Abstract
Ascidian embryos have been employed as model systems for studies of developmental biology for well over a century, owing to their desirable blend of experimental advantages, which include their rapid development, traceable cell lineage, and evolutionarily conserved morphogenetic movements. Two decades ago, the development of a streamlined electroporation method drastically reduced the time and cost of transgenic experiments, and, along with the elucidation of the complete genomic sequences of several ascidian species, propelled these simple chordates to the forefront of the model organisms available for studies of regulation of gene expression. Numerous ascidian sequences with tissue-specific enhancer activity were isolated and rapidly characterized through systematic in vivo experiments that would require several weeks in most other model systems. These cis-regulatory sequences include a large collection of notochord enhancers, which have been used to visualize notochord development in vivo, to generate mutant phenotypes, and to knock down genes of interest. Moreover, their detailed characterization has allowed the reconstruction of different branches of the notochord gene regulatory network. This chapter describes how the use of transgenic techniques has rendered the ascidian Ciona a competitive model organism for studies of notochord development, evolution, and gene regulation.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Ascidian
- Brachyury
- Ciona
- cis-Regulatory Module
- Electroporation
- Enhancer
- Notochord
- T-Box
- Tbx2/3
- Transcription factor
8.1 Introduction
The notochord (in Latin, chorda dorsalis) is the chief distinctive feature of the phylum Chordata, a large division of deuterostomes comprising two subphyla of mostly marine animals, Tunicates and Cephalochordates, in addition to the Vertebrates subphylum, which includes humans (Fig. 8.1). Owing to the scarcity of interpretable fossil records, the molecular mechanisms underlying the appearance of the notochord during the evolutionary history of multicellular animals are still under investigation (Satoh et al. 2012). Fossil remnants from the Middle Cambrian (~510–495 million years ago) have allowed the tentative identification of a structure similar to the notochord in Pikaia gracilens, and suggested that this extinct organism might represent the earliest stem-group chordate identified thus far (Morris and Caron 2012). However, other studies question the function of the putative notochord of Pikaia and the phylogenetic position of this animal within chordates (Lacalli 2012; Mallatt and Holland 2013).
The notochord in the three subphyla of chordates. Simplified drawings of representative animals from the three subphyla of the phylum Chordata. The notochord and notochord-derived structures are symbolized by red lines. (a) Lateral profile of the cephalochordate amphioxus (or lancelet). (b) Side view of an ascidian tailbud embryo; recent molecular phylogenies have indicated that tunicates are the closest living relatives of vertebrates (Delsuc et al. 2006). In (a) and (b) anterior is on the left. (c) Simplified drawings of developing mice at embryonic days ~E11.5 (left) and at ~E17 (right). As the backbone develops, the notochord regresses, and its remnants form the nuclei pulposi of the intervertebral discs (right)
In extant chordates, the notochord is an axial structure of mesodermal origin that provides support and patterning signals to the developing embryo and functions as a cornerstone for the organization of its body plan. The notochord induces the regionalization of the neural tube, patterns the paraxially located mesoderm, and influences the morphogenesis of structures ranging from endodermal derivatives to blood vessels (Fig. 8.1) (Cleaver and Krieg 2001; Reese et al. 2004; Stemple 2005). Importantly, in vertebrates, the notochord is replaced during embryonic development by the vertebral column, and its remnants form the central-most regions of the intervertebral discs, or nuclei pulposi (Fig. 8.1c) (Lawson and Harfe 2015). In cephalochordates and tunicates, the notochord is not replaced by the vertebral column, hence these groups are collectively known as invertebrate chordates (Fig. 8.1a, b). In the cephalochordate amphioxus (Fig. 8.1a), the notochord extends to the anterior-most region of the body (e.g., Holland et al. 2004), whereas in tunicates, this structure is confined to the tail (hence the alternative name Urochordates used for this subphylum; Fig. 8.1b). Among invertebrate chordates, ascidians offer a number of experimental advantages that render them amenable to studies of notochord development. Most solitary ascidians are available and fertile year-round and can be easily fertilized in vitro. The resulting embryos develop a notochord within less than one day, and as they are relatively translucent, their notochord cells can be visualized without histological staining (Fig. 8.2).
The notochord in the ascidian Ciona. (a, b) Ciona embryo electroporated at the one-cell stage with the Ci-Bra>green fluorescent protein (GFP) plasmid, incubated until the mid-tailbud II stage (Hotta et al. 2007a), fixed, and stained with rhodamine phalloidin. The 40 definitive notochord cells express GFP; all nuclei are stained with 4′,6-diamidino-2-phenylindole (DAPI; blue) and the cell contours are highlighted in red. (b) High-magnification view of the region boxed in (a); the notochord cells have a characteristic stack-of-coins arrangement and their nuclei are centrally located. (c) Ciona embryo electroporated at the one-cell stage with the Ci-Bra>GFP plasmid, raised until the hatching larva stage (Hotta et al. 2007a) and fixed for imaging without counterstaining. Half of the 40 notochord cells are fluorescent, owing to mosaic incorporation of the transgene. (d) High-magnification view of the notochord of another Ciona embryo, electroporated and raised in parallel with the embryo in (c). The notochord cells appear considerably stretched and “wrapped” around the central lumen. Scale bars: ~100 μm
The cell lineage of the notochord and all other larval tissues is nearly invariant and has been mapped for several cell divisions after fertilization (Conklin 1905; Ortolani 1954; Reverberi 1971; Nishida and Satoh 1983, 1985; Lemaire 2009). Moreover, tunicates feature the most compact chordate genomes. For example, the genome of the solitary ascidian Ciona intestinalis spans ~140 megabases (Dehal et al. 2002) and most tissue-specific enhancers, or cis-regulatory modules (CRMs), identified in this animal have been mapped within a few kilobases upstream of the genes that they control, or within their usually short introns (Passamaneck and Di Gregorio 2005; Stolfi and Christiaen 2012; Irvine 2013). Remarkably, orthologs of evolutionarily conserved regulators of notochord development, such as the transcription factors Brachyury and Foxa2, are present in single copy in Ciona (Corbo et al. 1997; Di Gregorio et al. 2001; Imai et al. 2004) and are part of relatively simplified gene regulatory networks (GRNs) (Imai et al. 2006; José-Edwards et al. 2013).
The notochord of most ascidian species is composed of 40 post-mitotic cells that stop dividing approximately around the end of neurulation, and form a definitive single-cell row in the center of the tail through convergent extension (Jiang and Smith 2007) (Fig. 8.2a). Tail elongation is achieved through extensive changes in the shape and dimensions of the 40 notochord cells (Fig. 8.2b, c). For comparison, the notochord of another tunicate, the larvacean Oikopleura, is initially composed of only 20 cells, but these cells continue to divide until they reach a final total of 120–160 by the third day after fertilization (Søviknes and Glover 2008). Remarkably, the ascidian species Molgula occulta and Molgula tectiformis are considered tailless, because they form only 20 and 10 definitive notochord cells respectively, which do not undergo convergent extension and are incapable of driving tail elongation (Takada et al. 2002).
Within the past two decades, the rapid and synchronized development of Ciona embryos and their amenability to transfection via a straightforward electroporation protocol have enabled the discovery of a multitude of notochord genes, in addition to live confocal imaging of notochord formation and functional studies of notochord genes. Transient transgenesis has allowed the identification of a surprising variety of cis-regulatory mechanisms controlling gene expression in the simple ascidian notochord. These findings, in turn, have ushered in the first comparative studies of notochord CRMs across chordates.
8.2 Identification of Novel Notochord Genes and Reconstruction of the Notochord Gene Regulatory Network
For her remarkable studies aimed at the creation of cell lineage maps, Ortolani relied upon her manual dexterity to label individual blastomeres of early ascidian embryos with minute grains of either charcoal or colored chalk, and used them to follow the localization of their daughter cells in embryonic tissues of transparent ascidians (Ortolani 1954). These studies expanded the research on cell lineage and fate determination that had been initiated by Conklin (1905) using the naturally pigmented muscle precursors of Styela partita to include nonpigmented ascidian embryos.
In the early 1980s, the intracellular microinjection of tracer enzymes, such as horseradish peroxidase, originally developed in leeches (Weisblat et al. 1978), was successfully used in ascidian embryos by Nishida and Satoh to determine accurate fate maps that extended to the early tailbud stage for most embryonic tissues (Nishida and Satoh 1983, 1985). The development of microinjection techniques paved the way for the first transgenic experiments in ascidians, which involved the microinjection of plasmids containing genomic fragments upstream of a muscle-specific actin fused to the LacZ reporter gene (Hikosaka et al. 1992, 1994). A few years later, the laboriousness of the microinjection techniques prompted the development of a simple and economical electroporation protocol. This method was first employed in Ciona for the identification and characterization of the notochord-specific cis-regulatory regions of Ciona intestinalis Brachyury (Ci-Bra), which encodes a transcription factor of the T-box family (Corbo et al. 1997) (Fig. 8.2) and of the Ciona ortholog of forkhead/HNF-3beta/Foxa2 (Ci-fkh/HNF-3b, aka Ci-FoxA.a), which encodes a transcription factor of the forkhead/winged-helix family (Di Gregorio et al. 2001; Imai et al. 2004).
In ascidians and in other chordates, Ci-FoxA.a is expressed in notochord and in regions of the nervous system, endoderm, and additional territories, similar to its mouse counterpart HNF-3beta (Sasaki and Hogan 1993).
In contrast to most other chordates analyzed thus far, Brachyury is notochord-specific in the ascidians Ciona and Halocynthia (Corbo et al. 1997; Yasuo and Satoh 1993). This restricted notochord-specific expression of Brachyury provides ascidian embryos with the rare advantage of enabling studies focused on the notochord-specific function and transcriptional targets of this factor.
In an experiment aimed at the identification of Ciona notochord genes controlled by Ci-Bra, the Ci-FoxA.a promoter region, which is active early in multiple embryonic territories, including notochord, endoderm, and nervous system, was used to direct the ectopic expression of the notochord-specific Ci-Bra transcriptional activator in endodermal and neural precursors.
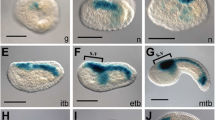
Transgenic embryos that ectopically express Ci-Bra in the endoderm and nervous system can be easily recognized because they display a reproducible phenotype consisting in the presence of a large mass of cells displaced to the ventral region of the tail from their normal destination. These embryos were collected alongside wild-type control embryos grown in parallel, and were subjected to RNA extraction followed by a subtractive hybridization screen (Takahashi et al. 1999; Fig. 8.3a). Whole-mount in-situ hybridizations were carried out for 501 complementary DNA (cDNA) clones, and a total of ~50 genes that are bona fide notochord transcriptional targets of Ci-Bra were identified (Fig. 8.3b–d); the function and regulation of several of these genes were analyzed in subsequent studies (Takahashi et al. 1999, 2010; Hotta et al. 1999, 2000, 2007b, 2008; Di Gregorio and Levine 1999; Dunn and Di Gregorio 2009; Katikala et al. 2013).
Use of transgenesis for the identification of novel notochord genes in Ciona. (a, e, i) Schematic representations of transgenic experiments aimed at the identification of novel notochord genes in Ciona. (b–d, h, k, l) Whole-mount embryos at the mid-tailbud II stage hybridized in situ with digoxygenin-labeled antisense RNA probes directed against the genes annotated in the top right corner of each panel. (f, g, j) Whole-mount in-situ hybridizations of embryos at the late gastrula, initial tailbud I, and mid-tailbud/late tailbud stages respectively. (a) Subtractive hybridization between transgenic embryos ectopically expressing Ci-Bra under the control of the Ci-FoxA.a promoter region, which is active in notochord, neural, and endodermal cells (Takahashi et al. 1999), and wild-type control embryos. The Ci-FoxA.a>Ci-Bra plasmid is abbreviated as fkh>Bra. (b–d) Representative expression patterns of genes that were identified through the experiment summarized in (a). (e) Dissociation of transgenic embryos carrying the Ci-Bra>GFP plasmid, shown here at the mid-tailbud stage for simplicity, followed by fluorescence-activated sorting (FACS) of notochord cells, RNA extraction and subtractive microarray screens, in parallel with RNAs extracted from whole embryos. (f–h) Representative expression patterns of genes that were identified through the experiment summarized in (e) and encode sequence-specific notochord transcription factors (José-Edwards et al. 2011). (i) Subtractive microarray screen between transgenic embryos carrying the Ci-Bra > Ci-Tbx2/3 DBD ::GFP plasmid, which expresses in the notochord a repressor form of Ci-Tbx2/3, and wild-type control embryos. (j) Whole-mount embryo hybridized in situ with a digoxygenin-labeled antisense RNA probe for Ci-Tbx2/3. (k, l) Two of the notochord-specific genes that were identified through the experiment are summarized in (i). Of note, these notochord-specific expression patterns, along with other tissue-specific patterns (not shown), suggest that Ci-Tbx2/3 might activate expression in non-notochord territories via tissue-specific co-activators (José-Edwards et al. 2013). Red font: genes encoding for transcription factors. Arrowheads: red, notochord; blue, nervous system; purple, mesenchyme; green, epidermis
A similar strategy was employed to identify notochord genes controlled by Ci-Tbx2/3, the only T-box transcription factor reportedly expressed in the ascidian notochord other than Brachyury (Imai et al. 2004). In this case, a subtractive microarray screen was carried out between embryos expressing in their notochord a putative repressor form of Ci-Tbx2/3, consisting of its DNA-binding domain fused to green fluorescent protein (GFP; Ci-Bra>Ci-Tbx2/3 DBD ::GFP (Fig. 8.3i) (José-Edwards et al. 2013). Control embryos were transfected with the Ci-Bra>GFP plasmid (Corbo et al. 1997). Approximately, 100–300 fluorescent transgenic embryos were selected under an epifluorescent microscope and subjected to RNA extraction, followed by hybridization to the Ciona Affymetrix GeneChip (Christiaen et al. 2008). Eighty-one putative Ci-Tbx2/3-downstream genes were identified, 20 of which (~29%) were expressed in the notochord, whereas others were expressed in other Ci-Tbx2/3 expression domains, including areas of the central nervous system (José-Edwards et al. 2013) (Fig. 8.3j–l). A few of these genes turned out to be shared targets of both Ci-Bra and Ci-Tbx2/3, including, in particular, Ci-Noto4, whose product is a protein containing a conserved phosphotyrosine binding domain required for notochord intercalation in Ciona embryos (Yamada et al. 2011). These results showed that in addition to regulating expression of a specific group of target genes, Ci-Tbx2/3 corroborates a crucial branch of the Ci-Bra-downstream GRN (José-Edwards et al. 2013).
Additional notochord transcription factors that had eluded previous searches were identified and provisionally positioned within the Ci-Bra-downstream gene battery through the use of a method previously developed for the isolation of heart precursors (Christiaen et al. 2008). This approach relies upon fluorescence-activated cell sorting (FACS) of the cells of interest for the identification of their specific transcriptomes, either through microarray screens, or more recently, through RNA sequencing. FACS-mediated isolation of fluorescent notochord cells was used in combination with microarray screens to identify novel notochord transcription factors, some of which are controlled either directly or indirectly by Ci-Bra (José-Edwards et al. 2011), in addition to numerous putative notochord genes (our unpublished results).
In a related approach, the cDNAs for several Ciona transcription factors, including Ci-Bra, were fused to the GFP coding region and cloned downstream of their respective promoters, with the goal of inducing the expression of GFP-tagged transcription factors in their native territories of activity. En-masse electroporations of these plasmids, followed by chromatin immunoprecipitation (ChIP) with an anti-GFP antibody and hybridization of the immunoprecipitated DNA to Ciona whole-genome microarrays (ChIP-chip), led to the identification of a very high number of genomic regions bound by each transcription factor at the 110-cell stage (Kubo et al. 2010). In particular, approximately 2,092 individual genes, including 194 transcription factors, were found to be occupied by the transgenic Ci-Bra-GFP protein in early embryos, and 3,653 were bound by Ci-FoxA.a-GFP (Kubo et al. 2010). Approximately 1,020 genes are shared between the two lists of putative targets, which suggests that these genes might be controlled synergistically by both transcription factors, as was previously suggested by the analysis of Ci-tune and related notochord CRMs (Passamaneck et al. 2009; José-Edwards et al. 2015) (discussed in Sect. 8.5.2).
The extensive studies of notochord genes in Ciona have successfully instructed related research in other chordates. A study of the expression patterns of mouse orthologs of Ciona notochord genes has shown that the expression of nine of these genes is conserved in the notochord cells of developing mouse embryos. These genes include the three mouse orthologs of the single-copy Ciona leprecan/prolyl 3-hydroxylase 1 (P3H1) gene (Fig. 8.4), named Leprecan/P3H1, Leprecan-like1/P3H2 and Leprecan-like2/P3H3. All three genes are expressed in notochord cells, although with different temporal onsets, and in various additional vertebrate-specific territories that are not present in ascidian embryos, such as portions of the vertebral cartilages (Capellini et al. 2008).
CRISPR/Cas9-mediated knockdown of the Ci-leprecan/P3H1 notochord gene. Specific oligonucleotide primers were designed in target sequences selected in exons one and two of the Ci-leprecan/P3H1 coding region, through the use of the CRISPOR software (http://crispor.tefor.net), and were used for one-step overlap (OSO) PCR. The resulting PCR products were cloned into plasmids under the control of the U6 promoter (Stolfi et al. 2014). Two of these plasmids were co-electroporated with a plasmid that expresses Cas9 in the notochord (Ci-Bra> Cas9), and with the Ci-Bra>GFP plasmid, to label notochord cells and monitor incorporation of the transgenes. For this experiment, 50 μg of each plasmid were employed. (a) Control embryo electroporated with 50 μg of the developmentally neutral Ci-Bra>GFP plasmid. Approximately half of the notochord cells display fluorescence, because of mosaic incorporation of the plasmid. (b–d) Embryos electroporated with the PCR products described above and the Ci-Bra >GFP plasmid. The shape of the notochord cells appears irregular (small orange arrowheads), and together with the loss of integrity of the notochordal sheath, which is predicted to be caused by the knockdown of Ci-leprecan/P3H1, is likely responsible for the reproducible bends in the tail (yellow arrowheads)
8.3 High-Resolution Imaging of Live Transgenic Notochord Cells
Owing to their natural translucency, most ascidian embryos are ideally suited to studies of notochord formation in vivo. In fact, notochord cells can be observed without any need for staining and/or sectioning, even though they are centrally located in the tail and are flanked by muscle cells and by the outermost epidermal cells. Moreover, the rapidity of ascidian embryogenesis allows the visualization of all the steps of notochord formation within less than one day, and to track morphogenetic movements as they unfold, through the use of routine differential interference contrast microscopy and time-lapse video recording. Time-lapse recording and bright-field microphotography were used in wild-type Ciona embryos to record morphogenesis and changes in cell shape that lead the six notochord precursors found in the 64-cell embryo to form a final rod-like structure composed of 40 post-mitotic cells (Miyamoto and Crowther 1985). In particular, these studies highlighted how the formation of cavities of increasing size between notochord cells and their progressive coalescence gradually give rise to a continuous lumen in the center of the tail (Fig. 8.2c, d; see also Figures in Chap. 15).
The lumen was described as a cavity surrounded by a basal lamina and a collagen-based notochordal sheath that is progressively built around the notochord through the extensive secretory activity of its cells (Cloney 1964). Indeed, subsequent studies proved that different collagen genes are expressed by notochord cells in Ciona (Wada et al., 2006). However, the low resolution of the imaging techniques and the lack of specific markers of cell boundaries led to the erroneous classification of the small cavities formed in between notochord cells as “intracellular vacuoles” (Miyamoto and Crowther 1985).
After the development of electroporation and the construction of the first plasmids able to induce expression of fluorescent proteins in the notochord, it was clarified through confocal imaging of fluorescent transgenic notochord cells that the “intracellular vacuoles” are rather “extracellular lumen pockets.” These extracellular pockets form in between notochord cells and are sealed by tight junctions in the regions where the apical domains of adjacent notochord cells are juxtaposed (Denker et al. 2015).
The phases of lumen growth have been identified and quantified (Denker and Jiang 2012; Denker et al. 2013, 2015), and the identities of the main molecular players responsible for this process and for the elongation of notochord cells in the absence of cell division have been elucidated. In particular, using a combination of in vivo confocal studies and morphometric analyses, it has been shown that cortical actin and ezrin-radixin-moesin (ERM; Figs. 8.3c and 8.5b) are required for lumen formation, along with a microtubule network that forms at the apical cortex of the notochord cells (Dong et al. 2011), and that an equatorially positioned actomyosin ring constricts the notochord cells and promotes their elongation in the absence of cell division (Sehring et al. 2014). The actomyosin complex competes with anteriorly localized Prickle and other components of the planar cell polarity pathway for the repositioning of the cytoskeleton (Jiang et al. 2005; Newman-Smith et al. 2015; Sehring et al. 2015).
Genomic location, organization, and activity of notochord cis-regulatory modules (CRMs) associated with Ci-Bra-downstream genes. Ciona embryos electroporated at the one-cell stage with plasmids containing the genomic regions symbolized by red boxes in the schematics below each microphotograph. Each of these regions was cloned upstream of the Ci-FoxA.a basal promoter fused to the LacZ reporter gene (Oda-Ishii and Di Gregorio 2007) (not depicted) and was found to act as a notochord CRM. All the respective genes are bona fide Ci-Bra-downstream transcriptional targets. (a) Among these notochord CRMs, Ci-thrombospondin 3A (Ci-thbs3A) contains multiple Ci-Bra binding sites (yellow vertical bars in the magnified region), with generic consensus sequence TNNCAC. (b) The 362-base pair (bp) Ci-ezrin-radixin-moesin (Ci-ERM) notochord CRM contains two functional Ci-Bra binding sites, both required for its activity. (c) The 248-bp long Ci-Noto9 notochord CRM relies upon an individual functional Ci-Bra binding site, and its corresponding gene is activated later during notochord development (middle-onset), compared with the early-onset genes Ci-thbs3A and Ci-ERM (Katikala et al. 2013). (d) Last, the 220-bp Ci-ACL notochord CRM is devoid of canonical Ci-Bra binding sites and relies upon a sequence that resembles a binding site for a homeodomain transcription factor (blue square). The corresponding gene, Ci-ACL, is a late-onset Ci-Bra-downstream notochord gene (Katikala et al. 2013). Red arrowheads: representative notochord cells
In parallel to these exquisitely detailed studies of individual notochord cells behaviors, the availability of different tissue-specific CRMs and confocal time–lapse recording allowed the visualization of notochord formation within its global embryonic context, through the labeling of muscle, notochord, and nervous system with multiple fluorophores (Rhee et al. 2005).
Last, the accurate visualization of developing notochord cells has been freed from mosaic incorporation of marker transgenes through the generation of stable transgenic Ciona lines. A stable transgenic line of Ciona savignyi was generated through the co-injection of the I-SceI endonuclease and a plasmid containing a modified Ci-Bra>GFP sequence (Corbo et al. 1997) flanked by the I-SceI recognition site (Deschet et al. 2003). This strategy induced the stable integration of multiple consecutive copies of the Ci-Bra>GFP transgene and the nonmosaic inheritance of the GFP labeling of notochord cells and their precursors. In turn, the pervasive and persistent fluorescence displayed by the notochord cells of stable Ci-Bra>GFP transgenics allowed the high-resolution visualization of the notochord defects that characterize animals heterozygous for the notochord mutation chongmague (chm) (Deschet et al. 2003). It was revealed through these analyses that embryos obtained by crossing animals from the chm mutant line with stable Ci-Bra>GFP transgenics do form the final 40 notochord cells; however, these cells fail to converge on the midline and to complete intercalation, because they continue to move and collide with each other (Deschet et al. 2003). A related transgenic line carrying the Ci-Bra>GFP construct was obtained in Ciona intestinalis through the use of the Minos transposon from Drosophila hydei and is publicly available for any future studies that might require accurate morphometric analyses of notochord development (Sasakura et al. 2010).
8.4 Functional Studies of Notochord Genes
Before the advent of transgenic techniques, functional studies of ascidian genes that required the overexpression or misexpression of genes of interest relied upon the microinjection of their corresponding in vitro synthesized messenger RNAs into eggs, zygotes or individual blastomeres. This technique has been perfected and has continued to yield several relevant results in the Japanese ascidian Halocynthia roretzi, where it was used, for example, to ectopically activate transcription of Hr-FoxA, the ortholog of Ci-FoxA.a, in cells of the animal region of the embryo. These studies demonstrated that Hr-FoxA is sufficient to direct ectopic expression of Hr-Bra, the Brachyury ortholog in this species (Kumano et al. 2006).
In parallel to these gain-of-function experiments, loss-of-function studies have been carried out by microinjecting morpholino oligonucleotides into fertilized eggs, in Halocynthia and in Ciona, even though the latter is characterized by smaller eggs that are more difficult to handle. A large-scale morpholino screen in Ciona led to the inactivation of numerous transcription factors and signaling molecules and shed light on the structure of the main tissue-specific GRNs that orchestrate development in these embryos (Imai et al. 2006). These laborious and time-consuming studies have been partly replaced by the development of plasmids able to direct notochord expression of chimeric or mutant proteins that can be simply introduced into a very large number of zygotes via electroporation.
An early example of a plasmid able to induce a gain-of-function phenotype is the fusion of the Ci-FoxA.a promoter region and the Ci-Bra coding region (Takahashi et al. 1999). As described in Sect. 8.2, electroporation of this plasmid in Ciona eggs elicited a dramatic rearrangement of the body plan, whereby endodermal and neural precursors that ectopically expressed Ci-Bra adopted a notochord-like phenotype (Takahashi et al. 1999; Fig. 8.3a). This suggested that Ci-Bra might play a major role in cell movements, similar to its counterparts from other chordates and from nonchordate animals (Nibu et al. 2013).
Conversely, electroporation of a plasmid that induced expression in Ciona notochord precursors of the Xenopus bix transcriptional repressor, which reportedly down-regulates XBra (Tada et al. 1998), caused a phenotype characterized by abnormally shaped notochord cells, a disorganized notochord and a very short tail, likely by causing the down-regulation of Ci-Bra (Di Gregorio et al. 2002). This phenotype is reminiscent of the loss of notochord identity observed in N-ethyl-N-nitrosourea--induced Ci-Bra −/− mutants (Chiba et al. 2009).
One experimental hindrance frequently encountered in these experiments is the mosaic incorporation of the plasmid(s) inducing expression of mutant forms of the proteins of interest. However, mosaic incorporation can be quite advantageous for functional studies, because it can induce the appearance of milder, intermediate phenotypes that are easier to interpret and can provide valuable information on the function of genes that are essential for notochord formation. For example, the expression of Xenopus bix in the notochord precursors of Ciona, caused by the Ci-Bra>bix plasmid, disrupts notochord formation; however, the mosaic incorporation of this plasmid allowed the formation of partial notochord fragments, and the development of a slightly longer tail (Di Gregorio et al. 2002). Similarly, the mosaic incorporation of a repressor form of Ci-Bra, obtained by fusing its DNA-binding domain with the Engrailed repression domain, enabled the detection of the down-regulation of the notochord transcription factor Ci-Fos-a in cells where Ci-Bra activity is reduced, and to use the wild-type notochord cells within the same embryos as controls. In turn, these results positioned Ci-Fos-a downstream of Ci-Bra in the notochord GRN (José-Edwards et al. 2011).
Loss-of-function experiments have also been carried out through the electroporation of plasmids able to cause formation of short hairpin RNAs (shRNAs) aimed at interfering with the translation of a specific gene product (Nishiyama and Fujiwara 2008). A notochord gene that was knocked down through this method is Ci-leprecan, which encodes prolyl 3-hydroxylase1 (P3H1) (Hotta et al. 2000; Dunn and Di Gregorio 2009). The shRNA-mediated loss-of-function of Ci-leprecan/P3H1 caused abnormal notochord formation and impaired tail elongation. In particular, the notochord displayed defects that ranged from the presence of one or more bends in the tail to a widened notochordal territory, whereby the notochord cells were misshapen and failed to intercalate (Dunn and Di Gregorio 2009). The function of Ci-leprecan/P3H1 was also analyzed through the electroporation of a plasmid that induced expression of a truncated form of Ci-leprecan/P3H1 lacking the iron-binding region of its catalytic domain and therefore is presumably unable to modify collagen. The mutant Ci-leprecan/P3H1 protein likely competes with the endogenous wild-type protein by sequestering its interacting proteins, Ci-CRTAP and Ci-CYPB, which are also expressed in the Ciona notochord (Myllyharju and Kivirikko 1997; Dunn and Di Gregorio 2009). These experiments reproduced the phenotypes observed in the shRNA-mediated interference studies (Dunn and Di Gregorio 2009).
A related strategy has been successfully employed to interfere with the function of notochord transcription factors. In this case, a truncated form of a transcription factor of interest containing only its DNA-binding domain was expressed in the developing notochord; alternatively, the DNA-binding domain was either fused to the Engrailed repression domain, or to the Drosophila Hairy repression domain (Corbo et al. 1998). These mutant proteins compete with their respective endogenous counterparts by occupying their target binding sites without activating gene expression, or by repressing transcription. This approach was used, for example, to interfere with the function of Ci-Tbx2/3 and to identify the notochord genes whose expression was affected (José-Edwards et al. 2013) (Sect. 8.2, Fig. 8.3i, k, l).
The most recently developed tool for functional studies of notochord genes involves genome editing through the use of clustered regularly interspaced short palindromic repeats and the Cas9 endonuclease (CRISPR/Cas9) (Stolfi et al. 2014; Sasaki et al. 2014). The combined use of two single guide RNAs designed to target the first two exons of Ci-leprecan/P3H1 produced different mutations, including a deletion that caused a truncation and a frame-shift in the predicted protein. Compared with control embryos (Fig. 8.4a), mutant Ci-leprecan/P3H1 embryos display a slightly shorter tail, with one or more bends. This predominant mild phenotype indicates that most notochord cells intercalate properly, although, occasionally, misshapen cells fail to intercalate and cause the formation of kinks in the tail (Fig. 8.4b, c). These results are consistent with the effects of the shRNA-induced knockdown and with the phenotypes induced by the expression of the mutant form of Ci-leprecan/P3H1 in the notochord (Dunn and Di Gregorio 2009; Sect. 8.2). Together, these studies suggest the working model that a reduction in Ci-leprecan/P3H1 function impairs the post-translational modification of collagen molecules that compose the notochordal sheath, thus causing a reduction in its rigidity. Consequently, notochord cells that are even slightly misshapen can leave the rod-like structure and force the neighboring notochord cells to deviate (Fig. 8.4b, c) (Pandey and Di Gregorio, unpublished results).
The CRISPR/Cas9 method was also used to impair the function of another notochord gene, Ciona fibronectin (Ci-FN1-containing) (José-Edwards et al. 2013), recently renamed Ci-Fn in Segade et al. (2016). The results of the knockdown of this gene indicated its functional requirement for the intercalation of notochord precursors (Segade et al. 2016). However, in contrast to the chm mutants, in which Ciona laminin alpha is mutated (Veeman et al. 2008), in Ci-Fn mutant embryos the notochordal sheath appears unaffected, as the notochord cells that are not completely incorporated in the definitive rod-like structure are unable to cross the boundaries of the notochord territory and do not invade adjacent tissues (Segade et al. 2016).
8.5 Identification of cis-Regulatory Mechanisms Controlling Gene Expression in the Notochord
Cis-regulatory modules (CRMs), or enhancers, contain crucial information required for appropriate spatiotemporal gene expression and morphogenesis (e.g., Levine 2010). Compared with protein-coding regions, however, these elements are usually difficult to identify, and their elusive nature has traditionally hindered their functional analysis, especially in the case of complex genomes, such as those of vertebrate animals. In addition to its initial identification, the functional characterization of an enhancer region requires additional in vivo experiments, such as mutational analyses, that can be very challenging, time-consuming, and expensive in most multicellular model organisms.
Within the compact genome of Ciona, most of the enhancer regions identified thus far have been found in the proximity of the coding regions that they control, either upstream of transcription units, or within their first introns (Irvine 2013; Katikala et al. 2013). This highly desirable genomic configuration, coupled with the rapid embryonic development and the ease of transgenesis, have greatly expedited the discovery of enhancers active in all larval tissues, and the elucidation of the minimal regulatory sequences required for their function (reviewed in Irvine 2013). In particular, nearly 40 notochord CRMs have been identified and thoroughly characterized, and their minimal functional sequences have been used to uncover the transcriptional activators controlling them (Corbo et al. 1997; Di Gregorio and Levine 1999; Anno et al. 2006; Christiaen et al. 2008; Dunn and Di Gregorio 2009; Passamaneck et al. 2009; Katikala et al. 2013; José-Edwards et al. 2013, 2015; Thompson and Di Gregorio 2015; Farley et al. 2016; Segade et al. 2016).
8.5.1 Temporal Regulation of Notochord Gene Expression by Ciona Brachyury
A subset of crucial transcriptional activators of notochord gene expression appears to be evolutionarily conserved across chordates and to be necessary for notochord formation. Among them is Brachyury (Greek for “short tail”), also known as “T” (for “tail”). The loss of Brachyury function in mouse embryos homozygous for a mutation in this locus causes severe defects in the formation of posterior mesoderm, hindgut, and allantois; in particular, the notochord of these mutants is described as “nearly completely absent” (Gluecksohn-Schoenheimer 1940). Mutations in Brachyury orthologs have been either identified or induced in different chordates, and they all severely impaired notochord formation (Smith 1999; Chiba et al. 2009; Nibu et al. 2013).
The impact of Brachyury mutations on notochord development can be unequivocally evaluated in ascidian embryos, because in the ascidian species analyzed thus far, this gene is notochord-specific (Yasuo and Satoh 1993; Corbo et al. 1997; Di Gregorio 2017). The product of Brachyury is a sequence-specific transcription factor that was first characterized in mouse and, through electrophoretic mobility assays, was found to bind a palindromic sequence (Kispert et al. 1995). Subsequent studies in Xenopus and Ciona demonstrated that Brachyury proteins can also recognize nonpalindromic half-sites with the generic sequence TNNCAC (Casey et al. 1998; Di Gregorio and Levine 1999).
The notochord-specific expression of Ci-Bra permitted the subtraction screen described above (Fig. 8.3a), which yielded numerous bona fide Ci-Bra-downstream notochord genes (Takahashi et al. 1999). These transcriptional targets of Ci-Bra are responsible for crucial steps of notochord development, such as cell division, convergent extension, and tubulogenesis (Hotta et al. 2008), and need to be deployed in a finely regulated temporal sequence during the ~14 h that elapse between the specification of the notochord precursors and the formation of the notochordal lumen. Interestingly, although many of these genes are expressed starting at early gastrula (early-onset notochord expression), others are detected around the neural plate stage (middle-onset expression), and a third group of these genes are not detectable in the notochord before the early tailbud stage (late-onset expression) (Hotta et al. 1999; Katikala et al. 2013). The molecular mechanisms responsible for these differences in the temporal read-out of Ci-Bra-downstream gene expression were investigated by taking advantage of the compact genome of Ciona.
A few notochord genes were selected as representatives of the early-, middle- and late-onset Ci-Bra targets, their notochord CRMs were identified, and the minimal sequences responsible for their activity were determined. In most cases, these sequences matched published Ci-Bra binding sites (Katikala et al. 2013). However, even within the same CRM, some of these putative binding sites turned out to be dispensable, whereas others were required for activity.
Further experiments indicated that notochord CRMs that require multiple functional Ci-Bra binding sites are associated with early-onset notochord genes, such as Ci-thrombospondin 3A (Ci-thbs3A), which encodes an evolutionarily conserved extracellular matrix glycoprotein that mediates cell adhesion and migration (Katikala et al. 2013; Urry et al. 1998) (Fig. 8.5a). Additional CRMs in this category are associated with Ci-fibrillar collagen 2A1 (CiFCol1), which in ascidians is a component of the notochordal sheath, and in vertebrates is co-opted to cartilage (Katikala et al. 2013; Cloney 1964; Wada et al. 2006), and Ci-ERM (Figs. 8.3c and 8.5b), which is required in notochord cells for the acquisition of their characteristic stack-of-coins organization and for lumen formation (Katikala et al. 2013; Hotta et al. 2007b; Dong et al. 2011).
Notochord CRMs that rely upon individual functional Ci-Bra binding sites accompany middle-onset notochord genes, such as Ci-Noto9/Rrbp1 (Figs. 8.3b and 8.5c). This gene is first detected in notochord cells by the neural plate/early neurula stage and encodes an evolutionarily conserved ribosome-binding protein that is also expressed in the notochord of Xenopus embryos (Katikala et al. 2013; Liu et al. 2016). In addition to the notochord CRMs directly bound by Ci-Bra, these studies identified notochord CRMs that are controlled by Ci-Bra indirectly, through a relay mechanism that involves Ci-Bra-downstream intermediary transcription factors. These CRMs are devoid of functional Ci-Bra binding sites and are associated with late-onset Ci-Bra target genes, which are first detected in notochord cells around the time of neurulation and include the ATP-citrate lyase, encoded by Ci-ACL (Figs. 8.3d and 8.5d), which is required for both the establishment of cell polarity along the medio-lateral axis and intercalation (Hotta et al. 2007b).
The Ci-ACL notochord CRM is controlled by Ci-Bra through a transcriptional activator of the homeodomain family (Fig. 8.5d), whereas another late-onset Ci-Bra target, Ci-beta4GalT, which encodes a beta1,4-galactosyltransferase, is controlled by Ci-Bra through a still unknown activator (Katikala et al. 2013).
Together, these results delineated a possible molecular mechanism that would ensure the appropriate timing of notochord gene expression and could be conserved in more complex chordates.
8.5.2 Synergistic Activation of Notochord CRMs by Ci-Bra and Ci-FoxA.a
In mouse and other vertebrate embryos, another major activator of notochord gene expression in addition to Brachyury is HNF-3beta/Foxa2, a member of the forkhead/winged-helix family of transcription factors (Friedman and Kaestner 2006).
Mice carrying a homozygous mutation in the Foxa2 locus lack an organized node and fail to develop a notochord (Ang and Rossant 1994). Subsequent studies have identified numerous mouse notochord genes whose expression is influenced by Foxa2, in addition to seven Foxa2-bound mouse genomic regions that can activate reporter gene expression in the zebrafish notochord (Tamplin et al. 2011). The Ciona counterpart of Foxa2, Ci-FoxA.a, similar to its vertebrate counterparts, is expressed in a wide embryonic territory, which encompasses notochord, neural tube, and endoderm (Jeffery et al. 1998; Di Gregorio et al. 2001).
Knockdown experiments carried out in Molgula oculata using antisense oligodeoxyribonucleotides have shown that one of the Molgula Fox genes, MocuFH1, is required for the movements of notochord and endodermal cells, and for axis formation (Olsen and Jeffery 1997).
In Ciona, morpholino oligonucleotide-mediated knockdown of Ci-FoxA.a indicated that this transcription factor controls expression of numerous genes, including Ci-Bra (Imai et al. 2006), and that in early embryos it occupies, among numerous others, the genomic loci of 245 target genes encoding transcription factors (Kubo et al. 2010). The analysis of Ciona notochord CRMs has indicated that Ci-FoxA.a can activate notochord gene expression by acting alone, as well as by synergizing with either Ci-Bra or with other unrelated transcription factors (José-Edwards et al., 2015). A notochord CRM located upstream of one of the Ci-ZicL genes, which encode zinc-finger transcription factors that are also involved in notochord gene expression, requires for its activity two binding sites for transcription factors of the Fox family (Anno et al. 2006), whereas a single Fox binding site is required for the activity of the Ci-quaking notochord CRM (José-Edwards et al. 2015, and our unpublished results).
In addition to working independently, Ci-FoxA.a can synergize with Ci-Bra and activate a subset of notochord CRMs that are equally dependent on binding sites for both transcription factors. Thus far, three examples of Ci-Bra/Ci-FoxA.a-dependent CRMs have been identified; Ci-tune, which encodes an ascidian-specific protein of unknown function (Passamaneck et al. 2009), Ci-CRM24, which is located upstream of the notochord gene discoidin domain receptor 1 (José-Edwards et al. 2015), and Ci-CRM96, which is associated with Ci-pavarotti-like, whose product is a kinesin-like protein that is still uncharacterized in ascidians, while in flies is required for proper formation of the mitotic spindle (José-Edwards et al. 2015; Adams et al. 1998). Additionally, the Ci-FN notochord CRM seems to rely upon a Fox binding site and a T-box binding site (Segade et al. 2016). This latter binding site might be used by Ci-Tbx2/3, as suggested by microarray results (José-Edwards et al. 2013).
8.6 Evolutionarily Conserved Features of Notochord CRMs: Chordate-Wide or Clade-Specific Mechanisms?
The availability of a large number of fully characterized Ciona notochord CRMs has prompted the first comparative study of their structural features, and the provisional categorization of these regulatory regions in Ciona. More importantly, this research compared and contrasted the architectural and functional requirements of notochord CRMs across chordates.
The notochord functions of Brachyury and Foxa2, in addition to their binding sites, are evolutionarily conserved across chordates. Similarly, despite the frequent lack of conservation in overall enhancer sequences, some of the notochord CRMs isolated from vertebrates rely upon Brachyury binding sites related to those found in Ciona CRMs. Thus far, notochord CRMs requiring either one or two Brachyury binding sites, similar to most Ciona notochord CRMs, have been found in the zebrafish Sonic hedgehog (Shh) ar-C intronic notochord and floor plate enhancer (Müller et al. 1999) and in the Xenopus eFGF promoter region (Casey et al. 1998), respectively.
Of note, in Ciona, the Ci-Ephrin3 notochord CRM requires, in addition to a functional Ci-Bra binding site, an (AC)6 microsatellite sequence (José-Edwards et al. 2015). Interestingly, this unusual association and interdependence of a functional Brachyury binding site with a repetitive sequence, which has been revealed through the analysis of the Ci-Ephrin3 notochord CRM, is paralleled by related findings in mice.
Studies carried out in mouse embryonic stem cells through ChIP-chip show that mouse Brachyury can bind (AC)≥6 microsatellite repeats (Evans et al. 2012). In vivo testing of the predicted enhancer activity of these genomic regions is required to determine whether these regions possess cis-regulatory activity and whether the functional association of Brachyury and the (AC)n microsatellite sequence uncovered in Ciona is conserved throughout the chordate spectrum.
Functional Foxa2 binding sites seem to be more frequent in the notochord enhancers (NOCEs) characterized from mouse embryos; however, this is likely because they were identified in screens focused on Foxa2 target genes (Tamplin et al. 2011). Notochord CRMs associated with the mouse genes Pkd1/1–1, Shh, Bicc1–1, and Sox9 contain five, three, two, and one Foxa2 binding sites respectively (Tamplin et al. 2011; Jeong and Epstein 2003; Bagheri-Fam et al. 2006). In zebrafish, a group of notochord CRMs have been shown to necessitate, in addition to individual Foxa2 binding sites, a sequence motif, named “motif 2,” located at variable distances from it (Rastegar et al. 2008). This configuration does not have a direct counterpart in Ciona; however, in one of the Ciona notochord CRMs, a Foxa2 binding site has been found to be working cooperatively with binding sites for transcription factors of the AP1 and homeodomain families (José-Edwards et al. 2015).
In addition to the CRMs that employ a shared repertoire of various configurations of generic T-box and Fox binding sites, other notochord CRMs seem to be requiring clade-specific transcription factors and their respective binding sites for their activity. In Ciona, at least three notochord CRMs rely for their activity upon minimal sequences related to binding sites for transcription factors of the Myb family, whereas one CRM requires a binding site for a basic helix-loop-helix (bHLH) transcription factor (José-Edwards et al. 2015). Furthermore, the notochord CRM of Ci-Bra requires for its function binding sites for the early activators ZicL (Yagi et al. 2004) and Ets (Matsumoto et al. 2007; Farley et al. 2016). Combinations of these binding sites have been found to be required for the activity of a notochord CRM associated with Ci-Mnx (Farley et al. 2016), which encodes an early notochord transcription factor of the homeodomain family (Imai et al. 2004), and for the activity of an additional notochord CRM located upstream of the main Ci-Bra enhancer/promoter region (Farley et al. 2016). Binding sites for transcription factors of the Zic and Ets families are yet to be reported as functional components required for the activation of notochord CRMs in vertebrates.
On the other hand, the presence of an orphan binding site (OBS) in the node and nascent NOCE of mouse Noto, a gene that encodes another evolutionarily conserved notochord transcriptional activator (Alten et al. 2012), indicates the existence of additional, still uncharacterized notochord activators. Together, the presence of OBS sequences in the mouse Foxa2 and Sox9 notochord CRMs and in four out of seven Foxa2-downstream notochord CRMs (Tamplin et al. 2011; Alten et al. 2012), together with the absence of canonical OBS sequences in minimal Ciona notochord CRMs, suggest that either the OBS-binding activator might be vertebrate-specific, or, alternatively, that an additional class of notochord CRMs might exist in Ciona and is yet to be found. In addition, another binding site present in the minimal Noto NOCE matches the consensus sequence bound by transcriptional activators of the Tead family. This result is in agreement with the previously described ability of Tead proteins to activate the Foxa2 CRM, in cooperation with a still unknown factor (Sawada et al. 2005), and suggests that binding sites for Tead activators might be additional recurring components of mouse notochord CRMs. BLAST searches suggest that the Ciona gene most highly related to vertebrate Tead genes might be Ci-scalloped/TEF1 (gene model KH.C14.426); however, this gene is reportedly expressed in neurons of the adhesive organ and in the primordium of the oral siphon, but is not detected in notochord cells (Imai et al. 2004).
Together, these results suggest that Tead transcription factors, alone or in synergy with additional activators, might be part of a vertebrate-specific molecular mechanism for the control of notochord gene expression that evolved after the separation of ascidians from the main chordate lineage. Alternatively, this and other seemingly vertebrate-specific strategies for the control of notochord gene expression could have been present in the common chordate ancestor and would have been selectively lost in ascidians.
As novel notochord CRMs continue to be identified in both ascidians and vertebrates, we expect the number of cis-regulatory strategies that are found to be conserved across divergent chordates to increase. In turn, the differences between notochord CRMs in ascidians and vertebrates should point out the clade-specific regulatory mechanisms responsible for the morphological and functional differences between the notochords of these animals, and ultimately shed light on the evolutionary origins of the backbone.
Abbreviations
- bp:
-
Base pair(s)
- cDNA:
-
Complementary DNA
- ChIP:
-
Chromatin immunoprecipitation
- CRM:
-
cis-regulatory module
- DAPI:
-
4′,6-diamidino-2-phenylindole
- FACS:
-
fluorescence-activated cell sorting
- GFP:
-
Green fluorescent protein
- GRN:
-
Gene regulatory network
- NOCE:
-
Notochord enhancer
- OBS:
-
Orphan binding site
- P3H1:
-
prolyl 3-hydroxylase1
- shRNA:
-
Short hairpin RNA
References
Adams RR, Tavares AA, Salzberg A, Bellen HJ, Glover DM (1998) Pavarotti encodes a kinesin-like protein required to organize the central spindle and contractile ring for cytokinesis. Genes Dev 12(10):1483–1494
Alten L, Schuster-Gossler K, Eichenlaub MP, Wittbrodt B, Wittbrodt J, Gossler A (2012) A novel mammal-specific three partite enhancer element regulates node and notochord-specific Noto expression. PLoS One 7(10):e47785
Ang SL, Rossant J (1994) HNF-3 beta is essential for node and notochord formation in mouse development. Cell 78:561–574
Anno C, Satou A, Fujiwara S (2006) Transcriptional regulation of ZicL in the Ciona intestinalis embryo. Dev Genes Evol 216(10):597–605
Bagheri-Fam S, Barrionuevo F, Dohrmann U, Günther T, Schüle R, Kemler R, Mallo M, Kanzler B, Scherer G (2006) Long-range upstream and downstream enhancers control distinct subsets of the complex spatiotemporal Sox9 expression pattern. Dev Biol 291(2):382–397
Capellini TD, Dunn MP, Passamaneck YJ, Selleri L, Di Gregorio A (2008) Conservation of notochord gene expression across chordates: insights from the Leprecan gene family. Genesis 46(11):683–696
Casey ES, O'Reilly MA, Conlon FL, Smith JC (1998) The T-box transcription factor Brachyury regulates expression of eFGF through binding to a non-palindromic response element. Development 125(19):3887–3894
Chiba S, Jiang D, Satoh N, Smith WC (2009) Brachyury null mutant-induced defects in juvenile ascidian endodermal organs. Development 136(1):35–39
Christiaen L, Davidson B, Kawashima T, Powell W, Nolla H, Vranizan K, Levine M (2008) The transcription/migration interface in heart precursors of Ciona intestinalis. Science 320(5881):1349–1352
Cleaver O, Krieg PA (2001) Notochord patterning of the endoderm. Dev Biol 234(1):1–12
Cloney RA (1964) Development of the ascidian notochord. Acta Embryol Morphol Exp 7:111–130
Conklin EG (1905) The organization and cell-lineage of the ascidian egg. J Acad Nat Sci 13:1–119
Corbo JC, Levine M, Zeller RW (1997) Characterization of a notochord-specific enhancer from the Brachyury promoter region of the ascidian, Ciona intestinalis. Development 124(3):589–602
Corbo JC, Fujiwara S, Levine M, Di Gregorio A (1998) Suppressor of hairless activates brachyury expression in the Ciona embryo. Dev Biol 203(2):358–368
Dehal P, Satou Y, Campbell RK, Chapman J, Degnan B, De Tomaso A, Davidson B, Di Gregorio A et al (2002) The draft genome of Ciona intestinalis: insights into chordate and vertebrate origins. Science 298(5601):2157–2167
Delsuc F, Brinkmann H, Chourrout D, Philippe H (2006) Tunicates and not cephalochordates are the closest living relatives of vertebrates. Nature 439(7079):965–968
Denker E, Jiang D (2012) Ciona intestinalis notochord as a new model to investigate the cellular and molecular mechanisms of tubulogenesis. Semin Cell Dev Biol 23(3):308–319
Denker E, Bocina I, Jiang D (2013) Tubulogenesis in a simple cell cord requires the formation of bi-apical cells through two discrete par domains. Development 140(14):2985–2996
Denker E, Sehring IM, Dong B, Audisso J, Mathiesen B, Jiang D (2015) Regulation by a TGFβ-ROCK-actomyosin axis secures a non-linear lumen expansion that is essential for tubulogenesis. Development 142(9):1639–1650
Deschet K, Nakatani Y, Smith WC (2003) Generation of ci-Brachyury-GFP stable transgenic lines in the ascidian Ciona savignyi. Genesis 35(4):248–259
Di Gregorio A (2017) T-box genes and developmental gene regulatory networks in ascidians. Curr Top Dev Biol 122:55–91
Di Gregorio A, Levine M (1999) Regulation of Ci-tropomyosin-like, a Brachyury target gene in the ascidian, Ciona intestinalis. Development 126(24):5599–5609
Di Gregorio A, Corbo JC, Levine M (2001) The regulation of forkhead/HNF-3beta expression in the Ciona embryo. Dev Biol 229(1):31–43
Di Gregorio A, Harland RM, Levine M, Casey ES (2002) Tail morphogenesis in the ascidian, Ciona intestinalis, requires cooperation between notochord and muscle. Dev Biol 244(2):385–395
Dong B, Deng W, Jiang D (2011) Distinct cytoskeleton populations and extensive crosstalk control Ciona notochord tubulogenesis. Development 138(8):1631–1641
Dunn MP, Di Gregorio A (2009) The evolutionarily conserved leprecan gene: its regulation by Brachyury and its role in the developing Ciona notochord. Dev Biol 328(2):561–574
Evans AL, Faial T, Gilchrist MJ, Down T, Vallier L, Pedersen RA, Wardle FC, Smith JC (2012) Genomic targets of Brachyury (T) in differentiating mouse embryonic stem cells. PLoS One 7(3):e33346
Farley EK, Olson KM, Zhang W, Rokhsar DS, Levine MS (2016) Syntax compensates for poor binding sites to encode tissue specificity of developmental enhancers. Proc Natl Acad Sci U S A 113(23):6508–6513
Friedman JR, Kaestner KH (2006) The Foxa family of transcription factors in development and metabolism. Cell Mol Life Sci 63(19–20):2317–2328
Gluecksohn-Schoenheimer S (1940) The effect of an early lethal (t) in the house mouse. Genetics 25(4):391–400
Hikosaka A, Kusakabe T, Satoh N, Makabe KW (1992) Introduction and expression of recombinant genes in ascidian embryos. Develop Growth Differ 34:631–638
Hikosaka A, Kusakabe T, Satoh N (1994) Short upstream sequences associated with the muscle-specific expression of an actin gene in ascidian embryos. Dev Biol 166:763–769
Holland LZ, Laudet V, Schubert M (2004) The chordate amphioxus: an emerging model organism for developmental biology. Cell Mol Life Sci 61(18):2290–2308
Hotta K, Takahashi H, Erives A, Levine M, Satoh N (1999) Temporal expression patterns of 39 Brachyury-downstream genes associated with notochord formation in the Ciona intestinalis embryo. Develop Growth Differ 41(6):657–664
Hotta K, Takahashi H, Asakura T, Saitoh B, Takatori N, Satou Y, Satoh N (2000) Characterization of Brachyury-downstream notochord genes in the Ciona intestinalis embryo. Dev Biol 224(1):69–80
Hotta K, Mitsuhara K, Takahashi H, Inaba K, Oka K, Gojobori T, Ikeo K (2007a) A web-based interactive developmental table for the ascidian Ciona intestinalis, including 3D real-image embryo reconstructions: I. From fertilized egg to hatching larva. Dev Dyn 236(7):1790–1805
Hotta K, Yamada S, Ueno N, Satoh N, Takahashi H (2007b) Brachyury-downstream notochord genes and convergent extension in Ciona intestinalis embryos. Develop Growth Differ 49(5):373–382
Hotta K, Takahashi H, Satoh N, Gojobori T (2008) Brachyury-downstream gene sets in a chordate, Ciona intestinalis: integrating notochord specification, morphogenesis and chordate evolution. Evol Dev 10(1):37–51
Imai KS, Hino K, Yagi K, Satoh N, Satou Y (2004) Gene expression profiles of transcription factors and signaling molecules in the ascidian embryo: towards a comprehensive understanding of gene networks. Development 131(16):4047–4058
Imai KS, Levine M, Satoh N, Satou Y (2006) Regulatory blueprint for a chordate embryo. Science 312(5777):1183–1187
Irvine SQ (2013) Study of cis-regulatory elements in the Ascidian Ciona intestinalis. Curr Genomics 14(1):56–67
Jeffery WR, Ewing N, Machula J, Olsen CL, Swalla BJ (1998) Cytoskeletal actin genes function downstream of HNF-3beta in ascidian notochord development. Int J Dev Biol 42(8):1085–1092
Jeong Y, Epstein DJ (2003) Distinct regulators of Shh transcription in the floor plate and notochord indicate separate origins for these tissues in the mouse node. Development 130(16):3891–3902
Jiang D, Smith WC (2007) Ascidian notochord morphogenesis. Dev Dyn 236(7):1748–1757
Jiang D, Munro EM, Smith WC (2005) Ascidian prickle regulates both mediolateral and anterior-posterior cell polarity of notochord cells. Curr Biol 15(1):79–85
José-Edwards DS, Kerner P, Kugler JE, Deng W, Jiang D, Di Gregorio A (2011) The identification of transcription factors expressed in the notochord of Ciona intestinalis adds new potential players to the brachyury gene regulatory network. Dev Dyn 240(7):1793–1805
José-Edwards DS, Oda-Ishii I, Nibu Y, Di Gregorio A (2013) Tbx2/3 is an essential mediator within the Brachyury gene network during Ciona notochord development. Development 140(11):2422–2433
José-Edwards DS, Oda-Ishii I, Kugler JE, Passamaneck YJ, Katikala L, Nibu Y, Di Gregorio A (2015) Brachyury, Foxa2 and the cis-Regulatory Origins of the Notochord. PLoS Genet 11(12):e1005730
Katikala L, Aihara H, Passamaneck YJ, Gazdoiu S, José-Edwards DS, Kugler JE, Oda-Ishii I, Imai JH, Nibu Y, Di Gregorio A (2013) Functional Brachyury binding sites establish a temporal read-out of gene expression in the Ciona notochord. PLoS Biol 11(10):e1001697
Kispert A, Koschorz B, Herrmann BG (1995) The T protein encoded by Brachyury is a tissue-specific transcription factor. EMBO J 14(19):4763–4772
Kubo A, Suzuki N, Yuan X, Nakai K, Satoh N, Imai KS, Satou Y (2010) Genomic cis-regulatory networks in the early Ciona intestinalis embryo. Development 137(10):1613–1623
Kumano G, Yamaguchi S, Nishida H (2006) Overlapping expression of FoxA and Zic confers responsiveness to FGF signaling to specify notochord in ascidian embryos. Dev Biol 300(2):770–784
Lacalli T (2012) The Middle Cambrian fossil Pikaia and the evolution of chordate swimming. Evodevo 3(1):12
Lawson L, Harfe BD (2015) Notochord to nucleus pulposus transition. Curr Osteoporos Rep 13(5):336–341
Lemaire P (2009) Unfolding a chordate developmental program, one cell at a time: invariant cell lineages, short-range inductions and evolutionary plasticity in ascidians. Dev Biol 332(1):48–60
Levine M (2010) Transcriptional enhancers in animal development and evolution. Curr Biol 20(17):R754–R763
Liu GH, Mao CZ, Wu HY, Zhou DC, Xia JB, Kim SK, Cai DQ, Zhao H, Qi XF (2016) Expression profile of rrbp1 genes during embryonic development and in adult tissues of Xenopus laevis. Gene Expr Patterns 23–24:1–6
Mallatt J, Holland N (2013) Pikaia gracilens Walcott: stem chordate, or already specialized in the Cambrian? J Exp Zool B Mol Dev Evol 320:247–271
Matsumoto J, Kumano G, Nishida H (2007) Direct activation by Ets and Zic is required for initial expression of the Brachyury gene in the ascidian notochord. Dev Biol 306(2):870–882
Miyamoto DM, Crowther RJ (1985) Formation of the notochord in living ascidian embryos. J Embryol Exp Morphol 86:1–17
Morris SC, Caron JB (2012) Pikaia gracilens Walcott, a stem-group chordate from the middle Cambrian of British Columbia. Biol Rev Camb Philos Soc 87:480–512
Müller F, Chang B, Albert S, Fischer N, Tora L, Strähle U (1999) Intronic enhancers control expression of zebrafish sonic hedgehog in floor plate and notochord. Development 126(10):2103–2116
Myllyharju J, Kivirikko KI (1997) Characterization of the iron- and 2-oxoglutarate-binding sites of human prolyl 4-hydroxylase. EMBO J 16(6):1173–1180
Newman-Smith E, Kourakis MJ, Reeves W, Veeman M, Smith WC (2015) Reciprocal and dynamic polarization of planar cell polarity core components and myosin. Elife 13(4):e05361
Nibu Y, José-Edwards DS, Di Gregorio A (2013) From notochord formation to hereditary chordoma: the many roles of Brachyury. Biomed Res Int 2013:826435
Nishida H, Satoh N (1983) Cell lineage analysis in ascidian embryos by intracellular injection of a tracer enzyme. I. Up to the eight-cell stage. Dev Biol 99:382–394
Nishida H, Satoh N (1985) Cell lineage analysis in ascidian embryos by intracellular injection of a tracer enzyme. II. The 16- and 32-cell stages. Dev Biol 110:440–454
Nishiyama A, Fujiwara S (2008) RNA interference by expressing short hairpin RNA in the Ciona intestinalis embryo. Develop Growth Differ 50(6):521–529
Oda-Ishii I, Di Gregorio A (2007) Lineage-independent mosaic expression and regulation of the Ciona multidom gene in the ancestral notochord. Dev Dyn 236(7):1806–1819
Olsen CL, Jeffery WR (1997) A forkhead gene related to HNF-3beta is required for gastrulation and axis formation in the ascidian embryo. Development 124(18):3609–3619
Ortolani G (1954) Risultati definitive sulla distribuzione dei territory presuntivi degli organi nel germe di Ascidie allo stadio VIII, determinati con le marche al carbone. Pubbl Staz Zool Napoli 25:161–187
Passamaneck YJ, Di Gregorio A (2005) Ciona intestinalis: chordate development made simple. Dev Dyn 233(1):1–19
Passamaneck YJ, Katikala L, Perrone L, Dunn MP, Oda-Ishii I, Di Gregorio A (2009) Direct activation of a notochord cis-regulatory module by Brachyury and FoxA in the ascidian Ciona intestinalis. Development 136(21):3679–3689
Rastegar S, Hess I, Dickmeis T, Nicod JC, Ertzer R, Hadzhiev Y, Thies WG, Scherer G, Strähle U (2008) The words of the regulatory code are arranged in a variable manner in highly conserved enhancers. Dev Biol 318(2):366–377
Reese DE, Hall CE, Mikawa T (2004) Negative regulation of midline vascular development by the notochord. Dev Cell 6(5):699–708
Reverberi G (1971) Ascidians. In: Reverberi G (ed) Experimental embryology of marine and fresh-water invertebrates. North-Holland, Amsterdam, pp 507–550
Rhee JM, Oda-Ishii I, Passamaneck YJ, Hadjantonakis AK, Di Gregorio A (2005) Live imaging and morphometric analysis of embryonic development in the ascidian Ciona intestinalis. Genesis 43(3):136–147
Sasaki H, Hogan BL (1993) Differential expression of multiple fork head related genes during gastrulation and axial pattern formation in the mouse embryo. Development 118(1):47–59
Sasaki H, Yoshida K, Hozumi A, Sasakura Y (2014) CRISPR/Cas9-mediated gene knockout in the ascidian Ciona intestinalis. Develop Growth Differ 56(7):499–510
Sasakura Y, Suzuki MM, Hozumi A, Inaba K, Satoh N (2010) Maternal factor-mediated epigenetic gene silencing in the ascidian Ciona intestinalis. Mol Gen Genomics 283(1):99–110
Satoh N, Tagawa K, Takahashi H (2012) How was the notochord born? Evol Dev 14(1):56–75
Sawada A, Nishizaki Y, Sato H, Yada Y, Nakayama R et al (2005) Tead proteins activate the Foxa2 enhancer in the node in cooperation with a second factor. Development 132:4719–4729
Segade F, Cota C, Famiglietti A, Cha A, Davidson B (2016) Fibronectin contributes to notochord intercalation in the invertebrate chordate, Ciona intestinalis. EvoDevo 7(1):21
Sehring IM, Dong B, Denker E, Bhattachan P, Deng W, Mathiesen BT, Jiang D (2014) An equatorial contractile mechanism drives cell elongation but not cell division. PLoS Biol 12(2):e1001781
Sehring IM, Recho P, Denker E, Kourakis M, Mathiesen B, Hannezo E, Dong B, Jiang D (2015) Assembly and positioning of actomyosin rings by contractility and planar cell polarity. Elife 21(4):e09206
Smith J (1999) T-box genes: what they do and how they do it. Trends Genet 15(4):154–158
Søviknes AM, Glover JC (2008) Continued growth and cell proliferation into adulthood in the notochord of the appendicularian Oikopleura dioica. Biol Bull 214(1):17–28
Stemple DL (2005) Structure and function of the notochord: an essential organ for chordate development. Development 132:2503–2512
Stolfi A, Christiaen L (2012) Genetic and genomic toolbox of the chordate Ciona intestinalis. Genetics 192(1):55–66
Stolfi A, Gandhi S, Salek F, Christiaen L (2014) Tissue-specific genome editing in Ciona embryos by CRISPR/Cas9. Development 141(21):4115–4120
Tada M, Casey ES, Fairclough L, Smith JC (1998) Bix1, a direct target of Xenopus T-box genes, causes formation of ventral mesoderm and endoderm. Development 125(20):3997–4006
Takada N, Satoh N, Swalla BJ (2002) Expression of Tbx6, a muscle lineage T-box gene, in the tailless embryo of the ascidian Molgula tectiformis. Dev Genes Evol 212:354–356
Takahashi H, Hotta K, Erives A, Di Gregorio A, Zeller RW, Levine M, Satoh N (1999) Brachyury downstream notochord differentiation in the ascidian embryo. Genes Dev 13(12):1519–1523
Takahashi H, Hotta K, Takagi C, Ueno N, Satoh N, Shoguchi E (2010) Regulation of notochord-specific expression of Ci-Bra downstream genes in Ciona intestinalis embryos. Zool Sci 27(2):110–118
Tamplin OJ, Cox BJ, Rossant J (2011) Integrated microarray and ChIP analysis identifies multiple Foxa2 dependent target genes in the notochord. Dev Biol 360(2):415–425
Thompson JM, Di Gregorio A (2015) Insulin-like genes in ascidians: findings in Ciona and hypotheses on the evolutionary origins of the pancreas. Genesis 53(1):82–104
Urry LA, Whittaker CA, Duquette M, Lawler J, DeSimone DW (1998) Thrombospondins in early Xenopus embryos: dynamic patterns of expression suggest diverse roles in nervous system, notochord, and muscle development. Dev Dyn 211:390–407
Veeman MT, Nakatani Y, Hendrickson C, Ericson V, Lin C, Smith WC (2008) Chongmague reveals an essential role for laminin-mediated boundary formation in chordate convergence and extension movements. Development 135(1):33–41
Wada H, Okuyama M, Satoh N, Zhang S (2006) Molecular evolution of fibrillar collagen in chordates, with implications for the evolution of vertebrate skeletons and chordate phylogeny. Evol Dev 8:370–377
Weisblat DA, Sawyer RT, Stent GS (1978) Cell lineage analysis by intracellular injection of a tracer enzyme. Science 202:1295–1298
Yagi K, Satou Y, Satoh N (2004) A zinc finger transcription factor, ZicL, is a direct activator of Brachyury in the notochord specification of Ciona intestinalis. Development 131(6):1279–1288
Yamada S, Ueno N, Satoh N, Takahashi H (2011) Ciona intestinalis Noto4 contains a phosphotyrosine interaction domain and is involved in the midline intercalation of notochord cells. Int J Dev Biol 55(1):11–18
Yasuo H, Satoh N (1993) Function of vertebrate T gene. Nature 364(6438):582–583
Acknowledgements
Thanks to all present and past laboratory members and collaborators. We are particularly indebted to Drs. Diana José-Edwards, Lavanya Katikala, Izumi Oda-Ishii, and Yale Passamaneck for their original microphotographs. Research in our laboratory is supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number R01GM100466.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Maguire, J.E., Pandey, A., Wu, Y., Di Gregorio, A. (2018). Investigating Evolutionarily Conserved Molecular Mechanisms Controlling Gene Expression in the Notochord. In: Sasakura, Y. (eds) Transgenic Ascidians . Advances in Experimental Medicine and Biology, vol 1029. Springer, Singapore. https://doi.org/10.1007/978-981-10-7545-2_8
Download citation
DOI: https://doi.org/10.1007/978-981-10-7545-2_8
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-7544-5
Online ISBN: 978-981-10-7545-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)