Abstract
Chitosan, a deacetylated chitin, is one of the few natural polymers similar to glycosaminoglycans (GAGs) widely distributed throughout connective tissues. It has been believed that the excellent biocompatibility of chitosan is largely attributed to this structural similarity. Chitosan is also known to possess biodegradability, antimicrobial activity and low toxicity and immunogenicity which are essential for scaffolds. In addition, the existence of free amine groups in its backbone chain enables further chemical modifications to create the additional biomedical functionality. For these reasons, chitosan has found a tremendous variety of biomedical applications in recent years. This chapter introduces the basic contents of chitosan and discusses its applications to artificial skin, artificial bone, and artificial cartilage in tissue engineering purpose.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Scaffolds
Tissue engineering is an emerging multidisciplinary approach that incorporates biology, medicine and engineering [7]. As a field of study, the discipline of tissue engineering aims to understand the relationship between structure and function in cell and tissue and to develop biological substitutes that can repair or replace the dead or damaged tissues, organs and/or parts of the human body. The success of tissue engineering may depend on a harmonious interplay of three components; cells for neo-tissue formation; biomaterials to act as scaffolds; biological signaling molecules that instruct cells to form desired tissue type [51]. Among the components, scaffolds play a pivotal role in the field of modern regenerative medicine, because they provide an architectural context in which cells and growth factors can cooperate and represent a wide range of morphological and geometric possibility for suitable clinical application [32]. So far, many biomaterials of natural and synthetic origin have been adapted for the manufacture of scaffolds with various fabrication techniques to create three-dimensional (3-D) environment mimicking extracellular matrix (ECM) [6, 70], many of which have been the subject of practical development efforts [16, 58]. As natural polymers, collagen and hyaluronic acid can meet the several requirements for scaffold, therefore, have been extensively studied and currently being employed in clinical trials [8, 55]. However, it is crucial that there exists the imbalance between supply and demand in natural polymers because of natural inconsistency in the in vivo source; the lot-to-lot variability is always a concern [24]. The additional drawbacks of natural polymers could be the potential impurities that may result in unwanted immune reaction and the difficulties in control mechanical properties [35, 55]. Meanwhile, the main advantage of synthetic polymers over natural polymers is the suffcient availability on demand with constant quality supporting industrial-scale production. Therefore, numerous attempts have been made to use synthetic biodegradable polyesters, such as polylactic acid (PLA), polyglycolic acid (PGA) and their copolymer (PLGA) as the substitute for natural polymeric scaffolds, however, their lack of cell recognition site for cell adhesion, migration and subsequent cellular behaviors often limits applications [32, 65, 69]. Consequently, both natural and synthetic materials have their own merits and demerits have to be complemented.
2 Chitosan
In addition to collagen and hyaluronic acid, a candidate of interest as natural polymeric material for scaffold preparation would be chitin and chitosan. Chitin is the second abundant biopolymer on earth, exceeded only by cellulose [15]. Chitin can be found widely in the exoskeletons of arthropods, shells of crustaceans, and the cuticles of insects [18]. Chitosan, a deacetylated chitin, is one of the few natural polymers that has free amine groups in its backbone chain, thus has the characteristics of a polymeric hydrogel owing to a high water absorption capacity [34]. It is also known to possess biodegradability, antimicrobial activity and low toxicity and immunogenicity which are essential for scaffolds [29, 67]. For these reasons, chitosan has found a tremendous variety of biomedical applications in recent years.
2.1 Chemical Structure
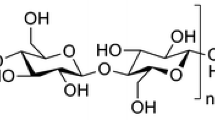
Chitosan, produced by deacetylation of chitin, is a linear polysaccharide composed of β-(1→4)-linked D-glucosamine and N-acetyl-D-glucosamine. The deacetylation process of chitin can not only control degree of deacetylation (DD) but also change the average molecular weight of chitosan. In general, the weight-average molecular weight (Mw) of chitin is in the range from 1.03 to 2.5 × 106 g/mole, but the deacetylation process of chitin results in reduced Mw of chitosan to range from 1 to 5 × 105 g/mole [62]. Despite the loss in molecular weight of polymer, the main reason for manufacturing chitosan can be the poor solubility of chitin.
In the beginning, because the chemical structure of chitin is very similar to that of cellulose, the studies on solvents for chitin have been carried out together with the development of cellulose. Chitin is a long chain polysaccharide, like cellulose, that shows the degree of polymerization around 7000~15,000 [66]. The inter- and intra-molecular hydrogen bond due to the presence of acetyl amino and hydroxyl bond makes chitin highly aggregated and insoluble in most of organic solvents. The solvents for chitin reported by far include the concentrated salt solutions such as LiCNS, Ca(CNS)2, CaI2, the strong acids such as HCl, H2SO4 and H3PO4 and other kinds of acids containing carboxylic group such as formic acid, dichloroacetic acid, and trichloroacetic acid, however, in most cases chitin showed very slow dissolution rate accompanied by severe level of molecular decomposition [64]. Recently, N,N-dimethylacetamide, N-methyl-2-pyrollidone and their mixture in the presence of 5% LiCl are known to be a suitable solvent system for cellulose [76]. The main principle is similar to cellulose xanthate, that is Li+ ions formed in DMA and NMP solutions bind to the hydroxyl group of cellulose to break the original strong interactions between cellulose chains resulting in dissolution. The same system has been used to solubilize chitin, however, there still exist number of problems awaiting solutions [14, 66]. Chitosan, on the other hand, is easily dissolved in a dilute acid solution in the form of an ammonium salt and has functionality of amino groups, primary and secondary hydroxyl groups for further chemical modifications [5].
2.2 Nomenclature
Because deacetylated unit (D-glucosamine) and acetylated unit (N-acetyl-D-glucosamine) is randomly distributed in chitosan, and because the composition of two residues is entirely dependent upon deacetylation process, nomenclature of chitosan is still controversial. A group of deacetylated chitin whose D-glucosamine residues over 50% (or 60%) is often referred to as chitosan, however, there is no boundary in the nomenclature distinguishing chitin from chitosan [23]. This misunderstanding is probably caused by the fact that the % of DD in commercial chitosan ranges from 60 to 99%. As mentioned above, the important factor in naming ‘chitin or chitosan’ is the solubility in dilute aqueous acid solutions. That is, regardless of the % of DD, if a deacetylated chitin is insoluble, it cannot be classified into chitosan [64]. In addition to DD, the Mw of chitosan is another important characterization parameter because the application field of chitosan can be widely varied with the distribution of Mw. For biological and functional applications of chitin and chitosan, the international official standard methods to determine DD and Mw of chitin and chitosan, ASTM F2260–03 and ASTM F2606–13, have been provided to researchers and manufacturers.
2.3 Distribution of N-Acetyl-D-Glucosamine and D-Glucosamine Units
From chemical point of view, either acids or alkalis can be used to deacetylate chitin, however, alkaline deacetylation is preferred, because glycosidic bonds are very susceptible to acid. As the alkaline deacetylation of chitin, either heterogeneous or homogeneous hydrolysis has been being employed. Heterogeneous hydrolysis employs the severe conditions with hot concentrated NaOH solution within few hours. By this heterogeneous hydrolysis, the deacetylated chitin whose DD up to 80% can be obtained, but they are insoluble. On the contrary, homogeneous hydrolysis using very mild condition at 25 °C of deacetylation temperature produces a soluble chitosan, even though the range of DD is 48–55 [36]. This can be attributed that deacetylation reaction performed under heterogeneous conditions gives an irregular distribution of N-acetyl-d-glucosamine and d-glucosamine residues with some block-wise acetyl group distribution along polymeric chains [2]. Thus, solubility and degree of aggregation of chitosan can vary in aqueous solutions leading to changes in their native characteristics. For instance, physico-chemical properties of such chitosans may differ from those of randomly acetylated chitosans obtained under homogeneous conditions.
2.4 Biocompatible Factors
In addition to good solubility, chitosan has a variety of biocompatible factors compared to chitin. The chemical structure of chitosan is very close to hyaluronic acid, the fourth class and non-sulfated GAG widely distributed throughout connective tissues. It has been believed that the excellent biocompatibility of chitosan is largely attributed to this structural similarity, therefore, numerous attempts have been made to prepare chitosan based scaffolds for tissue engineering applications [37].
The biodegradability is an essential factor for scaffold preparation because the degradation of scaffold material is a very important process in the tissue remodeling. In the case of chitosan, lysozyme plays a leading role in degradation in vivo, and degradation rate is inversely proportional to the degree of crystallinity, which is greatly influenced on DD [73]. Ren et al. reported that each reacetylated chitosan matrices with deacetylation degree of 52.6%, 56.1%, and 62.4% has weight half-lives of 9.8 days, 27.3 days, and above 56 days, respectively, with mean molecular weights of 8.4%, 8.8%, and 20.0%, respectively. They also reported that each reacetylated chitosan matrices with deacetylation degree of 71.7%, 81.7%, and 93.5% has slow degradation rates, and half-lives of above 84 days both weight and average molecular weight [63].
When chitosan is dissolved, the free amine group of chitosan chain becomes charged as positive, in turn produce the dielectric interactions with negatively charged biologics including the growth factors and the cytokines. The primary amine group can also be utilized as the coupling site for conjugation with biologics in order to build stable interaction. These modifications provide further improvements to chitosan in its biomedical applications [48].
Chitosan is largely known to have a broad antimicrobial activity to which gram-positive, gram-negative and fungi are highly susceptible [61]. Although the precise mechanism for this action has not fully established yet, the most acceptable antimicrobial mechanism includes the presence of positively charged groups in backbone chain and their interactions to negatively charged bacterial wall. This ionic interaction leads the changes in cytoplasmic permeability of bacteria, results in cell death. Chitosan, however, shows its antibacterial activity only in acidic circumstances because of its poor solubility above pH 6.5. In this regards, Kim originally produced the water soluble chitosan derivatives with ammonium salts and showed their broader spectra of antimicrobial activities [30].
3 Tissue Engineering Applications
For the construction of tissue-engineered organ, three main elements are required; the scaffold, a source of cells and the bio-signaling. 3-D scaffold with various forms takes a role of ECM that function as structural templates for tissue regeneration. For this purpose, the scaffold should have adequate porosity and morphology for transporting of cells, gases, metabolites, nutrients and signal molecules both within the scaffold and between the scaffold and the local environment. In the scaffold with higher porosity and pore size, efficient nutrient supply, diffusion of gas and secretion of metabolites are promoted, however, the interactions between cell-cell become decrease because of low cell attachment. In contrast, lower porosity and pore size results in adverse effects [72]. Therefore, it is necessary to produce scaffolds with appropriate pore size distribution and porosity depending on the cells and tissues.
By virtue of good solubility, chitosan can be manufactured into various forms of scaffolds including fibers, sponges and hydrogels. Madihally prepared chitosan scaffolds of controlled microstructure in several tissue-relevant geometries using freezing and lyophilization technique [48]. Mean pore diameters could be controlled within the range of 1–250 μm. This could be a starting point for design and preparation of chitosan based scaffold materials. Years later, 3-D interconnected open porous chitosan scaffold with controlled pore distribution was prepared [10]. Alcohols were used as non-solvent to induce the liquid-liquid and liquid-solid phase separation via demixing solution. This method enabled to produce the controlled homogeneous micropores and the improved interconnectivity between pores with minimum surface skin layer formation. This interconnectivity of chitosan scaffold provided the efficient transportation of the substances for cell, therefore, enhanced adhesion as well as proliferation rates of fibroblasts around two folds.
In the meantime, the modifications with ECM components or growth factors to chitosan based scaffolds have been conducted to further increase cell adhesion, proliferation and differentiation through modulation of cellular responses [13, 53]. As the major ECM protein, collagen has been used to enhance cell adhesion to chitosan scaffold in the form of blender of two polymers [49]. Fibronectin as well as laminin have been employed to chitosan for mimicking the biological function of the ECM through immobilization or carbodiimide based crosslinking [12, 27]. Instead of using these macromolecules, there also have been other attempts to make use of small adhesive molecules such as motifs. Many research groups including Ho and Hansson have functionalized chitosan scaffold with arginine-glycine-aspartic acid (RGD) and showed successful cell-scaffold interactions [20, 22].
Proteins and glycoprotein, collagen, laminin and fibronectin, and their amino acid sequence such as RGD, GFOGER and so on are all known to induce cell adhesion and migration through integrin mediated focal adhesion, rather than proliferation and differentiation [21, 38, 68]. There exist, in deed, numerous report that scaffold with ECMs or motifs increases cell proliferation and differentiation, however, the elements that dominate these cellular events are growth factors and cytokines related to receptor tyrosine kinases (RTKs) signaling pathway [41]. A comparative study of cell adhesive peptide and growth factor using chitosan based scaffold also showed the same consequences as mentioned above. Tiğli prepared two kinds of chitosan based scaffolds modified either with RGD or epidermal growth factor (EGF), and found the proliferation trend of ATDC5 murine chondrogenic cells on EGF-chitosan was superior compared to chitosan and RGD-chitosan; although, there was no significant effect on cell attachment [71]. Hence, various types of growth factors including basic fibroblast growth factor (bFGF), transforming growth factor-β1 (TGF-β1), platelet-derived growth factor-BB (PDGF-BB), and epidermal growth factor (EGF) have been currently introduced to chitosan based tissue engineering scaffold for skin, cartilage and bone [33, 34, 42, 71, 77].
3.1 Skin
Numerous efforts have been made to develop chitosan based skin substitute because chitosan may play a key role in wound healing phases: blood clotting, inflammation, tissue growth and remodeling. First of all, chitosan has very strong hemostatic activity which is independent on the classical coagulation cascade [60, 78]. Polycations of chitosan bind with host plasmas, cells and tissues inherently charged as negative when come in contact to traumatic wounds. This includes RBCs agglutination, that is, positively charged glucosamine on chitosan strongly attracts negatively charged RBCs to agglutinate; therefore, produce instantaneous clotting together with plasma sorption. The systemic hemostasis activation through platelet adhesion, aggregation and activation follows this fast clot formation. So far, more than 10 chitosan based wound dressing materials including HemCon®, Chitoflex® and Chitoseal® have been commercialized and used as hemostatic dressing [60].
Inflammation is a protective response to eliminate the cause of injury, clear out necrotic cells and tissues through the process of phagocytosis, in turn initiates tissue repair [17]. During proliferation, the factors for tissue regeneration such as, angiogenesis, collagen deposition, granulation and epithelialization occur [52]. Among the cells involved in wound healing process, macrophages may perform indispensable functions in inflammation as well as tissue repair [44, 54]. As a host defender, macrophages recognize and destroy foreign organisms, debride dead and damaged tissue components (classical activation, M1), and produce cytokines, growth factors, and angiogenic factors, which regulate tissue growth and remodeling (alternative activation, M2) [46]. An important point regarding macrophages function is that chitosan induces both classical and alternative activation in macrophages by the receptor mediated stimulatory effect of chitosan in macrophages, suggesting that chitosan can be one of the functional biomaterials that are responsible for wound healing [26, 74]. Therefore, chitosan scaffolds with various forms that include cross-linked hydrogels, nano-fibrous structures, ion-etched films and so on, fabricated and applied to traumatic or burn wound [1, 28, 47].
In tissue engineering, the focal adhesion is the primary requirement in which cells are communicated. In the case of chitosan, the increase in the content of free amine group increases the attachment of fibroblast but rather decreases the migration and the proliferation [9]. This implies that strong electrostatic interaction between cells and free amine groups in chitosan hiders the cell attachment through the focal adhesion. Kim et al. [31] leveled down this electrostatic property and improved biocompatibility of chitosan through the rigorous dry heat treatment at 110 °C. They had controlled the DD of chitosan based scaffold from 85 to 30% with increase heat treatment time.
The poor focal adhesion capability of chitosan can be enhanced by the addition of ECM components. Ma et al. [45] prepared porous scaffold with the mixture of collagen and chitosan, and found good cytocompatibility to effectively accelerate cell infiltration and proliferation. In addition, much attention has been focused on the use of the growth factor functionalized and/or cell based skin graft. Obara et al. [56] and Alemdaroğlu et al. [3] prepared FGF-2 and EGF incorporated chitosan hydrogel, respectively, and most recently, Yang et al. [74] produced dual growth factors releasing chitosan based hydrogels for accelerated wound healing. Altman et al. [4] had seeded human adipose derived stem cells on chitosan based scaffold and transplanted to wound bed using a murine soft tissue injury model. They found Green Fluorescent Protein (GFP)-positive stem cells on chitosan scaffolds have differentiated into variety of lineages for soft tissue restoration including fibrovascular, endothelial and epithelial cells up to 4 weeks.
3.2 Bone
For bone regeneration, hydroxyapatite (HA) and/or tricalcium phosphate (TCP) have been widely employed with polymeric scaffolds because of their unique osseointegrative properties. Lee et al. [40] prepared platelet-derived growth factor (PDGF) loaded chitosan/TCP sponge type scaffold and implanted calvarial defect of rat. The results showed that the addition of PDGF to the scaffold further enhanced bone regeneration. In order to treat large scale bone defect, Ge et al. [19] proposed chitin-HA composite scaffold as a promising candidate to form a structural framework for bone regeneration. They have demonstrated that chitin-HA scaffold provided many requirements for bone tissue regeneration by responding physiological and biological changes and by remodeling the ECM to integrate with surrounding tissue.
Recently, liquid phase chitosan has gained popularity as an injectable scaffold to carry osteoinductive and/or osteoconductive material and to fill out bone defect area for minimally invasive technique. Liu et al. [43] prepared a novel injectable bone substitute material consists of chitosan solution as the liquid phase and TCP powder as the solid phase. The mixture of two components became bone cement upon immersion in SBF, and showed good compressive strength, bioactivity and cytocompatibility enough to have prospect for orthopedic applications. As another approach of injectable scaffold, Park et al. [59] have produced chitosan/alginate based composite that carries recombinant human bone morphogenetic protein-2 (BMP-2) with mesenchymal stem cells and subcutaneously transplanted into the space on the dorsum of nude mice. They have found the trabecular type new bone formation and concluded that this chitosan/alginate composite could become clinically useful injectable scaffold.
3.3 Cartilage
In tissue engineering of articular cartilage, the round morphology of chondrocyte represents the maintenance of differentiated chondrocytic phenotype. However, this phenotype is unstable in culture, because chondrocytes may undergo de-differentiation that involves gradual shift from the synthesis of type II to type I and III collagen, in turn provides the inferior fibrocartilaginous circumstances [75]. This is the major restriction to form hyaline cartilage in cell therapy for repair full thickness destructive cartilage. Therefore, the ideal scaffold that closely mimics the naturally occurring environment in the cartilage matrix is required to stimulate and support chondrogenesis in vitro and in vivo. GAGs are known to stimulate the chondrogenesis, therefore, use of chitosan as an analog of GAG appears to be ideal for scaffold material of chondrogenesis. In this regard, Lahiji et al. [37] and Iwasaki et al. [25] hypothesized that chitosan based scaffold can support the function and expression of ECM components in chondrocytes, and demonstrated that chitosan leads chondrocytes to have continued expression of collagen II and to maintain their characteristic round morphology. Cui et al. [11] used chitosan to modify poly (L-lactic acid), biodegradable aliphatic polyester, for the purpose of improving cytocompatibility. The bovine articular cartilage chondrocytes cultured on the chitosan modified surface showed beneficial effects on adhesion, proliferation and function. Oliveira et al. [57] have designed and prepared a novel HA/chitosan based bilayered hybrid scaffold using a combination method of sintering and a freeze-drying technique for osteochondral tissue-engineering applications. Both HA and chitosan layer provided an adequate support for osteogenecity and chodrogenecity to seeded MSCs, respectively. Chitosan have been also employed to deliver the growth factors and morphogenetic proteins for further enhanced chondrogenesis in the field of cartilage engineering ([33, 34, 39, 50]).
4 Future Perspective
With rapid advances and developments of modern sciences and technologies, a new era in tissue engineering and regenerative medicine where scientists with different backgrounds work together to cope with their multidisciplinary has established. For decades, a remarkable achievement has been made to take a major step forward to regenerate skin, cartilage, bone, liver and nervous system. As the second abundant biopolymer on earth, chitosan has also been widely applied to tissue engineering because of its biodegradability, antimicrobial activity and low toxicity and immunogenicity which are essential for scaffolds. However, there still remain problems. Chitosan, similar to the other natural products, has brittleness that limits its practical application; therefore, further efforts are needed to improve mechanical strength. Regarding most of studies using chitosan have been carried out in vitro, the additional comprehensive studies using animal models are required to figure out the precise relationship between chitosan and cells or tissues of various organs, Fortunately, HemCon Medical Technologies of the United States commercialized the chitosan based hemostatic bandages for military and emergency use, and hemostatic agents for dentistry. In canada, Biosyntech developed chitosan based injectable hydrogels, for skin (BST-DermOn), for cartilage (BST-CarGel) and for bone (BST-Ossifil). They are all in clinical trials for FDA approval. These activities truly lead chitosan based scaffolds to a step closer to the practical applications for tissue engineering purpose.
References
Ahmadi F, Oveisi Z, Samani SM, Amoozgar Z (2015) Chitosan based hydrogels: characteristics and pharmaceutical applications. Res Pharm Sci 10(1):1–16
Aiba S-I (1991) Studies on chitosan: 3. Evidence for the presence of random and block copolymer structures in partially N-acetylated chitosans. Inter J Biol Macromol 13(1):40–44. https://doi.org/10.1016/0141-8130(91)90008-I
Alemdaroğlu C, Değim Z, Celebi N, Zor F, Oztürk S, Erdoğan D (2006) An investigation on burn wound healing in rats with chitosan gel formulation containing epidermal growth factor. Burns 32(3):319–327. https://doi.org/10.1016/j.burns.2005.10.015
Altman AM, Yan Y, Matthias N, Bai X, Rios C, Mathur AB, Song YH, Alt EU (2009) IFATS collection: human adipose-derived stem cells seeded on a silk fibroin-chitosan scaffold enhance wound repair in a murine soft tissue injury model. Stem Cells 27(1):250–258. https://doi.org/10.1634/stemcells.2008-0178
Athanasiou KA, Shah AR, Hermandez RJ, LeBaron RG (2001) Basic science of articular cartilage repair. Clin Sports Med 20(2):223–247. https://doi.org/10.1016/S0278-5919(05)70304-5
Barnes CP, Sell SA, Boland ED, Simpson DG, Bowlin GL (2007) Nanofiber technology: designing the next generation of tissue engineering scaffolds. Adv Drug Deliv Rev 59(14):1413–1433. https://doi.org/10.1016/j.addr.2007.04.022
Bernhard P (2003) Tissue engineering. CRC Press, Boca Raton
Bowman S, Awad ME, Hamrick MW, Hunter M, Fulzele S (2018) Recent advances in hyaluronic acid based therapy for osteoarthritis. Clin Transl Med 7:6. https://doi.org/10.1186/s40169-017-0180-3
Chatelet C, Damour O, Domard A (2001) Influence of the degree of acetylation on some biological properties of chitosan films. Biomaterials 22(3):261–268. https://doi.org/10.1016/S0142-9612(00)00183-6
Chun HJ, Kim G-W, Kim C-H (2008) Fabrication of porous chitosan scaffold in order to improve biocompatibility. J Phys Chem Solids 69(5–6):1573–1576. https://doi.org/10.1016/j.jpcs.2007.10.104
Cui YL, Qi AD, Liu WG, Wang XH, Wang H, Ma DM, Yao KD (2003) Biomimetic surface modification of poly(L-lactic acid) with chitosan and its effects on articular chondrocytes in vitro. Biomaterials 24(21):3859–3868. https://doi.org/10.1016/S0142-9612(03)00209-6
Custódio CA, Alves CM, Reis RL, Mano JF (2010) Immobilization of fibronectin in chitosan substrates improves cell adhesion and proliferation. J Tissue Eng Regen Med 4(4):316–323. https://doi.org/10.1002/term.248
Cuy JL, Beckstead BL, Brown CD, Hoffman AS, Giachelli CM (2003) Adhesive protein interactions with chitosan: consequences for valve endothelial cell growth on tissue-engineering materials. J Biomed Mater Res A 67(2):538–547. https://doi.org/10.1002/jbm.a.10095
de Vasconcelos CL, Bezerril PM, Pereira MR, Ginani MF, Fonseca JL (2011) Viscosity-temperature behavior of chitin solutions using lithium chloride/DMA as solvent. Carbohydr Res 346(5):614–618. https://doi.org/10.1016/j.carres.2010.12.016
Durkin CA, Mock T, Armbrust EV (2009) Chitin in diatoms and its association with the cell wall. Eukaryot Cell 8(7):1038–1050. https://doi.org/10.1128/EC.00079-09
Ellis CE, Ellis LK, Korbutt RS, Suuronen EJ, Korbutt GS (2015) Development and characterization of a collagen-based matrix for vascularization and cell delivery. BioRes Open Access 491:188–197. https://doi.org/10.1089/biores.2015.0007
Ferrero-Miliani L, Nielsen OH, Andersen PS, Girardin SE (2007) Chronic inflammation: importance of NOD2 and NALP3 in interleukin-1β generation. Clin Exp Immunol 147(2):227–235. https://doi.org/10.1111/j.1365-2249.2006.03261.x
Freier T, Montenegro R, Shan Koh H, Shoichet MS (2005) Chitin-based tubes for tissue engineering in the nervous system. Biomaterials 26(22):4624–4632. https://doi.org/10.1016/j.biomaterials.2004.11.040
Ge Z, Baguenard S, Lim LY, Wee A, Khor E (2004) Hydroxyapatite-chitin materials as potential tissue engineered bone substitutes. Biomaterials 25(6):1049–1058. https://doi.org/10.1016/S0142-9612(03)00612-4
Hansson A, Hashom N, Falson F, Rousselle P, Jordan O, Borchard G (2012) In vitro evaluation of an RGD-functionalized chitosan derivative for enhanced cell adhesion. Carbohydr Polym 90(4):1494–1500. https://doi.org/10.1016/j.carbpol.2012.07.020
Hersel U, Dahmen C, Kessler H (2003) RGD modified polymers: biomaterials for stimulate cell adhesion and beyond. Biomaterials 24(24):4385–4415. https://doi.org/10.1016/S0142-9612(03)00343-0
Ho MH, Wang DM, Hsieh HJ, Liu HC, Hsien TY, Lai JY, Hou LT (2005) Preparation and characterization of RGD-immobilized chitosan scaffolds. Biomaterials 26(16):3197–3206. https://doi.org/10.1016/j.biomaterials.2004.08.032
Hudson SM, Smith C (1998) Polysaccharide: chitin and chitosan: chemistry and technology of their use as structural materials. In: Kaplan DL (ed) Biopolymers from renewable resources. Springer-Verlag, Berlin Heidelberg
Ige OO, Umoru LE, Aribo S (2012) Natural products: a minefield of biomaterials. ISRN Mater Sci 2012:983062. https://doi.org/10.5402/2012/983062
Iwasaki N, Yamane S-T, Majima T, Kasahara Y, Minami A, Harada K, Nonaka S, Maekawa N, Tamura H, Tokura S, Shiono M, Monde K, Nishimura S-I (2004) Feasibility of polysaccharide hybrid materials for scaffolds in cartilage tissue engineering: evaluation of chondrocyte adhesion to polyion complex fibers prepared form alginate and chitosan. Biomacromolecules 5(3):828–833. https://doi.org/10.1021/bm0400067
Jeon IH, Mok JY, Park KH, Hwang HM, Song MS, Lee D, Lee MH, Chai KY, Jang SI (2012) Inhibitor effect of dibutyryl chitin ester on nitric oxide and prostaglandin E2 production in LPS-stimulated RAW 264.7 cells. Arch Pharm Res 35(7):1287–1292. https://doi.org/10.1007/s12272-012-0720-8
Junka R, Valmikinathan CM, Kalyon DM, Yu X (2013) Laminin functionalized biomimetic nanofibers for nerve tissue engineering. K=J Biomater Tissue Eng 3(4):494–502. https://doi.org/10.1166/jbt.2013.1110
Kang YO, Yoon IS, Lee SY, Kim DD, Lee SJ, Park WH, Hudson SM (2010) Chitosan-coated poly(vinyl alcohol) nanofibers for wound dressings. J Biomed Mater Res B Appl Biomater 92(2):568–576. https://doi.org/10.1002/jbm.b.31554
Khor E, Lm LY (2003) Implantable applications of chitin and chitosan. Biomaterials 24(13):2339–2349. https://doi.org/10.1016/S0142-9612(03)00026-7
Kim CH, Choi JW, Chun HJ, Choi KS (1997) Synthesis of chitosan derivatives with quaternary ammonium salt and their antibacterial activity. Polym Bull 38(4):387–393. https://doi.org/10.1007/s002890050064
Kim CH, Park H-S, Gin YJ, Son Y, Lim S-H, Choi YJ, Park K-S, Park CW (2004) Improvement of the biocompatibility of chitosan dermal scaffold by rigorous dry heat treatment. Macromol Res 12(4):367–373. https://doi.org/10.1007/BF03218413
Kim MS, Kim JH, Min BH, Chun HJ, Han DK, Lee HB (2011) Polymeric scaffolds for regenerative medicine. Polym Rev 51(1):23–52. https://doi.org/10.1080/15583724.2010.537800
Kim SE, Park JH, Cho YW, Chung H, Jeong SY, Lee EB, Kwon IC (2003a) Porous chitosan scaffold containing microspheres loaded with transforming growth factor-beta1: implications for cartilage tissue engineering. J Control Release 91(3):365–374. https://doi.org/10.1016/S0168-3659(03)00274-8
Kim SJ, Park SJ, Kim SI (2003b) Swelling behavior of interpenetrating polymer network hydrogels composed of poly(vinyl alcohol) and chitosan. React Funct Polym 55(1):53–59. https://doi.org/10.1016/S1381-5148(02)00214-6
Ko H-F, Sfeir C, Kumta PN (2010) Novel synthesis strategies for natural polymer and composite biomaterials as potential scaffolds for tissue engineering. Philos Trans A Math Phys Eng Sci 368(1917):1981–1997. https://doi.org/10.1098/rsta.2010.0009
Kurita K, Sannan T, Iwakura Y (1977) Studies on chitin, 4. Evidence for formation of block and random copolymers of N-acetyl-D-glucosamine and D-glucosamine by hetero- and homogeneous hydrolyses. Die Makromolekulare Chemie 178(12):3197–3202. https://doi.org/10.1002/macp.1977.021781203
Lahiji A, Sohrabi A, Hungerford DS, Frondoza CG (2000) Chitosan supports the expression of exracellular matrix proteins in human osteoblasts and chondrocytes. J Biomed Mater Res 51(4):586–595. https://doi.org/10.1002/1097-4636(20000915)51:4<586::AID-JBM6>3.0.CO;2-S
Lam J, Sequra T (2013) The modulation of MSC integrin expression by RGD presentation. Biomaterials 34(16):3938–3947. https://doi.org/10.1016/j.biomaterials.2013.01.091
Lee JE, Kim KE, Kwon IC, Ahn HJ, Lee SH, Cho H, Kim HJ, Seong SC, LEE MC (2004) Effect of the controlled-released TGF- beta 1 from chitosan microspheres on chondrocytes cultured in a collagen/chitosan/glycosaminoglycan scaffold. Biomaterials 25(18):4163–4173. https://doi.org/10.1016/j.biomaterials.2003.10.057
Lee YM, Park YJ, Lee SJ, Ku Y, Han SB, Klokkevold PR, Chung CP (2000) The bone regenerative effect of platelet-derived growth factor-BB delivered with a chitosan/tricalcium phosphate sponge carrier. J Periodontol 71(3):418–424. https://doi.org/10.1902/jop.2000.71.3.418
Lemmon MA, Schlessinger J (2010) Cell signaling by receptor tyrosine kinases. Cell 141(7):1117–1134. https://doi.org/10.1016/j.cell.2010.06.011
Liu H, Fan H, Cui Y, Chen Y, Yao K, Goh JC (2007) Effects of the controlled-released basic fibroblast growth factor from chitosan-gelatin microspheres on human fibroblasts cultured on a chitosan-gelatin scaffold. Biomacromolecules 8(5):1446–1455. https://doi.org/10.1021/bm061025e
Liu H, Li H, Cheng W, Yang Y, Zhu M, Zhou C (2006) Novel injectable calcium phosphate/chitosan composites for bone substitute materials. Acta Biomater 2(5):557–565. https://doi.org/10.1016/j.actbio.2006.03.007
Liu Y, Stewart KN, Bishop E, Marek CJ, Kluth DC, Rees AJ, Wilson HM (2008) Unique expression of suppressor of cytokine signaling 3 is essential for classical macrophage activation in rodents in vitro and in vivo. J Immunol 180(9):6270–6278. https://doi.org/10.4049/jimmunol.180.9.6270
Ma L, Gao C, Mao Z, Zhou J, Shen J, Hu X, Han C (2003) Collagen/chitosan porous scaffolds with improved biostability for skin tissue engineering. Biomaterials 24(26):4833–4841. https://doi.org/10.1016/S0142-9612(03)00374-0
Macedo L, Pinhal-Enfield G, Alshits V, Elson G, Cronstein BN, Leibovich SJ (2007) Wound healing is impaired in MyD88-deficient mice: a role for MyD88 in the regulation of wound healing by adenosine A2A receptors. Am J Pathol 171(6):1774–1788. https://doi.org/10.2353/ajpath.2007.061048
Madaghiele M, Demitri C, Sannino A, Ambrosio L (2014) Polymeric hydrogels for burn wound care: advanced skin wound dressings and regenerative templates. Burns Trauma 2:20040153. https://doi.org/10.4103/2321-3868.143616
Madihally S, Matthew HW (1999) Porous chitosan scaffolds for tissue engineering. Biomaterials 20(12):1133–1142. https://doi.org/10.1016/S0142-9612(99)00011-3
Martínez A, Blanco MD, Davidenko N, Cameron RE (2015) Tailoring chitosan/collagen scaffolds for tissue engineering: effect of composition and different crosslinking agents on scaffold properties. Carbohydr Polym 132:606–619. https://doi.org/10.1016/j.carbpol.2015.06.084
Mattioli-Belmonte M, Gigante A, Muzzarelli RAA, Politano R, De Benedittis A, Specchia N, Buffa A, Biagini G, Greco F (1999) N,N-dicarboxymethyl chitosan as delivery agent for bone morphogenetic protein in the repair of articular cartilage. Med Biol Eng Comput 37(1):130–134. https://doi.org/10.1007/BF02513279
Mhanna R, Hasan A (2017) 1. Introduction to tissue engineering. In: Hasan A (ed) Tissue engineering for artificial organs: regenerative medicine, smart diagnostics and personalized medicine. WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
Midwood KS, Williams LV, Schwarzbauer JE (2004) Tissue repair and the dynamics of the extracellular matrix. Int J Biochem Cell Biol 36(6):1031–1037. https://doi.org/10.1016/j.biocel.2003.12.003
Mochizuki M, Kadoya Y, Wakabayashi Y, Kato K, Okazaki I, Yamada M, Sato T, Sakairi N, Nishi N, Nomizu M (2003) Laminin-1 peptide-conjugated chitosan membranes as a novel approach for cell engineering. FASEB J 17(8):875–877. https://doi.org/10.1096/fj.02-0564fje
Nair MG, Cochrane DW, Allen JE (2003) Macrophages in chronic type 2 inflammation have a novel phenotype characterized by the abundant expression of YM1 and Fizz1 that can be partly replicated in vitro. Immunol Lett 85(2):173–180. https://doi.org/10.1016/S0165-2478(02)00225-0
O’Brien FJ (2011) Biomaterials & scaffolds for tissue engineering. Mater Today 14:88–95. https://doi.org/10.1016/S1369-7021(11)70058-X
Obara K, Ishihara M, Ishizuka T, Fujita M, Ozeki Y, Maehara T, Saito Y, Hirofumi Y, Matsui T, Hattori H, Kikuchi M, Kurita A (2003) Photocrosslinkable chitosan hydrogel containing fibroblast growth factor-2 stimulates wound healing in healing-impaired db/db mice. Biomaterials 24(20):3437–3444. https://doi.org/10.1016/S0142-9612(03)00220-5
Oliveira JM, Rodrigues MT, Silva SS, Malafaya PB, Gomes ME, Viegas CA, Dias IR, Azevedo JT, Mano JF, Reis RL (2006) Novel hydroxyapatite/chitosan bilayered scaffold for osteochondral tissue-engineering applications: scaffold design and its performance when seeded with goat bone marrow stromal cells. Biomaterials 27(36):6123–6137. https://doi.org/10.1016/j.biomaterials.2006.07.034
Ortega-Paz L, Brugaletta S, Sabaté M (2018) Impact of PSP technique on clinical outcomes following bioresorbable scaffolds implantation. J Clin Med 7(2):27. https://doi.org/10.3390/jcm7020027
Park DJ, Choi BH, Zhu SJ, Huh JY, Kim BY, Lee SH (2005) Injectable bone using chitosan-alginate gel/mesenchymal stem cells/BMP-2 composites. J Craniomaxillofac Surg 33(1):50–54. https://doi.org/10.1016/j.jcms.2004.05.011
Pogorielov M, Kalinkevich O, Deineka V, Garbuzova V, Solodovnik A, Kalinkevich A, Kalinichenoko T, Gapchenko A, Sklyar A, Danilchenko S (2015) Haemostatic chitosan coated gauze: in vitro interaction with human blood and in-vivo effectiveness. Biomater Res 19:22. https://doi.org/10.1186/s40824-015-0044-0
Rabea EI, Mohamed ETB, Christian VS, Guy S, Walter S (2003) Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules 4(6):1457–1465. https://doi.org/10.1012/bm034130m
Ravi Kumar MNV (2000) A review of chitin and chitosan applications. React Funct Polym 46(1):1–27. https://doi.org/10.1016/S1381-5148(00)00038-9
Ren D, Yi H, Wang W, Ma X (2005) The enzymatic degradation and swelling properties of chitosan matrices with different degree of N-acetylation. Carbohydr Res 340(15):2403–2410. https://doi.org/10.1016/j.carres.2005.07.022
Roberts GAF (1992) Derivatives of chitin and chitosan. In: Chitin chemistry. Palgrave, London. https://doi.org/10.1007/978-1-349-11545-7_4
Roh H, Yang DH, Chun HJ, Khang G (2015) Cellular behavior of hepatocyte-like cells from nude mouse bone marrow-derived mesenchymal stem cells on galactosylated poly(D,L-lactic-co-glycolic acid). J Tissue Eng Regen Med 9(7):819–825. https://doi.org/10.1002/term.1771
Roy JC, Salaün F, Giraud S, Ferri A (2017) Solubility of chitin: solvents, solution behaviors and their related mechanisms. In: Xu Z (ed) Solubility of polysaccharides. InTechOpen. https://doi.org/10.5772/intechopen.71385
Shahidi F, Abuzaytoun R (2005) Chitin, chitosan, and co-products: chemistry, production, applications, and health effects. Adv Food Nurt Res 49:93–135. https://doi.org/10.1016/S1043-4526(05)
Shen B, Delaney MK, Du X (2012) Inside-out, outside-in, and inside-outside-in: G protein signaling in integrin-mediated cell adhesion, spreading, and retraction. Curr Opin Cell Biol 24(5):600–606. https://doi.org/10.1016/j.ceb.2012.08.011
Shin YM, Shin HJ, Yang DH, Koh YJ, Shin H, Chun HJ (2017) Advance capability of radially aligned fibrous scaffolds coated with polydopamine for guiding directional migration of human mesenchymal stem cells. J Mater Chem B 5:8725–8737. https://doi.org/10.1039/C7TB01758H
Tibbitt MW, Anseth KS (2009) Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol Bioeng 103(4):655–663. https://doi.org/10.1002/bit.22361
Tiğli RS, Gümüşderelioğlu M (2008) Evaluation of RGD- or EGF-immobilized chitosan scaffolds for chondrogenic activity. Int J Biol Macromol 43(2):121–128. https://doi.org/10.1016/j.ijbiomac.2008.04.003
van Tienen TG, Heijkants GJC, Buma P, de Groot JH, Pennings AJ, Veth RPH (2002) Tissue ingrowth and degradation of two biodegradable porous polymers with different porosities and pore sizes. Biomaterials 23(8):1731–1738. https://doi.org/10.1016/S0142-9612(01)00280-0
Vårum KM, Myhr MM, Hjerde RJ, Smidsrød O (1997) In vitro degradation rates of partially N-acetylated chitosans in human serum. Carbohydr Res 299(1–2):99–101. https://doi.org/10.1016/S0008-6215(96)00332-1
Yang DH, Seo DI, Lee DW, Bhang SH, Park K, Jang G, Kim CH, Chun HJ (2017) Preparation and evaluation of visible-light cured glycol chitosan hydrogel dressing containing dual growth factors for accelerated wound healing. J Ind Eng Chem 53:360–370. https://doi.org/10.1016/j.jiec.2017.05.007
Yoon Y-M, Kim S-J, Oh C-D, Ju J-W, Song WK, Yoo YJ, Hun T-L, Chun J-S (2002) Maintenance of differentiated phenotype of articular chondrocytes by protein kinase C and extracellular signal-regulated protein kinase. J Biol Chem 277:8412–8420. https://doi.org/10.1074/jbc.M110608200
Zhang C, Liu R, Xiang J, Kang H, Liu Z, Huang Y (2014) Dissolution mechanism of cellulose in N,N-dimethylacetamide/lithium chloride: revisiting through molecular interactions. J Phys Chem B 118(31):9507–9514. https://doi.org/10.1021/jp506013c
Zhang Y, Wang Y, Shi B, Cheng X (2007) A platelet-derived growth factor releasing chitosan/coral composite scaffold for periodontal tissue engineering. Biomaterials 28(8):1515–1522. https://doi.org/10.1016/j.biomaterials.2016.11/040
Zhou JC, Zhang JJ, Zhang W, Ke ZY, Zhang B (2017) Efficacy of chitosan dressing on endoscopic sinus surgery: a systematic review and meta-analysis. Eur Arch Otorhinolaryngol 274(9):3269–3274. https://doi.org/10.1007/s00405-017-4584-x
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Kim, CH., Park, S.J., Yang, D.H., Chun, H.J. (2018). Chitosan for Tissue Engineering. In: Chun, H., Park, K., Kim, CH., Khang, G. (eds) Novel Biomaterials for Regenerative Medicine. Advances in Experimental Medicine and Biology, vol 1077. Springer, Singapore. https://doi.org/10.1007/978-981-13-0947-2_25
Download citation
DOI: https://doi.org/10.1007/978-981-13-0947-2_25
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-0946-5
Online ISBN: 978-981-13-0947-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)