea, pigeon pea and poplar (details: Table 9.1), independently on concentration or state of oxidation of As provided in particular study, was significantly decreased (Wang et al. 2011b; Pandey et al. 2013; Srivastava and Singh 2014; Garg et al. 2015).
9.5 Concluding Remarks
Review of recent reports on microorganism-plant interactions showed that the cooperation between microbes and plants particularly under stress condition is beneficial or even indispensable. These interrelationships are intensively explored last years, starting from screening and discovering organisms involved in different interactions, via isolation of microbes, and their characteristics and analyses of their effect on plant physiology, ending with molecular signalling pathways underlying the interrelationship in terms of its beneficial role for both involved sides, however focusing on the plant. Research in this relatively novel topic joins interests of microbiology and plant biochemistry and physiology.
In all discussed cases, experiments comparing physiological and biochemical properties and/or activity of microbial inoculates in treated and in untreated plants exposed to As stress demonstrated, that the presence of As-resistant microorganisms brings irrefutable benefits to colonized plant (Table 9.1). Investigation of such parameters as total biomass of leaves, shoots and roots, as well as fronds or rhizomes (Pteris genus) of plant organism interacting with primarily bacteria but also fungi including yeasts under As exposure demonstrated that beyond plant or symbiotic microorganism genus/species, each interrelationship results in increase of physiological potential of studied plant to diminish efficiently deleterious As effect, survive and grow, comparing to plants uninoculated with microorganisms. In several cases studied plants even reached the extent of measured parameters comparable to controls without As and microorganisms.
It was proved that endosymbionts and beneficial rhizosphere bacteria support nutrient uptake by plant releasing such elements as P or Fe from insoluble minerals, thus enabling their sequestration (usually facilitated by siderophores) and transport into root tissue. Another benefit for the plant is that activity of microorganisms efficiently involved in oxidation/reduction of As(III)/As(V) reactions functionally decreases the concentration of As available for plant or contributes its efflux from tissues.
Biochemical analyses of enzyme activity increasing physiologically under stress conditions, such as SOD, CAT and POD, demonstrated that the activity of the enzymes protecting cells from oxidative stress, except for POD activity, increases with the concentration of As(III) or As(V) plant exposure; however when the concentration of As exceeds critical value (depending on species and other factors), inhibition of the activity starts. Colonization of rhizosphere and plant tissues with rhizospheric and endosymbiotic bacteria expands the range of the concentration and decreases the value of the concentration-deactivating enzyme. On the other hand, microorganisms efficiently synthesize compounds such as IAA or ACC deaminase, which support root cell division and nitrogen uptake by the plant, thus directly contributing to plant development. The biodiversity of microorganisms, amongst which there are representatives of bacteria and fungi including yeasts (Ramos-Garza et al. 2015), creating unique and specific for their host microbiomes indicate, that they are responsible for a variety of functions supporting plant organism like another higher organism.
Reviewed results reflect previous knowledge about the role of rhizospheric and endosymbiotic microbes of plants, especially in terms of As uptake, transformation and translocation within tissues and beneficial effect on plant physiology under stress conditions, such as As contamination, therefore changing the significance of those microorganisms towards plant organism, including human interests in terms of potential bio-phytoremediation application.
References
Ahmed E, Holmström SJM (2014) Siderophores in environmental research: roles and applications. Microb Biotechnol 7:196–208. https://doi.org/10.1111/1751-7915.12117
Awasthi S, Chauhan R, Srivastava S et al (2017) The journey of arsenic from soil to grain in rice. Front Plant Sci 8:1007. https://doi.org/10.3389/fpls.2017.01007
Azeem M, Riaz A, Chaudhary AN et al (2014) Microbial phytase activity and their role in organic P mineralization. Arch Agron Soil Sci 61:751–766. https://doi.org/10.1080/03650340.2014.963796
Bais HP, Weir TL, Perry LG et al (2006) The role of root exudates in rhizosphere interactions with plants and other organisms. Annu Rev Plant Biol 57:233–266. https://doi.org/10.1146/annurev.arplant.57.032905.105159
Batool K, Zahra tuz F, Rehman Y (2017) Arsenic-redox transformation and plant growth promotion by purple nonsulfur bacteria Rhodopseudomonas palustris CS2 and Rhodopseudomonas faecalis SS5. Bio Med Res Int 2017:1–8. https://doi.org/10.1155/2017/6250327
Blute NK, Brabander DJ, Hemond HF et al (2004) Arsenic sequestration by ferric iron plaque on cattail roots. Environ Sci Technol 38:6074–6077. https://doi.org/10.1021/es049448g
Bulgarelli D, Schlaeppi K, Spaepen S et al (2013) Structure and functions of the bacterial microbiota of plants. Annu Rev Plant Biol 64:807–838. https://doi.org/10.1146/annurev-arplant-050312-120106
Busby PE, Soman C, Wagner M et al (2017) Research priorities for harnessing plant microbiomes in sustainable agriculture. PLoS Biol 15(3):e2001793. https://doi.org/10.1371/journal.pbio.2001793
Chen Y, Fu JW, Han YH et al (2016) High As exposure induced substantial arsenite efflux in As-hyperaccumulator Pteris vittata. Chemosphere 144:2189–2194. https://doi.org/10.1016/j.chemosphere.2015.11.001
Coats VC, Rumpho ME (2014) The rhizosphere microbiota of plant invaders: an overview of recent advances in the microbiomics of invasive plants. Front Microbiol 5:368. https://doi.org/10.3389/fmicb.2014.00368
Das J, Sarkar P (2018) Remediation of arsenic in mung bean (Vigna radiata) with growth enhancement by unique arsenic-resistant bacterium Acinetobacter lwoffii. Sci Total Environ 624:1106–1118. https://doi.org/10.1016/j.scitotenv.2017.12.157
Garg N, Aggarwal N (2012) Effect of mycorrhizal inoculations on heavy metal uptake and stress alleviation of Cajanus cajan (L.) Millsp. Genotypes grown in cadmium and lead contaminated soil. Plant Growth Regul 66:9–26. https://doi.org/10.1007/s10725-011-9624-8
Garg N, Bhandari P (2012) Influence of cadmium stress and arbuscular mycorrhizal fungi on nodule senescence in Cajanus cajan (L.) Millsp. Int J Phytoremediation 14:62–74. https://doi.org/10.1080/15226514.2011.573822
Garg N, Singla P, Bhandari P (2015) Metal uptake, oxidative metabolism, and mycorrhization in pigeonpea and pea under arsenic and cadmium stress. Turk J Agric For 39:234–250. https://doi.org/10.3906/tar-1406-121
Ghosh P, Rathinasabapathi B, Ma LQ (2011) Arsenic-resistant bacteria solubilized arsenic in the growth media and increased growth of arsenic hyperaccumulator Pteris vittata L. Bioresour Technol 102:8756–8761. https://doi.org/10.1016/j.biortech.2011.07.064
Ghosh P, Rathinasabapathi B, Ma LQ (2015) Phosphorus solubilization and plant growth enhancement by arsenic-resistant bacteria. Chemosphere 134:1–6. https://doi.org/10.1016/j.chemosphere.2015.03.048
Gupta M, Khan E (2015) Mechanism of arsenic toxicity and tolerance in plants: role of silicon and signalling molecules. In: Tripathi B, Müller M (eds) Stress responses in plants. Springer, Cham, pp 143–157. https://doi.org/10.1007/978-3-319-13368-3_6
Han YH, Fu JW, Chen Y (2016a) Arsenic uptake, arsenite efflux and plant growth in hyperaccumulator Pteris vittata: role of arsenic-resistant bacteria. Chemosphere 144:1937–1942. https://doi.org/10.1016/j.chemosphere.2015.10.096
Han YH, Yang GM, Fu JW et al (2016b) Arsenic-induced plant growth of arsenic-hyperaccumulator Pteris vittata: impact of arsenic and phosphate rock. Chemosphere 149:366–372. https://doi.org/10.1016/j.chemosphere.2016.01.118
Han YH, Jia MR, Liu X et al (2017a) Bacteria from the rhizosphere and tissues of As-hyperaccumulator Pteris vittata and their role in arsenic transformation. Chemosphere 186:599–606. https://doi.org/10.1016/j.chemosphere.2017.08.031
Han YH, Liu X, Rathinasabapathi B (2017b) Mechanisms of efficient As solubilization in soils and As accumulation by As-hyperaccumulator Pteris vittata. Environ Pollut 227:569–597. https://doi.org/10.1016/j.envpol.2017.05.001
Hu H, Zhang J, Wang H et al (2013) Effect of silicate supplementation on the alleviation of arsenite toxicity in 93–11 (Oryza sativa L.). Environ Sci Pollut Res 20:8579–8589. https://doi.org/10.1007/s11356-013-1811-x
Hu M, Li F, Liu C et al (2015) The diversity and abundance of As(III)-oxidizers on root iron plaque is critical for arsenic bioavailability to rice. Sci Rep 5:13611. https://doi.org/10.1038/srep13611
Jia Y, Huang H, Hen Z et al (2014) Arsenic uptake by rice is influenced by microbe-mediated arsenic redox changes in the rhizosphere. Environ Sci Technol 48:1001–1007. https://doi.org/10.1021/es403877s
Kong W, Liu F, Zhang C et al (2016) Non-destructive determination of malondialdehyde (MDA) distribution in oilseed rape leaves by laboratory scale NIR hyperspectral imaging. Sci Rep 14:35393. https://doi.org/10.1038/srep35393
Latowski D, Kowalczyk A (2016) Biochemical pathways of arsenic uptake from the environment to human cells. Mol Biophys Biochem 1:9–16. https://doi.org/10.15761/MBB.1000103
Lehmann T, Hoffmann M, Hentrich M et al (2010) Indole-3-acetamide-dependent auxin biosynthesis: a widely distributed way of indole-3-acetic acid production? Eur J Cell Biol 89:895–905. https://doi.org/10.1016/j.ejcb.2010.06.021
Liu X, Yang GM, Guan DX et al (2015) Catecholate-siderophore produced by as-resistant bacterium effectively dissolved FeAsO4 and promoted Pteris vittata growth. Environ Pollut 206:376–381. https://doi.org/10.1016/j.envpol.2015.07.034
Liu X, Fu JW, Guan DX et al (2016) Arsenic induced phytate exudation, and promoted FeAsO4 dissolution and plant growth in As-hyperaccumulator Pteris vittata. Environ Sci Technol 50:9070–9077. https://doi.org/10.1021/acs.est.6b0066
Liu X, Fu JW, Da Silva E et al (2017a) Microbial siderophores and root exudates enhanced goethite dissolution and Fe/As uptake by As-hyperaccumulator Pteris vittata. Environ Pollut 223:230–237. https://doi.org/10.1016/j.envpol.2017.01.016
Liu X, Fu JW, Tang N et al (2017b) Phytate induced arsenic uptake and plant growth in arsenic-hyperaccumulator Pteris vittata. Environ Pollut 226:212–218. https://doi.org/10.1016/j.envpol.2017.04.021
Mallick I, Bhattacharyya C, Mukherji S et al (2018) Effective rhizoinoculation and biofilm formation by arsenic immobilizing halophilic plant growth promoting bacteria (PGPB) isolated from mangrove rhizosphere: a step towards arsenic rhizoremediation. Sci Total Environ 610:1239–1250. https://doi.org/10.1016/j.scitotenv.2017.07.234
Martin F, Kohler A, Murat C et al (2016) Unearthing the roots of ectomycorrhizal symbioses. Nat Rev Microbiol 14(12):760–773. https://doi.org/10.1038/nrmicro.2016.149
Martin FM, Uroz S, Barker DG (2017) Ancestral alliances: plant mutualistic symbioses with fungi and bacteria. Science 356:eaad4501. https://doi.org/10.1126/science.aad4501
Mathews S, Ma LQ, Rathinasabapathi B et al (2010) Arsenic transformation in the growth media and biomass of hyperaccumulator Pteris vittata L. Bioresour Technol 101:8024–8030. https://doi.org/10.1016/j.biortech.2010.05.042
Mora Y, Díaz R, Vargas-Lagunas C (2014) Nitrogen-fixing rhizobial strains isolated from common bean seeds: phylogeny, physiology, and genome analysis. Appl Environ Microbiol 80:5644–5654. https://doi.org/10.1128/AEM.01491-14
Müller B, Vogel C, Bai Y et al (2016) The plant microbiota: systems-level insights and perspectives. Annu Rev Genet 50:211–234. https://doi.org/10.1146/annurev-genet-120215-034952
Pandey S, Ghosh PK, Ghosh S et al (2013) Role of heavy metal resistant Ochrobactrum sp. and Bacillus spp. strains in bioremediation of a rice cultivar and their PGPR like activities. J Microbiol 51:11–17. https://doi.org/10.1007/s12275-013-2330-7
Ramos-Garza J, Bustamante-Brito R, Ángeles de Paz G et al (2015) Isolation and characterization of yeasts associated with plants growing in heavy-metal- and arsenic-contaminated soils. Canadian J Microbiol 62:307–319. https://doi.org/10.1139/cjm-2015-0226
Schneider J, Stürmer SL, Guilherme LR et al (2013) Arbuscular mycorrhizal fungi in arsenic-contaminated areas in Brazil. J Hazard Mater 262:1105–1115. https://doi.org/10.1016/j.jhazmat.2012.09.063
Sharma VK, Sohn M (2009) Aquatic arsenic: toxicity, speciation, transformations, and remediation. Environ Int 35:743–759. https://doi.org/10.1016/j.envint.2009.01.005
Singh N, Marwa N, Mishra SK et al (2016) Brevundimonas diminuta mediated alleviation of arsenic toxicity and plant growth promotion in Oryza sativa L. Ecotoxicol Environ Saf 125:25–34. https://doi.org/10.1016/j.ecoenv.2015.11.020
Srivastava S, Singh N (2014) Mitigation approach of arsenic toxicity in chickpea grown in arsenic amended soil with arsenic tolerant plant growth promoting Acinetobacter sp. Ecol Eng 60:146–153. https://doi.org/10.1016/j.ecoleng.2014.05.008
Wang X, Ma LQ, Rathinasabapathi B et al (2011a) Mechanisms of efficient arsenite uptake by arsenic hyperaccumulator Pteris vittata. Environ Sci Technol 45:9719–9725. https://doi.org/10.1021/es2018048
Wang Q, Xiong D, Zhao P et al (2011b) Effect of applying an arsenic-resistant and plant growth-promoting rhizobacterium to enhance soil arsenic phytoremediation by Populus deltoides LH05-17. J Appl Microbiol 111:1065–1074. https://doi.org/10.1111/j.1365-2672.2011.05142.x
Xu XY, McGrath SP, Zhao FJ (2007) Rapid reduction of arsenate in the medium mediated by plant roots. New Phytol 176:590–599. https://doi.org/10.1111/j.1469-8137.2007.02195.x
Zgadzaj R, Garrido-Oter R, Jensen D et al (2016) Root nodule symbiosis in Lotus japonicus drives the establishment of distinctive rhizosphere, root, and nodule bacterial communities. Proc Natl Acad Sci U S A 113:7996–8005. https://doi.org/10.1073/pnas.1616564113pmid:27864511
Zhao FJ, Ma JF, Meharg AA et al (2009) Arsenic uptake and metabolism in plants. New Phytol 181:777–794. https://doi.org/10.1111/j.1469-8137.2008.02716.x
Zhu LJ, Guan DX, Luo J et al (2014) Characterization of arsenic-resistant endophytic bacteria from hyperaccumulators Pteris vittata and Pteris multifida. Chemosphere 113:9–16. https://doi.org/10.1016/j.chemosphere.2014.03.081
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Kowalczyk, A., Latowski, D. (2018). Role of Plant-Microorganism Interactions in Plant Tolerance to Arsenic. In: Hasanuzzaman, M., Nahar, K., Fujita, M. (eds) Mechanisms of Arsenic Toxicity and Tolerance in Plants. Springer, Singapore. https://doi.org/10.1007/978-981-13-1292-2_9
Download citation
DOI: https://doi.org/10.1007/978-981-13-1292-2_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-1291-5
Online ISBN: 978-981-13-1292-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)