Abstract
Mullerian ducts are the root of female reproductive organs. Any defect during embryogenesis can lead to a variety of structural anomalies, which usually manifest as repeated abortions, preterm labor, etc. Uterine septum is one of the commonest Mullerian anomalies. Hysteroscopic septal resection is now the accepted treatment of choice for symptomatic septate uterus. The procedure is minimally invasive and can be performed in an outpatient setting, with a rapid recovery and no prolonged postoperative delay in conception. Correct understanding of the technique of metroplasty is important to avoid complications and to get the best surgical outcome.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
7.1 Introduction
Congenital uterine anomalies are developmental defects of Mullerian ducts during embryogenesis. These are categorized into defects of genesis and fusion.
References regarding the existence of Mullerian defects date back to antiquity, around 300 BC. Columbo reported the first documented case of vaginal agenesis (uterus and vagina) in the sixteenth century [1].
The exact incidence of congenital uterine anomalies is unknown because most women with such anomalies remain asymptomatic. Most of the studies measuring prevalence were conducted in a small population of women who have experienced a pregnancy loss; hence the actual prevalence in general population remains undetermined. Uterine anomalies occur in 2–4% of infertile women and fertile women with normal reproductive outcomes. The incidence is higher, however, among women with recurrent first-trimester miscarriage or late first- or second-trimester miscarriage/preterm delivery. Arcuate uterus, often diagnosed in women without any reproductive problems, is the most common uterine anomaly (5%), followed by septate uterus (3%) and bicornuate uterus (0.5%) [2].
Mullerian anomalies may present with a variety of gynecological and obstetrical problems [2, 3]. Uterine septum is the commonest uterine anomaly, responsible for approximately 80–90% of congenital uterine malformations [2]. Among all uterine malformations, uterine septum is the only one that can be treated and corrected by hysteroscopic surgery [3].
7.2 Etiology
The underlying etiology of congenital Mullerian defects is not well understood. The karyotype of women with these anomalies is usually normal. It is the process of embryogenesis that governs the malformations of the genital tract. The development of the uterus starts at around 8–16 weeks of fetal life from the paired paramesonephric (Mullerian) ducts. These ducts pass through three phases of development [4]:
-
Organogenesis: Both paramesonephric ducts begin to develop.
-
Fusion: Upper part of the vagina, cervix, and uterus are formed by lateral fusion of the lower Mullerian ducts. The upper cranial part of the Mullerian ducts remains unfused and forms the fallopian tubes.
-
Septal absorption: Central septum left after the fusion of lower Mullerian ducts eventually gets absorbed after 9 weeks leading to single uterus and cervix.
A defect in the subperitoneal fibro-muscular tissue, which normally pulls the Mullerian duct together, an unusually thick round ligament or a tough vesicorectal fold, may contribute to developmental defects.
There is another set of ducts called mesonephric or Wolffian ducts which are crucial for renal development and also induce female reproductive tract development [5]. As a result, any abnormalities of mesonephric maldevelopment may also have an effect on genital tract formation [6]. Hence it is important to look for congenital abnormalities of renal system in patients where any genital anomaly is detected [7].
7.3 Classification
The most widely accepted classification of Mullerian duct anomalies is designed by American Fertility Society (AFS) (1988) (Table 7.1) [8]. Although this system is based on the clinically useful scheme of Buttram and Gibbons which combines the degree of developmental failure with clinical manifestations [9], it does not fully cover associated anomalies in the vagina, cervix, fallopian tubes, and renal system.
This system focuses largely on vertical fusion defects. Associated anomalies are not fully included, and a note of these should always be made. Detailed discussion of the classification would be beyond the prerogative of this chapter.
7.4 Signs and Symptoms
Complains suggestive of a uterine anomaly include dysmenorrhea, menstrual abnormalities (amenorrhea, hypomenorrhea), hematocolpos, recurrent miscarriage, malpresentation, and preterm delivery. The ability to achieve a clinical pregnancy is not typically impaired.
A clinical examination may reveal a vaginal septum, double cervix, an unusually deviated uterus, or a very wide uterus to raise a suspicion of an anomaly.
7.5 Mullerian Anomalies and Reproductive Outcome
Different types of congenital uterine malformations may have different effects on reproductive performance [10]. Unicornuate uterus and uterus didelphys usually have a similar effect on pregnancy outcome [10,11,12]. Bicornuate and septate uterus both have incomplete absorption of septum as the etiological cause [11,12,13,14]. In patients with septate uteri, the reported incidence of abortion is 67%, prematurity 33%, and live births 28% [11].
Among various mechanisms proposed to explain the adverse effect of septate uterus on pregnancy outcome, the diminished size of uterine cavity and cervical incompetence have been suggested as the most probable etiological factors [13, 15]. Septum consisting of fibroelastic tissue with inadequate vascularization and having altered relations between myometrial and endometrial vessels can exert a negative effect on fetal placentation [12, 14, 16]. A contrasting study by Dabirashrafi et al. found significantly less connective tissue, a higher amount of muscle tissue, and more vessels in the septum. They suggested that pregnancy wastage is caused by poor decidualization and placentation. Reduced amounts of connective tissue and increased muscle content are said to cause uncoordinated contractility [15].
7.6 Diagnosis
For definitive diagnosis of Mullerian anomalies, one has to visualize both the internal and external uterine contour. Various diagnostic modalities have been utilized for uterine anomalies, each one having its own advantages and limitations. Two-dimensional ultrasonography or hysterosalpingography is an acceptable first-line screening tool.
7.6.1 Ultrasonography
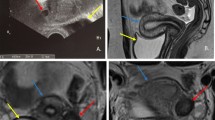
Ultrasonography (USG) is useful for evaluating the kidneys, detecting hematometra or hematocolpos, and confirming the presence of ovaries in women with primary amenorrhea or diagnosis of septate uterus or agenesis. It also provides information about uterine contour, internal and external. In the secretory phase of the menstrual cycle, there is improved visualization of the endometrium. A 3D ultrasound can visualize the uterine cavity, myometrium, and the outer contour of the uterus in a single image, such as a coronal view. It is a noninvasive, reproducible, reliable method of differentiating the septate from the bicornuate uteri [16,17,18] (Fig. 7.1).
7.6.2 Hysterosalpingography
Hysterosalpingography (HSG) is a cost-effective modality of evaluating the uterine cavity for diagnosis of uterine anomalies. It is widely available even in low-cost settings. The advantage of hysterosalpingography is that it provides additional information of fallopian tube patency. The drawback of HSG is that it does not give any information of external uterine contour. To overcome this, additional modalities may be required for making a definitive diagnosis (Figs. 7.2 and 7.3).
7.6.3 Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) can provide excellent delineation of internal as well as external contour of the uterus, without exposure to ionizing radiations. It can also measure the intercornual diameter (>4 cm suggests a bicornuate/didelphys uterus and <2 cm suggests a septate uterus; measurements of 2–4 cm are indeterminate). MRI can distinguish between myometrial and fibrous septum of a bicornuate uterus and septate uterus, respectively. MRI can visualize the extent of the septum in both anomalies [19]. MR imaging may also be helpful in detecting a uterine horn and visualizing an endometrial stripe.
7.6.4 Hysteroscopy and Laparoscopy
Hysteroscopy enables direct visualization of the intrauterine cavity and ostia, hence very helpful in establishing correct diagnosis in suspected cases with abnormal HSG [20,21,22]. However, hysteroscopy has a limitation of inability to visualize the external contour of the uterus and is therefore often inadequate in differentiating between different types of malformations. Consequently, patients with a septum-like structure on HSG need a combined approach with diagnostic laparoscopy to differentiate bicornuate and septate uteri. In spite of newer technologies available, this combination (hysteroscopy/laparoscopy) is considered to be the gold standard in evaluating congenital uterine anomalies [20,21,22,23].
Hysteroscopy with laparoscopy offers the added advantage of concurrent treatment, as in the case of a uterine septum resection and other fertility enhancing surgery.
Hysteroscopy has the drawback of being an invasive procedure. With advancement in technology, smaller diameter telescopes, inbuilt cameras, and illumination systems, hysteroscopy nowadays is often performed under local anesthesia, in an office setting. Serious complications such as air embolism or uterine perforation can occur rarely [24].
7.7 Treatment
Most of the Mullerian anomalies are accidentally diagnosed during an infertility workup, during pregnancy or at childbirth. Restoration of normal uterine architecture and preservation of fertility are the goals of surgical treatment of uterine anomalies.
The commonest anomaly is a septate uterus. Surgical intervention for a septate uterus is needed only in patients with recurrent miscarriages. Infertility remains a controversial indication for performing septum resection.
7.8 Hysteroscopy in Mullerian Anomalies
Depending upon the degree of fusion defect, Mullerian anomalies can be classified as arcuate, partial, and complete septum.
7.8.1 Arcuate Uterus
Arcuate uterus is a condition in which the myometrium dips at the fundus and may form a small septation into the cavity. It is also defined as any fundal protrusion into the cavity that has an angle of more than 90°. The demarcation between an arcuate and a septate uterus remains undefined. Arcuate uterus is considered to be a normal variant in uterine shape, and most patients with an arcuate uterus do not require any surgery. In patients with repeated pregnancy loss suspected to be due to an arcuate uterus, hysteroscopic resection can be considered, although there are no conclusive studies on the same (Fig. 7.4).
7.8.2 Uterine Septum
Uterine septum is the only Mullerian anomaly where hysteroscopy has a definite role in diagnosis and management. There is no doubt about the therapeutic effects of hysteroscopic metroplasty in patients with recurrent abortions and normal fertility. In patients with secondary infertility and recurrent spontaneous abortions, hysteroscopic metroplasty is applied as a treatment for their poor reproductive performance [25]. But the role of hysteroscopic septal resection as a prophylactic procedure is always debatable in patients presenting with primary unexplained infertility [14, 26].
Abdominal metroplasty by Jones and Tompkins technique for the incision of septum has been completely abandoned in favor of hysteroscopy, which has a much lower morbidity.
Timing of surgery: Best time to perform surgery is the postmenstrual or early follicular phase because the surrounding endometrium is thin and least vascular at this phase. Ostia which serve as important landmark are also clearly visible at this time.
Preoperative evaluation: Septal resection should be offered if there is history of poor reproductive performance rather than mere presence of a septate uterus [27]. Ideal candidates for surgery include women who had recurrent spontaneous abortions, a single second-trimester loss, or history of preterm delivery [28, 29].
Although few authors have tried pretreatment with gonadotropin-releasing hormone agonists to make the endometrium thin, there doesn’t seem to be any definitive role in improving the surgical outcome.
Instrumentation: Hysteroscope 30°, 4 mm (widely available), and 2.9 mm (preferred for office procedures) (Figs. 7.5 and 7.6).
Distention medium: Normal saline can be used with bipolar instruments or when operative sheath and scissor are being used. It prevents complications of fluid overload. Glycine is required when monopolar current is being used.
Technique: Surgery begins with the insertion of a diagnostic hysteroscope, to confirm the diagnosis and to assess the extent and thickness of septum. This initial assessment helps in selection of the correct instrument and distension medium for the therapeutic procedure. Although it is preferred to use the narrowest sheath through vaginoscopy, one should not hesitate in dilating the cervix, if needed. Forceful entry through the cervix increases the risk of perforation or creation of a false passage. Serial dilatation with lubricated ends of dilators prevents this complication.
Septum may be complete reaching up to the os and may have a cervical and vaginal component or partial (subseptate). It may have a broad or a narrow base at the fundus.
Combining laparoscopy is still considered to be the most accepted method to differentiate with bicornuate uterus. With advancements in technology, it is possible to confidently make a diagnosis of septum by a preoperative MRI or 3D scan. Hence in modern settings, laparoscopy may not be essential, just for confirmation of diagnosis of a bicornuate uterus.
A thin septum (<3 cm at fundus) can be easily cut with either a scissor through an operative sheath or Collin’s knife through a resectoscope, starting from the most proximal (or caudal) end by straight incising movements. Once the fundus is approaching, the septum usually becomes broad. Here it is important to convert a broad septum into a narrow one, by incising the lateral sides first (Figs. 7.7, 7.8, and 7.9).
For a broad septum equal to or more than 3 cm at the fundus, the incision should begin at the lower most part, with scissors directed superiorly along the lateral margins of the septum, until it is incised up to 0.5 cm from its junction with the normal myometrium. The opposite lateral margin should be similarly incised.
This process is repeatedly performed alternatively on each side until the original V-shaped septum is reconfigured into a short, broad notch between the tubal ostia. The notch is incised horizontally by a Collin’s knife starting near one tubal ostium and progressing to the opposite side.
Usually the tendency of operator is to drift posteriorly during the dissection; hence it is important to consciously remain equidistant between the anterior and the posterior wall. Intermittent withdrawal of the hysteroscope to reorient in the cavity is an essential step to prevent perforation.
If two cervices are noted, a Foley balloon may be inserted into one os to prevent leakage of the distension media. Septum is incised at a point above the internal cervical os till the Foley’s catheter is visualized. Uterine septum is then incised in the usual way. There is a controversy about increasing the risk of cervical incompetence, by cutting the cervical septum after unification of the cavity. There is a school of thought that it is better not to remove the cervical part in order to prevent cervical incompetence [30, 31]. Author does not support this hypothesis as also the work done by Donnez and Nisolle [32].
It can be difficult to recognize the end point of septum dissection. One approach is to continue cutting until increased bleeding is noted, since the septum often has poor blood supply compared to the myometrium, but this approach will not work if a coagulative instrument is used.
If laparoscopy is performed at the same time, the laparoscope can be used to visualize when the hysteroscopic resection is getting too close to the uterine serosal surface, especially if the laparoscopic light is dimmed so that the hysteroscopic light can be appreciated.
On completion of the procedure, the surgeon should be able to visualize the fundus and sweep easily between ostia. The uterine cavity should appear normal.
7.9 Complications
7.9.1 Perforation
Three important landmarks in any hysteroscopic surgery are the internal os and the two ostia. Proper orientation by intermittent withdrawal of the hysteroscope up to the os and taking a panoramic view of the cavity help in preventing perforation.
The tendency to go too deep into the myometrium to complete the procedure needs to be discouraged. It is better to underdo the septum than overdoing it. Leaving behind a residual fundal notch up to 1 cm is accepted as a complete procedure.
7.9.2 Fluid Overload
General principles of quick surgery, maintaining input-output balance and using adequate pressure settings on hysteromat, prevent this complication. Although saline is safer than glycine, absorption of too much of any fluid should be avoided.
7.9.3 Residual Septa
Residual septa are a part of septa which persist post-surgery. Fedele L found incidence of residual septum of 44.1% in their study and concluded that the residual septum of <1 cm does not adversely affect on reproductive performance, and repeat surgery is therefore not indicated [33].
7.10 Postoperative Care
No further treatment is required postoperatively. Intrauterine devices, Foley balloons, high-dose estrogen, and antibiotics are not necessary [6]. Formation of intrauterine synechiae is rare, as are postoperative infections. Endogenous estrogen is sufficient to promote new endometrium within 2 months of hysteroscopic metroplasty [7]. In a randomized study by Dabirashrafi et al., there was no benefit of estrogen therapy after hysteroscopic metroplasty [33]. In spite of this, many surgeons still prefer to give conjugated estrogens 1.25 mg/day for 25 days and progesterone 10 mg/day added on days 21–25 after surgery to assist epithelialization.
An HSG should be performed 2 months after surgery to assess success. Typically, over 90% of the septum is removed during the procedure. Occasionally, further repairs of the septum are required, again in an ambulatory setting [6, 34]. In one series, a residual fundal notch >1 cm on follow-up hysteroscopy was considered as an indication for repeating septoplasty [35]. Attempts at pregnancy may begin 2 months postoperatively if the procedure is deemed adequate.
7.12 Hysteroscopy in Other Uterine Anomalies
Most of the Mullerian anomalies are diagnosed preoperatively by imaging. Sometimes in an unexpected case, if an anomaly is diagnosed during a hysteron-laparoscopy, one may use this modality to assess the size of the cavities. Didelphys and bicornuate uteri even though have a slightly smaller cavity, they do not need any surgical intervention.
Unicornuate uterus is a rare uterine malformation, with an incidence of 1 in 1,00,000. It is an outcome of incomplete development of one of the Mullerian ducts. Hence, a unicornuate uterus may not necessarily be associated with a rudimentary horn. Most of the rudimentary horns are non-communicating and are mostly connected with the uterus through a fibrous band.
The endometrium in the rudimentary horn may be functional or nonfunctional. Rarely an ectopic pregnancy may occur in the non-communicating rudimentary horn through transperitoneal migration of sperms or fertilized ovum from the contralateral tube. Unlike tubal ectopic pregnancy, which usually ruptures in first trimester, about 90% of these pregnancies culminate in rupture mostly in the second trimester. This is because the myometrium supporting and surrounding the gestational sac can expand with the growing fetus but only up to a certain extent. A functional rudimentary horn may develop endometriosis leading to severe menstrual pain. Treatment consists of excision of rudimentary horn (Figs. 7.10 and 7.11).
In a small study, hysteroscopic drainage of a hematometra in a functional non-communicating accessory horn of a unicornuate uterus was performed by using electrocautery to create a communication between the horns. At 1 month follow-up, a single uterine cavity was identified, and the symptoms were completely relieved. Further studies are required before these treatment modalities are widely accepted.
Conclusion
Mullerian anomalies are a diverse group of developmental defects of the female genital tract. Establishing an accurate diagnosis is essential to decide if any surgical intervention is required. Further management strategies will depend upon the patient’s symptoms and the type of malformation. Ultimate goal of treatment is to achieve an anatomically and physiologically normal genital tract to fulfill healthy sexual relations and to achieve successful reproductive outcomes for the patient.
Hysteroscopic septal resection with concurrent laparoscopy is the treatment of choice for symptomatic septate uterus. Unlike the transabdominal approach, it is a safe and effective method of achieving normal or near-normal uterus, in an outpatient setting. There is minimal risk of intrauterine adhesions with rapid recovery. With hysteroscopic metroplasty, patient can immediately plan conception postoperatively and has lower risk of uterine rupture during pregnancy when compared to abdominal approach, and vaginal delivery is possible, avoiding subsequent cesarean delivery.
References
Steinmetz GP. Formation of artificial vagina. West J Surg. 1940;48:169–3.
Zhu L, Wong F, Lang JH. Minimally invasive surgery and map for female genital abnormalities, vol. 150. Beijing: People’s Medical Publishing House; 2010.
Cao ZY. Chinese J Obstet Gynecol. (clinical edition) Beijing: People’s Medical Publishing House. 2010;374
Braun P, Grau FV, Pons RM, Enguix DP. Is hysterosalpingography able to diagnose all uterine malformations correctly? A retrospective study. Eur J Radiol. 2005;53:274–9.
Hannema SE, Hughes IA. Regulation of Wolffian duct development. Horm Res. 2007;67:142–51.
Acien P, Acien M, Sanchez-Ferrer M. Complex malformations of the female genital tract. New types and revision of classification. Hum Reprod. 2004;19:2377–84.
Oppelt P, von Have M, Paulsen M, Strissel P, Strick R, Brucker S, Wallwiener S, Beckmann M. Female genital malformations and their associated abnormalities. Fertil Steril. 2007;87:335–42.
The American Fertility Society classifications of adnexal adhesions, distal tubal occlusion, tubal occlusion secondary to tubal ligation, tubal pregnancies, müllerian anomalies and intrauterine adhesions. Fertil Steril. 1988;49(6):944–55.
Buttram VC Jr, Gibbons WE. Müllerian anomalies: a proposed classification. (An analysis of 144 cases). Fertil Steril. 1979;32(1):40–6.
Moutos MD, Damewood DM, Schlaff DW, Rock AJ. A comparison of the reproductive outcome between women with a unicornuate uterus and women with a didelphic uterus. Fertil Steril. 1983;58:88–93.
Buttram CV. Mullerian anomalies and their management. Fertil Steril. 1983;40:159–63.
Marcus S, Al-Shawaf T, Brinsden P. The obstetric outcome of in vitro fertilization and embryo transfer in women with congenital uterine malformation. Am J Obstet Gynecol. 1996;175:85–9.
Fedele L, Bianchi S. Hysteroscopic metroplasty for septate uterus. Obstet Gynecol Clin North Am. 1995;22:473–89.
Fedele L, Dorta M, Brioschi D, et al. Pregnancies in septate uteri: outcome in relation to site of uterine implantation as determined by sonography. Am J Roentgenol. 1989;152:781–4.
Dabirashrafi H, Bahadori M, Mohammad K, et al. Septate uterus: new idea on the histologic features of the septum in this abnormal uterus. Am J Obstet Gynecol. 1995;172:105–7.
Wu MH, Hsu CC, Huang KE. Detection of congenital müllerian duct anomalies using three-dimensional ultrasound. J Clin Ultrasound. 1997;25:487.
Jurkovic D, Geipel A, Gruboeck K, et al. Three-dimensional ultrasound for the assessment of uterine anatomy and detection of congenital anomalies: a comparison with hysterosalpingography and two-dimensional sonography. Ultrasound Obstet Gynecol. 1995;5:233.
Bermejo C, Martínez Ten P, Cantarero R, et al. Three-dimensional ultrasound in the diagnosis of Müllerian duct anomalies and concordance with magnetic resonance imaging. Ultrasound Obstet Gynecol. 2010;35:593.
Leung JW, Hricak H. Role of magnetic resonance imaging in the evaluation of gynecologic disease. In: Callen PW, editor. Ultrasonography in obstetrics and gynecology. 4th ed. Philadelphia, PA: WB Saunders; 2000. p. 940.
Soares SR, Barbosa dos Reis MM, Camargos AF. Diagnostic accuracy of sonohysterography, transvaginal sonography, and hysterosalpingography in patients with uterine cavity diseases. Fertil Steril. 2000;73:406.
Homer HA, Li TC, Cooke ID. The septate uterus: a review of management and reproductive outcome. Fertil Steril. 2000;73:1–14.
Taylor E, Gomel V. The uterus and fertility. Fertil Steril. 2008;89:1–16.
Pellerito JS, McCarthy SM, Doyle MB, et al. Diagnosis of uterine anomalies: relative accuracy of MR imaging, endovaginal sonography, and hysterosalpingography. Radiology. 1992;183:795.
Kupesic S. Clinical implications of sonographic detection of uterine anomalies for reproductive outcome. Ultrasound Obstet Gynecol. 2001;18:387–400.
March MC, Israel R. Hysteroscopic management of recurrent abortion caused by septate uterus. Am J Obstet Gynecol. 1987;156:834–42.
Rock A, Schlaff DW. The obstetric consequences of uterovaginal anomalies. Fertil Steril. 1985;43:681–91.
Heinonen PK, Saarikoski S, Pystynen P. Reproductive performance of women with uterine anomalies. An evaluation of 182 cases. Acta Obstet Gynecol Scand. 1982;61(2):157–62.
Simon C, Martinez L, Pardo F, et al. Mullerian defects in women with normal reproductive outcome. Fertil Steril. 1991;56(6):1192–3.
Fischetti SG, Politi G, Lomeo E, Garozzo G. Magnetic resonance in the evaluation of Mullerian duct anomalies. Radiol Med (Torino). 1995;89(1-2):105–11.
Daly CD, Maier D, Soto-Albors C. Hysteroscopic metroplasty: six years experience. Obstet Gynecol. 1989;73:201–5.
Römer T, Lober R. Hysteroscopic correction of a complete septate uterus using a balloon technique. Hum Reprod. 1997;12:478–9.
Donnez J, Nisolle M. Endoscopic laser treatment of uterine malformations. Hum Reprod. 1997;12(7):1381.
Fedele L, Bianchi S, Marchini M, Mezzopane R, Di Nola G, Tozzi L. Residual uterine septum of less than 1 cm after hysteroscopic metroplasty does not impair reproductive outcome. Hum Reprod. 1996;11(4):727–9.
Dabirashrafi H, Mohammad K, Moghadami-Tabrizi N, Zandinejad K, Moghadami-Tabrizi M. Is estrogen necessary after hysteroscopic incision of the uterine septum? J Am Assoc Gynecol Laparosc. 1996;3(4):623–5.
Barakat AJ. Association of unilateral renal agenesis and genital anomalies. Case Rep Clin Pract Rev. 2002;3:57–60.
Valle RF, Sciarra JJ. Hysteroscopic treatment of the septate uterus. Obstet Gynecol. 1986;67(2):253–7.
De Cherney AH, Russell JB, Graebe RA, Polan ML. Resectoscopic management of mullerian fusion defects. Fertil Steril. 1986;45(5):726–8.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Mishra, J. (2018). Role of Hysteroscopy in Mullerian Anomalies. In: Jain, S., Inamdar, D. (eds) Manual of Fertility Enhancing Hysteroscopy. Springer, Singapore. https://doi.org/10.1007/978-981-10-8028-9_7
Download citation
DOI: https://doi.org/10.1007/978-981-10-8028-9_7
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-8027-2
Online ISBN: 978-981-10-8028-9
eBook Packages: MedicineMedicine (R0)