Abstract
Stroke is caused by a lack of blood supply in a particular region of the brain that may be a consequence of clot formation or rupture of the blood vessel. It presents high incidence, generating permanent neurological sequelae, which leads to motor impairment and functional limitations. It is necessary to identify techniques of motor rehabilitation that favour the cortical reorganization and the functional recovery of affected individuals. Motor imagery (MI) is a technique used in motor rehabilitation of individuals with motor deficit, defined as mental simulation of movements without movement actually occurring. MI activates the same cortical regions as the execution of the movement, especially the motor area and the somatosensory cortex. The objective of this study was to investigate changes in cortical activity related to MI rehabilitation (during 1 month—three session/week) in hemiparetic individuals after stroke. EEG signals from eight post-stroke individuals were registered one week before MI rehabilitation (BMI) and 1 month after rehabilitation (Follow-up), during motor task execution (MOV) and motor task imagination (IMG). Magnitude squared of coherence (MSC) was evaluated, using 50 epochs of the EEG signal considered without artefact. The delta band presented the highest MSC values in both conditions (MOV and IMG), mainly in frontal and central regions. Only two volunteers presented MSC values in the Follow-up period higher than in the period before mental rehabilitation, mainly on electrodes F3 (p = 0.04), C3 (p = 0.036) and F4 (p = 0.02).
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Stroke affects about 15 million people annually, with one-third of individuals showing some sort of permanent neurological sequelae. This disease presents high incidence and prevalence, generating sequels that lead to motor impairment and functional limitations. Due to the clinical relevance of this condition, it is necessary to identify motor rehabilitation techniques that improve cortical reorganization and functional recovery of these individuals [1, 2]. Motor imagery (MI) is one of these techniques for rehabilitation after stroke [3].
The definition of MI is a mental simulation of movements without actual movement [3]. MI promotes activation of motor and somatosensory cortex areas, which are the same cortical regions activated during movement [4]. Studies considered 1st person MI (MI kinesthetic) an important therapeutic tool in motor recovery of post-stroke individuals, mainly due to hemiparesis, which affects active movement, making it difficult for motor rehabilitation [2, 5].
Electroencephalography (EEG) signals are used in the evaluation of sensory and cognitive processes. One of the main methods of electrophysiological analysis is the identification of process-related or event-related cortical activation by EEG signal analysis. Magnitude-Squared Coherence is a technique used in the analysis of somatosensory responses in individuals without neurological dysfunctions [6]. The MSC evaluates the linear dependence between the harmonic components of the stimulus (or motor task) and the brain response. In post-stroke individuals, cortical patterns have been poorly evaluated, requiring further studies to understand how and to what degree the MI influences the brain electrical activity of these individuals [5].
The objective of this study was to investigate changes in cortical activity based on MSC of EEG signals related to MI rehabilitation in hemiparetic individuals after stroke.
2 Methodology
2.1 Volunteers
The EEG signals of post-stroke individuals were recorded following the protocol approved by the Ethics Committee of the Federal University of Minas Gerais (ETIC 467/08). Signal analysis was realized at the Laboratory of Signal Processing and Medical Imaging (LAPIS) at the Federal University of Rio de Janeiro.
The sample consisted of nine volunteers (5 women) with mean age of 42.2 ± 12.2 years (between 23 and 54 years) and injury time ranging from 7 to 24 months (mean of 13 ± 6.5 months), with 5 right-handed individuals. Inclusion criteria: age between 20 and 60 years; diagnosis of unilateral stroke for more than 6 months; participation in a physiotherapy program for strengthening and stretching the muscles of the paretic upper limb since the acute phase of the stroke; commitment of its dominant side; without cognitive deficit (score > 18 in Mini Mental Health Examination—MESM); capability to perform active flexion of at least 10° from the neutral wrist, metacarpophalangeal and interphalangeal position of the thumb [2]. Participants did not use any medication that would interfere with the EEG signal. Exclusion criteria: Difficulty in performing motor imagery, identified by score ≤ 4 in the questions of the revised motor imagination questionnaire (MIQ-RS) (Gregg et al. 2007).
Motor rehabilitation consisted of conventional physiotherapeutic intervention (CPI) associated with MI. A physical therapy practitioner performed CPI in post stroke subjects for 4 weeks (30 min per session, 3 times per week). The CPI emphasized stretching of the flexor muscles and strengthening of the flexor and extensor muscles of the shoulder, elbow and wrist, in addition to the scapular muscles. The IM tasks were in first-person perspective (where the individual imagines to perform the movements) and selected according to the individual’s goal, being specific to each one. For example, individual #1 focused on picking glass and can (1st week), manipulating objects (2nd week), using cutlery (3rd week) and combing hair (4th week), while individual #9 focused on opening and closing jars to direct, to use cutlery and to button clothes and shoes in the first, second, third and fourth weeks, respectively. The intensity of the tasks was progressive, starting with simpler tasks and evolving to more complex tasks, focusing on the reach and grasp of certain objects [2]. Further details of the rehabilitation process can be found at [2].
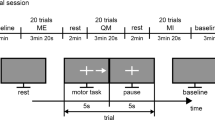
Data acquisition. The experiment started with volunteers positioned in a comfortable armchair, with supported upper limbs. EEG signals acquired in two periods: a week before MR (BMR) and 1 month after MR (Follow-up). At each recording session, there were collected: (1) 15 min of spontaneous EEG; (2) 15 min of grasping movement in dominant hand (MOV) and (3) 15 min of grasping MI in dominant hand (IMG). EEG signal was recorded at 17 electrodes placed according to the international 10–20 system. A biological signal amplifier (BrainNET BNT-36) was used, presenting bandpass filter between 0.1 and 100 Hz, notch filter at 60 and 600 Hz sampling frequency.
The EEG signal was recorded using a protocol similar to that in Santos Filho et al. (2009). The red LED was triggered 4 s before the motor task, remaining until the beginning of the task. The yellow LED was activated 3 s after the red LED was, remaining on for 1 s until the motor task start. The LEDs had different functions in the protocol: the red LED was used to focus the attention of the participant in the procedure and the yellow LED indicated preparation for task. After the 4-s interval, both LEDs were turned off indicating the order of accomplishment of the task. Individuals performed the task after the shutdown of both LEDs.
EEG signals were filtered with a 2nd-order Butterworth bandpass (0.1–40 Hz) and segmented into 14-s epochs (−4 to 10 s), synchronized with the motor task execution (t = 0 s). The interval (−1 and 3 s) was selected, where the response related to the motor task would be expected [6]. Epochs considered to have artefacts (visually identified) were rejected. Finally, Magnitude-Squared Coherence (MSC) was estimated for each volunteer in both conditions MOV and IMG. EEG signal from one of the individuals was excluded because it was highly contaminated by artefacts. For statistical analysis, the Wilcoxon test was used to compare the BMR and Follow-up periods for each volunteer, both in the MOV and IMG conditions, considered as statistically significant values of p < 0.05.
Magnitude-Squared Coherence (MSC). Technique proposed to test for the presence of evoked responses in EEG signal [6, 7]. Coherence between two signals x(n) and y(n) is defined as the cross-correlation spectrum between these signals and quantifies the linear dependence between their harmonic components. Assuming x(n) to be the EEG signal registered from the scalp and y(n) identical in all epochs of these signal (i.e. an intermittent stimulation or given task), MSC can be estimated as:
where Xi(f) is the Discrete Fourier Transform (DFT) of the i-th epoch of x(n) and M, the number of epochs in the signal. For the case of lack of coherence between the signals x(n) and y(n) (null hypothesis), MSC follows a beta distribution with the following parameters [8]:
where β(1,M−1) is the standard beta distribution with parameters 1 and M − 1. The critical value of MSC for a significance level α, considering the null hypothesis test for the absence of stimulus, is calculated by [8]:
In the absence of response to the event, MSC tends asymptotically to 0 when M tends to infinity. At frequency f, the presence of synchronized response to the event is expected for MSC(f) > MSCcrit.
3 Results
The number of epochs without artefacts was 50 (M = 50), leading to MSCcrit equal to 0.0593 (Eq. 3). The delta band (<4 Hz) presented higher MSC values, especially in frontal and central cortical regions in both conditions, MOV and IMG (Fig. 1). In this band, the Follow-up and BMR periods were compared, for each volunteer, in each conditions MOV and IMG.
Three volunteers (#1, #5 and #8) presented statistically significant differences (p < 0.05) in MSC values between periods Follow-up and BMR (Table 1). In volunteers #1 and #8, F3 (Fig. 1a) and C3 (Fig. 1b) electrodes in condition IMG and F4 electrode in condition MOV (Fig. 1c) had higher MSC values in period Follow-up. On the other hand, volunteer #5 exhibited opposite behaviour (i.e., BMR > Follow-up) in C3 and F3 electrodes in condition MOV and F3 electrode in condition IMG.
4 Discussion
Acute influence of motor rehabilitation with MI on cortical activity has been the focus of recent studies [9, 10]. In volunteers without neurological dysfunctions, Paz et al. [10] found increased values of MSC in the delta band (≤4 Hz) after MI training [10]. The delta band is related to the accomplishment of mental tasks, mainly to the difficulty of the task and to the inhibition of stimuli unrelated to the performance of the task [11]. The increase in neural efficiency and the favoring of motor learning are related to higher values of coherence in the delta band [10].
Our work showed that, during the execution of the motor task in individuals after stroke, the delta band had the highest MSC values. The initial hypothesis, in relation to the conditions studied (BMR and Follow-up), was that the effect of training with MI would be similar to that occurring in normal volunteers (MSC in Follow-up > MSC in BMR) [10].
Post-stroke volunteers showed motor improvement after MI training, assessed by the Minnesota Manual Dexterity Test (MMDT) and gait speed [2]. However, despite motor improvement, the MSC of 5 volunteers did not present statistically significant differences between BMR and Follow-up conditions. Only Two volunteers presented MSC values in the Follow-up period higher than in BMR (p < 0.05) at F3, C3 and F4 electrodes. The other volunteer presented opposite results, exhibiting higher values in the BMR period (p < 0.05), at C3 and F3 electrodes.
In the volunteers who presented difference, the results suggest that the motor task in Follow-up was more synchronized to the stimulus than in BMR because it presented higher MSC values. However, in most individuals, there was no difference between the periods studied. It is speculated that the brain region and extension of the lesion may influence this aspect. Damage to the sensorimotor cortex due to a cortical stroke can result in a reduction in the size of the sensorimotor cortex and thus to the processing capacity of the cortex, as assessed by neurophysiological markers [12].
Future studies will be needed to improve results. Evaluation of individuals (e.g., laterality and stroke extension), in addition to EEG signal analysis by other processing techniques (e.g., Event-related EEG synchronization and desynchronization and Entropy) will be important for understanding the physiological mechanisms involved.
References
WHO Stroke, Cerebrovascular accident, http://www.who.int/topics/cerebrovascular_accident/en/, last accessed 2016/11/15.
Paz, CCSC., Teixeira-Salmela, LF., Tierra- Criollo, CJ. The addition of functional task-oriented mental practice to conventional physical therapy improves motor skills in daily functions after stroke. Braz J Phys Ther. 17(6):564–571 (2013).
Jeannerod, M. The representing brain: Neural correlates of motor intention and imagery. Behavioral and Brain Sciences, 17 (2):187–245 (1994).
Jackson PL et al. Potential role of mental Practice using motor imagery in neurologic rehabilitation. Archives of Physical Medicine Rehabilitation. 82:1133–41 (2001).
Sharma, N., Pomeroy, V.M., Baron, J.C. Motor Imagery A Backdoor to the Motor System After Stroke? Stroke 37:1941–1952 (2006).
Santos Filho et al. Magnitude Squared of Coherence to Detect Imaginary Movement. EURASIP Journal on Advances in Signal Processing. 2009(1): 1–10 (2009).
Gregg et al. The MIQ-RS: A Suitable Option for Examining Movement Imagery Ability. eCAM Advance Access (2007).
Miranda de Sá, AM., Felix, LB., Infantosi AF. A matrix-based algorithm for estimating multiple coherence of a periodic signal and its application to the multichannel EEG during sensory stimulation. IEEE Trans Biomed Eng. 51(7):1140–6 (2004).
Page et al. Cortical plasticity following motor skill learning during mental practice in stroke. Neurorehabil Neural Repair. 23(4):382–8 (2009).
Paz CCSC et al. A influência aguda da prática mental sobre as oscilações corticais delta – um estudo piloto. Rev. Bras. Eng. Biom., 28(4): 375–386 (2012).
Harmony T et al. Do specific EEG frequencies indicate different processes during mental calculation? Neuroscience Letters. 266:25–8 (1999).
Campfens at al. Poor motor function is associated with reduced sensory processing after stroke. Experimental Brain Research. 233(4):1339–1349 (2015).
Acknowledgements
This research was supported by the Brazilian development agencies Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
The authors declare that they have no conflict of interest. And the Federal University of Minas Gerais (UFMG) Ethics Committee approved the project.
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
da Silva, L.C.P., Paz, C.C.S.C., Miranda de Sá, A.M.F.L., Tierra-Criollo, C.J. (2019). EEG Coherence Analysis in Subjects After Rehabilitation from Stroke with Motor Imagery. In: Lhotska, L., Sukupova, L., Lacković, I., Ibbott, G. (eds) World Congress on Medical Physics and Biomedical Engineering 2018. IFMBE Proceedings, vol 68/2. Springer, Singapore. https://doi.org/10.1007/978-981-10-9038-7_61
Download citation
DOI: https://doi.org/10.1007/978-981-10-9038-7_61
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-9037-0
Online ISBN: 978-981-10-9038-7
eBook Packages: EngineeringEngineering (R0)