Abstract
The hot springs are populated by mesophilic, thermotolerant, and hyperthermophilic bacteria. These populations are diverse, and some of them show combinations of other extreme conditions, for example, acidic, alkaline, high pressure, and high concentrations of salts and heavy metals. Anaerobes inhabiting hot springs are considered to be the closest living descendants of the earliest living forms on earth, and their study offers understandings about the origin and evolution of life. In this chapter, thermal spring bacterial diversity from Pakistani ecology is reviewed. The bacterial populations in Pakistani hydrothermal vent environments showed a great genetic diversity, and most members of these populations appeared to be uncultivated and unidentified organisms. Analysis suggested that some microorganisms of novel phylotypes predicted by molecular phylogenetic analysis were likely present in thermal spring environments. Libraries were predominantly composed of rare phylotypes that appeared to be unclassified, and the number and type of phylotypes observed were correlated with biogeography as well as biogeochemistry. These findings broaden our opinion of the genetic diversity of bacteria in hot water spring environments. The global-scale bacterial diversity of other hot water spring environments, on the other hand, may be beyond present proficiencies for authentic study.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Thermophiles
- Thermal springs
- Bacterial diversity
- Taxonomy
- Biogeography
- Biogeochemistry
- Unculturable methods
1.1 Introduction
Temperature as an environmental factor compels all living microorganisms. In contrast to the upper temperature boundaries, the lower temperature boundaries for growth among microorganisms are not well defined (Russell et al. 1990). Thermophiles are the microorganisms that “love” heat. A word of caution is necessary regarding the use of the term “thermophilic.” The term means different temperature ranges for different groups of microorganisms. For example, Candida thermophile is described as a thermophilic yeast with a maximum growth temperature of 51 °C. The optimal growth temperature for this microorganism is 30–35 °C (Shin et al. 2001). Among bacteria, this would be a thermotolerant species. The record for the widest temperature span for growth is held by Methanothermobacter thermautotrophicus that able to grow from 22 to 75 °C (Gerday and Glansdorff 2007).
Strain 121, a Fe(III)-reducing archaea isolated from a hydrothermal vent along the Juan de Fuca Ridge, is reported to have a doubling time of 24 h at 121 °C and remains viable after exposure to temperatures as high as 130 °C (Kashefi and Lovley 2003). The most heat-resistant spore is held by Moorella thermoacetica strain JW/DB-4. Under autotrophic conditions at 60 °C, this bacterium forms spores with a decimal reduction time of 2 h at 121 °C. A subpopulation of spores apparently requires 1 h at 100 °C to become fully activated before germinating (Byrer et al. 2000).
Thermus aquaticus, an aerobic, thermophilic bacterium, was isolated from Yellowstone National Park, Wyoming, in the late 1960s (Brock and Freeze 1969), and the microorganism’s Taq DNA polymerase has become an essential component of molecular biology. Brock (1997) stated that among different types of research methods, the one done by individual scientist usually returns late. Thermus aquaticus, an anaerobic bacterium was isolated from Yellowstone National Park, Wyoming, in the late 1960s by Thomas Brock (Brock and Freeze 1969). That lead to the discovery of Taq polymerase which become a breakthrough in molecular biology. Cosmopolitan microorganisms from thermal environments include Methanothermobacter thermautotrophicus, Thermoanaerobacter thermohydrosulfuricus, Thermoanaerobacterium thermosaccharolyticum, and Geobacillus stearothermophilus.
The interaction correlation between biogeography and biogeochemistry in thermal environments is also worthy. As an example, three combinations can be defined by Engle et al. (1995):
Relaxed biogeography and biogeochemistry: For example, Thermoanaerobacterium thermosaccharolyticum and Thermobrachium celere have a relaxed biogeography and biogeochemistry. They have been isolated from a variety of environments from several locations including thermobiotic, mesobiotic, slightly alkaline, and acidic environments.
Relaxed biogeography and restricted biogeochemistry: For example, Clostridium paradoxum and Clostridium thermoalcaliphilum are isolated from sewage sludge on four different continents, but only from sewage sludge.
Restricted biogeography and relaxed biogeochemistry: That is, Anaerobranca horikoshii has only been isolated from a specific area behind the old faithful ranger station in Yellowstone National Park, but from several pools in that area, representing a spectrum of pH values from acidic (pH 5) to alkaline (pH 8.5). Although relatively easy to isolate, strains of A. horikoshii have not been obtained from other areas of Yellowstone National Park or other countries, nor has its sequence been found in environmental 16S rRNA gene libraries.
Strazzulli et al. (2017) studied that spots of volcanic activity exist all over the Earth’s surface and under the sea. They offer a variety of different environments for extremophilic microorganisms. Hot springs are always full of hyperthermophiles, the majority of which belong to the domain of archaea. Combination of extreme temperature with other physicochemical parameters, i.e., acidic, alkaline, high pressure, and high concentrations of salts and heavy metals, also selects specific classes of bacteria and discourages remaining classes for which these conditions are not favorable (Cowan et al. 2015). Archaea which resides in hot springs are claimed to be the closest living descendants of the primitive living forms on earth and are considered as models to study origin and evolution of life (Olsen et al. 1994).
1.2 History
It is well established that a number of archaeal and bacterial species live under extreme environmental conditions, which include pressure, high temperature, UV light, ionizing radiation, very low levels of nutrients, and low or high levels of pH (Gerday and Glansdorff 2007). Cavicchioli (2002) suggested the possibility that while considering these extremophiles as models, we can get insights into the lifestyle at celestial habitat.
It would be highly significant to establish identification between ancient and primitive organisms. It has been observed that cladistically ancient organisms are located near the root of universal rRNA-based trees, but they do not own primitive molecular genetic apparatus, nor they are more basic in their metabolic abilities than their aerobic equivalents (Islas et al. 2003). Pre-RNA worlds are the foundation of primitive living systems, in which life may have been based on polymers using backbones other than ribose phosphate and possibly the bases different from guanine, adenine, uracil, and cytosine (Levy and Miller 1998), followed by a stage in which life was based on RNA both as the genetic material and as catalysts (Joyce 2002). Only very few facts support hyperthermophilic origin of life. Firstly, the deepest, branches of rRNA-based molecular phylogenies are full of hyperthermophiles (Pace 1991, 1997). Secondly, immediately after earth formation, the surface of the earth was extremely hot and planet remained molten for some time after its formation. About 4.6 × 109 years ago, life on earth and only hyperthermophilic life were possible (Wiegel 1998). The biphasic temperature-growth curves of many thermophiles growing at elevated temperatures and the existence of cryptic thermophiles are considered as additional arguments for the start of life in the range of 60–90 °C and that hyperthermophiles as well as mesophiles and psychrophiles are adaptations to changed environments.
While some antagonists say that the earth’s surface speedily lost temperature to provide mesophilic origin of life (Wilde et al. 2001). Chemical decomposition of recognized biochemical compounds, i.e., amino acids, nucleobases, RNA, and thermolabile molecules, has half-lives for decomposition at temperatures between 250 and 350 °C at the most a few minutes (Miller and Bada 1988). Another theory that supports mesophilic origin of life came from Gulen et al. (2016). Petrov et al. (2015) believe that the property of ribosome that shields it in high temperature, e.g., RNA foldings, evolved slowly during evolution. Hyperthermophilic microbial lifestyles are the product of secondary adaptations that developed during early stages of cell evolution, but we do not have an information on the composition of the terrestrial atmosphere during the period of the origin of life or on the temperature, ocean pH values, and other general and local environmental conditions that were important for the emergence of living systems (Lazcano and Bada 2003). Delaye et al. (2005) believe that the origin of the mutant sequences ancestral to those found in all existing species and the divergence of the bacteria, archaea, and eukarya were not synchronous events, i.e., the separation of the primary domains took place later, perhaps even much later, and then the appearance of the genetic components of their least common ancestors. The cenancestor is thus one of the last evolutionary outcomes of a series of ancestral events, including lateral gene transfer, gene losses, and paralogous duplications that took place before the separation of bacteria, archaea, and eukarya. Dworkin et al. (2002) and Forterre et al. (2002) believes that if hyperthermophile is not truly primordial, then heat-loving lifestyles may be remainders of a secondary adaptation that evolved after the origin of life and before or soon after the separation of the major lineages. Forterre et al. (2002) believe in adaptation of bacteria to extreme environments by lateral transfer of reverse gyrase and other thermo-adaptive traits from heat-loving archaea. Dworkin et al. (2002) believe that outcompetition of older mesophiles by hyperthermophiles originally adapted to stress-inducing conditions other than high temperatures.
Wilson (1992) created the term biodiversity and wrote The Diversity of Life. At that time, there were 4800 species described in the “kingdom” Monera. Currently more than 30,000 whole genomes have been submitted from all three domains of life bacteria, archaea, and eukarya and are available in the Joint Genome Institute’s Integrated Microbial Genomes database (Hug et al. 2016). Recently, Hug et al. (2016) gave new tree of life (Fig. 1.1) in which 92 phyla which are representing total bacterial eukaryotic and archaeal diversity and includes 92 phyla belonging to bacteria, 26 of archaea five of the eukaryotes. Genome-resolved metagenomics and single-cell genomics of hundreds of genomes revealed that all members have comparatively small genomes and most of them have restricted metabolic capacities or are symbionts. Therefore, all cells either lack thorough citric acid cycles or respiratory chains, and furthermost few have limited or no ability to synthesize nucleotides and amino acids. It is presumed that these reduced metabolisms resulted from either super phylum-wide damages or inherited characteristics. If its result of inherited characteristics, then symbiotic lifestyles were secondary adoptions from once more complex organisms appeared.
Figure adopted from Hug et al. (2016) is showing tree of life. All major lineages are highlighted with genome-wide depiction, but mostly are phylum-level branches. Major lineages are allocated random colors and named, with the published and described names, in italics. Uncultured lineages are highlighted with red dots and are nonitalic. Brackets around Tenericutes and Thermodesulfobacteria show that these are subbranches of Firmicutes and Deltaproteobacteria, respectively. Phylum like Proteobacteria which is not monophyletic (Alphaproteobacteria, Betaproteobacteria, Deltaproteobacteria, Gammaproteobacteria) is shown separately. The candidate phyla radiation (CPR) is assigned a single color because all are uncultured and unclassified and are still in the process of description at lower taxonomic levels. Further analysis by ribosomal proteins as well as by primitive genetic code showed that there is vast difference in composition of three domains, i.e., thermophilic, mesophilic, and halophilic domains (®Macmillan Publishers Limited, 2017)
1.3 Thermal Environments and Biodiversity
What makes thermal environment a popular model to test biogeographical hypotheses is its island-like nature. Using similar strains of the thermophilic archaeon Sulfolobus originating from hot springs in Yellowstone National Park and Italy, Zillig et al. (1980) formulated the hypothesis that “geographical barriers between habitats of the same type do not exist for microorganisms.” This hypothesis also corresponds to the oft-quoted hypothesis that “everything is everywhere and the environment selects” (Beijerinck 1913). However, Whitaker et al. (2003) attribute genetic divergence detected by multilocus sequence analysis of strains of Sulfolobus solfataricus from five sites to geographic isolation.
Recent reports on bacterial diversity of hot water springs revealed that it is difficult to propose the reasons for the presence of specific bacterial species in a thermal spring because these ecosystems are always deviate when influenced by an outside influence, for example, Hu et al. (2017) reported that in acidic thermal springs in New Zealand temperature (range 30–80 °C) was the only significant variable associated with community turnover. Near 40 °C, chemolithoautotrophs were dominant, whereas, at temperatures >65 °C, the microbial community was dominated approximately solely by sulfur-oxidizing archaea. At mesophilic temperatures, the community structure was diverse, encompassing both archaea and bacteria but dominated mainly by chemolithotrophic sulfur and hydrogen oxidizers. In another report, Jiang and Takacs-Vesbach (2017) revealed that despite similar pH of all studied sites of Yellow Stone National Park, bacterial diversity varied a lot. Lowest-temperature site predominant phyla were Chloroflexi, Bacteroidetes, Proteobacteria, and Firmicutes. Metagenome study revealed that all genes related to energy production were present, i.e., transcription, carbohydrate transport, genes related to sulfate reduction, dissimilatory nitrogen reduction, and H2S. In another study, Merkel et al. (2017) on Kamchatka Peninsula hot springs revealed dominance of sulfur-oxidizing bacteria of genus Sulfurihydrogenibium which were followed by the second most dominant anaerobic bacteria of genus Caldimicrobium. At high-temperature sites, archaea of the genus Vulcanisaeta were abundant, and at acidic springs Nanoarchaeota and uncultured Thermoplasmataceae A10 were also present.
1.4 General Features and Geography of Pakistan Hot Water Springs
The Main Mantle Thrust and the Main Karakoram Thrust (MKT) in Chilas and Hunza areas of Northern Pakistan are host to many hydrothermal activity with numerous thermal springs distributed between latitude 30°–37° N and longitude 73°–77° E (Bakht 2000). One of the ways to address the woes of energy crisis effectively in the developing world is through the use of geothermal energy resources (Gondal et al. 2017). There are seven hot springs in Murtazaabad which lie along the Main Karakoram Thrust in Northern Areas of Pakistan with the surface temperature range of 47–92 °C. All the thermal waters of Pakistan are formulated from NaHCO3. Tattapani and Tato thermal springs along the Main Mantle Thrust have a surface temperature from 48 to 92 °C. These are also NaHCO3 type. Geothermal springs of Chagai are related to the youngest volcano (Koh-I-Sultan) of Pakistan. The northern areas having geothermal fields at Tattapani, Tato, and Murtazaabad are located between the latitudes 35°20′ N–36°30′ N and longitudes 74°E–76°E with sheer topography and U-shaped valleys, which are drained by the rivers Indus, Gilgit, and Hunza, while the rivers Shigar, Shyok, Ishkuman, and Yasin form the major branches to these rivers.
Other important mountain ranges of the area are the Kailas, Rakaposhi, and Masherbrum. Rainfall in these areas is light, and the geotectonic development of the northern areas of Pakistan occurs during late Cretaceous to Cenozoic era. The creation involved three tectonic elements, i.e., the Indo-Pakistan shield and its northern sedimentary cover (Indian Mass), the rocks deposited on the southern part of the Eurasian Mass, and the Kohistan island arc sequence (Ahmad et al. 2015).
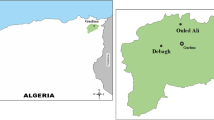
Amin et al. (2017b) reported bacterial diversity and ecological interactions with physicochemical parameters in 9 hot water springs scattered along Himalayan geothermal region where temperature ranges from 60 to 95 °C and pH from 6.2 to 9.4, and in mineralogy from HCO3 − (Tato field), sulfates (Tattapani) to mixed type (Fig. 1.2) (Murtazaabad).
Among various hot water springs present in Pakistan (Table 1.1), Chang et al. (2013) have reported on the chemical composition of Manghopir thermal spring for the year 2008. In our published report by Amin et al. (2017b), we revealed that among the studied sites (Table 1.1) were Tato field thermal springs that were bicarbonated in nature and had higher bicarbonates (525–610 mg L−1) than sulfates (410–460 mg L−1) and the Tattapani hot springs that were sulfate type considering bicarbonate level (133–159 mg L−1) was significantly lower than sulfates (545–684 mg L−1). The Murtazaabad thermal springs were mixed type with high level of both sulfates and bicarbonates (710–940 mg L−1). The levels of sulfates and bicarbonates in the Murtazaabad springs were also significantly higher than the other two sites.
1.5 Bacterial Diversity in Hot Water Springs of Pakistan
In order to scratch the surface in revealing a new vista, we designed a study to discover unique and undiscovered microbiota of hot water springs present in Pakistan by second-generation pyrosequencing and compared the gap between cultured and uncultured microbiota by assessing the relationships between microbial community compositions and environmental conditions (e.g., water geochemistry). Two new species of bacteria were characterized by polyphasic taxonomic approach for validation at species level Nocardioides pakistanensis sp. nov. and Streptomyces caldifontis sp. nov. (Amin et al. 2016, 2017a).
According to unculturable method, at 97% OTU (operational taxonomic unit) level, 5535 quality reads were distributed into 972 microbial genera and 53 phyla. OTUs of the phyla Proteobacteria and Chloroflexi were found to be dominantly present in all the sampling sites. Distinct phyla which seemed to outcompete were Proteobacteria, Chloroflexi, Thermotoga, Bacteriodetes, Deinococcus-Thermus, Nitrospirae, and Acidobacteria, and other well-reported thermal spring phyla were UT06, OP11, BRC1, OD1, OP8, OP1, OP3, OP9, OMAN, and NKB19. Environmental properties like pH, temperature, and sulfur influenced the community structure at most that was depicted by the presence of sulfur- and nitrate-reducing bacteria, but the influence of other factors on microbial community assemblage like anaerobic stress in deep water; methane, ammonia and presence of planktonic material were supported by the presence of Chloroflexi and haloanaerobia. Dominance of Chloroflexus and low number of order Aquificales were also studied by Skirnisdottir et al. (2000) at upper temperature of 88–90 °C that matched our results. High-temperature and low-oxygen site of Tattapani spring (TP-H3-c) had the largest OTUs for sulfur bacteria. Deltaproteobacteria purple sulfur bacteria were most dominant in sulfur-rich (Tattapani hot water spring) TP-H3 sites. The presence of Cyanobacteria at (Tato field hot water spring) TF-H2-a and (Tato field hot water spring) TF-H2-b affected number and diversity of purple sulfur bacteria even at high temperature. The highest numbers of OTUs for purple sulfur bacteria, that is, 217, were present in (Tattapani hot water spring samples) TP-H3-c which was a sulfur-rich site, and the second highest number was observed in (Tato field hot water spring) TF-H2 where sulfur level was much lower, but phylum Cyanobacteria population was present to support their growth. Another unique phylum UT06 was present with rich diversity in (Tattapani hot water spring) TP-H3 and (Murtazaabad hot water spring) MA-H4 and low in Tato field samples. Its diversity and number were evenly high in all samples of MA-H4, but in TP-H3 its distribution was not even. Similar physiochemical properties of hot water springs located at far distances and varying geographical locations were responsible for linked microbial community. The presence of closely related microbial species in neighboring hot water springs indicated that movement of water and soil was also responsible for designing microbial community structure in adjacent environments. These geothermal sites should be considered to explore natural biogeochemical cycles and role of specific microorganisms in energizing these cycles to exploit these potentials in the near future. In these hot water springs, enhanced conditions of high temperature, alkaline pH, and methanogenesis all met automatically, and genes for methanogenesis were switched in methanogens. Study suggested that methanotrophy in these thermal sites was not restricted to only one type of methanotroph, but members of type I methanotroph, aerobic methanotroph, Betaproteobacteria methanotroph, and type II methanotroph all collectively were responsible for methane cycle in thermal systems (Amin et al. 2017b).
1.6 Survival Mechanisms at Thermophilic Environment
Survival of thermophilic bacteria (Fig. 1.3), at high temperatures, is because of various adaptations in physiological systems and genetics as stress response to stabilize homeostasis (Wang et al. 2015). Few examples are production of DNA-binding proteins, activation of heat shock proteins, activation of reactive oxygen species, and efficient repair damage (Ranawat and Rawat 2017).
Other mechanisms involve amino acid substitutions (Arnórsdóttir et al. 2009), hydrophobic cores (Bezsudnova et al. 2012), interactions among subunits (Pang and Allemann 2007), and inactivation of spores by high hydrostatic pressure (Sarker et al. 2015) and by adjusting membrane fluidity after adjusting membrane fatty acid composition (Yoon et al. 2015) and also by maintaining membrane fluidity using various thermosensors, e.g., DesK (Cybulski et al. 2015).
Another worth-mentioning fact of thermophilic proteins is substantial rise in the proportion of alpha helices and beta strands, with a decline in irregular region; with moderately thermophilic proteins, alpha helical increase is dominant, whereas in extremely thermophilic ones, beta strand rise is more extensive (Chakravorty et al. 2017; Szilágyi and Závodszky 2000).
Another distinguishing factor among extreme thermophilic proteins and moderately thermotolerant proteins is amino acid composition. In moderately thermophilic proteins, lysine content is less than arginine content, but in extremely thermophilic proteins, lysine residues are more in number because of their requirement of stronger electrostatic interactions and lysine is a charged residue. Amino acid residues which are not high temperature tolerant, i.e., methionine and asparagine, are less in extreme thermophilic proteins (Mrabet et al. 1992). However, the important phenomena, i.e., lysine succinylation and lysine propionylation which are important for protein function, are not different in extremely thermophilic and moderately thermophilic proteins. These are common protein functions which are not dependent on temperature tolerance (Okanishi et al. 2017).
High temperature is also related to strong association between ions. The despairs for the ion pair decreases with an increase in temperatures. In thermostable proteins, internal water molecules (bridging water molecules) are present which ensures that ions are not fully desolated at high temperature. Hence it was established that salt bridges are very important in the design of thermostable proteins (Bikkina et al. 2017). Upregulation of proteins which are responsible for protection against heat stress, i.e., heat shock proteins (HSPs), is common in all thermophiles (Mizobata et al. 2000), i.e., upregulation of HSP60, HSP20, groEL-growES, hrcA-grpE-dnaJ, and dnaK-sHSP in Thermotoga maritima and sHSPs in Sulfolobus solfataricus during temperature rise (Shockley et al. 2003; Johnson et al. 2006). Other chaperon proteins which are upregulated in thermophilic strains Thermoanaerobacter tengcongensis and Thermotoga maritime in response to heat stress include GroEL, GroES, DnaK, and GrpE (Chen et al. 2012a, b; Wang et al. 2012).
1.7 Types of Thermal Environments
Various types of thermal environments exist including terrestrial, solar-heated, marine, subsurface, anthropogenic, temporary, and mesobiotic environments; a brief overview of these environments was reviewed below.
1.7.1 Terrestrial Thermal Environments
The northern areas of Pakistan have a large number of hot springs in the Gilgit, Hunza, and Yasin valleys. The Tattapani hot springs are located on Karakoram Highway at the right bank of Indus River. These springs are located at the altitude of 1200 m. There are two hot springs in Murtazaabad, located in the Hunza valley, downward near the bank of the Khunjerab River: Murtazaabad Zairen and Murtazaabad Balai hot springs. Murtazaabad Balai hot spring is located somewhat upper as compared to the Murtazaabad Zairen hot spring. Other hot springs are located 3.0 km earlier from Darkot Pass in Yasin valley upper to the Rawat base camp. It is situated at the height of about 4650 m from the sea level. Two hot springs are oozing out here, which seem to have the same origin (Ahmad et al. 2013). Shuja (1986) and Bakht (2000) have also found numerous hot springs along the Main Mantle Thrust and Main Karakoram Thrust in Chilas and Hunza areas, respectively. The geothermal system here is the result of the collision of the Indian and Eurasian plates. Hot springs are scattered and their temperature ranges up to 91 °C. Three parts of Pakistan, i.e., Kashmir, Khyber Pakhtunkhwa, and Baluchistan, are the potential zones where geothermal resources are located. Major tectonic elements during the Cenozoic and Mesozoic era have shaped the geological structures that are observed in Pakistan today. These structural elements are indicators for delineating and developing the potential geothermal resources of the country. Worldwide these environments are found at geysers, solfataras (mud or paint pots), and mud or paint pots in volcanically active regions throughout the world, including Iceland, Western North America, New Zealand, Japan, Eastern Russia, and the rest of the so-called Pacific Ring of Fire; major examples include Yellowstone National Park at North America which is being studied dating back to 1897 (Reysenbach and Shock 2002). Neutral to alkaline areas richer in chloride salts or carbonate were observed in areas of terrestrial environments (Zhao et al. 2005).
1.7.2 Solar-Heated Environments
Solar-heated environments may occur anywhere on earth receiving solar energy inputs. Such environments are likely inhabited by mesophilic, thermotolerant, and thermophilic microorganisms because solar energy can heat some soils to 60 °C and shallow waters to 50 °C at certain times of the day or year, as pointed out by Brock (2012). Thermal environments on the earth’s surface also experience evaporation, and thus many environments have elevated salinity and, therefore, halophilic inhabitants. For example, Thermohalobacter berrensis, a thermophilic and halophilic bacterium, was isolated from a solar slattern in France (Cayol et al. 2000). Halo-alkali-thermophiles and halophilic (up to 25% NaCl 4.5 M sodium ion as NaCl/Na2CO3), thermophilic (up to 75 °C), and alkaliphilic (up to pH 10.5) triple extremophiles, coined, have been isolated from dry salts from salt flats in Nevada and from sediments of athalassohaline lakes in Wadi El Natrun, Egypt (Mesbah and Wiegel 2005).
1.7.3 Marine Environments
Marine thermal environments may occur at Beaches: Hot Water Beach (Whitianga, New Zealand), Pozzuoli (Italy), Savusavu (Fiji Island) (Burgess 2009). Under 8 m of water: Vents off the coast of Mílos Island, Greece (Sievert et al. 2000). Under 2500 m: Abysmal of water, deep-sea hydrothermal vents first discovered in 1977 near the Galápagos Islands (Corliss et al. 1979).
Organisms inhabiting such environments face multiple challenges, i.e., venting water can exceed 300 °C, but in deep-sea vents, it cools quickly upon mixing with cold, deep-sea water; habitat types range from those preferred by hyperthermophiles to temperatures habitable by psychrophiles (Kelley et al. 2002), i.e., black smoker chimneys, associated with volcanic psychrophiles activity; and plate spreading zones generally are fueled by high concentrations of sulfides (Kelley et al. 2002). Serpentinite-hosted systems, like the Lost City hydrothermal field, are enriched in hydrogen and methane as energy sources (Kelley et al. 2005). Thermococcus barophilus, obtained from the snake pit region of the Mid-Atlantic Ridge, requires elevated pressure for growth at or above 95 °C (Marteinsson et al. 1999). Pyrococcus strain ES4 shows an extension of Tmax under increased pressure (Pledger et al. 1994; Summit et al. 1998).
Jolivet et al. (2003, 2004) reported that at hydrothermal vents, the level of natural radioactivity can be 100 times greater than that at the earth’s surface because of increased occurrence of elements such as Pb, Po, and Rn. For example, archaea Thermococcus gammatolerans was isolated from a hydrothermal site in Guaymas Basin, Thermococcus marinus from the snake pit hydrothermal site on the Mid-Atlantic Ridge, and Thermococcus radiotolerans from a hydrothermal site in the Guaymas Basin. Additionally, all organisms existing in marine environments also have some tolerance for moderate (around 3%) salinity.
1.7.4 Subsurface Environment
Subsurface thermal environments include petroleum reservoirs and geothermally heated lakes and aquifers. Activity in subsurface environments varies with the availability of nutrients, water, energy, depth, surrounding matrix, and source materials. Lethal temperatures may not occur until as much as 10,000 m below the surface (Pedersen 2000) with few exceptions, e.g., Uzon Caldera; temperatures well above 100 °C can occur at depths of only a few meters (Burgess 2009). A depth record for culturable life has been established at 5278 m (Szewzyk et al. 1994). Elevated temperatures found within petroleum reservoirs can be up to 130 °C (Grassia et al. 1996). The geochemical conditions in reservoirs are variable because of age, source material, and surrounding geology and prokaryote communities (Orphan et al. 2003). Takahata et al. (2000) have proposed that microorganisms in these environments may face oligotrophic conditions. Subsurface geothermal aquifers such as the well- known and expensive Great Artesian Basin of Australia are non-volcanically heated but experience temperatures up to nearly 103 °C.
1.7.5 Anthropogenic Environments
Anthropogenic habitats include household and water heaters and industrial process environments and thermal effluent from power plants (Brock 2012). One of the earliest well-known anaerobic thermophiles, Thermoanaerobacter (basonym Clostridium) thermohydrosulfuricus, was isolated from an Austrian sugar factory (Lee et al. 1993). Other thermophiles have been isolated from thermally polluted effluent from a carpet factory (Carreto et al. 1996), the smoldering slag heap of a uranium mine (Fuchs et al. 1996), and mushroom compost (Korn-Wendisch et al. 1995). Strains of Thermus aquaticus have been isolated from various anthropogenic thermal environments including hot tap water and greenhouse soil (Brock and Freeze 1969).
1.7.6 Temporary Environments and Mesobiotic Environments
Thermophiles can be isolated from various environments, such as animal droppings, manure piles, and compost, temporarily heated by biodegradation of organic material, sun-heated soils, and sediments at the edges of lakes and puddles which can have temperatures up to 50 °C but are frequently around 35–45 °C, whereas most of the thermophiles isolated from these environments are Firmicutes. One example is the archaeon Methanothermobacter thermautotrophicus. This species can be easily isolated from sun-heated black sediments of lakes and mesobiotic sewage plants, but it also has been isolated from sun-heated wood stumps in Georgia, United States (Luo et al. 2013), and mesobiotic environments such as cold stream sediments in Germany (Wiegel et al. 1981) or sediments of Lake Mendota, Wisconsin, for which temperatures have never reached 16 °C. In contrast, thermophiles, living in steady thermal environments such as thermal spring and sediments even if substrate concentration is low, do not have that selection pressure for very rapid growth as long as their residence time in the pool is longer than their doubling time (Fig. 1.4).
1.8 Diversity of Thermophiles
1.8.1 Cultural Diversity
Most of the microorganisms from nearly all environments inhabit are presently uncultured (Hugenholtz 2002). Considering the extreme conditions in which most thermophiles thrive, some require special handling or novel approaches for their enrichment, culturing, and isolation (Mesbah and Wiegel 2005). During our study on thermal springs of Pakistan, based on the 16S rRNA gene sequence similarities, we observed that 248 isolated strains belonged to 37 genera and 3 major phyla which were Proteobacteria, Firmicutes, and Actinobacteria. Of the potentially novel species of Actinobacteria and Bacteria, two were also characterized by polyphasic taxonomy. These strains were characterized as novel species of the genera Nocardioides and Streptomyces (Amin et al. 2016, 2017a).
Until now many species of thermophilic anaerobic bacteria have been isolated and described, and few anaerobic bacteria isolated and described include Thermoanaerobacter tengcongensis sp. nov. (Xue et al. 2001), Chlorobium tepidium sp. nov. (Wahlund et al. 1991), Pyrobaculum igneiluti sp. nov. (Lee et al. 2017), and Desulfuribacillus stibiiarsenatis sp. nov. (Abin and Hollibaugh 2017) (others are mentioned in Table 1.2). Their habitats include geothermal areas (Wiegel and Ljungdahl 1981) (Jessen and Orlygsson 2012) and deep-sea vents (Slobodkin et al. 1999). Low-oxygen concentrations are usually present in the habitats of anaerobes; hence most known thermophilic species are obligate or facultative anaerobes (Amend and Shock 2001).
1.8.2 Phylogenetic and Genetic Diversity
Amplification of 16S rRNA genes directly from environmental DNA has shown intense variation in amount of diversity among prokaryotes and novel lineages of thermophilic bacteria and archaea (Kimura et al. 2005; Burgess 2009; Burgess et al. 2007). Sequences from deep-sea hydrothermal vents led to the identification of novel lineages among archaea and bacteria (Reysenbach et al. 2000), but this is not an absolute fact because some thermal environment communities may contain only a few phylogenetic types; e.g., Reysenbach and Shock (2002) identified only three major phylogenetic groups out of 35 clones analyzed during a study on Yellowstone National Park. During a study on Pakistan hot water springs by unculturable technique, major phyla observed were Proteobacteria, Chloroflexi, Thermotoga, Bacteriodetes, Deinococcus-Thermus, Nitrospirae, and Acidobacteria, and other well-reported thermal spring phyla which are still unclassified were UT06, OP11, BRC1, OD1, OP8, OP1, OP3, OP9, OMAN, and NKB19 (Fig. 1.5). Presence of 40.1% unclassified OTUs clearly suggest the presence of many undiscovered and unexplored unique microbiota within these sites (Amin et al. 2017b).
Pie chart showing distribution of bacterial phyla and Actinobacteria in hot water springs of Pakistan by pyrosequencing approach of 16S rRNA gene. Values in brackets are showing an average number of OTUs (operational taxonomic units). Unclassified OTUs belong to UT06, Caldithrix_p, BRC1, OP8, OP11, MATCR, OMAN, Bacteria_uc, NKB19, JX105615_p, WS1, and O
While limited cultivation-based study of the geothermal springs in this region has been reported (Javed et al. 2012), a cultivation-independent study which provides a more comprehensive assessment of microbial diversity was still lacking before our studies. Great plate count anomaly illustrates that less than 1% of existing microorganisms are culturable. Under such conditions, culture-independent approaches facilitate the exploration of microbial diversity from diverse habitats (Hou et al. 2013). In Pakistan thermal springs, higher species richness and abundance in sediments of Tattapani than in sediments of Tato field and Murtazaabad were reported to be due to moderate temperature, high silicates, and high sulfate contents of Tattapani springs. Lau et al. (2009) and Yim et al. (2006) also reported the influence of temperature (<70 °C) for the presence of Cyanobacteria and Chloroflexi in hot springs.
Thermophilic and hyperthermophilic bacteria had been predominantly isolated from streamers with temperature above 75 °C and mainly comprised of the phyla Aquificae, Deinococcus-Thermus, Thermodesulfobacteria, and Thermotogae and some members of the phyla Proteobacteria and Firmicutes (Miller et al. 2009; Wang et al. 2013). Murtazaabad hot springs with relatively higher temperature (90–95 °C) favored the growth of thermophilic bacterial phylum Thermotogae. These domains were also detected in higher proportion in sites of Tata field and Tattapani, where average temperature is above 85 °C. However, OTUs of phyla Aquificae and Deinococcus-Thermus were more dominant in sites ranging in temperature from 70 to 85 °C. At sites with low silica and high temperature, OTUs belonging to phylum Chloroflexi were dominant. Kambura et al. (2016) believed that the existence of phyla Actinobacteria and Firmicutes was an adaptation in low-nutrient conditions of the hot springs.
1.8.3 Metabolic Diversity
Among all types of microbial metabolism from thermal environments, chemolithotrophy either autotrophy or heterotrophy is a foundation of hyperthermophilic communities in sunless and too hot environments which are not suitable for photoautotrophic production. Few chemolithoautotrophs, e.g., bacteria of the order Aquificales, are considered as primary producers in these ecosystems (Blank et al. 2002). Among bacteria are anaerobic Firmicutes such as the facultative chemolithoautotrophs Moorella thermoacetica that undergoes homoacetogenic fermentations from carbohydrates and the anaerobe Ammonifex degensii, capable of forming ammonium from nitrate via chemolithoautotrophic growth (Huber et al. 1996), Fe(III)-reducing Thermolithobacter ferrireducens, and Thermolithobacter carboxydivorans, a hydrogenic CO utilizer (Wiegel et al. 2003). Photoheterotrophic Chloroflexus aggregans, Chloroflexus aurantiacus, Heliobacterium modesticaldum, and Roseiflexus castenholzii (Hanada et al. 2002). In some examples, in situ geochemistry of thermal environments may be shaping the dominant metabolisms or perhaps is shaped by the dominant metabolisms (Orphan et al. 2003). Many thermal environments are enriched in elements that are toxic to humans, such as arsenic and selenium, and some microorganisms in these habitats use toxic, redox-active elements to gain energy, via either oxidation or reduction (Donahoe-Christiansen et al. 2004).
During a study by Amin et al. (2017b), Pakistan thermal springs were explored for bacterial diversity and it was reported that among Murtazaabad hot water spring, sulfur-reducing bacteria was extensively present in deep waters and Physiological functions revealed that in sulpur rich grothermal springs with anoxic waters, methane is produced by consortium of methanotophs and sulfur reducing bacteria (Tripathy et al. 2016a, b; Delgado-Serrano et al. 2014). Type I and II methanotrophs and SRB were major constituents among phylum Proteobacteria and likely involved in the mineral recycling under the low-oxygen conditions of hot springs, which in turn helped in energy production. In acidic hot springs, this metabolism of energy recycling was reported to be initiated by methane-oxidizing phylum Verrucomicrobia (Islam et al. 2008; Sharp et al. 2012).
Few strains which are dependent on varied metabolic classes isolated from the studied thermal springs included phylum Clostridia, which is obligatorily dependent on methanogens or on the presence of an external electron acceptor; thermophilic anaerobic, Mn(IV)- and Fe(III)-reducing Carboxydocella species Carboxydocella_uc and Carboxydocella manganica; and CO-assimilating chemolithoautotroph which survived under aerobic conditions by using CO dehydrogenases under anaerobic conditions. Genus Ammoniphilus were present in the three sites of Tattapani which were obligatory oxalotrophic and haloalkalitolerant bacteria and required a high concentration of ammonium ions and pH of 6.8–9.5. Unclassified species belonging to genera Anaerosporobacter and Nitratireductor were also detected which were in accordance to the study made by Stackebrandt (2014) who also reported oxidation of NO2 to nitrate by Nitrospira at high temperature and subsequent reduction of nitrate to nitrous oxide or complete oxidation to N2 by members of the order Thermales, Aquificales, and Bacillales (Nakagawa and Fukui 2002).
1.8.4 Ecological Diversity
The thermophilic prokaryotes have introduced us to novel modes of life because of biological interactions in geothermally heated environments. The discovery of deep-sea hydrothermal vent communities demonstrated that life can exist at temperatures 100 °C as well as at 2 °C on the basis of associated microbial vent community (Corliss et al. 1979). Novel symbioses between eukaryotes and prokaryotes have been identified at deep-sea vents, such as the association between the tube worm Riftia pachyptila and chemosynthetic, sulfur-oxidizing bacteria (Cavanaugh et al. 1981) or the thermotolerant Pompeii worm, which utilizes eurythermal enzymes of a community of prokaryotes living on its back (Chevaldonné et al. 2000). Fledgling field of microecology is rapidly expanding, and thermal environments are exemplary systems for it (Magurran 2013). In hot water springs, the diversity of microorganisms within mats of Cyanobacteria has been examined, and the importance of a prokaryote species is determined based on its role in their environments (Ward et al. 1998) and the effect of temperature on structuring community of prokaryotes through genetic parameters and the distribution of different metabolic types (Norris et al. 2002). FISH is also a helpful method that enables us to examine the structural distribution of microorganism of known phylogenetic affiliations (Nübel et al. 2002). Lipids present within the membranes of prokaryotes can be diagnostic for various types of microorganisms and have provided insight into the distribution of microorganisms among different environments. For example, analysis of glycerol dialkyl glycerol tetraethers (GDGTs) from selected hot springs in Nevada exposed the presence of the archaeal lipid crenarchaeol, which was believed to be present in low-temperature and marine environments. The second evidence came from the presence of DGGE band sequences of 16S rRNA genes from these springs which were related to thermophilic Crenarchaeota and confirmed that the presence of crenarchaeol is not exclusive to the cold-adapted, marine branch of the Crenarchaeota (Pearson et al. 2004).
1.9 Conclusion
In Pakistan to date, not a single study has been reported for bacterial diversity of hot water springs except studies by our group. Various other studies in which selective bacteria were isolated from hot water springs of Pakistan include isolation of strain Ralstonia sp. MRL-TL from hot water spring to check its ability to degrade poly(ε-caprolactone) (PCL) (Shah et al. 2015), Analysis of power generation from geothermal resources (Ahsan Mustaqeem et al. 2015), Euthermal hot water spring Mango Pir was studied for physicochemical and biological studies and Cyanophyta, Zooplankton, Bacillarophyta and Nematoda were isolated (Jahangir et al. 2001), freshwater spring was studied from Kohat, Pakistan, and the quality assessments of the drinking water were carried out by determining total coliform bacteria, total plate count, total fecal coliform and E. coli (Ahmad et al. 2013). Another study from Pakistan strain AK9 was isolated from hot water spring of Tattapani Azad Kashmir, Pakistan; cellulase enzyme was extracted and purified which reserved its activity from 50 to 70 °C and 3–7pH. They reported that B. amyloliquefaciens AK9 can be used in bioconversion of lignocellulosic biomass to fermentable sugar (Irfan et al. 2017).
Further we suggest that in the future we should focus to unravel all ignored geochemically important sites specially to fill gap of limited culturing techniques and substrates so far known to study these non-cultured bacteria. The taxonomic results obtained will provide information for exploration in this regime and thus to discover new whole area for further research in the subject of “extremophiles” which will lead to studies on controlled experiments which would help identify which factors influence species distribution at most. For example, Kumar et al. (2015), Boucher et al. (2011), and Porter et al. (2017) reported that core genomes of Rhizobium leguminosarum and Vibrio cholerae have high similarity, but the accessory genome is much varied (Porter et al. 2017). Specific classes of bacteria can be studied along with their biogeochemical cycles, i.e., methane cycle-related microorganisms, anaerobes, nitrogen cycle-related bacteria, sulfate-reducing bacteria, and archaea. Novel enzymes can be extracted from novel genomes by metagenomic studies and whole-genome analysis. Pasternak et al. (2013) stated that that impact of bacterial diversity and their abundance on nature can be explained only by using full-genome proteomic comparisons.
We have tried to highlight that microbial community composition varies with change in biogeography, biogeochemistry, temperature, pH, physicochemical parameters, and many other factors. Globally our results highlight the Pakistan thermal springs, in part the effects of changes in bacterial population in specific set of conditions, and particularly observed the microbial community differences, novel microbiota, and the need to further investigate them to cover all metabolic and genome-wide aspects. It will help researchers to extend this research for narrowing down habitat in genomic interconnected populations, discrete, particular bacterial lineages can be found in contrasting soil types, in case if genomic interconnections will be low, variations in the core and accessory genomes could be found to solve distribution of distinct biogeographical patterns.
References
Abin CA, Hollibaugh JT (2017) Desulfuribacillus stibiiarsenatis sp. nov., an obligately anaerobic, dissimilatory antimonate- and arsenate-reducing bacterium isolated from anoxic sediments, and emended description of the genus Desulfuribacillus. Int J Syst Evol Microbiol 67:1011–1017
Ahmad S, Ali A, Ullah I, Naz N, Ali A, Ali N (2013) Bacteriological and biochemical evaluation of the spring’s water of district Buner Khyber Pakhtunkhwa Pakistan. Int J Adv Res Technol 2:452–460
Ahmad M, Tasneem M, Rafique M, Akram W, Iqbal N, Rafiq M, Fazil M (2015) Identification of recharge sources and dating of groundwater using isotope and CFC techniques. In: Proceedings of the first international conference on environmentally sustainable development. 1slamabad, Pakistan. pp 1125–1135
Ahsan Mustaqeem S, Qureshi SR, Waqar A (2015) Exergetic and energetic analysis of power generation from geothermal resources in Pakistan. In: Proceedings of the World Geothermal Congress, Melbourne, Australia. pp 1–11
Al-Dhabi NA, Esmail GA, Duraipandiyan V, Arasu MV, Salem-Bekhit MM (2016) Isolation, identification and screening of antimicrobial thermophilic Streptomyces sp. Al-Dhabi-1 isolated from Tharban hot spring, Saudi Arabia. Extremophiles 20(1):79–90
Amend JP, Shock EL (2001) Energetics of overall metabolic reactions of thermophilic and hyperthermophilic Archaea and Bacteria. FEMS Microbiol Rev 25:175–243
Amin A, Ahmed I, Habib N, Abbas S, Xiao M, Hozzein WN, Li W-J (2016) Nocardioides pakistanensis sp. nov., isolated from a hot water spring of Tatta Pani in Pakistan. Antonie Van Leeuwenhoek 109:1101–1109
Amin A, Ahmed I, Khalid N, Osman G, Khan IU, Xiao M, Li W-J (2017a) Streptomyces caldifontis sp. nov., isolated from a hot water spring of Tatta Pani, Kotli, Pakistan. Antonie Van Leeuwenhoek 110:77–86
Amin A, Ahmed I, Salam N, Kim B-Y, Singh D, Zhi X-Y, Xiao M, Li W-J (2017b) Diversity and distribution of thermophilic bacteria in hot springs of Pakistan. Microbial Ecol 74:116–127
Arnórsdóttir J, Sigtryggsdóttir ÁR, Thorbjarnardóttir SH, Kristjánsson MM (2009) Effect of proline substitutions on stability and kinetic properties of a cold adapted subtilase. J Biochem 145:325–329
Bakht MS (2000) An overview of geothermal resources of Pakistan. In: Proceedings of the World Geothermal Congress, Kyushu, Japan. pp 77–83
Beijerinck M (1913) Jaarboek van de Koninklijke Akademie v. Wetenschoppen Muller, Amsterdam, The Netherlands. In Dutch
Bezsudnova EY, Boyko KM, Polyakov KM, Dorovatovskiy PV, Stekhanova TN, Gumerov VM, Ravin NV, Skryabin KG, Kovalchuk MV, Popov VO (2012) Structural insight into the molecular basis of polyextremophilicity of short-chain alcohol dehydrogenase from the hyperthermophilic archaeon Thermococcus sibiricus. Biochimie 94:2628–2638
Bikkina S, Bhati AP, Padhi S, Priyakumar UD (2017) Temperature dependence of the stability of ion pair interactions, and its implications on the thermostability of proteins from thermophiles. J Chem Sci 129:405–414
Blank CE, Cady SL, Pace NR (2002) Microbial composition of near-boiling silica-depositing thermal springs throughout Yellowstone National Park. Appl Environ Microbiol 68:5123–5135
Boucher Y, Cordero OX, Takemura A, Hunt DE, Schliep K, Bapteste E, Lopez P, Tarr CL, Polz MF (2011) Local mobile gene pools rapidly cross species boundaries to create endemicity within global Vibrio cholerae populations. MBio 2:e00335–e00310
Bravakos P, Kotoulas G, Skaraki K, Pantazidou A, Economou-Amilli A (2016) A polyphasic taxonomic approach in isolated strains of Cyanobacteria from thermal springs of Greece. Mol Phylogenet Evol 98:147–160
Brock TD (1997) The value of basic research: discovery of Thermus aquaticus and other extreme thermophiles. Genetics 146:1207–1210
Brock TD (2012) Thermophilic microorganisms and life at high temperatures. Springer, New York
Brock TD, Freeze H (1969) Thermus aquaticus gen. n. and sp. n., a nonsporulating extreme thermophile. J Bacteriol 98:289–297
Burgess EA (2009) Geomicrobiological description of two contemporary hydrothermal pools in Uzon Caldera, Kamchatka, Russia, as models for sulfur biogeochemistry. PhD Thesis, University of Georgia, USA
Burgess EA, Wagner ID, Wiegel J (2007) Thermal environments and biodiversity. In: Physiology and biochemistry of extremophiles. American Society of Microbiology, Washington, DC
Byrer DE, Rainey FA, Wiegel J (2000) Novel strains of Moorella thermoacetica form unusually heat-resistant spores. Arch Microbiol 174:334–339
Carreto L, Moore E, Nobre MF, Wait R, Riley PW, Sharp RJ, DA COSTA MS (1996) Rubrobacter xylanophilus sp. nov., a new thermophilic species isolated from a thermally polluted effluent. Int J Syst Evol Microbiol 46:460–465
Cavanaugh CM, Gardiner SL, Jones ML, Jannasch HW, Waterbury JB (1981) Prokaryotic cells in the hydrothermal vent tube worm Riftia pachyptila Jones: possible chemoautotrophic symbionts. Science 213:340–342
Cavicchioli R (2002) Extremophiles and the search for extraterrestrial life. Astrobiology 2:281–292
Cayol J-L, Ducerf S, Patel B, Garcia J-L, Thomas P, Ollivier B (2000) Thermohalobacter berrensis gen. nov., sp. nov., a thermophilic, strictly halophilic bacterium from a solar saltern. Int J Syst Evol Microbiol 50:559–564
Chakravorty D, Faheem Khan M, Patra S (2017) Thermostability of proteins revisited through machine learning methodologies: from nucleotide sequence to structure. Curr Biotechnol 6:39–49
Chang MS, Gachal GS, Qadri AH, Sheikh MY (2013) Ecological impacts on the status of Marsh Crocodiles in Manghopir Karachi. Int J Adv Res 1:42–46
Chen W, Wang BJ, Hong HZ, Yang H, Liu SJ (2012a) Deinococcus reticulitermitis sp. nov., isolated from a termite gut. Int J Syst Evol Microbiol 62:78–83
Chen Z, Wang Q, Lin L, Tang Q, Edwards JL, Li S, Liu S (2012b) Comparative evaluation of two isobaric labeling tags, DiART and iTRAQ. Anal Chem 84:2908–2915
Chevaldonné P, Fisher C, Childress J, Desbruyères D, Jollivet D, Zal F, Toulmond A (2000) Thermotolerance and the ‘Pompeii worms’. Mar Ecol Prog Ser 208:293–295
Corliss JB, Dymond J, Gordon LI, Edmond JM (1979) On the Galapagos Rift. Science 203:1073–1083
Cowan D, Ramond J, Makhalanyane T, De Maayer P (2015) Metagenomics of extreme environments. Curr Opin Microbiol 25:97–102
Cybulski LE, Ballering J, Moussatova A, Inda ME, Vazquez DB, Wassenaar TA, de Mendoza D, Tieleman DP, Killian JA (2015) Activation of the bacterial thermosensor DesK involves a serine zipper dimerization motif that is modulated by bilayer thickness. Proc Natl Acad Sci 112:6353–6358
Delaye L, Becerra A, Lazcano A (2005) The last common ancestor: what’s in a name? Orig Life Evol Biosph 35:537–554
Delgado-Serrano L, López G, Bohorquez LC, Bustos JR, Rubiano C, Osorio-Forero C, Junca H, Baena S, Zambrano MM (2014) Neotropical Andes hot springs harbor diverse and distinct planktonic microbial communities. FEMS Microbiol Ecol 89:56–66
Donahoe-Christiansen J, D’Imperio S, Jackson CR, Inskeep WP, McDermott TR (2004) Arsenite-oxidizing Hydrogenobaculum strain isolated from an acid-sulfate-chloride geothermal spring in Yellowstone National Park. Appl Environ Microbiol 70:1865–1868
Dong Y, Sanford RA, Boyanov MI, Kemner KM, Flynn TM, O’Loughlin EJ, Locke RA II, Weber JR, Egan SM, Fouke BW (2016) Tepidibacillus decaturensis sp. nov.: a microaerophilic, moderately thermophilic iron-reducing bacterium isolated from a depth of 1.7 km in the Illinois Basin, USA. Int J Syst Evol Microbiol 66(10):3964–3971
Dworkin JP, Lazcano A, Miller SL (2002) The roads to and from the RNA world. J Theor Biol 222:127–134
Engle M, Li Y, Woese C, Wiegel J (1995) Isolation and characterization of a novel Alkalitolerant Thermophile, Anaerobranca horikoshii gen. nov., sp. nov. Int J Syst Evol Microbiol 45:454–461
Fadhlaoui K, Hania WB, Postec A, Hamdi M, Ollivier B, Fardeau M-L (2016) Balneicella halophila gen. nov., sp. nov., an anaerobic bacterium, isolated from a thermal spring and description of Balneicellaceae fam. nov. Int J Syst Evol Microbiol 66(11):4692–4696
Forterre P, Brochier C, Philippe H (2002) Evolution of the Archaea. Theor Popul Biol 61:409–422
Fuchs T, Huber H, Teiner K, Burggraf S, Stetter K (1996) Metallosphaera prunae, sp. nov., a novel metal-mobilizing, thermoacidophilic Archaeum, isolated from a uranium mine in Germany. Syst Appl Microbiol 18:560–566
Gerday C, Glansdorff N (2007) Physiology and biochemistry of extremophiles. ASM Press, Washington, DC
Gondal IA, Masood SA, Amjad M (2017) Review of geothermal energy development efforts in Pakistan and way forward. Renew Sust Energ Rev 71:687–696
Grassia GS, McLean KM, Glénat P, Bauld J, Sheehy AJ (1996) A systematic survey for thermophilic fermentative bacteria and archaea in high temperature petroleum reservoirs. FEMS Microbiol Ecol 21:47–58
Gulen B, Petrov AS, Okafor CD, Vander Wood D, O’Neill EB, Hud NV, Williams LD (2016) Ribosomal small subunit domains radiate from a central core. Sci Rep 6:20885
Hanada S, Takaichi S, Matsuura K, Nakamura K (2002) Roseiflexus castenholzii gen. nov., sp. nov., a thermophilic, filamentous, photosynthetic bacterium that lacks chlorosomes. Int J Syst Evol Microbiol 52:187–193
Hou W, Wang S, Dong H, Jiang H, Briggs BR, Peacock JP, Huang Q, Huang L, Wu G, Zhi X (2013) A comprehensive census of microbial diversity in hot springs of Tengchong, Yunnan Province China using 16S rRNA gene pyrosequencing. PLoS One 8:e53350
Hu Z, Xu C, McDowell NG, Johnson DJ, Wang M, Luo Y, Zhou X, Huang Z (2017) Linking microbial community composition to C loss rates during wood decomposition. Soil Biol Biochem 104:108–116
Huber R, Rossnagel P, Woese C, Rachel R, Langworthy TA, Stetter KO (1996) Formation of ammonium from nitrate during chemolithoautotrophic growth of the extremely thermophilic bacterium Ammonifex degensii gen. nov. sp. nov. Syst Appl Microbiol 19:40–49
Hug LA, Baker BJ, Anantharaman K, Brown CT, Probst AJ, Castelle CJ, Butterfield CN, Hernsdorf AW, Amano Y, Ise K (2016) A new view of the tree of life. Nat Microbiol 1:16048
Hugenholtz P (2002) Exploring prokaryotic diversity in the genomic era. Genome Biol 3:REVIEWS0003
Ibrahim MH, Lebbe L, Willems A, Steinbüchel A (2016) Chelatococcusthermostellatus sp. nov., a new thermophile for bioplastic synthesis: comparative phylogenetic and physiological study. AMB Exp 6(1):1–9
Inan K, Ozer A, Guler HI, Belduz AO, Canakci S (2016) Brevibacillus gelatini sp. nov., isolated from a hot spring. Int J Syst Evol Microbiol 66(2):712–718
Islam T, Jensen S, Reigstad LJ, Larsen Ø, Birkeland N-K (2008) Methane oxidation at 55 C and pH 2 by a thermoacidophilic bacterium belonging to the Verrucomicrobia phylum. Proc Natl Acad Sci 105:300–304
Islas S, Velasco AM, Becerra A, Delaye L, Lazcano A (2003) Hyperthermophile and the origin and earliest evolution of life. Int Microbiol 6:87–94
Irfan M, Tayyab A, Hasan F, Khan S, Badshah M, Shah AA (2017) Production and characterization of organic solvent-tolerant cellulase from Bacillus amyloliquefaciens AK9 isolated from hot spring. Appl Biochem Biotechnol 182(4):1390–1402
Itoh T, Onishi M, Kato S, Iino T, Sakamoto M, Kudo T, Takashina T, Ohkuma M (2016) Athalassotoga saccharophila gen. nov., sp. nov., isolated from an acidic terrestrial hot spring, and proposal of Mesoaciditogales ord. nov. and Mesoaciditogaceae fam. nov. in the phylum Thermotogae. Int J Syst Evol Microbiol 66(2):1045–1051
Jahangir T, Khuhawar M, Leghari S, Leghari A (2001) Physico-chemical and biological study of Mangho Pir euthermal springs Karachi, Sindh, Pakistan. Online J Biol Sci 1:636–639
Javed M, Zahoor S, Sabar H, Haq I, Babar M (2012) Thermophilic bacteria from the hot springs of gilgit (Pakistan). J Anim Plant Sci 22:83–87
Jessen JE, Orlygsson J (2012) Production of ethanol from sugars and lignocellulosic biomass by Thermoanaerobacter J1 isolated from a hot spring in Iceland. Biomed Res Int 2012:86982
Jiang X, Takacs-Vesbach CD (2017) Microbial community analysis of pH 4 thermal springs in Yellowstone National Park. Extremophiles 21:135–152
Johnson M, Conners S, Montero C, Chou C, Shockley K, Kelly R (2006) The Thermotoga maritima phenotype is impacted by syntrophic interaction with Methanococcus jannaschii in hyperthermophilic coculture. Appl Environ Microbiol 72:811–818
Jolivet E, L’Haridon S, Corre E, Forterre P, Prieur D (2003) Thermococcus gammatolerans sp. nov., a hyperthermophilic archaeon from a deep-sea hydrothermal vent that resists ionizing radiation. Int J Syst Evol Microbiol 53:847–851
Jolivet E, Corre E, L’Haridon S, Forterre P, Prieur D (2004) Thermococcus marinus sp. nov. and Thermococcus radiotolerans sp. nov., two hyperthermophilic archaea from deep-sea hydrothermal vents that resist ionizing radiation. Extremophiles 8:219–227
Joyce GF (2002) The antiquity of RNA-based evolution. Nature 418:214–221
Kambura AK, Mwirichia RK, Kasili RW, Karanja EN, Makonde HM, Boga HI (2016) Bacteria and Archaea diversity within the hot springs of Lake Magadi and Little Magadi in Kenya. BMC Microbiol 16:136
Kashefi K, Lovley DR (2003) Extending the upper temperature limit for life. Science 301:934–934
Kelley DS, Baross JA, Delaney JR (2002) Volcanoes, fluids, and life at mid-ocean ridge spreading centers. Annu Rev Earth Planet Sci 30:385–491
Kelley DS, Karson JA, Früh-Green GL, Yoerger DR, Shank TM, Butterfield DA, Hayes JM, Schrenk MO, Olson EJ, Proskurowski G (2005) A serpentinite-hosted ecosystem: the Lost City hydrothermal field. Science 307:1428–1434
Kimura H, Sugihara M, Yamamoto H, Patel BK, Kato K, Hanada S (2005) Microbial community in a geothermal aquifer associated with the subsurface of the Great Artesian Basin, Australia. Extremophiles 9:407–414
Korn-Wendisch F, Rainey F, Kroppenstedt R, Kempf A, Majazza A, Kutzner H, Stackebrandt E (1995) Thermocrispum gen. nov., a new genus of the order Actinomycetales, and description of Thermocrispum municipale sp. nov. and Thermocrispum agreste sp. nov. Int J Syst Evol Microbiol 45:67–77
Kojima H, Umezawa K, Fukui M (2016) Caldimicrobium thiodismutans sp. nov., a sulfur-disproportionating bacterium isolated from a hot spring, and emended description of the genus Caldimicrobium. Int J Syst Evol Microbiol 66(4):1828–1831
Kojima H, Watanabe M, Fukui M (2017) Sulfuritortus calidifontis gen. nov., sp. nov., a novel sulfur oxidizer isolated from a hot spring microbial mat. Int J Syst Evol Microbiol 67(5):1355–1358
Khan IU, Hussain F, Tian Y, Habib N, Xian W-D, Jiang Z, Amin A, Yuan C-G, Zhou E-M, Zhi X-Y (2017) Tibeticola sediminis gen. nov., sp. nov., a thermophilic bacterium isolated from hot spring. Int J Syst Evol Microbiol 67(5):1133–1139
Kumar N, Lad G, Giuntini E, Kaye ME, Udomwong P, Shamsani NJ, Young JPW, Bailly X (2015) Bacterial genospecies that are not ecologically coherent: population genomics of Rhizobium leguminosarum. Open Biol 5:140133
Lau MC, Aitchison JC, Pointing SB (2009) Bacterial community composition in thermophilic microbial mats from five hot springs in Central Tibet. Extremophiles 13:139–149
Lazcano A, Bada JL (2003) The 1953 Stanley L. Miller experiment: fifty years of prebiotic organic chemistry. Orig Life Evol Biosph 33:235–242
Lee Y-E, Jain MK, Lee C, Zeikus JG (1993) Taxonomic distinction of saccharolytic thermophilic anaerobes: description of Thermoanaerobacterium xylanolyticum gen. nov., sp. nov., and Thermoanaerobacterium saccharolyticum gen. nov., sp. nov.; reclassification of Thermoanaerobium brockii, Clostridium thermosulfurogenes, and Clostridium thermohydrosulfuricum E100-69 as Thermoanaerobacter brockii comb. nov., Thermoanaerobacterium thermosulfurigenes comb. nov., and Thermoanaerobacter thermohydrosulfuricus comb. nov., respectively; and transfer of Clostridium thermohydrosulfuricum 39E to Thermoanaerobacter ethanolicus. Int J Syst Evol Microbiol 43:41–51
Lee JY, Iglesias B, Chu CE, Lawrence DJ, Crane EJ III (2017) Pyrobaculum igneiluti sp. nov., a novel anaerobic hyperthermophilic archaeon that reduces thiosulfate and ferric iron. Int J Syst Evol Microbiol 67:1714–1719
Levy M, Miller SL (1998) The stability of the RNA bases: implications for the origin of life. Proc Natl Acad Sci 95:7933–7938
López G, Cañas-Duarte S, Pinzón-Velasco A, Vega-Vela N, Rodríguez M, Restrepo S, Baena S (2017) Description of a new anaerobic thermophilic bacterium thermoanaerobacterium butyriciformans sp nov. Syst Appl Microbiol 40(2):86–91
Luo G, Wang W, Angelidaki I (2013) Anaerobic digestion for simultaneous sewage sludge treatment and CO biomethanation: process performance and microbial ecology. Environ Sci Technol 47:10685–10693
Magurran AE (2013) Measuring biological diversity. Wiley, Malden
Marteinsson VT, Birrien J-L, Reysenbach A-L, Vernet M, Marie D, Gambacorta A, Messner P, Sleytr UB, Prieur D (1999) Thermococcus barophilus sp. nov., a new barophilic and hyperthermophilic archaeon isolated under high hydrostatic pressure from a deep-sea hydrothermal vent. Int J Syst Evol Microbiol 49:351–359
Merkel AY, Pimenov NV, Rusanov II, Slobodkin AI, Slobodkina GB, Tarnovetckii IY, Frolov EN, Dubin AV, Perevalova AA, Bonch-Osmolovskaya EA (2017) Microbial diversity and autotrophic activity in Kamchatka hot springs. Extremophiles 21:307–317
Mesbah NM, Wiegel J (2005) Halophilic thermophiles: a novel group of extremophiles. Microbial diversity: current perspectives and potential applications. IK Publishing House, New Delhi, pp 91–118
Miller SL, Bada JL (1988) Submarine hot springs and the origin of life. Nature 334:609–611
Miller SR, Strong AL, Jones KL, Ungerer MC (2009) Bar-coded pyrosequencing reveals shared bacterial community properties along the temperature gradients of two alkaline hot springs in Yellowstone National Park. Appl Environ Microbiol 75:4565–4572
Mizobata T, Kagawa M, Murakoshi N, Kusaka E, Kameo K, Kawata Y, Nagai J (2000) Overproduction of Thermus sp. YS 8-13 manganese catalase in Escherichia coli. Eur J Biochem 267:4264–4271
Mrabet NT, Van den Broeck A, Stanssens P, Laroche Y, Lambeir A, Matthijssens G, Jenkins J, Chiadmi M, Van Tilbeurgh H (1992) Arginine residues as stabilizing elements in proteins. Biochemistry 31:2239–2253
Nakagawa T, Fukui M (2002) Phylogenetic characterization of microbial mats and streamers from a Japanese alkaline hot spring with a thermal gradient. J Gen Appl Microbiol 48:211–222
Norris TB, Wraith JM, Castenholz RW, McDermott TR (2002) Soil microbial community structure across a thermal gradient following a geothermal heating event. Appl Environ Microbiol 68:6300–6309
Nübel U, Bateson MM, Vandieken V, Wieland A, Kühl M, Ward DM (2002) Microscopic examination of distribution and phenotypic properties of phylogenetically diverse Chloroflexaceae-related bacteria in hot spring microbial mats. Appl Environ Microbiol 68:4593–4603
Okanishi H, Kim K, Fukui K, Yano T, Kuramitsu S, Masui R (2017) Proteome-wide identification of lysine succinylation in thermophilic and mesophilic bacteria. Biochim Biophys Acta 1865:232–242
Olsen GJ, Woese CR, Overbeek R (1994) The winds of (evolutionary) change: breathing new life into microbiology. J Bacteriol 176:1–6
Orphan V, Goffredi SK, Delong E, Boles J (2003) Geochemical influence on diversity and microbial processes in high temperature oil reservoirs. Geomicrobiol J 20:295–311
Pace NR (1991) Origin of life-facing up to the physical setting. Cell 65:531–533
Pace NR (1997) A molecular view of microbial diversity and the biosphere. Science 276:734–740
Pang J, Allemann RK (2007) Molecular dynamics simulation of thermal unfolding of Thermotoga maritima DHFR. Phys Chem Chem Phys 9:711–718
Pasternak Z, Pietrokovski S, Rotem O, Gophna U, Lurie-Weinberger MN, Jurkevitch E (2013) By their genes ye shall know them: genomic signatures of predatory bacteria. ISME J 7:756–769
Pearson A, Huang Z, Ingalls A, Romanek C, Wiegel J, Freeman K, Smittenberg R, Zhang C (2004) Nonmarine crenarchaeol in Nevada hot springs. Appl Environ Microbiol 70:5229–5237
Pedersen K (2000) Exploration of deep intraterrestrial microbial life: current perspectives. FEMS Microbiol Lett 185:9–16
Petrov AS, Gulen B, Norris AM, Kovacs NA, Bernier CR, Lanier KA, Fox GE, Harvey SC, Wartell RM, Hud NV (2015) History of the ribosome and the origin of translation. Proc Natl Acad Sci 112:15396–15401
Pérez-Rodríguez I, Rawls M, Coykendall DK, Foustoukos DI (2016) Deferrisoma palaeochoriense sp. nov., a thermophilic, iron (III)-reducing bacterium from a shallow-water hydrothermal vent in the Mediterranean Sea. Int J Syst Evol Microbiol 66(2):830–836
Pledger RJ, Crump BC, Baross JA (1994) A barophilic response by two hyperthermophilic, hydrothermal vent Archaea: an upward shift in the optimal temperature and acceleration of growth rate at supra-optimal temperatures by elevated pressure. FEMS Microbiol Ecol 14:233–241
Porter SS, Chang PL, Conow CA, Dunham JP, Friesen ML (2017) Association mapping reveals novel serpentine adaptation gene clusters in a population of symbiotic Mesorhizobium. ISME J 11:248–262
Ranawat P, Rawat S (2017) Stress response physiology of thermophiles. Arch Microbiol 199:391–414
Reysenbach A-L, Shock E (2002) Merging genomes with geochemistry in hydrothermal ecosystems. Science 296:1077–1082
Reysenbach A-L, Longnecker K, Kirshtein J (2000) Novel bacterial and archaeal lineages from an in situ growth chamber deployed at a Mid-Atlantic Ridge hydrothermal vent. Appl Environ Microbiol 66:3798–3806
Russell N, Harrisson P, Johnston I, Jaenicke R, Zuber M, Franks F, Wynn-Williams D (1990) Cold adaptation of microorganisms. Philos Trans R Soc Lond B 326:595–611
Salem RB, Abbassi MS, Cayol J-L, Bourouis A, Mahrouki S, Fardeau M-L, Belhadj O (2016) Thermophilic Bacillus licheniformis rbs 5 isolated from hot tunisian spring co-producing alkaline and thermostable [alpha]-amylase and protease enzymes. J Microbiol Biotechnol Food Sci 5(6):557
Sarker MR, Akhtar S, Torres JA, Paredes-Sabja D (2015) High hydrostatic pressure-induced inactivation of bacterial spores. Crit Rev Microbiol 41:18–26
Shah AA, Nawaz A, Kanwal L, Hasan F, Khan S, Badshah M (2015) Degradation of poly (ε-caprolactone) by a thermophilic bacterium Ralstonia sp. strain MRL-TL isolated from hot spring. Int Biodeterior Biodegrad 98:35–42
Sharp C, Stott M, Dunfield P (2012) Detection of autotrophic verrucomicrobial methanotrophs in a geothermal environment using stable isotope probing. Front Microbiol 3:303
Shin K-S, Shin YK, Yoon J-H, Park Y-H (2001) Candida thermophila sp. nov., a novel thermophilic yeast isolated from soil. Int J Syst Evol Microbiol 51:2167–2170
Shockley KR, Ward DE, Chhabra SR, Conners SB, Montero CI, Kelly RM (2003) Heat shock response by the hyperthermophilic archaeon Pyrococcus furiosus. Appl Environ Microbiol 69:2365–2371
Shuja TA (1986) Geothermal areas in Pakistan. Geothermics 15:719–723
Sievert SM, Kuever J, Muyzer G (2000) Identification of 16S ribosomal DNA-defined bacterial populations at a shallow submarine hydrothermal vent near Milos Island (Greece). Appl Environ Microbiol 66:3102–3109
Skirnisdottir S, Hreggvidsson GO, Hjorleifsdottir S, Marteinsson VT, Petursdottir SK, Holst O, Kristjansson JK (2000) Influence of sulfide and temperature on species composition and community structure of hot spring microbial mats. Appl Environ Microbiol 66:2835–2841
Slobodkin A, Tourova T, Kuznetsov B, Kostrikina N, Chernyh N, Bonch-Osmolovskaya E (1999) Thermoanaerobacter siderophilus sp. nov., a novel dissimilatory Fe (III)-reducing, anaerobic, thermophilic bacterium. Int J Syst Evol Microbiol 49:1471–1478
Slobodkina GB, Baslerov RV, Novikov AA, Viryasov MB, Bonch-Osmolovskaya EA, Slobodkin AI (2016) Inmirania thermothiophila gen. nov., sp. nov., a thermophilic, facultatively autotrophic, sulfur-oxidizing gammaproteobacterium isolated from a shallow-sea hydrothermal vent. Int J Syst Evol Microbiol 66(2):701–706
Stackebrandt E (2014) The family Gracilibacteraceae and transfer of the genus Lutispora into Gracilibacteraceae. In: The prokaryotes. Springer, Berlin, Heidelberg, pp 149–151
Strazzulli A, Iacono R, Giglio R, Moracci M, Cobucci-Ponzano B (2017) Metagenomics of hyperthermophilic environments: biodiversity and biotechnology. In: Microbial ecology of extreme environments. Springer, Cham, pp 103–135
Summit M, Scott B, Nielson K, Mathur E, Baross J (1998) Pressure enhances thermal stability of DNA polymerase from three thermophilic organisms. Extremophiles 2:339–345
Szewzyk U, Szewzyk R, Stenström T (1994) Thermophilic, anaerobic bacteria isolated from a deep borehole in granite in Sweden. Proc Natl Acad Sci 91:1810–1813
Szilágyi A, Závodszky P (2000) Structural differences between mesophilic, moderately thermophilic and extremely thermophilic protein subunits: results of a comprehensive survey. Structure 8:493–504
Takahata Y, Nishijima M, Hoaki T, Maruyama T (2000) Distribution and physiological characteristics of hyperthermophiles in the Kubiki oil reservoir in Niigata, Japan. Appl Environ Microbiol 66:73–79
Tripathi C, Mahato NK, Singh AK, Kamra K, Korpole S, Lal R (2016) Lampropedia cohaerens sp. nov., a biofilm-forming bacterium isolated from microbial mats of a hot water spring, and emended description of the genus Lampropedia. Int J Syst Evol Microbiol 66(3):1156–1162
Tripathy S, Padhi SK, Mohanty S, Samanta M, Maiti NK (2016a) Analysis of the metatranscriptome of microbial communities of an alkaline hot sulfur spring revealed different gene encoding pathway enzymes associated with energy metabolism. Extremophiles:1–12
Tripathy S, Padhi SK, Sen R, Maji U, Samanta M, Mohanty S, Maiti NK (2016b) Draft genome sequence of Brevibacillus borstelensis cifa_chp40, a Thermophilic Strain Having Biotechnological Importance. J Gen 4:4
Wahlund TM, Woese CR, Castenholz RW, Madigan MT (1991) A thermophilic green sulfur bacterium from New Zealand hot springs, Chlorobium tepidum sp. nov. Arch Microbiol 156(2):81–90
Wang Z, Tong W, Wang Q, Bai X, Chen Z, Zhao J, Xu N, Liu S (2012) The temperature dependent proteomic analysis of Thermotoga maritima. PLoS One 7:e46463
Wang S, Hou W, Dong H, Jiang H, Huang L, Wu G, Zhang C, Song Z, Zhang Y, Ren H (2013) Control of temperature on microbial community structure in hot springs of the Tibetan Plateau. PLoS One 8:e62901
Wang Q, Cen Z, Zhao J (2015) The survival mechanisms of thermophiles at high temperatures: an angle of omics. Physiology 30:97–106
Ward DM, Ferris MJ, Nold SC, Bateson MM (1998) A natural view of microbial biodiversity within hot spring cyanobacterial mat communities. Microbiol Mol Biol Rev 62:1353–1370
Whitaker RJ, Grogan DW, Taylor JW (2003) Geographic barriers isolate endemic populations of hyperthermophilic archaea. Science 301:976–978
Wiegel J (1998) Anaerobic alkalithermophiles, a novel group of extremophiles. Extremophiles 2:257–267
Wiegel J, Ljungdahl LG (1981) Thermoanaerobacter ethanolicus gen. nov., spec. nov., a new, extreme thermophilic, anaerobic bacterium. Arch Microbiol 128:343–348
Wiegel J, Braun M, Gottschalk G (1981) Clostridium thermoautotrophicum species novum, a thermophile producing acetate from molecular hydrogen and carbon dioxide. Curr Microbiol 5:255–260
Wiegel J, Hanel J, Aygen K (2003) Chemolithoautotrophic thermophilic iron (III)-reducer. In: Biochemistry and physiology of anaerobic Bacteria. Springer, New York, NY, pp 235–251
Wilde SA, Valley JW, Peck WH, Graham CM (2001) Evidence from detrital zircons for the existence of continental crust and oceans on the Earth 4.4 Gyr ago. Nature 409:175–178
Wilson EO (1992) The diversity of life. WW Norton & Company, New York
Xian W-D, Yin Y-R, Liu L, Yuan C-G, Hussain F, Khan I, Habib N, Zhou E-M, Li W-J (2016) Brevibacillus sediminis sp. nov., isolated from a hot spring. Int J Syst Evol Microbiol 66(2):548–553
Xue Y, Xu Y, Liu Y, Ma Y, Zhou P (2001) Thermoanaerobacter tengcongensis sp. nov., a novel anaerobic, saccharolytic, thermophilic bacterium isolated from a hot spring in Tengcong, China. Int J Syst Evol Microbiol 51:1335–1341
Yim LC, Hongmei J, Aitchison JC, Pointing SB (2006) Highly diverse community structure in a remote central Tibetan geothermal spring does not display monotonic variation to thermal stress. FEMS Microbiol Ecol 57:80–91
Yoon Y, Lee H, Lee S, Kim S, Choi K-H (2015) Membrane fluidity-related adaptive response mechanisms of foodborne bacterial pathogens under environmental stresses. Food Res Int 72:25–36
Zhao W-D, Romanek CS, Mills G, Wiegel J, Zhang C-L (2005) Geochemistry and microbiology of hot springs in Kamchatka. Russ Geol J China Univ 11:217–223
Zillig W, Stetter KO, Wunderl S, Schulz W, Priess H, Scholz I (1980) The Sulfolobus-“Caldariella” group: taxonomy on the basis of the structure of DNA-dependent RNA polymerases. Arch Microbiol 125(3):259–269
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Amin, A., Ahmed, I., Khalid, N., Zhang, Y., Xiao, M., Li, WJ. (2018). Insights into the Thermophile Diversity in Hot Springs of Pakistan. In: Egamberdieva, D., Birkeland, NK., Panosyan, H., Li, WJ. (eds) Extremophiles in Eurasian Ecosystems: Ecology, Diversity, and Applications. Microorganisms for Sustainability, vol 8. Springer, Singapore. https://doi.org/10.1007/978-981-13-0329-6_1
Download citation
DOI: https://doi.org/10.1007/978-981-13-0329-6_1
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-0328-9
Online ISBN: 978-981-13-0329-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)