Abstract
Background: Diffusion Weighted (DW) and Dynamic Contrast Enhanced (DCE) MRI have been used in therapy assessment of several tumors. Purpose: The aim of this paper is to explore the possibility to apply DW- and DCE-MRI in Locally Advanced Rectal Cancer (LARC) to predict complete pathological response (CPR) to short-course radio-therapy (SCRT). Methods: 34 patients with LARC underwent DCE and DW-MRI before and after SCRT. Afterwards, patients were surgically treated. Pathological responses were registered. Tumor Regression Grade (TRG) on a scale from 1 to 5 on the surgical sample was used to classify the response. Two groups (CPR = 1 complete pathological response and CPR = 0 partial response) were identified. The discriminative power of several features from DW and DCE MRI has been investigated. Specifically, diffusion features from the intra-voxel incoherent motion (IVIM) model have been computed by means of Siemens MR Body Diffusion ToolBox; in addition, diffusion kurtosis parameters have been estimated. As regards DCE features, we used the Standard Index of Shape (SIS) which our group has proposed in previous works. DW features and SIS have been evaluated on a statistical basis (Wilcoxon-Mann-Withney test for two independent samples, Area Under Curve of Receiver Operating Characteristics). Results: All diffusion features showed a significant variation after therapy; however, this difference was not significant to predict complete response. SIS was the only feature to show statistical significant difference between the two groups. ROC and tree-based analysis revealed an optimal cut-off of \( 78.26 \) with specificity and sensitivity of \( 0.93 \) and \( 0.86 \) respectively.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Rectal cancer is one of the most spread cancers among men [1]. Commonly used therapy involves radio-chemo therapy (CRT) followed by total mesorectal excision (TME) [2]. It has been observed that short course radiotherapy (SCRT) can have similar effects compared to conventional chemo-radio-therapy (CRT) [3].
In order to tailor the therapy to each specific patient is important to have non-invasive instruments for assessing the therapy response. In particular it is important to distinguish between complete response, which might lead to a conservative wait and see surgical approach, and partial response. MRI has been widely used in this field: specifically, diffusion weighted (DW-MRI) and dynamic contrast enhanced (DCE-MRI) have gained an increasing success [1, 4]. In fact, these two methods can provide useful ‘functional’ information not available with other methods.
In particular, DW-MRI gives information about the water diffusion (brownian motion). DW-MRI can be analyzed using a well known bi-exponential model known in literature as intra-voxel incoherent motion (IVIM) [5]. Model fitting provides an estimates of a few important tissue parameters. Another emerging model is the diffusion kurtosis which provides a measure of the non-gaussianity of the water diffusion [6].
As regards DCE-MRI, several methods have been proposed for analyzing time-intensity course of injected contrast agent: they can be subdivided into model-based [7] and model-free [8]. Our group has previously proposed a model-free based method for rectal cancer therapy evaluation which has been called standard index of shape (SIS) [9].
The aim of this study is to evaluate the discriminative power of IVIM and kurtosis parameters and SIS for the prediction of complete response to SCRT.
2 Methods
2.1 Patients
Thirty-four consecutive patients (age: 48–83, 26 male 8 female) at our institution have been included in the study. They all had a diagnosis of Locally Advanced Rectal Cancer (LARC). They underwent short-course radiotherapy before surgical resection: details in therapy can be found in [1]. Each patient underwent MR studies before and after SCR: baseline, on average 23.8 days before starting radiotherapy and delayed, on average 61.0 days after the end of SCR.
2.2 Specimens Evaluation
Hystopathological analysis has been performed using the Mandard system by an expert pathologist blinded to the results of MRI. Tumor Regression Grade has been assessed per each specimen. A score of TRG = 1 meant complete pathological response (CPR) i.e. absence of tumoral cells. TRG from 2 to 4 meant an increasing number of tumoral cells with respect to fibrotic tissue; TRG = 5 meant no therapy effect. More details on the scoring system can be found in [1].
2.3 Imaging
All examinations were performed at our institution using a Magnetom Symphony scanner 1.5T (Siemens Medical Systems, Erlangen, Germany).
2.3.1 Diffusion weighted MRI
DW-MRI imaging has been performed using the following DW-EPI sequence parameters: TR/TE = 2700/83 ms/ms, FOV = 136 × 160 mm × mm, matrix size = 230 × 272, ST = 4 mm (TR repetition time, TE echo time, FOV field of view, ST slice thickness). b-values were the following 0, 50, 100, 150, 300, 600, 800 s/mm2. Gradient directions were all three axis, direct trace imaging; voxel size was 1.7 × 1.7 × 4 mm3.
2.3.2 Dynamic Contrast Enhanced MRI
DCE-MRI imaging has been performed using the following FLASH 3D sequence parameters: TR/TE/FA = 9.8/4.76/25 ms/ms/deg, FOV = 330 × 247 mm×mm, ST = 3 mm. Temporal resolution was 35 s between two consecutive images. Voxel size 0.64 × 0.48 × 3 mm3.
2.4 Feature Extraction
Regions of interest (ROIs) were manually drawn appropriately by an expert radiologist. While IVIM analysis has been performed both before and after short-course radiotherapy, SIS has been evaluated only at the second MR examination because it involves a comparison of before—after therapy.
2.4.1 IVIM Parameters
Intravoxel incoherent motion parameters have been computed using the Siemens software MR Body Diffusion Toolbox. The IVIM model corresponds to the following bi-exponential equation [5]:
and the parameters have the following meanings: \( S(b) \) is the MR signal when a specific gradients \( b \)-value is used, \( f \) is the perfusion fraction (corresponding to the fraction of capillary vessels in the voxel), \( D \) is the diffusion coefficient (mm/s2) (Brownian motion coefficient), \( D^{*} \) is the pseudo-diffusion coefficient (mm/s2) (due to the motion of water in capillary vessels).
The Siemens software allowed ROI drawing and output ROI-averaged parameters.
2.4.2 Diffusion kurtosis Parameters
Under appropriate assumptions, DW signal can be modeled as [6]:
in which \( D \) is again the apparent diffusion coefficient and \( K \) is the kurtosis. Diffusion Kurtosis parameters have been computed using the Siemens software MR Body Diffusion Toolbox.
2.4.3 Standard Index of Shape
This model-free DCE parameter has been developed by our group in previous studies [1, 9,10,11]. The software for SIS computation has been developed at our institution (see Fig. 1); it is an Osirix plugin available upon request. SIS analysis involves the use of both before and after therapy MR exams. ROIs have been drawn manually by an expert radiologist. The software outputs ROI-averaged DCE-MRI semi-quantitative parameters and the SIS parameters which is a synthesis of the comparison between before/after therapy DCE-MRI.
2.5 Statistical Analysis
We performed mono- and multi-parametric analysis on each single DW feature for discriminating the two patient groups (CPR = 1 complete pathological response and CPR = 0 incomplete pathological response).
All tests have been considered significant for \( p\, < \,0.05 \). All statistical analysis have been performed in R [12].
2.5.1 Mono-parameter Analysis
First, the Wilcoxon-Mann-Withney test for two independent unmatched samples has been applied to each feature extracted from the before-therapy MR session. This has been done, in order to see if CPR could be predicted by some before-therapy feature. In this case we used only IVIM + kurtosis based features.
Second, we considered both before and after therapy features. Relative differences between before and after therapy MR have been evaluated using the following formula:
where \( X_{pre} \) is a generic feature evaluated before therapy while \( X_{post} \) is the same feature evaluated after therapy. In this case the Wilcoxon-signed-rank (paired data) test has been applied to relative differences.
ROC analysis was also made for the calculation of the best cut-off.
2.5.2 Multi-parametric Analysis
Further, classification and regression trees (CART) have been applied to all features [13]. Both the before-only and before-after features were analysed. CART is a particular type of non-linear classifier whose main advantage is that provides binary rules that are human interpretable.
3 Results
The result of the Wilcoxon-Mann-Withney test suggested that the groups CPR = 0 and CPR = 1 were not significantly different for all the IVIM + kurtosis features calculated on the pre-SCRT alone except the average and the standard deviation of \( D^{*} \). ROC analysis of these two features provided AUC 0.71 and 0.77, specificity and sensitivity 0.89, 0.57 and 0.51, 1.00 respectively.
Moreover, none of the relative differences pre-post of all IVIM + kurtosis features resulted significant at the Wilcoxon signed-rank test.
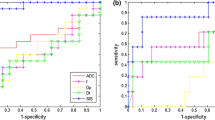
However, the SIS resulted in a significant difference between CPR = 0 and CPR = 1. In Fig. 2a the results of ROC analysis for SIS have been reported. The area under curve (AUC) was \( 0.89 \) while the best cut-off (Youden index) was \( 78.26 \) while specificity and sensitivity were \( 0.93 \) and \( 0.86 \) respectively. A further analysis based on CART provided results (see Fig. 2b) in line with the previous analysis and also the cut-off were coincident.
Receiver Operating Characteristic of the SIS parameter alone (a). Tree analysis reveals that SIS is indeed the most important feature in separating complete pathological response (CPR) from pathological response (PR) (b): each node of the tree reports the class contained in that node, the probability assigned to that class, the percentage of subjects in the node
4 Discussion
The aim of this study was to explore the possibility to predict, using DW- and DCE-MRI parameters, the Complete Pathologic Response of LARC patients to short-course radiotherapy.
Although all patients showed a modification in diffusion related parameters (IVIM + kurtosis) after therapy, the variation was not significant (with the exception of two features average and standard deviation of \( D^{*} \) with a low accuracy measured by ROC AUC) for distinguishing between CPR = 0 and CPR = 1.
In contrast, our previously proposed index, Standard index of Shape (SIS), showed a better behaviour: it was significantly different between CPR 0 and 1. Both conventional statistical analysis (Wilcoxon test) and tree based classification (CART) agreed upon the superiority of SIS.
One limit of this study is the set of \( b \)-values used for the diffusion kurtosis modelling: it should be pointed out that kurtosis imaging might require higher \( b \)-values; however, as this is an explorative study we attempted the kurtosis modelling aware of the possible limitations. In addition, although SIS has proven to be a relevant variable for complete response detection its interpretation is not completely clear, which will be the aim of future studies.
References
A. Petrillo, R. Fusco, V. Granata, S. V. Setola, M. Sansone, D. Rega, P. Delrio, F. Bianco, G. M. Romano, F. Tatangelo, A. Avallone, and B. Pecori, Mr imaging perfusion and diffusion analysis to assess preoperative short course radiotherapy response in locally advanced rectal cancer: Standardized index of shape by dce-mri and intravoxel incoherent motion-derived parameters by dw-mri. Med Oncol, vol. 34, p. 198, Nov 2017.
G. Tong, G. Zhang, J. Liu, Z. Zheng, Y. Chen, and E. Cui, A meta-analysis of short-term outcome of laparoscopic surgery versus conventional open surgery on colorectal carcinoma. Medicine (Baltimore), vol. 96, p. e8957, Dec 2017.
B. Ma, Q. Xu, Y. Song, P. Gao, and Z. Wang, Current issues of preoperative radio(chemo)therapy and its future evolution in locally advanced rectal cancer. Future Oncol, vol. 13, pp. 2489–2501, Nov 2017.
R. Fusco, M. Petrillo, V. Granata, S. Filice, M. Sansone, O. Catalano, and A. Petrillo, Magnetic resonance imaging evaluation in neoadjuvant therapy of locally advanced rectal cancer: A systematic review. Radiol Oncol, vol. 51, pp. 252–262, Sep 2017.
D. Le Bihan, E. Breton, D. Lallemand, P. Grenier, E. Cabanis, and M. Laval-Jeantet, Mr imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology, vol. 161, pp. 401–7, Nov 1986.
D. H. J. Poot, A. J. den Dekker, E. Achten, M. Verhoye, and J. Sijbers, Optimal experimental design for diffusion kurtosis imaging. IEEE Trans Med Imaging, vol. 29, pp. 819–29, Mar 2010.
S. P. Sourbron and D. L. Buckley, Classic models for dynamic contrast-enhanced mri. NMR Biomed, vol. 26, pp. 1004–27, Aug 2013.
A. Fabijańska, A novel approach for quantification of time-intensity curves in a dce-mri image series with an application to prostate cancer. Comput Biol Med, vol. 73, pp. 119–30, 06 2016.
A. Petrillo, R. Fusco, M. Petrillo, V. Granata, M. Sansone, A. Avallone, P. Delrio, B. Pecori, F. Tatangelo, and G. Ciliberto, Standardized index of shape (sis): a quantitative dce-mri parameter to discriminate responders by non-responders after neoadjuvant therapy in larc. Eur Radiol, vol. 25, pp. 1935–45, Jul 2015.
A. Petrillo, R. Fusco, M. Petrillo, V. Granata, P. Delrio, F. Bianco, B. Pecori, G. Botti, F. Tatangelo, C. Caracò, L. Aloj, A. Avallone, and S. Lastoria, Standardized index of shape (dce-mri) and standardized uptake value (pet/ct): Two quantitative approaches to discriminate chemo-radiotherapy locally advanced rectal cancer responders under a functional profile. Oncotarget, vol. 8, pp. 8143–8153, Jan 2017.
M. Petrillo, R. Fusco, O. Catalano, M. Sansone, A. Avallone, P. Delrio, B. Pecori, F. Tatangelo, and A. Petrillo, Mri for assessing response to neoadjuvant therapy in locally advanced rectal cancer using dce-mr and dw-mr data sets: A preliminary report. Biomed Res Int, vol. 2015, p. 514740, 2015.
R Development Core Team, R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria, 2008. ISBN 3-900051-07-0.
W.-Y. Loh, Classification and regression trees. Wiley Interdisciplinary Reviews: Data Mining and Knowledge Discovery, vol. 1, no. 1, pp. 14–23, 2011.
Acknowledgements
This work has been partially funded by the project n. BioMatMRI—000010–ALTRI-2017-M-SANSONE_001_001 at the Department of Electrical Engineering and Information Technologies (DIETI) of the University “Federico II”, Naples Italy.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Fusco, R., Sansone, M., Pepino, A., Petrillo, A. (2019). Diffusion and Perfusion MR Parameters in Locally Advanced Rectal Cancer Management: An Explorative Study. In: Lhotska, L., Sukupova, L., Lacković, I., Ibbott, G.S. (eds) World Congress on Medical Physics and Biomedical Engineering 2018. IFMBE Proceedings, vol 68/1. Springer, Singapore. https://doi.org/10.1007/978-981-10-9035-6_23
Download citation
DOI: https://doi.org/10.1007/978-981-10-9035-6_23
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-9034-9
Online ISBN: 978-981-10-9035-6
eBook Packages: EngineeringEngineering (R0)