Abstract
The emerging contaminants field suffers from insufficient global information, especially in Africa, and even less from Asia. Emerging contaminants constitute a group of natural, synthesised chemicals and microorganisms that have been proved to cause serious effects on the laboratory organisms and therefore a threat to human and aquatic species. They are presumed to be endocrine disruptors, carcinogenic, teratogenic, and interfere with the sexual and reproductive behaviour of some small aquatics. This being the case, the discovery of emerging contaminants stands to be the finest toxicological investigation owing to improved science and technology. Nevertheless, in future, the world will come up with new environmental discoveries because of the continuous production of materials, which by themselves may be harmful, or their life cycle may be a threat.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Emerging Contaminants (ECs)
- Nitrosodimethylamine (NDMA)
- Polybrominated Diphenyl Ethers (PBDEs)
- Dimethylnitrosamine
- Conventional Water Treatment Methods
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Emerging contaminants (ECs) are the potentially global, dynamic type of contaminants, representing unregulated chemical and microbial contaminants, which are anticipated to occur in the public water system, but lack public monitoring regulations. The circumstantial risks are uneven, vague and the advancement of science and technology extends the production of new contaminants that are expected to create more ecological risks. The limited traditional ecotoxicology approaches to environmental contaminants were not able to depict the occurrence of ECs until the discovery of advanced technologies, such as gas chromatography. These contaminants are not termed as emerging because they are new in terms of discovery, or the use of new advanced detection and treatment methods, but because they had been previously unrecognised, and there is a lack of standards and guidelines for their environmental monitoring. Recently, they have gained scientific attention because of their effects on health (Nosek et al. 2014).
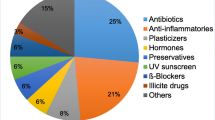
The public occurrence of ECs has being ongoing since the creation of the universe because of inorganic ECs such as cobalt, germanium and manganese. Nonetheless, reports on the scientifical discovery date back to 1735. Again, organic ECs such as industrial solvents, pharmaceuticals, pesticides, water disinfection by-products and perfluorinated surfactants were discovered after World War II. The first comprehensive research and reports of the environmental occurrence of ECs led by the US government under the environmental protection agency (US EPA) commenced in the 1970s (Snyder 2014). Significant amounts of ECs were reported worldwide, as summarised here under (Table 2.1).
The discovery of cobalt in 1735 for glasses and ceramics, germanium in 1886 for transistors, ziram in the 1940s as a pesticide, the first use of contraceptive pills in 1960 as an endocrine-disrupting hormone and the discovery of nanomaterials around the 1980s mark the potential history of the rise of ECs. However, in 1998, the first contaminant candidate list (CCL 1) was published by the US EPA with 10 microbes and 50 chemical contaminants. The next 2005 CCL 2 contained the same contaminants as CCL 1, but with integrated stakeholders’ recommendations. The CCL 3 of 2008 included 104 chemicals and 12 microbial contaminants (EPA 2009), whereas the latest CCL 3 of November 2016 contains about 94 chemicals and 12 microbial contaminants (EPA 2015).
The EC field suffers from insufficient global information, especially in Africa, and even less on Asia, the EU and later the US (Miraji et al. 2016). As for the USA, the EPA preferably uses the term “candidate” to express the essence of periodic (5 years) review of ECs based on their public health concern and requirements for regulatory decisions (US EPA 2016a). Apart from the scarcity of information some effects of ECs worth mentioning include hormonal interference in fishes, genotoxicity, carcinogenicity in laboratory animals, endocrine disruption and immune toxicity (Mortensen et al. 2014).
2 Sources of Emerging Contaminants
Subsequently, all sources of ECs, including military sites, mine tailings, agricultural fields, industrial units, waste treatment plants, pharmaceutical, clinical, construction and demolition waste, electronic waste and municipal wastewaters prevail in our environment; therefore, the public addressing of ECs is inevitable. Unforeseen sources of ECs are include landfills, accidental and intended spillage, improper disposal of cosmetics and waste drugs and swimming pools. To increase general ecological health and safety, and preventing future emergencies, the analytical world needs to develop analytical and clinical techniques for the identification of ECs in all matrices. Furthermore, by identifying the environmental occurrence, establishment, understanding of current and presumed future sources, transportation pathway and ecological risks upon exposure to ECs are needed. Later, we need to address eco-friendly techniques for the remediation of ECs in all environmental matrices.
In the traditional way, the natural and previously mentioned sources of ECs have lasted for decades to the point that they are common sources. In the modern way of classifying sources of ECs, the previously mentioned sources are termed as primary sources. Continuous release of ECs into the aquatic systems saturates absorbing sediments in the streams and receivers. Yet, the reversed process leads to the release of ECs from saturated sediments, thus becoming a secondary source, also referred as a sink (USDHHS 2015).
3 General Characteristics of Emerging Contaminants
Emerging contaminants (ECs) constitute a classical group of contaminants that have divergent chemistries, but share occurrences and lack primary regulations. The chemistry of ECs is characterised by slightly higher polarity, acidity, and alkalinity than natural environmental chemicals, thus making them unique. They are characterised by low levels, persistence, multiple sources, disease outbreaks, lack of standard biological tests, and entering the aquatic environment from the point and non-point sources. The organic nature of most ECs makes them hydrophobic; hence, they accumulate in the lipid-rich tissues (Ross and Ellis 2004). Their transport behavior and fate are not clear and policies to address the challenges are yet to be fixed. The previous and later arguments make ECs versatile to transportation through the ecological and food chains. In the presence of unpredicted human and natural changes in addition to yet to be discovered technologies such as zero emissions, environmental discoveries are expected. In such an unpredictable future, the next era of environmental pollutants resulting from the reactions among the existing and yet to be identified materials is to come soon or later. Conversely, once released into the environment, they undergo bioaccumulation, bioconcentration, persistence and wide range transport in the aquatic environment (Clarke and Cummins 2014). Despite the fact that there are no fully established national and international monitoring regulations, their suspected availability brings the potential for unclear health and ecological risks (Petrisor 2004; Richardson 2007; Lee et al. 2014; Qi et al. 2015).
4 Mobility of Emerging Contaminants
A typical non-polar compound such as some of the organic-type ECs is less reactive in aqueous media because of their equal sharing of electrons between atoms and its symmetrical arrangement of the polar bonds, making them hydrophobic (Stasinakis et al. 2013). Unless there are changes in the thermodynamic stability of a stable species, they will persist in the environment. Persistence is considered when the half-life of a chemical is more than 2 days in the air, 182 days in the water, 365 days in the sediments and 182 days in the soil (Government of Canada 1995). The persistence is associated with long periods of environmental existence, long-range transported bioaccumulation and thus adverse effects on the ecosystem (Bergman 2005).
The solubility facet of ECs, especially organic ones, is merely negligible; therefore, it plays a peculiar role in the persistence of ECs. Once in the aquatic system, most ECs remain suspended while moving with flowing water or remain still in the stagnant water. Surface adsorption on the rough sands, sediments, gravels, underlying bedrocks and settling at the bottom of stagnant water bodies are common scenarios. Surface adsorption on the aquatic and irrigated crops and absorption through direct feeding or through the skin of aquatic organisms are expected too. Human consumption of contaminated water through bathing, washing, and other anthropogenic needs, eating contaminated crops, fishes and animals results in accumulation in fatty tissues. Primary and secondary periodic releases, evaporations, sewage sludge and leaching re-concentrate the same contaminants as they circulate in the aquatic organisms, water, terrestrial organisms and soils, and then back to water (Clarke and Cummins 2014).
5 Ecological Risks of Emerging Contaminants
Simple scientific facts on the essence of ECs in the aquatic system may involve anti-malarial resistance to malaria parasites. Their DNA may have been altered by the presence of the type of drugs residual in the reproduction site. Likewise, continuous release of endocrine-disrupting hormones may alter the sexual behavior of aquatic organisms as the results interfere with breeding. Algal blooming resulted from nutrient pollution in the stagnant water releases algal toxins as an excretory waste, and when algae die their cells break down to release the same toxicant. The existing studies proved health effects in laboratory animals, meaning that short- or long-term exposure is a threat to humans too (Commettee Report 1999; Richardson et al. 2006; Guidotti 2009; Hansen 2007; Gothwal and Shashidhar 2014).
Conventional water treatment methods were considered effective to the point of releasing its treated water into rivers and public consumption before the discovery of ECs. In fact, there was no blame as the monitoring regulations accounted for only known contaminants. In such a situation, collected sludge was an effective source of farming nutrients. Unfortunately, the agricultural application of biosolids from wastewater treatment sludge causes the accumulation of ECs in the soil and then subsequently translocation into the food chain (Clarke and Cummins 2014). This state of affairs is similar to public treated drinking water, which was chlorinated, without knowing the presence of natural organic acids such as humic acids could result in the formation of harmful disinfestation by-products.
Once toxicants are in the human body, they undergo biotransformation via enzymatic oxidation, reduction and hydrolysis, followed by synthesis. In these processes, the body merely intends to detoxify, contrary to some resulting products being even more toxic than the original one. Consequently, they attack metabolic enzymes, cause cell membrane damage and uncoupling of oxidative phosphorylation (Bradberry et al. 2000). The extent of toxicity depends on the acute and chronic effects. In either case, the effect may be widespread or locally affect specific organs such as the central nervous system, circulatory system, liver, kidney, lungs, and skin. The biochemical attack varies among the toxicants, for example, the DNA-attacking agents such as benzopyrene and protein-attacking agents such as diclofenac can lead to mutation and/or activate tumor suppressors, which activate tumor followed by cancer. Apart from the above-mentioned effects, the failure of ATP synthesis and functioning is caused by cocaine slow-down or interfere with energy production in the body (Hussain 2013). Some toxicants may inactivate enzymes, attacking the immune system and leading to cellular changes.
6 Classification of Emerging Contaminants
Naturally occurring and synthetic ECs were all designed for a good ecological balance. The presence of guiding and monitoring standards is basically for environmental protection. Products such as prescribed drugs, biogenic hormones, nanomaterials, personal care products and artificial sweeteners are formulated for human, animal and plant consumption (Schultz et al. 2014). Unfortunately, the aquatic systems may contain chemical products such as perfluorinated surfactants, benzotriazoles, perchlorate, sunscreen/UV filters, flame reductants, algal toxins and emerging microbes that have accidentally arrived there.
Once in the environment, the classification of ECs brings challenges, merely because of the various classification approaches. For example, Madhumitha et al. (2013) classified ECS with regard to suspected health effects. A more comprehensive and supported approach is grouping them based on the sources, effects, uses and their chemistry (Richardson and Ternes 2011; Thomaidis 2012; Fawell and Ong 2012; Yang et al. 2014). Similarly applied to the US EPA, ECs are mainly classified into microbial contaminants and chemical contaminants. The latter can be sub-classified into inorganic- and organic-based contaminants. The organic forms of ECs are also classified into pharmaceuticals, industrial solvents, food stuffs such as artificial sweeteners etc.
In this context, the following groups of ECs are briefly covered: polybrominated diphenyl ethers (PBDEs), nitrosodimethylamine (NDMA), artificial sweeteners, personal care products, algal toxins, microbes, inorganic elements, illicit drugs, endocrine-disrupting hormones and pharmaceuticals.
6.1 Polybrominated Diphenyl Ethers
Polybrominated diphenyl ethers (PBDEs) are commercially synthesised by bromination of diphenyl ethers resulting in mixtures of brominated diphenyl ethers, with similar chemical structures to polychlorinated biphenyls (Fig. 2.1) (Siddiqi and Clinic 2003). The environmental toxicological effects of PBDEs include strong binding to the soil, dust and sediments; yet, they also reside in the aquatic environment (Bennett et al. 2015). They enter living organisms through dermal contact, inhalation, and ingestion and basically through the food chain gets, and are then deposited in the fatty tissues. They are likely carcinogenic to humans, as they have shown positive results in rats and mice.
Other potential adverse effects of PBDEs include endocrine/thyroid disruption, teratogenicity, weight-gain, decrease in glucose oxidation, interference with gene expression in a metabolic pathway, and other prenatally associated effects (Miraji et al. 2016; Chevrier et al. 2010; Vuong et al. 2016; US EPA 2014b). The use of PBDEs was banned and withdrawn from the market in Europe in 2003, followed by the USA in 2004 (USDHHS 2015).
6.2 Nitrosodimethylamine
Nitrosodimethylamine (NDMA) was at first synthesised and patented by (Richard and George 1957) through the reaction of dimethylamine and aqueous sodium nitrate in the presence of hydrochloric or sulfuric acid. NDMA is also produced as a by-product in wastewater chlorination and chlorination. NDMA is also industrially produced unintentionally in tanneries, pesticide, and rubber production through the reaction of alkylamines with nitrite salts and nitrous and nitrogen oxide (US EPA 2014a).
Apparently, NDMA is produced for research purposes; however, its natural production during industrial processes, metabolism and during water treatment contributes to environmental contamination. It is rarely found in the soil as it soon leaches into groundwater. Thus, its miscibility in water and nearly equal densities with water makes water the perfect residing medium. When exposed in the sunlight and/or biological processes, NDMA degrades with an estimated half-life of 16 min. Although NDMA is not persistent, it has been widely found in water sources (Richardson et al. 2006; Pal et al. 2014; Koumaki et al. 2015). It is through the food chain, direct food stuffs, drinking water, and whiskey, cosmetics such as shampoos and workplace exposure enable NDMA to get into the human body. Through studies in mice and rats, in which NDMA led to tumors in the liver, kidney, and lungs, NDMA is categorically considered carcinogenic, mutagenic and clastogenic to humans. That being the case, 0.7 ng/L concentration has been set as a cancer risk level for direct human consumption (Liteplo et al. 2002; US EPA 2014a; Ooka et al. 2016).
6.3 Artificial Sweeteners
Sucralose is a non-calorific artificial sweetener with a sweetness about 600 times that of sucrose, aspartame and saccharin (Fig. 2.2). It was discovered in 1976, synthesised in the laboratory by the conversion of sucrose, a refined form of sucrose, to sucrose-6 acetate, followed by selective chlorination and then deacetylation (Luo et al. 2008; Yu and Cn 2011; Wikipedia 2016a). The presence of three chlorine atoms, which replaced hydroxyl ions, make it indigestible in humans, mice, dogs and rats and is hence non-calorific.
Artificial sweeteners such as sucralose are widely consumed by diabetics. As they affect glucose regulation, they are presumed and associated with the development of metabolic imbalances that lead to obesity, cardiovascular disease, lymphomas, bladder and brain cancer, leukaemia and fatigue syndrome (Suez et al. 2014; Boullata and Mccauley 2008). They are also associated with premature birth and/or health risks to the mother or child, though these are yet to be confirmed (French Agency for Food 2015). Artificial sweeteners have proved to incur weight gain in cows and pigs. Some studies show no chronic or acute effects when some organisms were subjected to various doses of sucralose, just minor behavior changes in some aquatics (Bergheim et al. 2015; Perkola 2014). Conversely, these speculations have presented challenges in monitoring because of the need for long-term monitoring and metabolic and environmental variables (Shearer and Swithers 2016). Some literature has reported sucralose to be safe for consumption and that it lacks fatal effects (Brahmini et al. 2012). On the other hand, the presence of artificial sweeteners in the aquatic environment is a clear justification of fecal contamination, as sucralose is not metabolised in the body unless there is a direct disposal, which is less common.
6.4 Personal Care Products
Triclosan is a commercial additive chemical in domestic and cosmetic products such as soaps, detergents, toothpaste, hand washes, deodorants and toys purposely as an antifungal and antibacterial (Fig. 2.3). Its synthesis is a three-step process involving dehydration of 1-(2-hydroxyethyl)pyrrolidin-2-one with either zinc or calcium oxide to 1-vinylpyrrolidin-2-one, followed by reaction with 5-chloro-2-(2,4-dichlorophenoxy)phenyl acrylate in n-heptane to form triclosan (Wikipedia 2016b). Triclosan is a lipophilic white solid with a half-life of about 11 h from urine and 21 h from plasma, mostly detected in the urine rather than feces (Arbuckle et al. 2015). Triclosan enters the environment from a wide range of sources, including laundry and domestic waste waters, municipal waste, and commercial and out-of-use products (Chen et al. 2015). It is contained in sunscreens, where it protects human from exposure to harmful UV light up to 20% (Peng et al. 2016).
Toxicological investigation shows that triclosan interferes with oestrogen responses, thus it is speculated that it causes breast cancer. Experimental tests in rats indicated suppressed thyroid; therefore, being an endocrine disruptor it can induce female infertility in both test animals and humans (Yuan et al. 2015). Further investigations in the aquatic environment revealed that a concentration range of 0.26–0.54 mg/L is lethal to fishes, 0.13–0.39 mg/L is acute to crustaceans and 1.4–10 μg/L inhibits the growth of algae. Including its lipid solubility, triclosan undergoes bioaccumulation, and hence bioconcentration (Norwegian Scientific Committee for Food and Safety 2002). Following aerobic degradation, triclosan undergoes biotransformation into several products, including methyl-triclosan (Chen et al. 2015). No bacteria can biodegrade methyl-triclosan, making it more persistent than the parent form (Lozano et al. 2012). Finally, when triclosan merely resides in the sediments and soils in the aquatic systems owing to lipophilic and density factors, the quantity prevailing in the water is only in transit.
6.5 Algal Toxins
Algal toxins are varieties of toxicants either released from live cells or dead and ruptured cells, mostly occurring in the stagnant or slowly moving waters (US EPA 2016b). The blue–green algae (cyanobacteria) are the most infectious group of algae residing in the freshwaters (US EPA 2015). Other harmful species include Alexandrucitronella, which produces paralytic toxins to shellfish, Pseudo-nit zs chia, producing domoic acid, which is poisonous, Dinophysis, producing diarrhetic poisoning, Heterosigma, Ciguatera, Lingulodinium polyedrum and Gonyaulax spinifera (CIMT 2006). Harmful algal bloom releases algal toxins such as saxitoxins and anatoxins that are quietly found bioaccumulated in shellfish, and are responsible for acute neurotoxicity and possibly carcinogenicity (Zervou et al. 2016). The common exposure routes to humans include skin contact through swimming, inhalation, and ingestion of contaminated seafood and water. Fish toxicity, increased water viscosity, oxygen depletion, blocking sunlight, irritation of gills, food poisoning, bioaccumulation and public health concerns from consuming contaminated waters have occurred (UK Marine SACs Project 2001; Zhang and Zhang 2015).
6.6 Microbes
There are about 72 serotypes of enteroviruses isolated from the human body; yet, the only enteroviruses affecting humans that are homologous to the rhinovirus belongs to polioviruses, coxsackieviruses and enterocytopathic categories (Royston and Tapparel 2016). They enter the human body through the alimentary canal, via the fecal–oral route and contaminated food stuffs, and then reproduce in the gastrointestinal tract, followed by spreading and affecting body organs such as the nervous system, the heart and the skin. The incubation period ranges from 2 to 14 days. Epidemiological investigations revealed that enteroviruses have been responsible for type 1 diabetes as they were found in the pancreas of a diabetic person. These viruses are prospective suspects for damaging insulin-producing cells (Kondrashova 2014). Its world discovery is increasing tremendously; the same virus was detected in children with acute respiratory infections in the Philippines (Puppe et al. 1999; Imamura and Oshitani 2015). The environmental occurrence of enteroviruses had been reported (Kuroda et al. 2015; Han et al. 2015; Richardson and Ternes 2011), which can be quantified by using the EPA method 1615 (Fout et al. 2016).
6.7 Inorganic Elements
Some elements, including cobalt, germanium, strontium, tellurium and vanadium have been classified and categorised among ECs (EPA 2009). Ge and Te are diagonally related, V and Co are transition elements and Sr is an alkali earth metal. Co, Ge and V are under the same period 4, whereas Sr and Te are under period 5. Apart from industrial uses such as steel additive, pigment for ceramics and glasses, they play a dietary role in humans in very small amounts (Royal Society of Chemistry 2016; Yadav et al. 2017; Hare et al. 2017).
Inorganic elements can enter the environment through natural processes such as rock degradation, mining, wearing of metals, consumer products and domestic and municipal waste. Formerly, cobalt was used in drugs and as a germicide. Vanadium is widely used as a catalyst; therefore, chemical laboratory waste is among the sources. Electronic waste is a major source of germanium waste in the environment. Wash-outs from garages, car washes, and welding areas can be sources too. Vanadium is a water-soluble element that forms vanadium complexes. Vanadium oxide and vanadium chloride irritate the eyes and skin, are harmful on inhalation and contact with skin, and cause burns (Chemical Book 2016b). Germanium is flammable, irritating, and carries other properties of heavy metals (Chemical Book 2016a). These elements have dietary values; however, continuous exposure in various doses for a very long time may lead to bioaccumulation, which later results in unexpected chronic diseases.
6.8 Illicit Drugs
Illicit drugs are the classes of drugs that have been internationally banned from public use except for medical and research use. They include but are not limited to cocaine, heroin, marijuana, non-benzoylecgonine, methamphetamine and 3–4-methylendioxyamphetamine. The active component of marijuana is the cannabinoids, which, when abused, become illegal, but again they have medicinal value including decreasing pain, inflammation, controlling epileptic seizures and treating addiction and spasticity. The worse side of marijuana includes aggressive behavior, drugged drivers, impaired mental health, lung diseases, bladder cancer, delinquent behavior and low IQ in a baby when smoked by a pregnant woman. Collectively, wastewaters, urine, feces, runoffs from marijuana gardens and sewage networks are the primary sources of illicit drugs in the environment (Sarkar et al. 2009).
The pharmacological effects exerted by drugs of abuse represents contagious outcomes to humans and ecology as whole; therefore, further intensive research is needed. Drugs of abuse have been detected in the surface and wastewaters, which reflects its uses. Cultivation of coca and marijuana requires and destroys fertile lands, causes deforestation; destruction of protected lands, land cleaning, i.e. soil erosion. Utilisation of fertilisers and pesticides also causes a change in biodiversity. Nutrient runoffs cause algal bloom, which has devastating effects, as previously discussed (US Department of Justice 2014). Zebra mussels exposed to cocaine experienced oxidative stress, DNA damage and an inactive diffusive response (Pharand et al. 2015).
6.9 Endocrine-Disrupting Hormones
Estradiol and progesterone are sex hormones, but operate in the opposite sexes (Fig. 2.4). Its four-ring structure resembles steroids such as cholesterol, estradiol and testosterone (male sex hormone) (Hu et al. 2016). Steroids are responsible for membrane fluidity, carbohydrate regulation, inflammatory responses, bone metabolism, stress responses, cardiovascular fitness, behavior, mineral balancing and steroid receptors (Salimetrics Europe 2014). Not only in females, estradiol is also found in very small amounts in fishes, vertebrates, crustaceans, men and other animals.
Estradiol is a commercial hormonal replacement therapy, used in the treatment of infertility in women, stimulating female puberty and the treatment of prostate cancer. It comes in the form of oral, transdermal, topical, injectable and vaginal insertions. Its direct consumption together with its active metabolites may lead to a wide range of effects, including ovarian cancer, breast cancer, mood disturbances, increased blood pressure and many others (Linda 2013; Seeger and Mueck 2010). The most common routes of hormones in the environment include body excretions, dumping and accidental releases. As conventional water treatment methods were not established for removal of hormones, therefore these hormones may occur in the drinking water. Through food chain, they inter human body and then bioaccumulate in the liver. In addition to the food chains, they enter human bodies and then bioaccumulate in the liver (Narender and Cindy 2009). Early exposure to estradiol led to masculinisation in females and exposure to testosterone led to the expression of male genitalia in female offspring (Grober et al. 1998). The overall outcomes of hormonal releases in the environment are endocrine disruption, genotoxicity, metabolism alteration, cancerous cases, diabetes, birth defects, obesity, cardiopulmonary diseases, affected brain development and abnormal embryogenesis, which interferes with reproduction (Filby et al. 2007; Bellanger et al. 2015; Sanches et al. 2016; Jobling and Owen 2012).
6.10 Pharmaceuticals
Pharmaceuticals are materials or substances, which, when taken into the body by injection, inhalation, skin absorption or oral intake, change the physiology of the body. Their classification is too broad, ranging from their origin, mode of administration, type of diseases, therapeutic effects etc. Nearly all of them are organic and containing one or more cyclic rings. Not all consumed drugs are absorbed in the body; 30–90% of active drugs or metabolites are excreted (Heath et al. 2016; Jones et al. 2003).
Either in dissolved or suspended form, drugs are transported over a long distance and can be deposited in the lipid/fatty tissues, thus moving from one trophic level to another. Their environmental occurrence as an antibacterial, antidepressant, antihistamine, antiepileptic, hormone, lipid regulator, anti-malarial and/or analgesia in the aquatic environment present a threat to both aquatic and human lives. The receiving organism experiences physiological changes despite unnoticeable concentrations, as drugs are very active, even at very low concentrations (Donk et al. 2016). Some of the realistic effects include increased resistance of bacteria to antibiotics (Jones et al. 2003). Antibiotics can further affect cyanobacteria in the water, and thus have an effect on the food chains of all aquatic species. Research into the response to cancer drugs of zebra fish indicated histopathological changes, impaired kidney, liver and DNA, and interfered with plant reproduction capacity (Heath et al. 2016). Following exposure to complex mixtures of pharmaceuticals in the environment, worse chronic diseases can be promoted in human organs (National Toxics Network 2015).
7 Quantification Approaches
Emerging contaminants exist in the environment in very small quantities, which require effective methods for their isolation, identification and quantification. Solid phase extraction is a recent popular method widely used for the separation, extraction and pre-concentration of ECs. The adsorbent materials used bind reversibly with organic contaminants, which are then washed with polar solvent followed by analysis (Nosek et al. 2014; Matamoros et al. 2016; Hanigan et al. 2016). Ultrasound-assisted extraction is reported to be an effective technique for the extraction of pharmaceuticals and illicit drugs (Gago-ferrero and Thomaidis 2016). The analysis process is achieved by using high-resolution mass spectrometry (Zendonga et al. 2015), ultra-performance liquid chromatography-tandem mass spectrometry (Yan-long et al. 2016), inductively coupled plasma-mass spectrometry for the elemental determination of trace elements (EPA 1994) and gas chromatography mass spectrometry.
8 Environmental Remediation of Emerging Contaminants
8.1 Classical Water Treatments
The first recorded history of water treatment through filtration dates back to the seventeenth century when Sir Francis Bacon attempted to desalinate seawater, although it was unsuccessful. Since then, water treatment has employed physical, chemical, biological, instrumental or natural processes to remove natural and induced contaminants to meet specific requirements. The conventional and advanced water remediation approaches are intended to purify water for safe human consumption, ecological purposes and special uses such as medical or regulatory requirements. Traditional and household methods of water treatment include boiling, activated carbon filters, cloth filtration, solar disinfection and chlorination. Essential techniques for public water treatment shown in Table 2.2 are filtration, sedimentation, flocculation and chlorination, and sometimes activated carbons. Traditional methods simply move contaminants elsewhere and obviously create significant risks in the excavation, handling and transport of hazardous material. The isolated containment areas require monitoring and maintenance, which involves unnecessary costs.
Classical water treatment methods include but are not limited to activated sludge methods for the treatment of pharmaceuticals (Pei et al. 2015), carbon xerogels reported by (Álvarez et al. 2015) in the removal of ECs such as caffeine and diclofenac from aqueous solution, with a very promising output. Membrane bioreactors, ozonisation, photocatalysis (UV disinfection), artificial recharging, constructed wetlands, phytoremediation by using duckweeds (Allam et al. 2015), modified mesoporous silica (Ortiz-Martínez et al. 2015) and treatment with clay minerals (Styszko et al. 2015) have been reported to be effective approaches against ECs. Bioremediation, reverse osmosis and ultrafiltration membranes are useful too in decreasing the amount of ECs in the water (Table 2.3).
Public water treatment is a large scale project that requires intensive investments, commercial scale waste removal techniques, fast running procedures, state-of-art experienced workers and moreover retains value for money. Consequently, water containment, contaminants screening, pH adjustments, temporary storage and pre-chlorination to reduce fouling organisms are preliminary activities. Flocculation with alum (Al2SO4) or iron III salt (FeCl3) coagulates suspended particles that speed sedimentation. Accumulation of sediments at the bottom forms sludge, which is eventually removed periodically, either treated or without treatments. At a particular point, dissolved air flotation is used to remove fine particles that were not removed by flocculants. It is achieved by applying air at the bottom of the tank, creating bubbles, which trap fine particles, making them floating masses. Either rapid or slow sand filtration or wetlands are used to remove remaining suspended particles. At this point, conventional water treatment is mostly finalised by water disinfection. Yet, ECs that exist in very small concentrations are still there. Therefore, advanced remediation techniques are employed to remove ECs.
8.2 Modern Water Treatments
8.2.1 Bioremediation
Bioremediation is a cost-effective technique that uses the metabolic action of microorganisms to remove contaminants from environmental matrices. The method plays an effective role by removing hazardous organic and inorganic contaminants in the aquatic, sludge, soil, sediments and wastewater via eco-friendly, publically accepted and useful in onsite application. Microorganisms cannot remove inorganic contaminants from the environment; nevertheless, their oxidation reduction ability mobilises inorganic contaminants to less harmful waste. Bioremediation is therefore attained as the result of microorganisms using contaminants as their sources of food and energy. It requires less equipment, a small amount of labour and energy, and is thus very useful. Bioremediation has proved to be useful in the cleaning of blood, body fluids and communicable diseases at crime scenes (Bharagava et al. 2017).
8.2.1.1 Chemistry of Bioremediation
Biodegradation is a redox reaction occurring in organic matter facilitated by microbes. It is either a metabolic approach where bacteria consume released energy for its growth, or co-metabolism where the energy released during biodegradation is not consumed by bacteria for their growth. At lower carbon and contaminant concentrations oligotrophic bacteria dominate whereas eutrophic bacteria dominate at higher concentrations.
Bacteria speed up chemical reactions that transfer electrons from electron donors such as organic matter to electron acceptors. The energy released in redox reactions are consumed by bacteria for their growth. Some bacteria such as hydrogenotrophic and methanogens use inorganic contaminants through anaerobic respiration and fermentation to generate energy. Other examples are nitrifiers and sulphur-oxidising bacteria.
Simplified bioremediation equation:
Suitable conditions are not limited to:
-
The presence of suitable microbes
-
The availability of C, H, O2, N and P as nutrients
-
A temperature range between 15 and 45 °C
-
A pH range between 5.5 and 8.5
-
A moisture level between 40 and 80%,
-
An oxygen concentration greater than 2 mg/L
Under aerobic conditions, bacteria use oxygen to oxidise organic matters by removing electrons and converting them to carbon dioxide and water. In the absence of oxygen bacteria, nitrate, manganese (IV), iron (III), sulphate and carbonate are used as electron acceptors. Once electron acceptors are completely consumed, the bacteria switch to fermentation where organic wasters act as electron donor and acceptor (EPA 2013).
Bioremediation involves a combination of processes such as biostimulation for environmental modification to stimulate the bacteria responsible for bioremediation. Stimulation is achieved by adding nutrients and electron acceptors such as phosphorus, nitrogen, oxygen or carbon, in the form of molasses. When dealing with halogenated contaminants in anaerobic conditions, stimulation is achieved through the addition of electron donors such as organic substrates straw, sawdust, or corn cobs. Injection wells are used to accomplish the process. Bioaugumentation is employed to enhance biostimulation, which involves the introduction of specially prepared bacterial culture to increase the density of the bacteria, achieving a specific goal such as degrading complex organic compounds and increasing the overall removal rate. It is then followed by the bioaccumulation of live cells, biosorption of dead biomasses, phytoremediation by plants and then rhizoremediation through plant–microbe interactions. Other useful examples of bioremediation technologies are venting, bioleaching, land farming, bioreactors, composting and rhizofiltration.
Under suitable aerobic conditions such as balanced pH, moisture and temperature, microbes secrete enzymes that break down contaminants to a consumable level. While taking food and energy, they release water, carbon dioxide and non-harmful by-products such as amino acids. The performance of this technique is limited to the presence of microorganisms capable of degrading pollutants, the accessibility of contaminants to the microorganisms, the type of soil, temperature, pH, and the presence of oxygen or other electron acceptors and nutrients. Not all bacteria are capable of biodegradation, however. Some potential reportedly effective bacteria include Rhodococcus, Pseudomonas, Sphingomonas, Alcaligenes and Mycobacterium. Fungi such as the white rot fungus Phanerochaete chrysosporium have the ability to degrade persisting environmental pollutants, including polychlorinated biphenyls in river sediments, dechlorination of the solvent trichloroethylene and chloroform. Methylotroph is an aerobic bacterium that grows by using methane for carbon and energy. Sometimes an injection of air under pressure below the water table is performed to increase groundwater oxygen. This process is referred to as biosparging. Biopile treatment is a full-scale technology in which excavated soils are mixed with soil amendments, placed in a treatment area, and bioremediation takes place using forced aeration. Moisture, heat, nutrients, oxygen and pH are controlled to enhance biodegradation. Preliminarily, contaminated soils require excavation before being placed in the bioreactor (Ruiz-Aguilar et al. 2002). Bioreactors are special industrially manufactured controlled vessels in which microorganisms carry out biochemical reactions.
8.2.1.2 Evidence of Biodegradation
The ratio of biological oxygen demand and chemical oxygen demand are the key parameters for the establishment of biodegradation. Biological oxygen demand is a measure of oxygen required by microbes to break down a given organic contaminant in a sample of water. Chemical oxygen demand is an indirect measure of the amount of organic contaminant present in a sample of water. The method has proved useful upon remediation of ECs (Gothwal and Shashidhar 2014).
Membrane bioreactors constitute a wastewater remediation technique involving a combination of bioreactors and membranes in the remediation of water (Nguyen et al. 2013).
8.2.2 Membrane Filtration
Membrane filtration is a physical process that replaces traditional processes such as sedimentation, flocculation and adsorption through sand filters and active carbon filters, ion exchangers, extraction and distillation. The membrane is made up of semi-permeable materials that selectively allow the passage of water while particulates and microbes are retained with high productivity if suitable conditions prevail. It makes use of relatively low energy and no need for the addition of chemicals. Implementation of membrane filters can adapt either plate or tubular membrane systems.
Its mechanism of action can involve the maintenance of concentration gradients, the application of high pressure or the application of the electric potential. Either way, membrane filtration is divided into microfiltration (0.03–10 μm) and ultrafiltration (0.002–0.1 μm) for the removal of large particles, nanofiltration (0.001 μm) and reverse osmosis or hyperfiltration used for the removal of salts from water. the former methods principally rely on the pore sizes with less pressure while the latter depend on the diffusion and high pressure. Membrane filters present a reasonable opportunity for cost-effective and environmentally friendly approaches; yet, reversible or irreversible fouling caused by water quality, process design and control, membrane type and materials interferes. Eventually, membrane flushing, chemical cleaning or membrane replacement affects the outcome of the method.
The invention of membrane filtration technology around the 1960s changed the course of water treatment. The membranes are prepared from either polymeric organic material such as polypropylene, polyvinylchloride, polycarbonate, polyester, polysulphone, polytetrafluoroethylene, cellulose acetate of polysulphone, or from inorganic materials such as metals or commonly ceramics.
8.2.2.1 Operationalisation of Membranes
Various forms of energy are required for the proper functioning of membrane filters. These include:
-
Temperature gradient membranes such as membrane distillation, which allows only the passage of the vapour phase while blocking the liquid phase.
-
Pressure-operated membranes such as microfiltration, ultrafiltration, nanofiltration and reverse osmosis.
-
Electric potential-driven membranes such as membrane electrolysis, electrodialysis, electrodeionisation, electrofiltration and fuel cells.
-
Concentration-based membranes such as gas separation, forward osmosis, dialysis, pervaporation and artificial lungs.
Water purification and wastewater treatment plants widely use nanofiltration and reverse osmosis, whereas micro- and ultrafiltration membranes are commonly used in the food and beverage industries.
The membranes are porous sheets capable of selectively reclaiming portable water from microbes, organic materials that could react with disinfectants to form water disinfection by-products with rational outputs. Membrane fouling, production of polluted water via backwashing and regular replacements of the membrane are among hands-on challenges. However, a high-performance, space saving, simple operation and automatic disinfection create more opportunities.
Nanofiltration and reverse osmosis proved to remove sufficient amounts of ECs in the water (Snyder et al. 2007). In this case, there are possibilities of other remediation techniques to remove ECs from water, but it depends on what the analyst requires.
8.2.3 Ozonation
Ozonation is a chemical process of water treatment in an eco-friendly advanced technology. The mechanism of ozone treatment involves ozone decomposition to release hydroxyl radicals that react with organic particulate contaminants, which are then converted to small biodegradable molecules. In this process, hydrogen peroxide is useful in speeding up the process by adding extra hydroxyl radicals.
The ozonation process is an in-situ chemical oxidation process also known as advanced oxidation or UV disinfection. The production of radicals is the result of ozone and hydrogen peroxide decomposition, oxygen, an ultraviolet energy source or inorganic catalysts such as titanium oxide. Moreover, sulphate radical-based oxidation may appear during the treatment and therefore contribute to the reduction of pollutants. The method has been proven to remediate contaminants, including volatile organic compounds, pesticides and aromatics with high efficiency. This technique has been in existence since 1987; however, its commercial application is yet to be fully implemented because of the high running costs, despite its efficacy. Ozonation remediation, either in sludge or in water, has been proven to treat methyl tert-butyl ether, tetrachloroethene, NDMA, 1,4 dioxins and chlorinated organic compounds. Ozonation is less effective for the removal of pathogens because its half-life is short (Mckie et al. 2016).
8.2.3.1 Chemistry of Ozonation
Once ozone is generated in situ by using ozone generators, it is then released into the water to oxidise double bonds, amino groups and aromatic systems. Performance of this method depends on the amount of ozone, contact time and susceptibility of the contaminants. Ozone is very unstable, such that after its generation, it soon decomposes back to oxygen (Janna 2011). The mode of action includes:
-
Breakage of carbon–nitrogen bonds leading to depolymerisation
-
Reactions with radical by-products of ozone decomposition
-
Direct oxidation/destruction of the cell wall with leakage of cellular constituents outside of the cell
-
Damage to the constituents of the nucleic acids (purines and pyrimidines)
Disadvantages of Ozonation:
-
Ozonation is more complex than chlorination as it requires a special steel vessel
-
Ozone is very reactive and corrosive; hence the need for corrosion-resistant containers
-
Effective after secondary treatment,
-
Higher treatment costs than chlorine
-
Ozone is highly irritating
Advantages of Ozonation:
-
No harmful residual such as disinfection by-products formed after chlorination
-
Ozone is more effective at killing pathogens compared with chlorine
-
Short-term requirements
-
Ozonation increases the amount of dissolved oxygen
-
Ozone acts like a microflocculant
-
Ozone is generated in situ
-
There is no re-growth of microorganisms etc.
Snyder (2014) and Jobling and Owen (2012) reported effective ozonation treatment of water, despite the higher running costs. Ozonation is advantageous owing to its oxidative and disinfectant abilities (Snyder et al. 2003).
References
Allam A, Tawfik A, Negm A et al (2015) Treatment of drainage water containing pharmaceuticals using duckweed (Lemna Gibba). Energy Procedia 74:973–980. Available at https://doi.org/10.1016/j.egypro.2015.07.734
Álvarez S et al (2015) Chemical engineering research and design synthesis of carbon xerogels and their application in adsorption studies of caffeine and diclofenac as emerging contaminants. Chem Eng Res Des 95:229–238. Available at https://doi.org/10.1016/j.cherd.2014.11.001
Arbuckle TE et al (2015) Exposure to free and conjugated forms of bisphenol a and Triclosan among pregnant women in the MIREC cohort. Environ Health Perspect 123(4):277–284
Bellanger M et al (2015) Costs of exposure to endocrine-disrupting Chemicals in the European Union. J Clin Endocronol Metab 100(April):1256–1266
Bennett DH et al (2015) Polybrominated diphenyl ether (PBDE) concentrations and resulting exposure in homes in California: relationships among passive air, surface wipe and dust concentrations, and temporal variability. Indoor Air 25(2):220–229
Bergheim M et al (2015) Antibiotics and sweeteners in the aquatic environment: biodegradability, formation of phototransformation products, and in vitro toxicity. Environ Sci Pollut Res 22(22):18017–18030
Bharagava RN, Chowdhary P, Saxena G (2017) Bioremediation an eco-sustainable green technology, its applications and limitations. In: Bharagava RN (ed) Environmental pollutants and their bioremediation approaches. CRC Press, Taylor & Francis Group, Boca Raton, pp 1–22
Boullata J, Mccauley LA (2008) The potential toxicity of artificial sweeteners. Contin Educ 56(6):251–259
Bradberry SM et al (2000) Mechanisms of toxicity, clinical features, and management of acute chlorophenoxy herbicide poisoning: a review. J Toxicol Clin Toxicol 38(2):111–122. Available at http://www.ncbi.nlm.nih.gov/pubmed/10778907
Bradley PM, Journey CA (2014) Assessment of endocrine-disrupting chemicals attenuation in a coastal plain stream prior to wastewater treatment plant closure. J Am Water Resour Assoc 50(2):388–400. https://doi.org/10.1111/jawr.12165
Brahmini M et al (2012) Myths and facts about aspartame and sucralose: a critical review. IJRAP 3(3):373–375
Chemical Book (2016a) Germanium CAS#_ 7440–56-4. Public Domain. Available at http://www.chemicalbook.com/ProductChemicalPropertiesCB7733835_EN.htm#MSDSA
Chemical Book (2016b) VANADIUM (IV) OXIDE. Public Domain. Available at http://www.chemicalbook.com/ProductChemicalPropertiesCB7691007_EN.htm#MSDSA
Chen X et al (2015) Science of the total environment identification of triclosan-O-sulfate and other transformation products of triclosan formed by activated sludge. Sci Total Environ 505:39–46. Available at https://doi.org/10.1016/j.scitotenv.2014.09.077
Chevrier J et al (2010) Polybrominated diphenyl ether (PBDE) flame retardants and thyroid hormone during pregnancy. Environ Health Perspect 118(10):1444–1449
CIMT (2006) Harmful Algal Blooms (HABs). CeNCOOS, p 2
Clarke RM, Cummins E (2014) Evaluation of “classic” and emerging contaminants resulting from the application of biosolids to agricultural lands: a review. Hum Ecol Risk Assess Int J 21(2):492–513. Available at http://www.tandfonline.com/doi/abs/10.1080/10807039.2014.930295
Comero S et al (2013) EU-wide monitoring survey on emerging polar organic contaminants in wastewater treatment plant effluents. Water Res 47:6475–6487. https://doi.org/10.1016/j.watres.2013.08.024
Committee Report (1999) Emerging pathogens: viruses, protozoa and algal toxins. AWWA 91(9):110–121
EPA (1994) Determination of trace elements in waters and wastes by inductively coupled plasma – mass spectrometry. Available at https://www.epa.gov/sites/production/files/2015-08/documents/method_200-8_rev_5-4_1994.pdf
EPA (2009) Fact sheet: final third drinking water Contaminant Candidate List 3 (CCL 3)
EPA (2013) Introduction to in situ sioremediation of groundwater. Available at https://www.clu-in.org/download/remed/introductiontoinsitubioremediationofgroundwater_dec2013.pdf
EPA (2015) Fact sheet: drinking water contaminant candidate list 4 – Draft, Available at: http://www2.epa.gov/ccl
Fawell J, Ong CN (2012) Emerging contaminants and the implications for drinking water emerging contaminants and the implications for drinking water. Water Resour Dev 28(2):247–263. https://doi.org/10.1080/07900627.2012.672394
Filby AL et al (2007) Health impacts of estrogens in the environment, considering complex mixture effects. Environ Health Perspect 115(12):1704–1710
Fout GS et al (2016) EPA method 1615. Measurement of enterovirus and norovirus occurrence in water by culture and RT-qPCR. Part III Virus Detection by RT-qPCR. J Vis Exp 107(January):1–13. Available at http://www.jove.com/video/52646
French Agency for Food (2015) 2015 report on the safety of artificial sweeteners from the French Agency for Food, Environment and Occupational., (January), p 1. Available at http://www.medscape.com/viewarticle/839455
Gago-Ferrero P, Thomaidis NS (2016) Simultaneous determination of 148 pharmaceuticals and illicit drugs in sewage sludge based on ultrasound-assisted extraction and liquid chromatography – tandem mass spectrometry. Anal Bioanal Chem 407(15):4287–4297
Gothwal R, Shashidhar T (2014) Antibiotic pollution in the environment: a review. CLEAN Soil Air Water 42(9999):1–11
Government of Canada (1995) Toxic substances management policy Reprint of. Government of Canada, Ottawa. Available at http://publications.gc.ca/collections/Collection/En40-499-1-1995E.pdf
Green N, Bergman A (2005) Chemical reactivity as a tool for estimating persistence. Environ Sci Technol, 39(23), 23480A–23486A. Available at http://pubs.acs.org/doi/pdf/10.1021/es053408a
Grober MS et al (1998) The effects of estradiol on gonadotropin-releasing hormone neurons in the developing mouse brain. Gen Comp Endocrinol 112:356–363
Guidotti TL (2009) Emerging contaminants in drinking water: what to do? Arch Environ Occup Health 64(2):1–3
Han N, Gin KY, Hao H (2015) Science of the total environment fecal pollution source tracking toolbox for identification, evaluation and characterization of fecal contamination in receiving urban surface waters and groundwater. Sci Total Environ 538:38–57. Available at https://doi.org/10.1016/j.scitotenv.2015.07.155
Hanigan D et al (2016) Sorption and desorption of organic matter on solid-phase extraction media to isolate and identify N -nitrosodimethylamine precursors. J Sep Sci 9(14):2796–2805
Hansen P (2007) Risk assessment of emerging contaminants in aquatic systems. Trends Anal Chem 26(11):5
Hare V, Chowdhary P, Baghel VS (2017) Influence of bacterial strains on Oryza sativa grown under arsenic tainted soil: accumulation and detoxification response. Plant Physiol Biochem 119:93–102
Heath E et al (2016) Fate and effects of the residues of anticancer drugs in the environment. Environ Res Lett 23(15):14687–14691
Hu D et al (2016) Actions of estrogenic endocrine disrupting chemicals on human prostate stem/progenitor cells and prostate carcinogenesis. Open Biotechnol J 10(77):76–97
Hussain S (2013) Mechanisms of toxicity. University of California. Available at http://nature.berkeley.edu/~dnomura/pdf/Lecture6Mechanisms3.pdf. Accessed 13 Aug 2016
Imamura T, Oshitani H (2015) Global reemergence of enterovirus D68 as an important pathogen for acute respiratory infections. Rev Med Virol 25:102–114
Janna H (2011) Occurrence and removal of emerging contaminants in wastewaters. Brunel
Jobling S, Owen R (2012) 13 Ethinyl oestradiol in the aquatic environment
Jones OAH, Voulvoulis N, Lester JN (2003) Potential impact of pharmaceuticals on environmental health. Bull World Health Organ 81(10):768–769
Kaseva ME, Mwegoha WJS, Kihampa C, Matiko S (2008) Performance of a waste stabilization pond system treating domestic and hospital wastewater and its implications to the aquatic environment-a case study in Dar es Salaam, Tanzania. J Build Land Dev 15(1–2):14
Kolpin D, Furlong E, Zaugg S (2002) Pharmaceuticals, hormones and other organic wastewater contaminants in U. S. Streams, 1999–2000: a National Reconnaissance. US Geological Survey, pp 1999–2000
Kondrashova A, Hyöty H (2014) Role of viruses and other microbes in the pathogenesis of type 1 diabetes. Int Rev Immunol 33(4):284–295
Koumaki E et al (2015) Chemosphere degradation of emerging contaminants from water under natural sunlight: the effect of season, pH, humic acids and nitrate and identification of photodegradation by-products. Chemosphere 138:675–681. Available at https://doi.org/10.1016/j.chemosphere.2015.07.033
Kuroda K et al (2015) Science of the total environment pepper mild mottle virus as an indicator and a tracer of fecal pollution in water environments: comparative evaluation with wastewater-tracer pharmaceuticals in Hanoi, Vietnam. Sci Total Environ 506–507:287–298. Available at https://doi.org/10.1016/j.scitotenv.2014.11.021
Lee KE, Barber LB, Schoenfuss HL (2014) Spatial and temporal patterns of endocrine active chemicals in small streams. J Am Water Resour Assoc 50(2), 19
Linda G (2013) Steroids and hormonal science estradiol synthesis and metabolism and risk of ovarian cancer in older women taking prescribed or plant-derived estrogen supplementation. J Steroids Hormon Sci S12(3):2157–7536
Liteplo RG, Meek ME, Windle W (2002) Concise international chemical assessment document 38 N-nitrosodimethylamine first. Available at http://www.who.int/ipcs/publications/cicad/en/cicad38.pdf
Lozano N et al. (2012) Fate of triclosan and methyltriclosan in soil from biosolids application. Environ Pollut 160:103–108. Available at https://doi.org/10.1016/j.envpol.2011.09.020
Luo Y, Xu L, Sun X (2008) Synthesis of strong sweetener sucralose. Mod Appl Sci 2(3):13–15
Madhumitha R, Eden S, Catharine Mitchell, BW (2013) Contaminants of emerging concern in water, Arizona
Matamoros V, Rodríguez Y, Albaig J (2016) A comparative assessment of intensive and extensive wastewater treatment technologies for removing emerging contaminants in small communities. Water Res 88:777–785
McKie MJ, Andrews SA, Andrews RC (2016) Science of the total environment conventional drinking water treatment and direct biofiltration for the removal of pharmaceuticals and artificial sweeteners: a pilot-scale approach. Sci Total Environ 544:10–17. Available at https://doi.org/10.1016/j.scitotenv.2015.11.145
Miraji H et al 2016 Research trends in emerging contaminants on the aquatic environments of Tanzania. Scientifica 2016:7. Available at https://doi.org/10.1155/2016/3769690
Mortensen A et al (2014) Levels and risk assessment of chemical contaminants in byproducts for animal feed in Denmark levels and risk assessment of chemical contaminants in byproducts for animal feed in Denmark. J Environ Sci Health B 49:797–810
Munschy C et al (2013) Levels and trends of the emerging contaminants HBCDs (hexabromocyclododecanes) and PFCs (perfluorinated compounds) marine shellfish along French coasts. Chemosphere 91(2):233–240
Narender K, Cindy L (2009) Water quality guidelines for pharmaceutically-active-compounds (PhACs): 17α-ethinylestradiol (EE2), Provience of British Columbia
National Toxics Network (2015) Pharmaceutical pollution in the Environment: issues for Australia, New Zealand and Pacific Island countries, Australia
Nguyen LN et al (2013) Removal of emerging trace organic contaminants by MBR-based hybrid treatment processes. Int Biodeterior Biodegrad 85:474–482
Norwegian Scientific Committee for Food and Safety (2002) Risk assessment on the use of triclosan in cosmetics. In Risk assessment on the use of triclosan in cosmetics. pp 4–6
Nosek K, Styszko K, Golas J (2014) Combined method of solid-phase extraction and GC-MS for determination of acidic, neutral, and basic emerging contaminants in wastewater (Poland). Int J Environ Anal Chem 94(10):961–974
Ooka M et al (2016) Cytotoxic and genotoxic profiles of benzo[a]pyrene and N-nitrosodimethylamine demonstrated using DNA repair deficient DT40 cells with metabolic activation. Chemosphere 144:1901–1907
Ortiz-Martínez K et al (2015) Transition metal modified mesoporous silica adsorbents with zero microporosity for the adsorption of contaminants of emerging concern (CECs) from aqueous solutions. Chem Eng J 264:152–164
Pal A et al (2014) Emerging contaminants of public health significance as water quality indicator compounds in the urban water cycle untreated water sewer system. Environ Int 71:46–62. Available at https://doi.org/10.1016/j.envint.2014.05.025
Pei J et al (2015) Bioresource technology effect of ultrasonic and ozone pre-treatments on pharmaceutical waste activated sludge’s solubilisation, reduction, anaerobic biodegradability and acute biological toxicity. Bioresour Technol 192:418–423. Available at https://doi.org/10.1016/j.biortech.2015.05.079
Peng X et al (2016) Persistence, temporal and spatial profiles of ultraviolet absorbents and phenolic personal care products in riverine and estuarine sediment of the Pearl River catchment, China. J Hazard Mater. Available at https://doi.org/10.1016/j.jhazmat.2016.05.020
Perkola N (2014) Fate of artificial sweeteners and perfluoroalkyl acids in aquatic environment. University of Helsinki
Petrisor IG (2004) Emerging contaminants – the growing problem. Environ Forensic 5:183–184
Pharand P et al (2015) Effects of various illicit drugs on immune capacity of blue mussel (Mytilus edulis). J Xenobiotics 5(5770):1–3
Puppe W et al (1999) Rapid identification of nine microorganisms causing acute respiratory tract infections by single-tube multiplex reverse transcription-PCR: feasibility study. J Clin Microbiol 37(1):1–7
Qi W et al (2015) Elimination of polar micropollutants and anthropogenic markers by wastewater treatment in Beijing, China. Chemosphere 119:1054–1061. Available at https://doi.org/10.1016/j.chemosphere.2014.09.027
Richard D, George E (1957) Synthesis of nitrosodimethylamine. p 5. Available at https://www.google.com/patents/US3136821
Richardson SD (2007) Water analysis: emerging contaminants and current issues. Anal Chem 79(12):4295–4324
Richardson SD, Ternes TA (2011) Water analysis: emerging contaminants and current issues. Anal Chem 83:4614–4648
Richardson SD, Exposure N, Agency USEP (2006) Environmental mass spectrometry: emerging contaminants and current issues. Anal Chem 78(12):4021–4046
Rivera-utrilla J et al. (2013) Pharmaceuticals as emerging contaminants and their removal from water. A review. Chemosphere, in press(in press). https://doi.org/10.1016/j.chemosphere.2013.07.059
Ross PS, Ellis GM (2004) PBDEs, PBBs, and PCNs in three communities of free-ranging killer whales (Orcinus orca) from the northeastern Pacific Ocean. Environ Sci Technol 38(16):4293–4299
Royal Society of Chemistry (2016) Vanadium – element information, properties and uses. Public Domain. Available at http://www.rsc.org/periodic-table/element/23/vanadium. Accessed 25 Aug 2016
Royston L, Tapparel C (2016) Rhinoviruses and respiratory Enteroviruses: not as simple as ABC. Viruses 8(16):23
Ruiz-Aguilar GML et al (2002) Degradation by white-rot fungi of high concentrations of PCB extracted from a contaminated soil. Adv Environ Res 6(4):559–568
Salimetrics Europe (2014) High sensitivity salivary 12B–estradiol enzyme immunoassay kit
Sanches S et al (2016) Comparison of UV photolysis, nanofiltration, and their combination to remove hormones from a drinking water source and reduce endocrine disrupting activity. Environ Sci Pollut Res 23(11):11279–11288
Sarkar PK et al (2009) Toxicity and recovery studies of two ayurvedic preparations of iron. Indian J Exp Biol 47(12):987–992
Schultz AG et al (2014) Aquatic toxicity of manufactured nanomaterials: challenges and recommendations for future toxicity testing. Environ Chem 11(3):207–226. Available at http://www.publish.csiro.au/?paper=EN13221
Seeger H, Mueck AO (2010) Estradiol metabolites and their possible role in gynaecological cancer. J Reproduktionsmed Endokrinol 7(1):62–66
Shearer J, Swithers SE (2016) Artificial sweeteners and metabolic dysregulation: lessons learned from agriculture and the laboratory. Rev Endocr Metab Disord. Available at https://doi.org/10.1007/s11154-016-9372-1
Siddiqi MA, Clinic M (2003) Polybrominated diphenyl ethers (PBDEs): new pollutants – old diseases. Clin Med Res 1(4):281–290. Available at http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1069057/pdf/ClinMedRes0104-0281.pdf
Snyder SA (2014) Emerging chemical contaminants: looking for greater harmony. Am Water Works Assoc 108(8):14
Snyder SA et al (2003) Pharmaceuticals, personal care products and endocrine disruptors in Water : implications for the water industry. Environ Eng Sci 20(5):21
Snyder SA et al (2007) Role of membranes and activated carbon in the removal of endocrine disruptors and pharmaceuticals. Desalination 202:156–181
Sorensen JPR et al (2014) Emerging contaminants in urban groundwater sources in Africa. Water Res 72:1–13. https://doi.org/10.1016/j.watres.2014.08.002. Elseviere Ltd
Stasinakis AS et al (2013) Science of the total environment contribution of primary and secondary treatment on the removal of benzothiazoles, benzotriazoles, endocrine disruptors, pharmaceuticals and perfluorinated compounds in a sewage treatment plant. Sci Total Environ 463–464:1067–1075. Available at https://doi.org/10.1016/j.scitotenv.2013.06.087
Sturm R, Ahrens L (2010) Trends of polyfluoroalkyl compounds in marine biota and in humans. Environ Chem 7:457–484. https://doi.org/10.1071/EN10072
Styszko K et al (2015) Preliminary selection of clay minerals for the removal of pharmaceuticals, bisphenol A and triclosan in acidic and neutral aqueous solutions. C R Chim 18(10):1134–1142. Available at https://doi.org/10.1016/j.crci.2015.05.015
Suez J et al (2014) Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 514(7521):181–186. Available at https://doi.org/10.1038/nature13793
Thomaidis N(2012) Emerging contaminants: a tutorial mini-review. Global NEST …, 14(1), pp 72–79. Available at http://journal.gnest.org/sites/default/files/Journal Papers/72-79_823_Thomaidis_14–1.pdf. Accessed 13 Nov 2014
Tran NH et al (2014) Suitability of artificial sweeteners as indicators of raw wastewater contamination in surface water and groundwater. Water Res 48:443–456. https://doi.org/10.1016/j.watres.2013.09.053. Elsevier Ltd
UK Marine SACs Project (2001) Toxic substance profile_ Algal toxins and algae-related fish kills. Public Domain. Available at http://www.ukmarinesac.org.uk/activities/water-quality/wq8_51.htm. Accessed 22 Aug 2016
Urtiaga AM et al (2013) Removal of pharmaceuticals from a WWTP secondary effluent by ultrafiltration/reverse osmosis followed by electrochemical oxidation of the RO concentrate. Desalination 331:26–34. https://doi.org/10.1016/j.desal.2013.10.010. Elsevier B.V
US Department of Justice (2014) The dangers and consequences of marijuana abuse. (May), p 45. Available at www.DEA.gov
US EPA (2014a) Technical fact sheet – N-nitroso-dimethylamine. Available at https://www.epa.gov/sites/production/files/2014-03/documents/ffrrofactsheet_contaminant_ndma_january2014_final.pdf
US EPA (2014b) Technical fact sheet – polybrominated diphenyl ethers (PBDEs) and polybrominated biphenyls (PBBs) technical fact sheet – PBDEs and PBBs at a glance
US EPA (2015) Algal toxin risk assessment and management strategic plan for drinking water, Kansas. Available at www.waterone.org
US EPA (2016a) Basic information on the CCL and regulatory determination drinking water contaminant candidate list (CCL) and regulatory determination _ US EPA. EPA
US EPA (2016b) The effects human health nutrient pollution. Public Domain. Available at: https://www.epa.gov/nutrientpollution/effects. Accessed 22 August 2016
USDHHS (2015) Draft: toxicological profile for polybrominated diphenyl ethers (PBDEs), Atlanta. Available at http://www.atsdr.cdc.gov/toxprofiles/tp207.pdf
Voloshenko-Rossin A, Gasser G, Cohen K, Gun J, Cumbal-Flores L, Parra-Morales W, Sarabia F, Ojeda F, Lev O (2015) Emerging pollutants in the Esmeraldas watershed in Ecuador: discharge and attenuation of emerging organic pollutants along the San Pedro–Guayllabamba–Esmeraldas rivers. Environ Sci Process Impacts Roy Soc Chem 17(1):41–53 https://doi.org/10.1039/C4EM00394B.
van Donk E et al (2016) Pharmaceuticals may disrupt natural chemical information flows and species interactions in aquatic systems: ideas and perspectives on a hidden global change. Rev Environ Contam Toxicol 235:15
Vuong AM et al (2016) Prenatal polybrominated diphenyl ether exposure and body mass index in children up to 8 years of age. Environ Health Perspect, (February). Available at http://ehp.niehs.nih.gov/wp-content/uploads/advpub/2016/6/EHP139.acco.pdf
Wikipedia (2016a) Sucralose
Wikipedia (2016b) Triclosan
Wu J, Zhang L, Yang Z (2010) A review on the analysis of emerging contaminants in aquatic environment. Crit Rev Anal Chem 40:234–245. https://doi.org/10.1080/10408347.2010.515467
Yadav A, Chowdhary P, Kaithwas G, Bharagava RN (2017) Toxic metals in environment, threats on ecosystem and bioremediation approaches. In: Das S, Dash HR (eds) Handbook of metal-microbe interactions and bioremediation. CRC Press, Taylor & Francis Group, Boca Raton, p 813
Yang G, Fan M, Zhang G (2014) Emerging contaminants in surface waters in China – a short review. Environ Res Lett 74018:13
Yan-long W et al (2016) Determination of eight typical lipophilic algae toxins in particles suspended in seawater by ultra performance liquid chromatography – tandem mass spectrometry. Chin J Anal Chem 44(3):335–341. Available at https://doi.org/10.1016/S1872-2040(16)60911-8
Yu Y, Cn N (2011) Method of sucralose synthesis yield. Patent 2(12):4
Yuan M et al (2015) Preimplantation exposure to bisphenol a and Triclosan may lead to implantation failure in humans. Biomed Res Int 2015:9
Zendonga Z, McCarronb P, Christine Herrenknecht MS, Amzila Z, Coled RB, Hess P (2015) High resolution mass spectrometry for quantitative analysis and untargeted screening of algal toxins in mussels and passive samplers. J Chromatogr A 1416:10–21
Zervou S et al (2016) New SPE-LC-MS/MS method for simultaneous determination of multi-class cyanobacterial and algal toxins. J Hazard Mater. Available at: https://doi.org/10.1016/j.jhazmat.2016.07.020
Zhang C, Zhang J (2015) Environmental analytical chemistry current techniques for detecting and monitoring algal toxins and causative harmful algal blooms. J Environ Anal Chem 2(1):1–12
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Hossein, M. (2019). Toxicological Aspects of Emerging Contaminants. In: Bharagava, R., Chowdhary, P. (eds) Emerging and Eco-Friendly Approaches for Waste Management . Springer, Singapore. https://doi.org/10.1007/978-981-10-8669-4_2
Download citation
DOI: https://doi.org/10.1007/978-981-10-8669-4_2
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-8668-7
Online ISBN: 978-981-10-8669-4
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)