Abstract
Magnetotactic bacteria swim along the magnetic field using a unique bacterial organelle termed the magnetosome. Magnetosomes are comprised of nano-sized crystals of magnetite or greigite enclosed within lipid bilayer membrane vesicles and specifically associated proteins. The integration of magnetosomes in motility allows magnetotactic bacteria to orient themselves to find a favorable microaerobic habitat. Most of the magnetosome-associated proteins are encoded in gene clusters within a “magnetosome island.” Additionally, these proteins are highly conserved and essential for the synthesis and maintenance of magnetosomes. In this chapter, we will briefly introduce general insight into magnetotactic bacteria, and then, we will present recent research progress on magnetosome structure and function.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Magnetotactic bacteria are widespread aquatic eubacteria that utilize organelles known as magnetosomes to navigate along Earth’s magnetic field (reviewed in Bazylinski and Frankel 2004; Faivre and Schüler 2008; Komeili 2012; Lin et al. 2013; Schüler 2008). The unique properties of these bacteria regarding magnetic sensing, magnetosome formation, and biomineralization have garnered much multidisciplinary interests from fields such as microbiology, geology, chemistry, physics, mineralogy, astrobiology, synthetic biology, bioengineering, materials science, chemical engineering, medicine, and bionanotechnology. In this first section, we will provide a general overview of magnetotactic bacteria.
1.1 Discovery of Magnetotactic Bacteria
Two scientists, Richard Blakemore and Salvatore Bellini, independently and serendipitously discovered magnetotactic bacteria (Frankel 2009). Magnetotactic bacteria were first demonstrated by Salvatore Bellini, a medical doctor at the University of Pavia in Italy. He found magnetotactic bacteria in freshwater samples collected from Pavia and studied them from the late 1950s until 1963. His work was published in 1963 but was not widely disseminated. However, his findings have recently come to light, and his papers were translated to English and subsequently published in an international journal (Bellini 2009a, b). Richard Blakemore rediscovered magnetotactic bacteria in 1974 (Blakemore 1975). While observing sediment samples collected near Woods Hole, Massachusetts to study microbes enriched in sulfide mud, he found a group of highly motile bacteria that swam continuously in the same geographic direction (Blakemore 1982). These bacteria altered their swimming direction by placing a bar magnet near the microscope slide, and he deduced that these bacteria swam along the magnetic field. Blakemore demonstrated that these bacteria contained chains of iron-rich magnetic crystals in their cells, thus providing an explanation for their ability to respond to magnetic fields (Blakemore 1975). He termed such cell motility directed by a magnetic field as “magnetotaxis” (Blakemore 1975).
1.2 Isolation of Magnetotactic Bacteria
Magnetotactic bacteria are ubiquitous, widely distributed in a diverse array of aquatic environments such as fresh, marine, brackish, and hypersaline water or in chemically stratified water columns (Lefèvre and Bazylinski 2013). These bacteria have also been found on all continents (Lin et al. 2013; Lefèvre and Wu 2013). Although most magnetotactic bacteria dwell in aquatic habitats at ambient temperature and neutral pH, some species are extremophiles. Lefèvre et al. recently reported moderately thermophilic magnetotactic bacteria in hot springs (Lefèvre et al. 2010) and obligate magnetotactic alkaliphiles magnetotactic bacteria living in alkaline lakes (Lefèvre et al. 2011b).
Magnetotactic bacteria are either microaerobes or anaerobes and are found at the oxic-anoxic interface or anoxic region of aquatic habitats, respectively, in sediments or at the bottom of water column. Here we briefly mention a simple method to isolate magnetotactic bacteria from freshwater sediments. Sediment (0–3 cm deep) and surface water are collected from near the edge of a shallow freshwater pond using a scooper and placed into tightly capped 0.5 L glass bottle. The magnetotactic bacteria are concentrated by attaching a bar magnet to the outside of the bottle just above the sediment/water interface. North-seeking magnetotactic bacteria (swimming toward the magnetic S-pole) dominate in the Northern Hemisphere, while south-seeking magnetotactic bacteria (swimming toward the magnetic N-pole) dominate in the Southern Hemisphere. Therefore, for example, the S pole of the bar magnet is attached to the glass bottle in case of observation in the North Hemisphere. The bottle is kept in the dark for up to 3 h to allow magnetotactic cells to swim toward the magnet. A pipet is then used to collect magnetotactic bacteria. A drop of the magnetotactic bacteria is placed onto a slide, and the S-pole of bar magnet is placed on the microscope stage near the drop. The magnetotactic bacteria will swim to the edge of the drop near the magnet (Fig. 1.1a). When the bar magnet is rotated 180°, the cells will rotate and swim away from the magnet. Figure 1.1b shows examples of magnetotactic bacteria that were collected from a freshwater pond in Kanazawa, Japan. Morphologically diverse magnetotactic bacteria were observed from this collection, with chains of magnetosomes to help the bacteria move along the magnetic field.
Isolation of magnetotactic bacteria. (a) Phase contrast microscopic image of magnetotactic bacteria in a water drop. The S-pole of the bar magnet was placed on the right side of the image. Magnetotactic bacteria accumulated at the edge of the water drop. (b) Transmission electron microscopic (TEM) images of isolated magnetotactic bacteria from a freshwater pond in Kanazawa, Japan. Note that all of the cells possess chains of opaque structures, magnetosomes (arrows), to facilitate navigations along the magnetic field. Bar: 1 μm
1.3 Magnetotactic Bacteria Diversity
Magnetotactic bacteria are a physiologically, morphologically, and phylogenetically diverse group of Gram-negative bacteria. Many bacteria from this group have been isolated from various aquatic environments; however, the surface of magnetotactic bacteria diversity has barely been scratched due to difficulty in obtaining axenic cultures. Despite their ubiquitous presence, magnetotactic bacteria are fastidious and grow slowly. Recently, there has been much improvement in isolation and culturing methods, and employing current genetic techniques, such as metagenomics and single-cell genome analysis, has substantially provided insight about the diversity of magnetotactic bacteria. Known magnetotactic bacteria belong to different Proteobacteria phylum subgroups, including at least alpha-, gamma-, and delta-subgroups; the Nitrospirae phylum; and the OP3 of the PVC (Planctomycetes, Verrucomicrobia, and Chlamydiae) superphylum (reviewed in Lefèvre and Bazylinski 2013) (Table 1.1). Thus, magnetotactic bacteria do not form a phylogenetically coherent group.
With over 40 years of sustained efforts to date, more than 20 species of axenic cultures have been obtained. To note, species belonging to Alphaproteobacteria, e.g., Magnetospirillum magnetotacticum MS-1, M. magneticum AMB-1, M. gryphiswaldense MSR-1, Magnetococcus marinus MC-1, and Magnetovibrio blakemorei MV-1; Gammaproteobacteria, e.g., strain BW-2 and SS-5; and Deltaproteobacteria, e.g., Desulfovivbrio magneticus RS-1, have been cultivated (Table 1.1). Specifically, M. magneticum AMB-1 and M. gryphiswaldense MSR-1 are used as model species for the genetic and biochemical dissection of magnetosome formation and function. However, species belonging to Nitrospirae, and the PVC superphylum have yet to be cultured.
1.4 Magnetotaxis
Magnetotaxis is thought to facilitate cell location and maintenance cells within the microaerobic zones on the bottom of chemically stratified natural waters or in sediments (Frankel et al. 1997). The intracellular magnetic dipoles of magnetosomes enable bacteria to orient themselves to swim parallel to the geomagnetic field (Fig. 1.2). Due to the inclination of the Earth’s magnetic field (white dash arrows, Fig. 1.2), magnetotactic bacteria can swim unidirectionally along the vertical axis in aquatic habitats. Interestingly, north-seeking bacteria occur predominantly in the Northern Hemisphere, while south-seeking bacteria are predominantly in the Southern Hemisphere. In addition, the abundance of north- and south-seeking bacteria is distributed equally at the equator (Blakemore 1975, 1982). As a consequence, both of Northern Hemisphere magnetotactic bacteria and Southern Hemisphere magnetotactic bacteria swim to regions of low oxygen. Thus, magnetotactic bacteria swim along the geomagnetic field by using magnetotaxis. These bacteria maintain their position within the microaerobic environment via aerotaxis. This behavior is collectively termed “magneto-aerotaxis” (Frankel et al. 1997).
2 Magnetosome Structure and Protein Localization
Magnetosomes contain membrane-enclosed regular-sized magnetite (Fe3O4) or greigite (Fe3S4) crystals. Individual magnetosome particles are aligned into one or multiple chains and localize along the long axis of the cell, thereby facilitating cellular orientation along the geomagnetic field and the search for a microaerobic environment. Magnetosomes consist of the Mam and Mms proteins, which are specifically localized. These proteins function in magnetosome formation and are critical for precise control of the biomineralization of magnetite crystals and magnetic reception. In this section, we present our recent studies dissecting the roles of magnetosome-associated proteins MamA and MamK in maintenance of magnetosome structure.
2.1 Magnetosome-Associated Proteins
Figure 1.3a shows magnetosomes isolated from M. magneticum AMB-1, and Fig. 1.3b shows the SDS-PAGE gel profile of magnetosome-associated proteins. In 2004, Grünberg et al. identified key proteins to magnetosome function via proteomics and found that magnetosome-associated proteins are encoded in a specific genomic region called the magnetosome island (MAI) (Fig. 1.4) (Schübbe et al. 2003; Ullrich et al. 2005; Jogler et al. 2009; Fukuda et al. 2006). All magnetotactic bacterial genomes identified thus far have MAIs. The MAI of Magnetospirillum species encodes magnetosome-associated proteins in four operons, mamGFDC, mms6, mamAB, and mamXY (Fig. 1.4) (Matsunaga et al. 2005). Magnetosome-associated proteins comprise various kinds of proteins, such as TPR protein, transporter, PDZ domain-containing proteins, cytoskeletal proteins, heme proteins, serine proteases, and hypothetical proteins. These proteins show no or very low homology to known proteins and thus specific to magnetosome function, such as magnetosome biogenesis (Richter et al. 2007).
2.2 Tetratricopeptide Repeat (TPR) Protein MamA
MamA (Mam22) was the first identified magnetosome-associated protein (Okuda et al. 1996; Okuda and Fukumori 2001). MamA is also one of the most abundant proteins and conserved in MAIs of all known magnetotactic bacteria (Lefèvre et al. 2013). Taken together, MamA may have an essential role in the magnetosome. The primary structure of MamA consists of five TPR motifs and one putative TPR motif (Okuda and Fukumori 2001). A single TPR motif adopts a helix-turn-helix fold, while several adjacent TPR motifs create antiparallel α-helices that form a superhelix structure. This yields a pair of concave and convex curved surfaces that display binding sites capable of forming a multiprotein complex (Zeytuni and Zarivach 2012). Yamamoto et al. and Zeytuni et al. determined that MamA proteins interact with themselves to form an oligomeric complex (Yamamoto et al. 2010; Zeytuni et al. 2011). According to atomic force microscopic (AFM) observation, MamA forms spherical-shaped oligomers, and the size of the oligomer ranged from 4.5 to 6.5 nm in height and 14 to 20 nm in diameter (Yamamoto et al. 2010). Recently, X-ray crystal structures of MamA from M. magneticum AMB-1 (Zeytuni et al. 2011), M. gryphiswaldense MSR-1 (Zeytuni et al. 2011), Ca. Magnetobacterium bavaricum Candidatus Magnetobacterium bavaricum (Zeytuni et al. 2012), and Desulfovibrio magneticus RS-1 (Zeytuni et al. 2015) were determined. Zeytuni et al. proposed that MamA contains at least three protein binding sites: a putative TPR binding site, a concave binding site, and a convex binding site (Zeytuni et al. 2011). MamA structural insight suggested that such protein-protein interactions are critical to its function in the magnetosome.
2.3 MamA and the Magnetosome Matrix
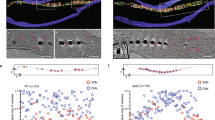
We observed isolated magnetosomes chains via transmission electron microscopy (TEM) and found that magnetosome vesicles are covered by a proteinaceous layer known as the magnetosome matrix (Taoka et al. 2006). We investigated MamA localization in magnetosomes using immunogold staining. Interestingly, MamA proteins localized to the magnetosome matrix. To further understand magnetosome structure and MamA localization, the use of AFM is employed. AFM is used to visualize organic samples under physiological conditions (Dufrêne 2008; Engel and Muller 2000). In AFM, the surface profile of the sample is imaged by detecting the interaction between the sample and the AFM stylus during the raster scanning of the sample. Figure 1.5a, b show AFM images of isolated magnetosomes. Based on AFM measurements, a magnetite crystal is surrounded by organic material with a ∼7 nm thickness (Yamamoto et al. 2010). Upon labeling magnetosomes with anti-MamA antibodies and visualizing via AFM, we observed dense packing of anti-MamA antibodies on the magnetosomes (Fig. 1.5c–f), which indicated that MamA was located at the outermost layer of the magnetosomes to form the magnetosome matrix (Fig. 1.5g). Moreover, we used AFM to observe chains of magnetosomes with and without MamA, and we proposed that MamA is anchored to the magnetosome membrane and may stabilize the structure of magnetosome chain (Yamamoto et al. 2010). Figure 1.5g shows a proposed model of magnetosome organization. In this model, MamA oligomers bind to the surface of magnetosome vesicles and form a proteinaceous layer to stabilize magnetosome chain arrangement. Recently, we reported that the magnetosome membrane protein Mms6 binds to MamA oligomers in vitro, suggesting that MamA and Mms6 interact each other in the magnetosome (Nguyen et al. 2016).
AFM observations of isolated magnetosome. (a, b) AFM images of magnetosomes chains. (c, d) AFM images of immunolabeled magnetosomes. Magnetosomes labeled with (c) anti-MamA antibodies and (d) preimmune serum. (e, f) Surface profiles along the lines indicated in panels c and d. (g) Model of magnetosome organization
2.4 Cytoskeletal Filaments Associated with Magnetosome Chains
In 2006, two research groups independently confirmed the existence of cytoskeletal structures associated with magnetosome chains using cryo-electron tomography. Scheffel et al. found a network of cytoskeleton-like filamentous structures extending up to the cell pole of M. gryphiswaldense MSR-1 (Scheffel et al. 2006). The magnetosome vesicles were arranged along the filamentous structure located close to the cytoplasmic membrane. Komeili et al. also found networks of filaments running parallel to individual M. magneticum AMB-1 magnetosomes (Komeili et al. 2006). Furthermore, based on a mamK deletion mutant, filaments appeared to be comprised of MamK, a bacterial homolog of actin (Komeili et al. 2006).
MamK is found only in magnetotactic bacteria, unlike other well-known bacterial actin-like proteins MreB and ParM (Shaevitz and Gitai 2010). MreB is generally conserved in rod- and spiral-shaped bacteria and associated with most spatially organized cellular processes, including cell morphogenesis, cellular polarity, protein localization, cell division, cell differentiation, and chromosome segregation. ParM is a plasmid-encoded actin-like protein and functions in the plasmid segregation process. MamK, MreB, and ParM are predicted to form phylogenetically and functionally distinct groups of prokaryotic actin-like proteins (Derman et al. 2009). The amino acid sequence similarity is >50% among the MamK proteins of different magnetotactic bacteria species. On the other hand, amino acid identity between M. magnetotacticum MS-1 MamK and MreB is 24% and 17% between M. magnetotacticum MS-1 MamK and human actin. The actin protein superfamily has five conserved sequence motifs that form the ATP-binding pocket and the interdomain hinge region (Bork et al. 1992). MamK proteins also have five conserved motifs, suggesting that MamK has biochemical properties similar to those of other actin homologs (Fig. 1.6). MamK is encoded by the mamAB operon of MAI (Fig. 1.4) and is conserved in most identified magnetotactic bacterial genomes. The mamAB operon is essential for magnetosome biosynthesis (Lohße et al. 2011; Murat et al. 2010), suggesting that MamK is important for magnetosome organization.
Conserved motifs within the actin superfamily. (a) Schematic drawing of the five motifs in MamK that exhibit similarity to conserved motifs in proteins with an actin fold. Numbers indicated are amino acids in M. magnetotacticum MS-1 MamK. (b) Sequence alignments of the five conserved MamK motifs with MreB and eukaryotic actin proteins. Conserved amino acids are indicated in bold
2.5 MamK Localization
We compared the localization of MamK and MreB in early stationary phase M. magnetotacticum MS-1 cells to further characterize this cytoskeletal protein. The intracellular localization of MamK in M. magnetotacticum MS-1 was examined by both immunofluorescence and immunoelectron microscopy. Immunofluorescence microscopy with an anti-MamK antibody revealed a linear distribution of MamK from pole to pole along the vertical axis of the cells (Fig. 1.7a, b). In contrast, immunofluorescence microscopy using an anti-MreB antibody showed that MreB formed either spiral filamentous structures (Fig. 1.7c) or was located in the septa as paired dots (Fig. 1.7d). This distribution of MreB is consistent with that of other bacteria, such as Bacillus subtilis (Jones et al. 2001) and E. coli (Kruse et al. 2003). The results demonstrated that two kinds of actin-like proteins assemble as different filamentous structures in magnetotactic bacteria. Immunogold labeling of ultrathin sections confirmed the locality of MamK as shown in Fig. 1.7e.
Localization of MamK. (a, b) Immunofluorescence images using the anti-MamK antibody. (c, d) Immunofluorescence images using the anti-MreB antibody. (e) Immunogold labeling of an ultrathin section using anti-MamK indicating that MamK is distributed from pole to pole along the long axis of the cell (arrows indicate 15 nm gold particles). (f–h) Immunoelectron microscopic images of purified magnetosomes using anti-MamK antibodies that indicates the 5 nm gold particles (arrows) are distributed in a line along the surfaces of several magnetite particles (arrowheads). (h) Immunoelectron microscopic image of magnetosomes with negative staining (4% uranyl acetate). Scale bars (a–d): 1 μm
To assess whether MamK filament is comprised of a single or multi-arranged protofilaments, the cellular abundance of the MamK protein was estimated by quantitative immunoblotting. The amount of MamK was estimated to be 26,000 ± 6000 (n = 8) molecules per cell in early stationary phase. Because of amino acid similarity and comparable molecular mass of MamK with MreB and ParM, the longitudinal monomer spacing of the MamK protein may be approximately that of MreB (51 Å) (van den Ent et al. 2001) and ParM (49 Å) (van den Ent et al. 2002). Thus, enough MamK is most likely available to accommodate a double helical filament of 14–22 times the length of the cell (mean length: 3.6 μm). These findings strongly suggest that MamK filaments exist as a bundle of multiple protofilaments or as a network structure in the cell. Additionally, in our analyses, MreB was estimated to be 5000 ± 1000 (n = 6) molecules per M. magnetotacticum cell. Thus, the difference in the estimated values of MamK and MreB molecules in a single cell suggested that their expression is independently regulated.
To elucidate the localization of MamK in the magnetosome chain, we performed immunogold staining with isolated magnetosomes from M. magnetotacticum MS-1. Immunoelectron microscopy showed that the 5 nm gold particles were distributed in a line along the surfaces of several magnetite particles and appeared to remain on one side of the magnetosome chain with magnetite particles (Fig. 1.7f–h). These linear distributions of the gold particles suggested that MamK is filamentous.
2.6 MamK Polymerization
Next, we characterized MamK protein from M. magnetotacticum MS-1 in vitro (Taoka et al. 2007). The recombinant MamK protein was purified from E. coli and was added at a final concentration of 10 μM in a total volume of 30 μl polymerization buffer, which contained 100 mM Tris-HCl (pH 7.0), 14 mM MgCl2, 100 mM NaCl, and 30 mM KCl. The mixture was then centrifuged at 150,000 × g at 4 °C for 1 h to remove aggregated MamK. After the addition of 2 mM of the non-hydrolyzable ATP analog ATP-γ-S, the mixture was incubated at 30 °C for 5 min. The sample was then loaded on Formvar- and carbon-coated grids and negatively stained with 4% uranyl acetate for TEM observation. MamK polymerized into well-developed, filamentous, straight bundles (Fig. 1.8). The bundles were comprised of fine filaments, smaller than 20 nm wide. Scanning TEM images of the filaments revealed that each filament was made up of two small helical filaments (Fig. 1.8). The mean crossover distance of the two helical filaments was 23 nm, and the helical filament was approximately 6 nm wide. Additionally, the helical filament was thus presumed to be a protofilament constructed with a single strand of MamK molecules. In vitro polymerized MamK filaments were observed using MamK protein from different species, M. gryphiswaldense MSR-1 (Sonkaria et al. 2012), M. magneticum AMB-1 (Ozyamak et al. 2013), and Ca. Magnetobacterium casensis (Deng et al. 2016). Recently, a high-resolution structure (~6.5 Å) of the MamK filament was determined using cryo-electron microscopy. Accordingly, the MamK filament was of double-stranded and non-staggered architecture, with monomeric MamK protein contacts apparent (Bergeron et al. 2016). The longitudinal contacts along each MamK strand most closely resembled those of eukaryotic actin; however, the cross-strand interface is novel among actin-like proteins and is responsible for the non-staggered architecture (Bergeron et al. 2016).
TEM images of in vitro polymerized MamK filaments. (a) Low magnification of a MamK filamentous bundle. (b) Two filaments are indicated in the bundle. (c) Scanning TEM images of in vitro polymerized MamK filaments. A fine filament in the MamK filamentous bundle demonstrates that the filament is made up of two smaller helical filaments. Crossovers of the two helical filaments are indicated by the arrows. The mean crossover distance is 23 nm. The helical filament is approximately 6 nm wide (Scale bars, a 200 nm; b 20 nm, c 20 nm)
2.7 Recent Advances in Understanding the MamK Cytoskeleton
Scheffel et al. reported that M. gryphiswaldense MSR-1 cells in which the acidic magnetosome protein MamJ was deleted did not assemble linear chains of magnetosomes. Rather, magnetosomes aggregated into three-dimensional clusters (Scheffel et al. 2006). Furthermore, using cryo-electron tomography, they demonstrated that in ∆mamJ cells, magnetosome vesicles were detached from cytoskeletal filaments. These observations led to the development of a model in which MamJ connects magnetosome vesicles to the MamK filament, which serves as a scaffold for stabilizing magnetosome chains (Scheffel et al. 2006). The results of a bacterial two-hybrid study indicated that MamJ interacts with MamK in vivo, and the C- and N-terminal sequence regions of MamJ mediated these interactions (Scheffel and Schüler 2007).
Draper et al. demonstrated that M. magneticum AMB-1 cells deficient in both MamJ and LimJ, a paralog of MamJ, formed a chain of magnetosomes in contrast to the aggregated magnetosomes observed in M. gryphiswaldense MSR-1 (Draper et al. 2011). Cryo-electron tomography observations of the ∆mamJ ∆limJ strain revealed chains with 100- to 250-nm gaps, which were devoid of magnetosomes (Draper et al. 2011). Interestingly, the distribution of magnetosome-associated MamK filaments was drastically altered in the ∆mamJ ∆limJ strain. To note, the filaments were observed as concentrated bundles within the gaps of the magnetosome chains. Furthermore, using the fluorescence recovery after photobleaching (FRAP) assay, they demonstrated that MamK forms dynamic filaments in M. magneticum AMB-1 (Draper et al. 2011). MamK mutants of essential residues for ATP hydrolysis activity (MamKD161A and MamK143A) did not display such dynamics, thereby demonstrating that MamK ATPase activity is required for own dynamics. Strikingly, in the ∆mamJ ∆limJ strain, MamK-GFP was static as assessed by FRAP, suggesting that MamJ and LimJ promote MamK filament dynamics. Taken together, these results indicate that MamK dynamics require MamJ and LimJ for the assembly and maintenance of the magnetosome chain.
Philippe and Wu, using a bimolecular fluorescence complementation assay, demonstrated that Amb0994, the MAI-encoded methyl-accepting chemotaxis protein, interacts with MamK at the cell poles where methyl-accepting chemotaxis proteins usually cluster (Philippe and Wu 2010). They also demonstrated that Amb0994 impacts effective magnetic sensing of M. magneticum AMB-1 (Zhu et al. 2014). They proposed that this interaction plays a key role in magnetotaxis. Moreover, two-hybrid genomic screenings suggested that FliM, a flagella motor switch protein; Amb0854, another methyl-accepting chemotaxis protein; and Amb3568 and GGDEF domain-containing protein interact with MamK (Pan et al. 2012). These findings may lead to a novel hypothesis for magnetotaxis.
3 Conclusions
In the past, bacterial cells were thought of as “bags of enzymes.” However, bacteria have been eloquently shown to possess highly ordered, compartmented organelles purposed for specific functions such as photosynthesis, nitrogen metabolism, and magnetic sensing. However, little is known about the molecular mechanisms governing the biogenesis and function of these bacterial organelles. Interestingly, the magnetosome possesses many features of a eukaryotic organelle. Particularly, the magnetosome is a membrane-enclosed subcellular compartment equipped with specialized proteins and cytoskeletal filaments to facilitate magnetic sensing. Additionally, magnetosomes are passed on to daughter bacterial cells. Thus, the magnetosome is an outstanding model to elucidate the molecular landscape of bacterial organelle organization. Further studies about the specific roles of magnetosome-associated proteins are warranted to shed light on magnetosome biogenesis and function.
References
Abreu F, Martins JL, Silveira TS, Keim CN, de Barros HG, Filho FJ, Lins U (2007) ‘Candidatus Magnetoglobus multicellularis’, a multicellular, magnetotactic prokaryote from a hypersaline environment. Int J Syst Evol Microbiol 57(Pt 6):1318–1322. https://doi.org/10.1099/ijs.0.64857-0
Bazylinski DA, Frankel RB (2004) Magnetosome formation in prokaryotes. Nat Rev Microbiol 2(3):217–230. https://doi.org/10.1038/nrmicro842
Bazylinski DA, Frankel RB, Jannasch HW (1988) Anaerobic magnetite production by a marine, magnetotactic bacterium. Nature 334:518–519
Bellini S (2009a) Further studies on “magnetosensitive bacteria”. Chin J Oceanol Limnol 27(1):6–12. https://doi.org/10.1007/s00343-009-0006-2
Bellini S (2009b) On a unique behavior of freshwater bacteria. Chin J Oceanol Limnol 27(1):3–5. https://doi.org/10.1007/s00343-009-0003-5
Bergeron JR, Hutto R, Ozyamak E, Hom N, Hansen J, Draper O, Byrne ME, Keyhani S, Komeili A, Kollman JM (2016) Structure of the magnetosome-associated actin-like MamK filament at subnanometer resolution. Protein Sci. https://doi.org/10.1002/pro.2979
Blakemore R (1975) Magnetotactic bacteria. Science 190(4212):377–379
Blakemore RP (1982) Magnetotactic bacteria. Annu Rev Microbiol 36:217–238. https://doi.org/10.1146/annurev.mi.36.100182.001245
Blakemore RP, Maratea D, Wolfe RS (1979) Isolation and pure culture of a freshwater magnetic spirillum in chemically defined medium. J Bacteriol 140(2):720–729
Bork P, Sander C, Valencia A (1992) An ATPase domain common to prokaryotic cell-cycle proteins, sugar kinases, actin, and Hsp70 heat-shock proteins. Proc Natl Acad Sci U S A 89(16):7290–7294. https://doi.org/10.1073/pnas.89.16.7290
Deng A, Lin W, Shi N, Wu J, Sun Z, Sun Q, Bai H, Pan Y, Wen T (2016) In vitro assembly of the bacterial actin protein MamK from ‘Candidatus Magnetobacterium casensis’ in the phylum Nitrospirae. Protein Cell 7(4):267–280. https://doi.org/10.1007/s13238-016-0253-x
Derman AI, Becker EC, Truong BD, Fujioka A, Tucey TM, Erb ML, Patterson PC, Pogliano J (2009) Phylogenetic analysis identifies many uncharacterized actin-like proteins (Alps) in bacteria: regulated polymerization, dynamic instability and treadmilling in Alp7A. Mol Microbiol 73(4):534–552. https://doi.org/10.1111/j.1365-2958.2009.06771.x
Draper O, Byrne ME, Li Z, Keyhani S, Barrozo JC, Jensen G, Komeili A (2011) MamK, a bacterial actin, forms dynamic filaments in vivo that are regulated by the acidic proteins MamJ and LimJ. Mol Microbiol 82(2):342–354. https://doi.org/10.1111/j.1365-2958.2011.07815.x
Dufrêne YF (2008) Towards nanomicrobiology using atomic force microscopy. Nat Rev Microbiol 6(9):674–680. https://doi.org/10.1038/nrmicro1948
Engel A, Müller DJ (2000) Observing single biomolecules at work with the atomic force microscope. Nat Struct Biol 7(9):715–718. https://doi.org/10.1038/78929
Faivre D, Schüler D (2008) Magnetotactic bacteria and magnetosomes. Chem Rev 108(11):4875–4898. https://doi.org/10.1021/cr078258w
Flies CB, Peplies J, Schüler D (2005) Combined approach for characterization of uncultivated magnetotactic bacteria from various aquatic environments. Appl Environ Microbiol 71(5):2723–2731. https://doi.org/10.1128/Aem.71.5.2723-2731.2005
Frankel RB (2009) The discovery of magnetotactic/magnetosensitive bacteria. Chin J Oceanol Limnol 27(1):1–2. https://doi.org/10.1007/s00343-009-0001-7
Frankel RB, Bazylinski DA, Johnson MS, Taylor BL (1997) Magneto-aerotaxis in marine coccoid bacteria. Biophys J 73(2):994–1000
Fukuda Y, Okamura Y, Takeyama H, Matsunaga T (2006) Dynamic analysis of a genomic island in Magnetospirillum sp. strain AMB-1 reveals how magnetosome synthesis developed. FEBS Lett 580(3):801–812. https://doi.org/10.1016/j.febstet.2006.01.003
Gorlenko VM, Dzyuba MV, Maleeva AN, Panteleeva AN, Kolganova TV, Kuznetsov BB (2011) Magnetospirillum aberrantis sp. nov., a new freshwater bacterium with magnetic inclusions. Microbiology 80(5):692–702. https://doi.org/10.1134/S0026261711050055
Jogler C, Lin W, Meyerdierks A, Kube M, Katzmann E, Flies C, Pan YX, Amann R, Reinhardt R, Schüler D (2009) Toward cloning of the magnetotactic metagenome: identification of magnetosome island gene clusters in uncultivated magnetotactic bacteria from different aquatic sediments. Appl Environ Microbiol 75(12):3972–3979. https://doi.org/10.1128/Aem.02701-08
Jones LJF, Carballido-López R, Errington J (2001) Control of cell shape in bacteria: helical, actin-like filaments in Bacillus subtilis. Cell 104(6):913–922. https://doi.org/10.1016/S0092-8674(01)00287-2
Kolinko S, Jogler C, Katzmann E, Wanner G, Peplies J, Schüler D (2012) Single-cell analysis reveals a novel uncultivated magnetotactic bacterium within the candidate division OP3. Environ Microbiol 14(7):1709–1721. https://doi.org/10.1111/j.1462-2920.2011.02609.x
Kolinko S, Wanner G, Katzmann E, Kiemer F, Fuchs BM, Schüler D (2013) Clone libraries and single cell genome amplification reveal extended diversity of uncultivated magnetotactic bacteria from marine and freshwater environments. Environ Microbiol 15(5):1290–1301. https://doi.org/10.1111/1462-2920.12004
Komeili A (2012) Molecular mechanisms of compartmentalization and biomineralization in magnetotactic bacteria. FEMS Microbiol Rev 36(1):232–255. https://doi.org/10.1111/j.1574-6976.2011.00315.x
Komeili A, Li Z, Newman DK, Jensen GJ (2006) Magnetosomes are cell membrane invaginations organized by the actin-like protein MamK. Science 311(5758):242–245. https://doi.org/10.1126/science.1123231
Kruse T, Møller-Jensen J, Løbner-Olesen A, Gerdes K (2003) Dysfunctional MreB inhibits chromosome segregation in Escherichia coli. EMBO J 22(19):5283–5292. https://doi.org/10.1093/emboj/cdg504
Lefèvre CT, Bazylinski DA (2013) Ecology, diversity, and evolution of magnetotactic bacteria. Microbiol Mol Biol Rev: MMBR 77(3):497–526. https://doi.org/10.1128/MMBR.00021-13
Lefèvre CT, Wu LF (2013) Evolution of the bacterial organelle responsible for magnetotaxis. Trends Microbiol 21(10):534–543. https://doi.org/10.1016/j.tim.2013.07.005
Lefèvre CT, Bernadac A, Yu-Zhang K, Pradel N, Wu LF (2009) Isolation and characterization of a magnetotactic bacterial culture from the Mediterranean Sea. Environ Microbiol 11(7):1646–1657. https://doi.org/10.1111/j.1462-2920.2009.01887.x
Lefèvre CT, Abreu F, Schmidt ML, Lins U, Frankel RB, Hedlund BP, Bazylinski DA (2010) Moderately thermophilic magnetotactic bacteria from hot springs in Nevada. Appl Environ Microbiol 76(11):3740–3743. https://doi.org/10.1128/Aem.03018-09
Lefèvre CT, Frankel RB, Abreu F, Lins U, Bazylinski DA (2011a) Culture-independent characterization of a novel, uncultivated magnetotactic member of the Nitrospirae phylum. Environ Microbiol 13(2):538–549. https://doi.org/10.1111/j.1462-2920.2010.02361.x
Lefèvre CT, Frankel RB, Pósfai M, Prozorov T, Bazylinski DA (2011b) Isolation of obligately alkaliphilic magnetotactic bacteria from extremely alkaline environments. Environ Microbiol 13(8):2342–2350. https://doi.org/10.1111/j.1462-2920.2011.02505.x
Lefèvre CT, Menguy N, Abreu F, Lins U, Pósfai M, Prozorov T, Pignol D, Frankel RB, Bazylinski DA (2011c) A cultured greigite-producing magnetotactic bacterium in a novel group of sulfate-reducing bacteria. Science 334(6063):1720–1723. https://doi.org/10.1126/science.1212596
Lefèvre CT, Viloria N, Schmidt ML, Posfai M, Frankel RB, Bazylinski DA (2012) Novel magnetite-producing magnetotactic bacteria belonging to the Gammaproteobacteria. ISME J 6(2):440–450. https://doi.org/10.1038/ismej.2011.97
Lefèvre CT, Trubitsyn D, Abreu F, Kolinko S, de Almeida LGP, de Vasconcelos ATR, Lins U, Schüler D, Ginet N, Pignol D, Bazylinski DA (2013) Monophyletic origin of magnetotaxis and the first magnetosomes. Environ Microbiol 15(8):2267–2274. https://doi.org/10.1111/1462-2920.12097
Li WB, Yu LJ, Zhou PP, Min Z (2007) Isolation of magnetotactic bacterium WM-1 from freshwater sediment and phylogenetic characterization. Arch Microbiol 188(1):97–102. https://doi.org/10.1007/s00203-007-0231-z
Lin W, Li JH, Pan YX (2012) Newly isolated but uncultivated magnetotactic bacterium of the phylum Nitrospirae from Beijing, China. Appl Environ Microbiol 78(3):668–675. https://doi.org/10.1128/Aem.06764-11
Lin W, Bazylinski DA, Xiao T, Wu LF, Pan Y (2013) Life with compass: diversity and biogeography of magnetotactic bacteria. Environ Microbiol 16(9):2646–2658. https://doi.org/10.1111/1462-2920.12313
Lin W, Deng AH, Wang Z, Li Y, Wen TY, Wu LF, Wu M, Pan YX (2014) Genomic insights into the uncultured genus ‘Candidatus Magnetobacterium’ in the phylum Nitrospirae. ISME J 8(12):2463–2477. https://doi.org/10.1038/ismej.2014.94
Lohße A, Ullrich S, Katzmann E, Borg S, Wanner G, Richter M, Voigt B, Schweder T, Schüler D (2011) Functional analysis of the magnetosome island in Magnetospirillum gryphiswaldense: the mamAB operon is sufficient for magnetite biomineralization. PLoS One 6(10):e25561. https://doi.org/10.1371/journal.pone.0025561
Matsunaga T, Sakaguchi T, Tadokoro F (1991) Magnetite formation by a magnetic bacterium capable of growing aerobically. Appl Microbiol Biotechnol 35(5):651–655
Matsunaga T, Okamura Y, Fukuda Y, Wahyudi AT, Murase Y, Takeyama H (2005) Complete genome sequence of the facultative anaerobic magnetotactic bacterium Magnetospirillum sp. strain AMB-1. DNA Res 12(3):157–166. https://doi.org/10.1093/dnares/dsi002
Meldrum FC, Mann S, Heywood BR, Frankel RB, Bazylinski DA (1993a) Electron microscopy study of magnetosomes in two cultured vibrioid magnetotactic bacteria. Proc R Soc Lond B Biol Sci 251(1332):237–242
Meldrum FC, Mann S, Heywood BR, Frankel RB, Bazylinski DA (1993b) Electron-microscopy study of magnetosomes in a cultured coccoid magnetotactic bacterium. Proc R Soc B-Biol Sci 251(1332):231–236. https://doi.org/10.1098/rspb.1993.0034
Murat D, Quinlan A, Vali H, Komeili A (2010) Comprehensive genetic dissection of the magnetosome gene island reveals the step-wise assembly of a prokaryotic organelle. Proc Natl Acad Sci U S A 107(12):5593–5598. https://doi.org/10.1073/pnas.0914439107
Nguyen HV, Suzuki E, Oestreicher Z, Minamide H, Endoh H, Fukumori Y, Taoka A (2016) A protein-protein interaction in magnetosomes: TPR protein MamA interacts with an Mms6 protein. Biochem Biophys Rep 7:39–44. https://doi.org/10.1016/j.bbrep.2016.05.010
Okuda Y, Fukumori Y (2001) Expression and characterization of a magnetosome-associated protein, TPR-containing MAM22, in Escherichia coli. FEBS Lett 491(3):169–173. https://doi.org/10.1016/S0014-5793(01)02178-0
Okuda Y, Denda K, Fukumori Y (1996) Cloning and sequencing of a gene encoding a new member of the tetratricopeptide protein family from magnetosomes of Magnetospirillum magnetotacticum. Gene 171(1):99–102. https://doi.org/10.1016/0378-1119(95)00008-9
Ozyamak E, Kollman J, Agard DA, Komeili A (2013) The bacterial actin MamK: in vitro assembly behavior and filament architecture. J Biol Chem 288(6):4265–4277. https://doi.org/10.1074/jbc.M112.417030
Pan WD, Xie CL, Lv J (2012) Screening for the interacting partners of the proteins MamK & MamJ by two-hybrid genomic DNA library of Magnetospirillum magneticum AMB-1. Curr Microbiol 64(6):515–523. https://doi.org/10.1007/s00284-012-0099-2
Petersen N, Weiss DG, Vali H (1989) Magnetic bacteria in lake sediments. Geomagnetism and palaeomagnetism, The NATO ASI Series, vol 261. Springer, Berlin, pp 231–241
Philippe N, Wu LF (2010) An MCP-like protein interacts with the MamK cytoskeleton and is involved in magnetotaxis in Magnetospirillum magneticum AMB-1. J Mol Biol 400(3):309–322. https://doi.org/10.1016/j.jmb.2010.05.011
Revathy T, Jacob JJ, Jayasri MA, Suthindhiran K (2016) Isolation and characterization of Magnetospirillum from saline lagoon. World J Microbiol Biotechnol 32(7):109. https://doi.org/10.1007/s11274-016-2075-7
Richter M, Kube M, Bazylinski DA, Lombardot T, Glöckner FO, Reinhardt R, Schüler D (2007) Comparative genome analysis of four magnetotactic bacteria reveals a complex set of group-specific genes implicated in magnetosome biomineralization and function. J Bacteriol 189(13):4899–4910. https://doi.org/10.1128/Jb.00119-07
Sakaguchi T, Burgess JG, Matsunaga T (1993) Magnetite formation by a sulfate-reducing bacterium. Nature 365(6441):47–49. https://doi.org/10.1038/365047a0
Scheffel A, Schüler D (2007) The acidic repetitive domain of the Magnetospirillum gryphiswaldense MamJ protein displays hypervariability but is not required for magnetosome chain assembly. J Bacteriol 189(17):6437–6446. https://doi.org/10.1128/Jb.00421-07
Scheffel A, Gruska M, Faivre D, Linaroudis A, Plitzko JM, Schüler D (2006) An acidic protein aligns magnetosomes along a filamentous structure in magnetotactic bacteria. Nature 440(7080):110–114. https://doi.org/10.1038/nature04382
Schleifer KH, Schüler D, Spring S, Weizenegger M, Amann R, Ludwig W, Kohler M (1991) The genus Magnetospirillum gen. nov. description of Magnetospirillum gryphiswaldense sp. nov. and transfer of Aquaspirillum magnetotacticum to Magnetospirillum magnetotacticum comb. nov. Syst Appl Microbiol 14(4):379–385
Schübbe S, Kube M, Scheffel A, Wawer C, Heyen U, Meyerdierks A, Madkour MH, Mayer F, Reinhardt R, Schüler D (2003) Characterization of a spontaneous nonmagnetic mutant of Magnetospirillum gryphiswaldense reveals a large deletion comprising a putative magnetosome island. J Bacteriol 185(19):5779–5790. https://doi.org/10.1128/Jb.185.19.5779-5790.2003
Schüler D (2008) Genetics and cell biology of magnetosome formation in magnetotactic bacteria. FEMS Microbiol Rev 32(4):654–672. https://doi.org/10.1111/j.1574-6976.2008.00116.x
Shaevitz JW, Gitai Z (2010) The structure and function of bacterial actin homologs. Cold Spring Harb Perspect Biol 2(9):a000364. https://doi.org/10.1101/cshperspect.a000364
Sonkaria S, Fuentes G, Verma C, Narang R, Khare V, Fischer A, Faivre D (2012) Insight into the assembly properties and functional organisation of the magnetotactic bacterial actin-like homolog, MamK. PLoS One 7(5):e34189. https://doi.org/10.1371/journal.pone.0034189
Taoka A, Asada R, Sasaki H, Anzawa K, Wu L, Fukumori Y (2006) Spatial localizations of Mam22 and Mam12 in the magnetosomes of Magnetospirillum magnetotacticum. J Bacteriol 188(11):3805–3812. https://doi.org/10.1128/JB.00020-06
Taoka A, Asada R, Wu L-F, Fukumori Y (2007) Polymerization of the actin-like protein MamK, which is associated with magnetosomes. J Bacteriol 189(23):8737–8740. https://doi.org/10.1128/JB.00899-07
Taoka A, Kondo J, Oestreicher Z, Fukumori Y (2014) Characterization of uncultured giant rod-shaped magnetotactic Gammaproteobacteria from a freshwater pond in Kanazawa, Japan. Microbiol-SGM 160:2226–2234. https://doi.org/10.1099/mic.0.078717-0
Thrash JC, Ahmadi S, Torok T, Coates JD (2010) Magnetospirillum bellicus sp. nov., a novel dissimilatory perchlorate-reducing Alphaproteobacterium isolated from a bioelectrical reactor. Appl Environ Microbiol 76(14):4730–4737. https://doi.org/10.1128/Aem.00015-10
Ullrich S, Kube M, Schübbe S, Reinhardt R, Schüler D (2005) A hypervariable 130-kilobase genomic region of Magnetospirillum gryphiswaldense comprises a magnetosome island which undergoes frequent rearrangements during stationary growth. J Bacteriol 187(21):7176–7184. https://doi.org/10.1128/Jb.187.21.7176-7184.2005
van den Ent F, Amos LA, Löwe J (2001) Prokaryotic origin of the actin cytoskeleton. Nature 413(6851):39–44. https://doi.org/10.1038/35092500
van den Ent F, Møller-Jensen J, Amos LA, Gerdes K, Löwe J (2002) F-actin-like filaments formed by plasmid segregation protein ParM. EMBO J 21(24):6935–6943. https://doi.org/10.1093/emboj/cdf672
Wang YZ, Lin W, Li JH, Zhang TW, Li Y, Tian JS, Gu LX, Heyden YV, Pan YX (2015) Characterizing and optimizing magnetosome production of Magnetospirillum sp XM-1 isolated from Xi’an City Moat, China. FEMS Microbiol Lett 362(21):fnv167. https://doi.org/10.1093/femsle/fnv167
Wenter R, Wanner G, Schüler D, Overmann J (2009) Ultrastructure, tactic behaviour and potential for sulfate reduction of a novel multicellular magnetotactic prokaryote from North Sea sediments. Environ Microbiol 11(6):1493–1505. https://doi.org/10.1111/j.1462-2920.2009.01877.x
Williams TJ, Lefèvre CT, Zhao WD, Beveridge TJ, Bazylinski DA (2012) Magnetospira thiophila gen. nov., sp. nov., a marine magnetotactic bacterium that represents a novel lineage within the Rhodospirillaceae (Alphaproteobacteria). Int J Syst Evol Microbiol 62:2443–2450. https://doi.org/10.1099/ijs.0.037697-0
Yamamoto D, Taoka A, Uchihashi T, Sasaki H, Watanabe H, Ando T, Fukumori Y (2010) Visualization and structural analysis of the bacterial magnetic organelle magnetosome using atomic force microscopy. Proc Natl Acad Sci U S A 107(20):9382–9387. https://doi.org/10.1073/pnas.1001870107
Zeytuni N, Zarivach R (2012) Structural and functional discussion of the tetra-trico-peptide repeat, a protein interaction module. Structure 20(3):397–405. https://doi.org/10.1016/j.str.2012.01.006
Zeytuni N, Ozyamak E, Ben-Harush K, Davidov G, Levin M, Gat Y, Moyal T, Brik A, Komeili A, Zarivach R (2011) Self-recognition mechanism of MamA, a magnetosome-associated TPR-containing protein, promotes complex assembly. Proc Natl Acad Sci U S A 108(33):E480–E487. https://doi.org/10.1073/pnas.1103367108
Zeytuni N, Baran D, Davidov G, Zarivach R (2012) Inter-phylum structural conservation of the magnetosome-associated TPR-containing protein, MamA. J Struct Biol 180(3):479–487. https://doi.org/10.1016/j.jsb.2012.08.001
Zeytuni N, Cronin S, Lefèvre CT, Arnoux P, Baran D, Shtein Z, Davidov G, Zarivach R (2015) MamA as a model protein for structure-based insight into the evolutionary origins of magnetotactic bacteria. PLoS One 10(6):e0130394. https://doi.org/10.1371/journal.pone.0130394
Zhang WY, Zhou K, Pan HM, Yue HD, Jiang M, Xiao T, Wu LF (2012) Two genera of magnetococci with bean-like morphology from intertidal sediments of the yellow sea, China. Appl Environ Microbiol 78(16):5606–5611. https://doi.org/10.1128/Aem.00081-12
Zhou K, Zhang WY, Yu-Zhang K, Pan HM, Zhang SD, Zhang WJ, Yue HD, Li Y, Xiao T, Wu LF (2012) A novel genus of multicellular magnetotactic prokaryotes from the Yellow Sea. Environ Microbiol 14(2):405–413. https://doi.org/10.1111/j.1462-2920.2011.02590.x
Zhu KL, Pan HM, Li JH, Yu-Zhang K, Zhang SD, Zhang WY, Zhou K, Yue HD, Pan YX, Xiao TA, Wu LF (2010) Isolation and characterization of a marine magnetotactic spirillum axenic culture QH-2 from an intertidal zone of the China Sea. Res Microbiol 161(4):276–283. https://doi.org/10.1016/j.resmic.2010.02.003
Zhu X, Ge X, Li N, Wu LF, Luo C, Ouyang Q, Tu Y, Chen G (2014) Angle sensing in magnetotaxis of Magnetospirillum magneticum AMB-1. Integr Biol 6(7):706–713. https://doi.org/10.1039/c3ib40259b
Acknowledgments
This work was supported by MEXT KAKENHI (24117007 and 23111508), JSPS KAKENHI (17H03791, 16 K07661, 25850051, and 22780063), the Human Frontier Science Program (RGP0035/2004-C104), and the Institute for Fermentation, Osaka (IFO).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Taoka, A., Fukumori, Y. (2018). Structure and Function of Aligned Magnetic Crystals in Magnetotactic Bacteria. In: Matsunaga, T., Tanaka, T., Kisailus, D. (eds) Biological Magnetic Materials and Applications. Springer, Singapore. https://doi.org/10.1007/978-981-10-8069-2_1
Download citation
DOI: https://doi.org/10.1007/978-981-10-8069-2_1
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-8068-5
Online ISBN: 978-981-10-8069-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)