Abstract
Magnetotactic bacteria are a group of prokaryotes capable of sensing and navigating along the earth’s magnetic field. The linear alignment of magnetosomes, which acts as a compass needle for orientation, is dependent on the proteins MamJ (amb0964) & MamK (amb0965). We constructed Magnetospirillum magneticum AMB-1 two-hybrid DNA libraries by fusing the random genomic fragments of AMB-1 to the N-terminal domain of the α-subunit of RNA polymerase in vector pTRG and used as preys. The genes mamJ & mamK were cloned in frame with the λ repressor protein (λ cI) in vector pBT and used as baits for screening the binding partners. After preliminary screening, we further confirmed the candidate interactions between selected protein pairs. The results showed that there were relatively strong interactions between MamK versus Amb3498 (flagella motor switch protein fliM), versus Amb0854 MCPs (signal domain of methyl-accepting chemotaxis protein) and versus Amb3568 (GGDEF domain-containing protein), respectively. MamJ versus Amb1722 (hypothetical protein), MamJ versus MamK, and MamK versus Amb1807 (cation transport ATPase) exhibited low level of interaction. Although the TPR repeat protein MamA (amb0971) showed no interaction with either MamJ or MamK, the TPR repeat protein Amb0024 with more motif sequences exhibited relatively strong interaction with MamK. Among the identified proteins, all categorized as signal transduction-related displayed interaction only with MamK and without MamJ, suggesting that magnetotaxis via MamK in Magnetospirillum magneticum AMB-1 might be somehow concerned with the widely accepted chemotaxis mechanism in bacteria.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Magnetotactic bacteria (MTB) are a group of prokaryotes capable of magnetic field orientation, which are featured by intracellular structures named magnetosomes comprising magnetic iron mineral crystals and enveloped by a phospholipids bilayer membrane [1, 2]. The synthesis of magnetosome chains involves several genetically controlled biochemical processes including invagination of cytoplasmic membranes, biomineralization of magnetite crystals and assembly of chains [3–5]. During the process, the acidic protein MamJ (amb0964) and actin-like protein MamK (amb0965) were described to be responsible for magnetosome chain formation [6, 7]. It was reported that the MamJ-deficient mutant from Magnetospirillum gryphiswaldense MSR-1 no longer produced linear chains of magnetosomes, but that magnetite crystals were instead arranged in compact three-dimensional clusters [7]. MamK, a homolog of the bacterial actin-like protein MreB, formed a network of cytoskeletal filaments both in vivo and in vitro [8, 9]. Compared to MreB, which appeared to form helical filaments adjacent to the cell membrane [10], MamK-GFP assembled in straight lines extending across most of the cell approximately along its inner curvature [11]. In a knockout mamK mutant, long highly organized magnetosome chains were no longer seen [6]. The widely accepted mechanism for magnetosome chain assembling was that MamK encoded the cytoskeletal magnetosome filaments to which MamJ connected the magnetosome vesicles [12]. Although a protein–protein interaction between MamJ and MamK was detected in MSR-1, the regions critical for binding MamK were not highly conserved in Magnetospirillums [13]. For better elucidating the roles of MamJ or MamK in magnetosome chain assembling in magnetotactic bacteria, we constructed two-hybrid genomic DNA library of Magnetospirillum magneticum AMB-1 for screening protein–protein interactions with either protein MamJ or MamK by using BacterioMatch II two-hybrid system.
Materials and Methods
Bacterial Strains and Growth Conditions
E. coli strains DH5α [F−φ80 lacZΔM15Δ (lacZYA-argF) U169 endA1 recA1 hsdR17 (r −k m +k ) supE44λ− thi-1 gryA96 relA1 phoA] were routinely grown in Luria–Bertani (LB) medium at 37°C. Medium and culture conditions for the E. coli strains XL1-Blue MRF’ Kan and BacterioMatch II Reporter strain (Stratagene, La Jolla, CA, USA) was prepared according to the instruction manual of BacterioMatch II Two-Hybrid kit. Liquid culture of M. magneticum strain AMB-1 was grown in enriched MSGM medium containing per liter: 5 ml Wolfe’s mineral solution, 0.68 g potassium phosphate, 0.12 g sodium nitrate, 0.74 g succinic acid, 0.05 g l-cysteine, 0.20 g polypeptone, 0.10 g yeast extract, with the final pH adjusted to 6.75 before autoclaving. After autoclaving, 10 ml of Wolfe’s vitamin solution was added [14]. The culture was maintained at 28°C in 50-ml bottles containing 40 ml of medium for 3 days, and R mag was measured to check the magnetism of magnetotactic bacteria [15].
Construction of Bait Clones and Prey Library
MamJ or MamK fusion expressed with bacteriophage-repressor protein (λ cI) in pBT was served as bait and fragments of AMB-1 genome DNA fusion expressed with alpha subunit of E. coli RNA polymerase (RNAP-α) in pTRG were served as prey library. Total DNA from M. magneticum AMB-1 used in this study was isolated as described previously [16]. Primers used in this study were deduced from GenBank reference sequences NC_007626.1, designed with Primer Premier 5 (PREMIER Biosoft) and shown in Table S1 in the supplemental material.
For bait clones, full-length of wild-type gene fragments of mamJ or mamK were obtained by PCR amplification from AMB-1 genomic DNA using gene specific primer pairs J pbt F/R and K pbt F/R, respectively. The resulting bait plasmids allowed bait inserts fusion expressed with the full-length λ repressor protein (λ cI) and were transformed into BacterioMatch II Reporter Strain. Expression of pBT encoding MamJ or MamK fusion constructs was confirmed by immunoblotting using polyclonal antibodies raised against λ cI at a dilution of 1:750.
The prey library was constructed from random genomic fragments of AMB-1. EcoRI/XhoI double digested pTRG was blunted with T4 DNA polymerase (Takara) and then dephosphorylated with alkaline phosphatase (Takara) to avoid self-ligation. Genomic DNA was fragmented by sonication (2 times, 200w, 2s, XinZhi JY92-IIN Φ2), and 0.5–2.0 kb DNA fragments were concentrated, end-repaired, dephosphorylated and cloned into pTRG (prey). The ligations were then transformed into host strain XL1-Blue using standard electroporation procedure. A total of 47,000 individual transformants were collected and pooled as the prey library. 12 Clones were tested by colony PCR with primer pairs pTRG F/R to estimate the average size of inserts. The function of fx = INT(RAND () × (4559 − 1) + 1), in which INT and RAND denotes integer and random number, respectively, was used to produce random numbers between 1 and 4559 for numbering the 4559 ORFs of AMB-1 genome. 15 Gene fragments were chosen randomly from 4559 ORFs of AMB-1 genome for evaluating the representation of the library.
Two-Hybrid Screening and Verification of Interactions
Detection of protein–protein interactions was based on transcriptional activation of the HIS3 reporter gene, which allowed growth in the presence of 3-amino-1,2,4-triazole (3-AT). Prior to screening, the self-activity of each bait clone was tested by co-transforming the empty prey vector into the bait vector-harboring reporter strain and plating on selective screening medium supplemented with 5 mM 3-AT. The bait reporter strain was then transformed with prey library plasmids and plated on both selective and non-selective medium according to the manufacturer’s instructions. Putative positive colonies were validated using streptomycin resistance as a secondary reporter. Aliquots of cultured positive colonies were used for sequencing the prey inserts, and were stored at −80°C. The sequence tags were subsequently subjected to a BLAST search against GenBank. Sequences corresponding to the sense strand of ORFs were selected and candidates of interest were chosen for further verification.
According to the prey sequence tags, full-length or conservative domain of candidate genes as well as mamA, mamE & mamK were cloned and in frame inserted into pTRG as to testify the protein–protein interactions between a pair of proteins in BacterioMatch II two-hybrid system.
Cross-Linking Analyses for Fully Identification of Interactions
In order to further demonstrate the related interactions, cross-linking experiments were performed in vitro to characterize the complex formation between MamK and Amb0854 MCPs, and between MamJ and Amb1722 CAS, respectively. The interactions between MamK & MamJ and MamA were also detected by using the cross-linking assay. For cloning and expression of the proteins MamK, MamJ, and MamA, full-length of wild-type genes were obtained by PCR amplification from Magnetospirillum Magneticum AMB-1 genomic DNA using an incorporated EcoRI (forward) and BamHI (reverse) recognition sites. For expression of Amb0854 MCPs and Amb1722 CAS, the signal domain of methyl-accepting chemotaxis protein from full-length of gene amb0854 (MCPs, nucleotide 1445-2025) and the CAS domain from full-length of gene amb1722 (nucleotide 1262-1962) were chosen. The cloning was performed using expression plasmid pGEX-4T-1 (GE Healthcare) and recombinant plasmids were transformed into E. coli BL21 (DE3) cells for expression. The optimal expression conditions chosen for recombinant MamK, MamA, and Amb0854 MCPs in this study were 0.1 mM of IPTG for 3 h at 37°C as well as 0.5 mM of IPTG for 3 h at 25°C for MamJ and Amb1722 CAS. The resulting supernatants from cell lysates were prepared by sonication. Glutathione Sepharose 4B in 50% (v/v) slurry was prepared and packed in a disposable 5 ml column. The supernatant containing the fusion products as well as other non-specific proteins were circulated through the column at 4°C and overnight to allow binding of GST fusin protein to the glutathione Sepharose beads. Subsequently, unbound proteins were washed out from the column with ten bed volumes of 1× PBS. For removal of GST from the fusion protein, an “on-column” (GSTrap FF) thrombin digestion was performed to cleave the GST tag from the fusion protein. The fusion products were incubated with thrombin (1 U/μl) at room temperature for 16 h. After the column was washed with approximately three bed volumes of 1× PBS, the flowthrough containing cleaved GST-free target protein and thrombin was collected, leaving the GST moiety bound to the Sepharose. The thromin was removed eventually using a HiTrap Benzamidine FF (high sub) column.
The rabbit anti-Amb0854 MCPs, anti-Amb1722 CAS, and anti-MamA serum were produced in this laboratory. The 2.5 μg of each protein was used in a final volume of 50 μl of PBS and incubated for 2 h on ice. The bis (sulfosuccinimidyl) suberate (BS3; Pierce) was added to a final concentration of 1 mM and the mixture was incubated for 1 h at room temperature. The reaction was quenched by the addition of Tris–HCl, pH 7.4 (20 mM final concentration). After boiling for 5 min, the proteins were separated by using a 10% SDS/PAGE gel under reducing conditions and blotted on nitrocellulose. The cross-linked proteins were detected by using the rabbit anti-Amb0854 MCPs, anti-Amb1722 CAS, and anti-MamA, respectively. For cross-linking studies, a molar ratio of 1:4 between MamK and Amb0854 MCPs, a molar ratio of 1:6 between MamJ and Amb1722 CAS, and a molar ratio of 1:1 between MamK & MamJ and MamA were selected, respectively.
Results
Construction of Two-Hybrid Library
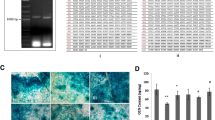
Insert DNA was prepared by ultrasonication and fragments size ranging from 0.5 to 2.0 kb were separated and enriched by electrophoresis. Electroporation competent of host strain XL1-Blue was prepared for harboring insert-pTRG plasmids and an efficiency of approximately 2 × 103 cfu/μl ligation reaction (ligation of the insert DNA into the pTRG plasmid) was achieved. 30 Transformations were carried out to scale up the library capacity and a total of 47,000 transformants were obtained. Inserts in individual colonies can be examined by PCR using pTRG-specific primers. The size of inserts from 12 randomly selected colonies was checked and estimated as 818, 1216, 699, 1726, 1567, 664, 972, 1843, 1093, 1104, 1387, and 1853 bp (Fig. 1a). The representativeness of the library was checked using 15 randomly selected genomic fragments ranging from 168 to 516 bp (Fig. 1b). Except the Amb4296, all the other 14 fragments could be identified by PCR from the library.
Construction of Magnetospirillum magneticum AMB-1 two-hybrid prey library. a Evaluation of average size of DNA inserts in AMB-1 two-hybrid prey library. Insert fragments were amplified by colony PCR from 12 random selected colonies (lane 1–12). PCR amplification from colony which harbored empty plasmid pTRG was served as negative control (lane ck−). Sizes of DNA markers were given on the left. b Evaluation of the representativeness of AMB-1 two-hybrid prey library. Selected genomic fragments were amplified by PCR from purified prey library plasmids (with filled square beneath the lane). Genes ID of the fragments were shown on the top. Amplification from AMB-1 genomic DNA served as positive control (with nothing beneath the lane)
Preliminary Screening of Library
MamJ or MamK was used as the bait for protein–protein interactions. Fusion expression of MamJ or MamK with λ cI was detected by western blot using antibody raised against λ cI (Fig. 2a). Among 5.47 × 105 screened co-transformants of the bait MamK and prey library plasmid, 164 positive colonies were validated with both 5 mM 3-AT and the secondary reporter aadA gene (Smr). As to MamJ, 2.23 × 105 co-transformants were screened and 50 colonies were validated with streptomycin. The typical screening plates for MamJ & MamK interacting partners were shown in Fig. 2b(1) and b(2). Among the total 214 clones, only 34 insert genomic fragments fusion expressed with RNAP-α were shown to be consistent with the reading frame as predicted on NCBI, while 3 clones were tested to have multiple inserts (Table 1). Of the 34 candidates, 9 inserts were interacting partners of MamJ and 25 inserts were interacting partners of MamK. Interestingly, fragment of Amb3520 (with MamK) and Amb2452 (with MamJ) were detected twice in sequenced clones. A small polypeptide of 38 amino acid residues, “GTIMVEQQCGAGKGVQPLGPQDQHGPPSLRGLRPGFFP,” which corresponds to the nucleotide sequence of frameshifted amb3894 might derive from randomly cut AMB-1 genomic DNA. The peptide shared no significant similarity with any known protein and was detected to interact with both MamJ and MamK. Based on COG database, proteins encoded by insert genes were categorized as signal transduction (7 clones), cell motility (5 clones), cell wall/membrane/envelope biogenesis (3 clones), and etc (Table 1). Seven of 34 gene fragments were annotated as hypothetical or of unknown function.
Western blot detection of MamJ & MamK fusion expression with λ cI and typical screening plates for interactions. a Immunodetection of MamJ & MamK fusion expressed with λ cI in reporter strain. Reporter strain harboring an empty vector (pBT) or a vector containing mamJ/mamK fused to λ cI at the C-terminus were grown with (+) or without (−) IPTG (0.1 mM). Crude extracts were resolved by SDS-PAGE and detected with anti-λ cI antibody. b The typical screening plates for MamJ & MamK interacting partners. CK+ co-transformants harboring pBT-LGF2 and pTRG-Gal11P as positive controls. MamJ & MamK-CK− co-transformants harboring pBT-mamJ or pBT-mamK and empty pTRG as negative controls (denoted by the two arrows in b2 and position-corresponding colonies in b1). Each colony representing a MamJ or MamK interacting protein corresponds to each other in both plates. 1 Nonselective screening plate without streptomycin and 3-amino-1,2,4-triazole. 2 Dual selective screening plate plus 12.5 μg/ml streptomycin and 5 mM 3-amino-1,2,4-triazole
Further Verification of Interactions
The interaction candidates were further validated by two-hybrid protein–protein interaction analysis between selected protein pairs (Fig. 3). The co-transformation of pBT-MamJ/MamK and pTRG (empty vector) was used as negative control and no colony growth was observed on selective screening medium, indicating that both MamJ and MamK were suitable for the two-hybrid system. Relatively strong interactions were detected between MamK and Amb3498 (flagella motor switch protein fliM), Amb0854 MCPs (signal domain of methyl-accepting chemotaxis protein), and Amb3568 (GGDEF domain-containing protein), respectively. MamJ and Amb1722 (hypothetical protein), MamK and Amb1807 (cation transport ATPase) as well as MamJ and MamK exhibited low level of interaction. The Trypsin-like serine protease MamE (amb0963) was identified to interact with both MamJ and MamK. Although the TPR repeat protein MamA (amb0971) showed no interaction with either MamJ or MamK, the TPR repeat protein Amb0024 with more motif sequences exhibited relatively strong interaction with MamK.
Interaction level of MamJ & MamK with proteins of interest. The protein–protein interactions were assayed using two-hybrid system in which each gene of interest was inserted into the prey plasmid pTRG and the gene mamJ or mamK cloned into pBT was used as the bait. Co-transformants harboring pBT-LGF2 and pTRG-Gal11P were used as positive controls. The empty prey plasmid co-tranformed with MamJ or MamK showed no background level of interaction (0 number of colony). Since the actin-like protein MamK was demonstrated to interact with itself either in vivo or in vitro (see the references [8, 13]), the number of co-transformed colonies for MamK–MamK was used as standard of normalization. The bars represent the relative ratio of the number of selected interacting colonies normalized by the number of colonies for MamK–MamK. The experiments were repeated for three times. Data were shown as means ± SD. Amb1722 CAS CAS domain of full-length Amb1722 (nucleotide 1262-1962), Amb0854 MCPs MCP signal domain of full-length Amb0854 (nucleotide 1445-2025), Amb1963 CheR CheR domain of full-length Amb1963 (nucleotide 257-822), Amb2277 EAL EAL domain of full-length Amb2277 (nucleotide 1721-2442). Amb3489, Amb1807, Amb1699, Amb3568, Amb0024, MamA, MamE & MamK represent full-length of the proteins, respectively
In order to further demonstrate the related interactions, cross-linking experiments were performed in vitro to characterize the complex formation between MamK and Amb0854 MCPs, between MamK & MamJ and MamA, and between MamJ and Amb1722 CAS, respectively. MamK and MamJ were identified to be chemically cross-linked to Amb0854 MCPs and Amb1722 CAS, respectively. As shown in Fig. 4a, the cross-linking experiments revealed a complex of 58 and 78 KDa corresponding to cross-linked MamK and Amb0854 MCPs, and cross-linked MamJ and Amb1722 CAS, respectively. In contrast, after chemical cross-linking of MamK & MamJ with MamA, no complex consisting of cross-linked MamA either with MamK or MamJ was observed (Fig. 4b).
Cross-linking interactions of selected proteins. Amb0854 MCPs, Amb1722 CAS, MamK, MamJ, and MamA from Magnetospirillum magneticum AMB-1 were cloned, expressed and purified by using the glutathione S-transferase gene fusion system (GE Healthcare), and SDS-PAGE for purified proteins under reducing conditions was performed using 5% stacking gels and 12% separating gels (a). The gels were stained with coomassie brilliant blue R-250. Lane MW stands for molecular mass marker proteins as well as Lane Amb0854, Amb1722 CAS, MamK, MamJ, and MamA stand for each protein. Amb0854 MCPs was cross-linked to MamK and Amb1722 CAS was cross-linked to MamJ (b), followed by reducing SDS-PAGE. MamA was cross-linked to either MamK or MamJ (c). After blotting on nitrocellulose the cross-linked proteins were visualized by the rabbit anti-Amb0854 MCPs, anti-Amb1722 CAS, and anti-MamA, respectively. Amb0854 MCPs MCP signal domain of Amb0854, Amb1722 CAS CAS domain of Amb1722, BS3 bis (sulfosuccinimidyl) suberate
Discussion
Among the interacting candidates of MamK, Amb3568 contains a conserved GGDEF domain which is widely accepted as a putative signal transduction domain in regulating the production of second messenger molecule of bis-(3′–5′)-cyclic dimeric guanosine monophosphate (c-di-GMP) in bacteria. The GGDEF domain represents the dinucleotide cyclase to catalyze the production of c-di-GMP, while EAL domain represents most probably the cyclic dinucleotide phosphodiesterase to catalyze the degradation of c-di-GMP [17, 18]. The EAL domain of Amb2277 was cloned as prey and was detected to interact with MamK. The MCPs of Amb0854 was found out to be a binding partner of MamK in a way similar to the methyl-accepting chemotaxis protein Amb0994, which was demonstrated to interact with MamK via its C-terminal domain (signal domain) by bimolecular fluorescence complementation [19]. We detected the interaction between MCP signal domain and MamK by a totally different approach, suggesting that this interaction is robust in AMB-1 strain and of critical importance for MamK’s function. The CheR domain from methylase of chemotaxis methyl-accepting protein Amb1963 was another binding partner of MamK. Among the identified proteins, all categorized as signal transduction-related exhibited interaction only with MamK and without MamJ, indicating that magnetotaxis via MamK in Magnetospirillum magneticum AMB-1 might be linked somehow to the widely accepted chemotaxis mechanism in bacteria.
Three flagella motor-associated proteins (Amb1699, Amb1700 and Amb3498) were demonstrated to interact with MamK. The protein Amb1699 contains a MotA domain and a FliG-C domain in its N-terminal and C-terminal, respectively, and shows a higher similarity in sequence with flagella motor proteins in AMB-1 strain. Similarly, the protein Amb1700 contains two MotA domains and a FliG-C domain in its N-terminal which was also detected to interact with MamK. Amb3498 is a flagella motor switch protein and the SpoA domain in its C-terminal is a consensus domain of flagella motor proteins FliM and FliN. It was known that MotA and MotB could form ion-conducting complexes and act as the stator. The proteins FliN, FliM, and FliG as components of the rotor could form C-ring and function in flagella assembly, torque generation and direction control [20]. Since the protein MamK was distributed in a line from pole to pole along the long axis of the AMB-1 strain [9], our work proposed the possibility that the magnetic torque produced by magnetosome chain might interfere with flagella rotation via direct interaction between MamK and the flagella motor proteins.
Identified as an interacting partner of MamJ, Amb1722 was predicted to be a hypothetical protein with a CAS domain on its C-terminal. CAS domain is referred to as caspase (cysteine-dependent aspartate-directed protease), a homologue of interleukin-1 beta converting enzyme (ICE) for mediating programmed cell death (apoptosis). Amb1722 was implicated to be involved in magnetite synthesis by transposon mutagenesis and it had no ortholog in other Magnetospirillums [21]. Although Amb1722 contained a domain related to subfamily of TPR repeats, it displayed less possibility of interacting with MamJ via the TPR domain.
Since the TPR repeat protein MamA (amb0971) was located at the outermost layer of magnetosomes and formed a high molecular mass complex around magnetosomes, it might act as a scaffold that linked between magnetosome vesicles and cytoplasmic components, contributing to the magnetosome chain assembly or magnetosome vesicles activation [22]. In a recent work by Zeytuni et al. [23], MamA folds as a sequential tetra-trico-peptide repeat (TPR) protein with a unique hook-like shape. Crystal structural analysis confirmed that the core of MamA is not affected by crystallization conditions and revealed three protein–protein interaction sites, namely a concave site, a convex site, and a putative TPR repeat. Therefore, it is supposed that a large homooligomeric scaffold derived from MamA’s concave site might interact with other magnetosome-associated proteins via the MamA convex site [23]. If a protein on magnetosomal membrane is responsible for connecting magnetosome to the bacterial actin filament, MamA would be a part of the intersection. However, due to the none interaction between MamA and MamK, it would speculate that MamA wraps magnetosomes hanging on the filamentous backbones like beads, or else links between magnetosome vesicles and cytoplasmic components to help stabilize the magnetosome chain and activate the magnetite formation. Different from MamA, the TPR repeat protein Amb0024 possesses a domain related to outer membrane biogenesis on its C-terminal. Since MamA showed no interactions with either MamJ or MamK, the domains on C-terminal of Amb0024 might be responsible for its interaction with MamK. On the other hand, the putative membrane-bound serine protease MamE was suggested to control the localization of other magnetosome proteins and alternatively to play a direct role in magnetosome crystal biomineralization [24–26]. Therefore, the detected interaction between MamE and MamJ was in accordance with MamE’s sorting function to some extent.
Although the preliminary screening of AMB-1 genomic DNA prey library with MamK-λ cI as bait could not identify MamK-Mamk interaction, it still could be detected with MamK-λ cI as bait and MamK-RNAP-α as an individual prey by using the bacterial two-hybrid system, which was consistent to the reports that MamK interacted with itself either in vivo or in vitro [6, 9]. Similarly, MamJ–MamK interaction could be detected by using the bacterial two-hybrid system, which was somehow consistent to the report that MamJ of MSR-1 strain interacted with MamK [13], despite the fact that MamJ of AMB-1 strain shares only 52 and 65% similarity with MamJ of MSR-1 strain in its N-terminal and C-terminal. The two acidic proteins of MamJ and LimJ in M. magneticum AMB-1 can promote the dynamic behavior of MamK filaments in wild-type cells [27]. The absence of both MamJ and LimJ produces static filaments, a disrupted magnetosome chain, and an anomalous build-up of cytoskeletal filaments between magnetosomes, suggesting that the dynamic behavior of MamK filaments are regulated by the acidic proteins MamJ and LimJ [27]. Also, magnetosome chains in M. gryphiswaldense underwent a dynamic pole-to-midcell translocation during cytokinesis and newly produced chains were recruited to division sites even in division-inhibited cells, but not in a mamK mutant, supporting an active role of MamK filaments engaged in magnetosome division and segregation [28]. Moreover, in order to test the related interactions independently, crosslinking protein interaction analyses were investigated using recombinant proteins between MamK and Amb0854 MCPs, and between MamJ and Amb1722 CAS separately. MamK and MamJ were identified to interact with Amb0854 MCPs and Amb1722 CAS, respectively, while MamA showed no complex formation with either MamJ or MamK, which were consistent somewhat to the results from the bacterial two-hybrid system.
Collectively, the present study provides a data source of protein–protein interactions with MamJ or MamK via the genomic screening in Magnetospirillum magneticum AMB-1. For a certain interaction candidate protein, further confirmation by other independent approaches would be needed to fully elucidate the in vivo processes associated with magnetosome chains assembling.
References
Blakemore RP (1975) Magnetotactic bacteria. Science 190:377–379
Blakemore RP (1982) Magnetotactic bacteria. Annu Rev Microbiol 36:217–238
Jogler C, Schüler D (2009) Genomics, genetics, and cell biology of magnetosome formation. Annu Rev Microbiol 63:501–521
Komeili A (2007) Molecular mechanisms of magnetosome formation. Annu Rev Biochem 76:351–366
Schüler D (2008) Genetics and cell biology of magnetosome formation in magnetotactic bacteria. FEMS Microbiol Rev 32:654–672
Komeili A, Li Z, Newman DK et al (2006) Magnetosomes are cell membrane invaginations organized by the actin-like protein MamK. Science 311:242–245
Scheffel A, Gruska M, Faivre D et al (2006) An acidic protein aligns magnetosomes along a filamentous structure in magnetotactic bacteria. Nature 440:110–114
Katzmann E, Scheffel A, Gruska M et al (2010) Loss of the actin like protein MamK has pleiotropic effects on magnetosome formation and chain assembly in Magnetospirillum gryphiswaldense. Mol Microbiol 77:208–224
Taoka A, Asada R, Wu LF et al (2007) Polymerization of the actin-like protein MamK, which is associated with magnetosomes. J Bacteriol 189:8737–8740
Graumann PL (2004) Cytoskeletal elements in bacteria. Curr Opin Microbiol 7:565–571
Pradel N, Santini CL, Bernadac A et al (2006) Biogenesis of actin-like bacterial cytoskeletal filaments destined for positioning prokaryotic magnetic organelles. Proc Natl Acad Sci USA 103:17485–17489
Frankel RB, Bazylinski DA (2006) How magnetotactic bacteria make magnetosomes queue up. Trends Microbiol 14:329–331
Scheffel A, Schüler D (2007) The acidic repetitive domain of the Magnetospirillum gryphiswaldense MamJ protein displays hypervariability but is not required for magnetosome chain assembly. J Bacteriol 189:6437–6446
Yang CD, Takeyama H, Tanaka T et al (2001) Effects of growth medium composition, iron sources and atmospheric oxygen concentrations on production of luciferase-bacterial magnetic particle complex by a recombinant Magnetospirillum magneticum AMB-1. Enzyme Microb Technol 29:13–19
Zhao LZ, Wu D, Wu LF et al (2007) A simple and accurate method for quantification of magnetosomes in magnetotactic bacteria by common spectrophotometer. J Biochem Biophys Methods 70:377–383
Marmur J (1961) A procedure for the isolation of deoxyribonucleic acid from microorganisms. J Mol Biol 3:208–218
Romling U, Gomelsky M, Galperin MY (2005) C-di-GMP: the dawning of a novel bacterial signalling system. Mol Microbiol 57:629–639
Simm R, Morr M, Kader A et al (2004) GGDEF and EAL domains inversely regulate cyclic di-GMP levels and transition from sessility to motility. Mol Microbiol 53:1123–1134
Philippe N, Wu LF (2010) An MCP-like protein interacts with the MamK cytoskeleton and is involved in magnetotaxis in Magnetospirillum magneticum AMB-1. J Mol Biol 400:309–322
Berg H (2003) The rotary motor of bacterial flagella. Annu Rev Biochem 72:19–54
Matsunaga T, Okamura Y, Fukuda Y et al (2005) Complete genome sequence of the facultative anaerobic magnetotactic bacterium Magnetospirillum sp. strain AMB-1. DNA Res 12:157–166
Yamamoto D, Taoka A, Uchihashi T et al (2010) Visualization and structural analysis of the bacterial magnetic organelle magnetosome using atomic force microscopy. Proc Natl Acad Sci USA 107:9382–9387
Zeytuni N, Ozyamak E, Ben-Harush K et al (2011) Self-recognition mechanism of MamA, a magnetosome-associated TPR-containing protein, promotes complex assembly. Proc Natl Acad Sci USA 108:13369–13370
Murat D, Quinlan A, Vali H et al (2010) Comprehensive genetic dissection of the magnetosome gene island reveals the step-wise assembly of a prokaryotic organelle. Proc Natl Acad Sci USA 107:5593–5598
Quinlan A, Murat D, Vali H et al (2011) The HtrA/DegP family protease MamE is a bifunctional protein with roles in magnetosome protein localization and magnetite biomineralization. Mol Microbiol 80:1087–1705
Yang W, Li R, Peng T et al (2010) mamO and mamE genes are essential for magnetosome crystal biomineralization in Magnetospirillum gryphiswaldense MSR-1. Res Microbiol 161:701–705
Draper O, Byrne ME, Li Z et al (2011) MamK, a bacterial actin, forms dynamic filaments in vivo that are regulated by the acidic proteins MamJ and LimJ. Mol Microbiol 82:342–354
Katzmann E, Müller FD, Lang C et al (2011) Magnetosome chains are recruited to cellular division sites and split by asymmetric septation. Mol Microbiol 82:1316–1329
Acknowledgment
This study was supported by research grants (No. 30670508 and No. 31070755) from the National Natural Science Foundation of China to Weidong Pan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Weidong Pan and Chunlan Xie share equal first authorship.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pan, W., Xie, C. & Lv, J. Screening for the Interacting Partners of the Proteins MamK & MamJ by Two-Hybrid Genomic DNA Library of Magnetospirillum magneticum AMB-1. Curr Microbiol 64, 515–523 (2012). https://doi.org/10.1007/s00284-012-0099-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-012-0099-2