Abstract
Depolymerization is a tertiary recycling technique which represents the transformation of polymer chain into monomer units along with oligomers. Depolymerization of polymers with heterofunctional groups can be targeted by microorganisms, especially by fungi. Polyethylene terephthalate (PET), polyurethane (PU), polyester are among such polymers. Hydrolysis is the main cause behind microbial degradation, and hydrophobic surface binding of involving enzyme is responsible for microscopic description. Microbial participation techniques can be modified by using a chemical-assisted hybrid technique, and even halogenated polymer is dechlorinated by this technique.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Microbial depolymerization of polymers includes various techniques that involve microbes for transformation of polymer chain back into its monomer units along with oligomers. Polymer degradation alters the polymer properties because of the bond scissions and successive transformations via chemical, physical, or biological reactions.
Depolymerization of plastics can be categorized as:
-
(i)
Photo-oxidative
-
(ii)
Thermal
-
(iii)
Mechanochemical
-
(iv)
Ozone-induced
-
(v)
Catalytic
-
(vi)
Biodegradation
Photo-oxidative degradation results in the decomposition of polymer by the action of light both UV and visible. The most damaging UV wavelength for a specific plastic depends on the bonds present, and therefore, the maximum degradation occurs at different wavelengths for different types of plastics, e.g., polyethylene (PE) degrades at around 300 nm, whereas polypropylene (PP) requires the wavelength of 370 nm for its degradation. The reaction initiates by the absorption of light energy by the suitable groups in the polymer chain; this results in chain scission leading to the formation of monomers and oligomers. Photodegradable polymers need to have a photo-responsive group (either already present in chain or by addition) (Kyrikou and Briassoulis 2007). Polymer degradation happens mainly in the ether parts of the soft segments, where photo-irradiation generates aldehyde, ester, propyl, formate, and end groups (Nagai et al. 2005). Both photochemical and thermal degradations are classified as oxidative degradation. The points of differences between these are (a) the sequence of initiation steps leading to auto-oxidation cycle and (b) thermal reactions occur throughout the bulk of the polymer sample, whereas photochemical reactions occur only on the surface (Singh and Sharma 2008). Many additional polymers depolymerize at high temperature, e.g., polymethylmethacrylate (PMMA) and PE have been converted almost quantitatively back to the monomer Thermal degradation (above 200 °C) leads to chain scission and mainly depends on impurities like head-to-head units, unsaturation sites (Ramis et al. 2004).
In mechanochemical degradation, breakdown of molecular chains under shear or mechanical force is often followed by a suitable chemical reaction. The mechanodegradation of polymers in melts occurs via free radical pathways. When polymers are subjected to shear, macroalkyl radicals are formed which accelerate oxidation. This may occur either in polymer melt during processing phase or under mechanical stress conditions (e.g., cross-linking rubber at lower temperatures). Under such conditions, chain-breaking electron acceptor (CB-A) antioxidants have noted to be relatively more effective than thermal oxidation (Scott 1984). When polymethylmethacrylate (PMMA) is degraded mechanochemically using nitroxides as chain termination agents, macroradicals are produced which are used in polymerization reactions (Schmidt‐Naake et al. 2002). PVC when dechlorinated mechanochemically, using oxide powders such as Fe2O3, CaO, SiO2 and Al2O3, in air, has shown to decrease the molecular weight of PVC (Inoue et al. 2004). Atmospheric ozone, even in every modest concentration, speeds up the aging of polymeric materials (Kefeli et al. 1971). When polymers are exposed to ozone, many carbonyl and unsaturated carbonyl products, based on ketones, aliphatic esters, and lactones as well as aromatic carbonyl associated with the styrene phase, are formed rapidly, followed by gradual formation of ether, hydroxyl, and terminal vinyl groups (Allen et al. 2003). Ozone mainly interacts with polymer chain aromatic rings, C=C bonds, or saturated hydrocarbon links. The degradation reaction proceeds via formation of unstable intermediates like peroxy radicals that can isomerize or degrade, thereby causing decomposition of macromolecules (Ghosh and Ray 2004). Ozone attacks polystyrene rather slowly compared to unsaturated polymers. Reaction products (i.e., 18% ketone, 35% peroxide, and 47% acid) are similar to those expected after free radical chain oxidation processes (Singh and Sharma 2008). The addition of a catalyst lowers the temperature of decomposition, improves the quality of products obtained from pyrolysis of plastic wastes, and also enables a given selectivity to a certain product to be achieved. Solid acid catalysts like zeolites favor hydrogen transfer reactions due to the presence of multiple active sites. The access of molecules to catalyst’s reactive sites is limited to the pore size as well as the growth of end products inside the pores. Zeolite catalysts, therefore, may produce molecular sieving and shape selectivity (Marcilla et al. 2003). Polyolefins are thermally or catalytically degraded into gases and oils. Zeolite, non-zeolite catalyst (Lin and Yen 2005), transition metal catalysts, i.e., Cr, Mo, Ni, Fe, Co supported over Al2O3 and SiO2 (Williams and Bargi 2004) and Pt–Co, Pt–Mo supported over SiO2 (Gimouhopoulos et al. 2000), are major catalytic combinations employed for degradation. Biodegradation involves biochemical transformation and mineralization of polymers using microorganisms. It is nowadays described as the sustainable technique due to its easy and environmental-friendly nature.
2 Microbial Participation
Biodegradation of a polymeric material is a degradation brought about by the enzymatic action of naturally occurring microorganisms like bacteria; fungi yeast etc. into metabolites such as H2O, CH4, CO2, biomass etc. (Lenz 1993; David et al. 1994; Chandra et al. 1998; Mohanty et al. 2000). In biodegradation of polymers, almost complete transformation of material happens, whereas in biodeterioration or biocorrosion, the change is observed only on polymer structure or in its composition (Gu et al. 2003). In both cases, structural integrity is lost due to reduction in molecular weight.
Microorganisms gain energy by catalyzing energy-producing chemical reactions that transport electrons away from the pollutant polymer. The organic part is oxidized, and the chemical that gains the electrons is reduced. The energy achieved from these electron transfers is then utilized along with some electrons and carbon from the contaminant, for the production of more cells. Several factors including accessibility to water, oxygen usage, minerals, temperature, pH, redox potential, carbon, and energy source influence the growth of microorganisms (Sand 2003) (Table 1).
2.1 Role of Enzymes
Enzymes are present in every living cell and therefore in all the microorganisms. Microorganisms are highly adaptive to environment and secrete both endo - enzymes and exo - enzymes that attack the substrate and cleave the molecular chains into segments (Lugauskas et al. 2003; Huang et al. 1990). Amounts of various enzymes produced by different microorganisms vary with species and even within the strains of the same species. The enzymes have proteins with complicated chemical structure, high molecular weights, and hydrophilic groups such as –OH, –COOH, and –NH2 (Potts 1978) which can attack and eventually destroy almost anything.
In presence of enzymes, accelerated reaction rates (of 106–1020) without creating production of undesirable products can be observed (Lenz 1993). Generally, over 2000 types of enzymes are present in a biological system, each one performing one specific chemical function. The following enzymatic factors greatly influence the susceptibility of polymers toward microbial attack:
-
(i)
enzyme availability;
-
(ii)
sites in the contaminant/polymer for enzyme attack;
-
(iii)
enzyme specificity for that contaminant;
-
(iv)
presence of co-enzyme if needed.
Microorganisms are incapable of directly transporting the lengthy and hydrophobic polymer chains into their cells via outer cell membranes. To utilize polymer molecules as carbon source, microbes excrete extracellular enzymes, which depolymerize the polymers outside the cells. Both extracellular and intracellular depolymerases enzymes are actively involved in biological degradation of polymers. A bacterium could constantly produce all of the enzymes required for degradation or else could start enzyme synthesis as required to metabolize when needed (Albertsson et al. 1987).
2.2 Degradation Mechanisms
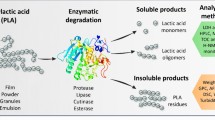
During degradation, exo-enzymes from microorganisms break down complex polymers into short chains or smaller hydrophilic molecules such as dimers, oligomers, and monomers, able to pass the outer semi-permeable bacterial membranes and to be utilized as carbon and energy sources (Gu 2003). The following are the steps toward any polymeric degradation and can work individually or jointly (Fig. 1).
2.2.1 Solubilization
Solubilization is dependent on the hydrophilicity of the polymer. Hydration of polymer chains takes place when structure stabilized by van der Waals forces and hydrogen bonds disrupt. After hydration, the polymer chains may become water soluble and/or the polymer backbone may be cleaved by chemical- or enzyme-catalyzed hydrolysis to result in the loss of polymer strength (Ishigaki et al. 1999). In case of cross-linked polymers, the polymer strength may be reduced by cleavage of either of the polymer backbone, cross-linker, or pendent chains. In non-swellable (non-water absorbing) polymer systems, the decrease in molecular weight may lead to the loss of coherence between polymer chains (Park et al. 2011). In a study exploring biodegradation of poly aromatic hydrocarbons (PAHs), enhancement of solubility and biodegradability of hydrophobic hydrocarbons was achieved using biosurfactants (Barkay et al. 1999)
2.2.2 Ionization
Some water-insoluble polymers can be solubilized by ionization or by protonation of a pendent group. Poly acids become soluble at high pH (Chambliss 1983), and poly (vinyl acetate phthalate) (PVAP) and hydroxyl propyl methyl cellulose phthalate are ionized at a lower pH (cellulose acetate phthalate becomes water soluble at a pH > 6).
2.2.3 Hydrolysis
Water-insoluble polymers with pendent anhydride and/or ester groups may be solubilized if anhydride or esters hydrolyze to form ionized acids on the polymer chain. For example, poly (methacrylate) and poly (methyl methacrylate) are water insoluble but become water soluble upon hydrolysis of the pendent esters and subsequent ionization of the carboxylic group (Murthy et al. 1986). For hydrolysis to occur, the polymer has to contain hydrolytically unstable bonds, reasonably hydrophilic for contact with water. Polyesters are degraded mainly by simple hydrolysis via non-enzymatic, random hydrolytic ester cleavage, and its duration is determined by the initial molecular weight and chemical structure of the polymer.
2.2.4 Enzyme-Catalyzed Hydrolysis
Enzymes act as catalysts for particular reactions or a series of reactions, such as reduction, oxidation, esterification, hydrolysis, synthesis, and molecular interconversions. The enzymatic degradation of some natural polymers occurs by the unzipping or chain-end degradation mechanism. For example, β-amylase degrades starch to maltose, beginning with the chain ends. In the lipase-catalyzed degradation of poly (vinyl acetate) (PVAc), the ester bonds in the side chains are broken particularly to yield oligomers with acid and alcohol groups (Singh and Sharma 2008). Poly (3-caprolactone) has been enzymatically degraded in supercritical carbon dioxide (scCO2) using Candida antarctica lipase to give oligo(3-caprolactone) (Takamoto et al. 2001). When the end products are inorganic species, e.g., CO2, H2O, or CH4, the degradation is also called mineralization. Mineralization can come about either aerobically or anaerobically. Anaerobic and aerobic biodegradation pathways are given (Fig. 2), with their corresponding final products (Gu 2003).
2.3 Aerobic Respiration
In aerobic respiration, microbes use O2 to oxidize part of the carbon in the polymer to CO2, and rest of the carbon is used to produce new cell mass. In the process, O2 gets reduced, producing H2O. Thus, the major by-products of aerobic respiration are carbon dioxide, water, and an increased population of microorganisms. The by-products produced during conversion which enter into the microbial cell can be utilized as the energy source (Shimao 2001).
2.4 Anaerobic Respiration
In anaerobic respiration, nitrate (NO3−), sulfate (SO4 2−) metals such as iron (Fe3+) and manganese (Mn4+), or even CO2 can accept electrons from the degraded contaminant (National Research Council 1993). Hence, anaerobic respiration uses inorganic chemicals as electron acceptors. By-products of anaerobic respiration are new cell matter, H2S, N2 gas, CH4, and reduced forms of metals.
Given that thermodynamically, O2 is a more efficient electron acceptor than both SO2 4−and CO2; aerobic processes yield much more energy and are capable of supporting a greater population of microorganisms than anaerobic processes. It is important to note that biodeterioration and degradation of polymer substrate can rarely arrive at 100% because a small fraction of the polymer will always be incorporated into microbial biomass, humus, and other natural products (Alexander 1977; Narayan 1993).
2.5 Formation of Biofilms
Microbes deteriorate and degrade both natural and synthetic polymers by adhering to and colonizing polymer surfaces forming biofilms (Gu et al. 2000; Mitchell 1996). The formation of a biofilm is a precondition for substantial corrosion and deterioration of these materials to take place. Polymers susceptible to biofilm formation include but not limited to paints, plastics, adhesives, sealants, lubricating materials, composites, fuels (Gross et al. 1995; Gross 1993; Lugauskas et al. 2003).
Biofilm is a slimy layer where bacterial cells can encase themselves in a hydrated matrix of polysaccharides and protein which is composed of water (80–95%), extracellular polymer substances that constitute 85–98% of the organic matter, the microorganisms, entrapped organic and inorganic particles (e.g., humic substances, debris, clay, silica, gypsum), substances sorbed to extracellular polymeric substances, cells or particles and substances dissolved in the interstitial water (Flemming 1998). The process of establishment of complex community of microorganisms on surface attachment as biofilm is known as biofouling or microfouling
Main mechanism (Flemming 1998) (both direct and indirect) through which the structure and function of synthetic polymeric materials can be damaged by biofilms includes as follows:
-
(i)
coating the surface, masking surface properties, and contaminating adjacent media such as H2O;
-
(ii)
increasing the leaching additives and monomers out of the polymer matrix;
-
(iii)
attack by enzymes or radicals of biological origin to polymer and additives, leading to both embrittlement and loss of mechanical stability;
-
(iv)
accumulating water and penetrating the polymer matrix with microbial filaments, causing swelling and increased conductivity;
-
(v)
excretion of lipophilic microbial pigments that lead to unwanted colors in the polymer.
2.6 Properties Deterioration
Mechanical properties of polymers are principally dictated by length of the polymer chains and are weakened by chain scissions. A single endo-cleavage in a polymer chain can reduce the molar mass up to 50% and hence cause significant alterations in the mechanical properties such as embrittlement, increased modulus, change in weight, dimensions. When plasticizers are removed from the plastic materials by microorganisms, embrittlement can also occur, whereas deterioration in electric properties such as dielectric constant, insulation resistance power factor, and dielectric strength are observed because of the surface growth and pH variations resulted by excreted metabolic products.
3 Biodegradation of Plastics Using Microorganisms
The potential of microbes for plastic degradation first came to light in 1961 when Fuhs reported that several microorganisms were capable of consuming paraffin as a carbon source. Plastic-degrading microbes were isolated from a multiple sources such as rhizosphere soil of mangroves, marine water, polythene buried in the soil, and soil at the dumping sites. Comparative study of polyethylene and paraffin degradation revealed that bacteria had utilized polyethylene as carbon source (Jen-hou and Schwartz 1961). The mechanism put forward by Gautam et al. (2007) reported that paraffin molecules first get converted to an alcohol by a monooxygenase enzyme; then alcohol is oxidized into an aldehyde by alcohol dehydrogenase enzyme. Further an aldehyde dehydrogenase coverts aldehyde to fatty acid, which then undergoes β-oxidation inside cells (Scheme 1).
Paraffin biodegradation pathway (Gautam et al. 2007)
3.1 Polyethylene (PE)
Hydrophobic nature of polyethylene (PE) restricts its bioavailability (Mahalakshmi et al. 2012). It was found that lower molecular hydrocarbon (less than 620) oligomers favor the growth of microorganisms while this is not possible in high molecular weight PE. To increase the effectiveness of the process, it is often coupled with some form of physical and chemical treatments like photo-oxidation, nitric acid oxidation, UV irradiation, and thermal treatment which favor its biodegradation (Hadad et al. 2005). PE molecules when treated with UV radiations form radical. Oxygen absorbed from atmosphere results in formation of hyperoxides which ultimately bring in C=O group in polymer (Scheme 2).
Biodegradation of PE (Gautam et al. 2007)
Various modifications affect PE biodegradation (Table 2). Chemical pretreatment of polyethylene with 0.5 molar nitric acid and sodium hydroxide speeds up the subsequent biodegradation using Pseudomonas sp. (Nwachkwu et al. 2010). Genera associated with PE biodegradation are Gordonia and Nocardia, Bacillus, Lysinibacillus, pseudomonas, staphylococcus, streptococcus, micrococcus, streptomyces, rhodococcus, proteus, listeria, vibrio, bravibacillus, serratia, nocardia, diplococcus, moraxella, penicillium, arthrobacter, aspergillus, phanerochaete, chaetomium, and Gliocladium (Bonhommea et al. 2003; Koutny et al. 2006a, b; Arutchelvi et al. 2008; Restrepo- Flórez et al. 2014; Grover et al. 2015). Non-ionic surfactants increase polymer hydrophilicity by providing better chances of surface adhesion to microbes (Hadad et al. 2005).
3.2 Nylon
Some types of nylon can be degraded by both fungi (Deguchi et al. 1997) and bacteria (Tomita et al. 2003). A number of studies for isolation, expression, and transformation of genes encoding nylon-degrading enzymes are available in the literature (Kakudo et al. 1993; Kanagawa et al. 1989). In a study, bacterium Geobacillus thermocatenulatus was effectively isolated and applied to degrade nylon 12 and nylon 66 (Tomita et al. 2003). This bacterium enzymatically hydrolyzed the amide bonds, which resulted in the production of 12-aminododecanoic acid (Scheme 3).
Lignin-degrading white rot fungus strain IZU-154 and extracellular enzyme fungus peroxidase from the same fungal strain degrade nylon 66 (Deguchi et al. 1997, 1998). Degradation products revealed the presence of CHO, NHCOH, CH3, and CONH2 end groups indicating the oxidative process (Scheme 4).
Bacterial biodegradation of nylon 66 has been noted to be a hydrolytic process. Proposed mechanism implied that initially, enzyme peroxidase oxidizes the methylene group next to N atom in polymer backbone forming –CH radical, which then undergoes oxidation (Nomura et al. 2001). The enzymes associated with nylon degradation are 6-aminohexanoate cyclic dimer hydrolase (F-EI), 6-aminohexanoate-dimer hydrolase (F-EII), and 6-aminohexanoate oligomer hydrolase (F-EIII) (extracted from Flavobacterium sp. KI72; Kakudo et al. 1993; Kinoshita et al. 1977, 1981; Negoro 1992).
3.3 Polyethylene Adipate (PEA) ([–OCH2CH2OOC(CH2)4CO–] n )
PEA with molecular weight 3000 can be degraded by using penicillium sp. as sole source of carbon. Out of the various other strains, 14-3 found to be showed greatest activities. This strain can also degrade aliphatic polyesters like polyethersulfone (PES), polybutylene succinate (PBS), and polybutylene adipate (PBA) (Tokiwa and Suzuki 1974). Furthermore, lipases from Rhizopus delemar, Rhizopus arrhizus, Achromobacter sp., and Candida cylindracea and esterase from hog liver are noted for showing PEA degradation (Tokiwa and Suzuki 1977a, b).
3.4 Polycaprolactone (PCL) ([–OCH2CH2CH2CH2CH2CO–] n )
Aliphatic ester linkages in PCL make it susceptible to enzymatic degradation, but hydrolysis of this polymer is time-consuming (Woodruff and Hutmacher 2010; Gajanand et al. 2014). Enzymatic cleavage of polymers is substrate specific (Shimao 2001; Murphy et al. 1996; Schink et al. 1992). Penicillium funiculosum and A. Flavus enzymatically hydrolyzed PCL in its amorphous region (Tokiwa et al. 2009b). PCL degradation was also achieved using enzyme esterases and lipases (Shimao 2001). Lipase-producing species such as R. delemar, R. Arrizus, Achromobacter sp., Candida cylindracea, Penicillium sp., have been reported for PCL degradation (Tokiwa et al. 2009). PCl when blended with % sebacic acid showed greater degradation rate (Salgado et al. 2012; Tokiwa et al. 2009). Aspergillus sp. strain ST-01 was used on multiple instances for PCL degradation (Tokiwa et al. 2009; Sanchez et al. 2000). Only 10% degradation of the polymer was noted by P. lilacinus (Oda et al. 1995). P. lilacinus D218 releases PCL depolymerase, whose optimum activity was observed at 30 °C in pH range of 3.5–4.5. Degradation under anaerobic conditions have also shown (Abou-Zeid et al. 2001; Yagi et al. 2009) good degradation results. Some other reported species for PCL degradation are Bacillus pumilus (Motiwalla et al. 2013); genus Alcanivorax, Tenacibaculum and Pseudomonas (isolated from marine environments) (Sekiguchi et al. 2009); yeast Cryptococcus laurentii (Benedict et al. 1983); Clostridium botulinum, Alcaligenes faecalis (Caruso 2015; Tokiwa et al. 2009). Activity of phytopathogenic fungi against PLC is suspected to be attributed to the presence of cutinases.
3.5 Polybutylene Succinate (PBS) ([–O(CH2)4OOC(CH2)2CO–] n ) and Polyethylene Succinate (PES) ([–O(CH2)2OOC(CH2)2CO–] n)
Strain Amycolatopsis sp. HT-6 has found to degrade PBS, PHB, PCL (Pranamuda et al. 1995). Actinomycetes (thermophilic) that degrade PBS and PES are Microbispora rosea, Excellospora japonica, Excellospora viridilutea (Jarerat and Tokiwa 2001; Duddu et al. 2015; Seretoudi et al. 2002; Hoang et al. 2007). Other microbes and genus reported are Pseudomonas sp. AKS2 strain (Qiu et al. 2003; Liu et al. 2012; Tribedi and Sil 2013), Saccharomonospora, Streptomyces, Microbispora, Thermoactinomyces, and Actinomadura (Tseng et al. 2007), Streptomyces sp. (Calabia and Tokiwa 2004) Bacillus sp. TT96 (Tokiwaet al. 2009), Mesophilic Bacillus, Paenibacillus, Bacillus pumilus, B. subtilis, and Paenibacillus amylolyticus (Tezuka et al. 2004), Rhizopus delemar (Seretoudi et al. 2002). Biostimulation helps in degradation by forming a new microbial community (Tribedi and Sil 2013). Hydrocarbon-degrading microbes from oil-contaminated marine environment have also been identified (Hazen et al. 2010). Microbial serine proteases have also been reported for PES biodegradation (Lim et al. 2005).
3.6 Poly (β-Propiolactone) (PPL) ([–OCH2CH2CO–] n )
Microbes belonging to Bacillus sp. have been employed for degrading this polymer (Nishida et al. 1998). Some other strains from thermophilic Streptomyces identified for PPL degradation are Variovorax paradoxus, Acidovorax sp, Sphingomonas paucimobilis, Rhizopus delemar (Tokiwa and Suzuki 1977a, b), poly (3-hydroxybutyrate) depolymerase (Tokiwa and Calabia 2006).
3.7 Aliphatic–Aromatic Copolyesters (AACs)
Aliphatic–aromatic copolyesters generally consisting of polyethylene terephthalate (PET), PCL, polyethylene isophthalate (PEIP), and polybutylene terephthalate were hydrolyzed by Rhizopus delemar lipase (Tokiwa and Suzuki 1981), although the susceptibility decreased with increased aromatic content. Kleeberg et al. (1998) reported Thermobifida fusca and its enzyme hydrolase for AAC degradation. This enzyme is highly similar to triacylglycerol lipase from Streptomyces albus G and triacylglycerol acylhydrolase from Streptomyces sp. M11 (Kleeberg et al. 2005). PET films were degraded when treated with (a) cutinase from Humilica insolens and Fusarium solani pisi (Donelli et al. 2010) observed 97% weight loss; (b) Pseudomonas mendocina with 5% weight loss (Heumann et al. 2006) and cracks on fiber surface (Zhang et al. 2004). Enzymes from Aspergillus sp., Beauveria sp., Thermobifida fusca, and commercial enzymes (TEXAZYME PES sp5 and Lipase PS) increased the hydrophilicity of PET fibers (Heumann et al. 2006; Ronkvist et al. 2009; Naharjan et al. 2006). Besides, the microbial capability of forming biofilm over PET film pointed out the possibility of degradation under natural conditions (Webb et al. 2009). Furthermore, to increase biodegradability of PET, studies have been done to modify the polymer in order to decrease the intermolecular cohesion. Nowak et al. (2011) modified PET films with Bionolle polyester in the presence of filamentous fungi Penicillium funiculosum. After 84-day incubation period, considerable reduction in quantity of aromatic rings was observed. In another study, series of copolyesters were prepared using waste PET beverage bottles and L-lactic acid. Samples were incubated in thermophilic sludge at 55 °C in alkaline environment for a period of 394 days. This modified copolyester was more inclined to hydrolytic biodegradation than PET waste. Copolyester mineralization gave methane-rich biogas in 69% and 34% of theoretical amount for two samples, respectively. The rate of anaerobic biodegradation decreased with higher starting content of aromatic sequences (Hermanová et al. 2015). The degradation of transparent PET sheets by microbes of Nocardia species and esterase was studied. Esterase was found to be involved in the biodegradation. Although the biodegradation was slow and weak, microbes and esterase could act on the polyethylene terephthalate (Sharon and Sharon 2017).
3.8 Poly (3-Hydroxybutyrate) (PHB) ([–O(CH3)CHCH2CO–] n )
First reports of PHB-degrading microbes were from Bacillus, Streptomyces, and Pseudomonas species (Chowdhury 1963). Mostly, microbes that utilize PHB do so at ambient temperatures and they have been isolated from soil Pseudomonas lemoigne, Comamonas sp., Acidovorax faecalis, Aspergillus fumigatus, Variovorax paradoxus, sludge Pseudomonas, Ilyobacter delafieldi, Alcaligenes faecalis, and sea and lake water Pseudomonas stutzeri, Comamonas testosterone (Lee 1996). A thermophilic Streptomyces sp. shows higher PHB-degrading activity than thermotolerant and thermophilic strains from same species (Calabia and Tokiwa 2004). Aspergillus sp. degraded 90% of PHB film in five (Sanchez et al. 2000). Actinomycetes associated with PHB, PCL, and PES depolymerization are members of the genera Streptomyces, Microbispora, Actinomadura, Saccharomonospora, and Thermoactinomyces (Tseng et al. 2007). Another study done to assess the biodegradation pattern of blends of polylactic acid (PLA) and polyhydroxybutyrate (PHB) at different weight ratios showed biodegradability of blends increased as ratio of PHB increases (Zhang and Thomas 2011). Biocomposites prepared form PHB and potato peel waste fermentation residue also showed complete degradation in eight months (Wei et al. 2015).
3.9 Polylactic Acid (PLA) ([–O(CH3)CHCO–] n )
Polylactic acid, a biodegradable plastic is used freely in medicine industry. T16-1 strain of Actinomadura keratinilytica NBRC 104111 has been illustrated for producing enzyme that degrades PLA. 60% degradation of PLA films have been observed by Amycolatopsis sp. over a period of fourteen days (Babu et al. 2013; Sukhumaporn et al. 2012; Garlotta 2002; Pranamudaet al. 1997). Bacillus amyloliquefaciens, a mesophilic bacteria, are also known to degrade PLA (Prema and Uma 2013). Masaki et al. (2005) used lipase enzyme purified from strain Cryptococcus sp. S-2 for PLA degradation. When dye having PLA films as a constituent was inspected, enzymes from Aneurinibacillus migulanus showed the same activity as proteinaseK for PLA degradation (Chaisu et al. 2015). PLA depolymerase enzyme is also reportedly produced by Pseudomonas tamsuii TKU015 (Liang et al. 2016).
3.10 Polyurethanes (PUR)
Polyurethanes are both polyether PUR and polyester PUR (Shimao 2001). Enzyme proteases, esterases, ureases, and lipases have been noted to bring about PUR hydrolysis. Aspergillus terreus, Trichoderma sp., and Chaetomium globosum have also been reported for the producing ureases and esterase that hydrolyze PUR (Bhardwaj et al. 2013; Howard 2012). A total of 22 fungal strains were screened, and up to 95% urease activity was found in most of them (Loredo-Trevino et al. 2012). Microbial species identified as polyurethanes degraders are Candida ethanolica, Fusarium solani (Schink et al. 1992; Zafar et al. 2013), Aspergillus fumigatus, Emericella, Arthrographis kalrae, Lichthemia, Fusarium solanii, Plectosphaerella, Phoma, Nectria, A. niger, Corynebacterium sp., Neonectria, Thermomyces, P. aeruginosa, Alternaria, Bacillus and Comamonas; C. acidovorans TB-35, Pestalotiopsis microspora (Bhardwaj et al. 2013; Howard 2012; Akutsu et al. 1998; Zafar 2013; Flavel et al. 2006; Shimao 2001). Proteolytic enzymes—urease and papain—also degrade polyurethane used in medical devices. Ma and Wong (2013) investigated the esterase activity of A. Flavus. Different experiments have illustrated the important role of GenespueA and pueB in PUR degradation (Howard 2012). pueA helps in enhancing PUR degradation by increasing the cellular density as shown by gene silencing experiments (Howard et al. 2007).
The various plastic-degrading microbes are summarized in Table 3.
4 Physiochemical Characteristics Influencing Depolymerization
Polymer biodegradation is a heterogeneous process since polymers are multicomponent systems. These components can provide nutrients for some microbes (Kyrikou and Briassoulis 2007). All polymers are biodegradable to some degree because of the organic fraction of their composition like resins, hardener. Polymeric material structures and compositions govern their biodegradation. Single polymer can contain different structural elements, e.g., random or alternating copolymers. Also, varying structures like branched network can directly impact the polymer chain cleavage. Substantial amount of qualitative and semi-quantitative information has been gathered documenting the factors that impact the rate of degradation of synthetic polymers in biological environments (Fig. 3).
4.1 Abiotic Factors
Environmental conditions such as temperature, humidity, pH, salinity, sunlight, water, presence/absence of oxygen, culture conditions, and stress significantly impact the degradation process and also control microbial population and enzyme activity (Gu 200). Increased temperature and moisture content increase the rate of hydrolysis and microbial activity, which in turn amplify the chain scissions subsequently increasing the available polymer sites for attack. This results in faster degradation (Ho et al. 1999). Chain scissions from photodegradation reduce the molecular weight of polymer and improve the approachability of microbes to the polymer (Stevens 2003).
4.2 Biotic Factors
4.2.1 Production of Extracellular Enzymes
Extracellular enzymes excreted by different microbes may have active sites with different structure/configuration and biodegrade certain polymers better than others. For example, Aspergillus niger and Aspergillus flavus fungi produce enzymes which are known to better digest 6-12 carbon di-acid-derived aliphatic polyesters as opposed to those from other monomers (Chandra and Rustgi 1997).
4.2.2 Enzyme–Substrate Complex Formation
Enzyme–substrate complex formation is generally explained by either lock and key fit or by induced - fit mechanisms (Donald et al. 2006; Koshland 1995). For a reaction to take place and enzyme–substrate complex to be formed, some geometrically similar pattern between enzyme and substrate is required. The lack of these similar geometric patterns between plastic substrate and available enzymes makes the succeeding biodegradation difficult.
4.2.3 Hydrophobicity
Although water solubility does not guarantee biodegradability, the particularly hydrophobic nature of plastics makes them harder to biodegrade. Enzymes and microbes often have complex metabolic pathways for mineralizing such extremely hydrophobic materials (Nakajima-Kambe et al. 1999).
4.3 Polymer Characteristics
4.3.1 Conformational Flexibility
Flexibility in a polymer makes it easily reachable for the microbes. Most polymers have highly cross-linked branched structures, which make formation of an enzyme–substrate complex harder. For example, flexible aliphatic polyesters are comparatively easier to biodegrade than rigid aromatics like polyethylene terephthalate (PET) (Omichi 1992).
4.3.2 Molecular Weight (MW)
Microorganisms can only digest lower molecular weight segments of polymer and convert them into metabolites. Synthetic polymers with M W > 1000 are found to be less susceptible to biodegradation than those with M W < 1000 (Suzuki et al. 1978).
4.3.3 Degree of Crystallinity
The amorphous part of polymeric chain is prone to enzymatic attack than the crystalline part because
-
(a)
molecules in amorphous part are freely packed, making the enzymatic attack easier and more susceptible. For example, the rate of polylactide acid degradation decreases with increasing crystallinity (Tsuji and Miyauchi 2001).
-
(b)
Amorphous regions show high permeability to O2. Oxidation rates depend on the reactivity of formed peroxy radicals and dissociation energies of available C–H bonds of polymer substrate. A polymer with unreactive methyl and phenyl groups or with no H at all shows resistance to oxidation (Bovey and Winslow 1979).
4.3.4 Melting Temperature (Tm)
Melting temperatures of polyesters is an important parameter for the enzymatic depolymerization. Higher melting temperatures imply lower polymer biodegradation (Tokiwa and Suzuki 1978, 1981; Tokiwa et al. 1979).
4.3.5 Size of the Polymer
In order to avoid mass transfer restrictions and to increase surface area available to microorganism for interaction, the polymer must be reduced in size before subjecting it to biodegradation.
4.3.6 Copolymers and Additives
Naturally occurring polymers are biodegradable but biodegradation of chemically tailored natural polymers is conditioned on type and degree of modification. Adding comonomers in a polymer increases chain irregularity, thereby reducing its crystallinity and providing easy access to microbes and H2O. Impurities such as catalytic residues, fillers, pigments, and altered products of additives also influence the resistance to degradation. Metals like Mn act as good pro-oxidants in polyolefins making them susceptible for thermo-oxidative degradation. After heat activation in the presence of oxygen, pro-oxidants produce free radicals on hydrocarbon chain which undergo oxidation (Orhan et al. 2004). Furthermore, the pro-oxidant catalyzes chain scissions giving low molecular mass oxidation products containing –OH, –COOH, C=O groups (Jakubowicz 2003). Traces of transition metals present also speed up thermal oxidative processes inducing hydroperoxide decomposition (Zheng et al. 2005, Zhang and Thomas 2011). For example, TiO2 delustrant makes polyamides more prone for light- and heat-induced oxidation. Making polyolefins blend with other polyester or starch also increases its susceptibility for degradation (Erlandsson et al. 1997).
4.3.7 Role of Heteroatom
It has been observed that polymers that include heteroatoms in the backbones such as polyamines and polyesters show higher susceptibility to degradation than polymers with pure carbon backbones (Zheng et al. 2005). Incorporating heterogroups like oxygen in polymer chains influences bond strength (of neighboring C–H bonds) promoting carbanion formation (e.g., in presence of bases) subsequently making them labile for thermal and biodegradation (Gowariker et al. 2000). Similarly, unsaturation in polymer chains makes polymer prone to oxidation, i.e., natural rubber is more vulnerable to degradation than polyethylene (Seymour 1971). Aromatic polyesters despite of having easily hydrolysable ester bonds have seen to be resistant to degradation. For example, PET despite having ester bonds in its polymer chains still remains non-degradable (in normal conditions) due to its aromatic content (Webb et al. 2012).
4.3.8 Introduction of Functionality
Introducing carbonyl groups like C=O in polyolefins makes them more vulnerable for photodegradation. Increase in the number of chromophores results in increased sites for photon absorption and hence increases the rate of photodegradation. Incorporation of additional chromophores like metal–metal bonds in polymer backbone too induces photodegradability. In such case, metal–metal bond can cleave homolytically when irradiated (Meyer and Caspar 1985).
4.3.9 Chemical Bonding
In thermoplastics, during addition polymerization head-to-head or tail-to-tail addition of monomers results in weak spots making plastic susceptible for degradation. For example, head-to-head linkage in poly (methyl methacrylate) improves its thermal degradation (Holland and Rae 1987). Similarly, branching in polymeric chains too increases thermal degradation (Gowariker et al. 2000).
4.3.10 Mode of Synthesis
Methods of synthesis exhibit an effect on the stability of the polymers (Hendrickson and Connole 1995), e.g., polystyrene (anionic polymerized) shows more photo-stability than its free radically formed equivalent because of the presence of peroxide residue in the latter, which is labile for photodegradation (Pospisil et al. 2006). Similarly, copolymerized polypropylene (PP) is less inclined to photodegradation than PP synthesized via bulk polymerization and by using Ziegler–Natta catalysts (Tang et al. 2005).
5 ASTM Standards for Plastic Biodegradation
The American Society for Testing and Materials (ASTM) has recognized an assortment of scientific and technological tests to assess true biodegradation in numerous plastic articles under varying environments using different analytical methods (Table 4). ASTM (standard D-5488-94d) recognizes biodegradation as “process which is capable of decomposition of materials into carbon dioxide, methane, inorganic compounds or biomass and water in which the predominant mechanism is the enzymatic action of microorganism that can be measured as standard tests, in a specified period of time, reflecting available disposal conditions.” Taking into account the fact that plastics more often than not have multifaceted compositions and are generally degraded via heterogeneous surface mechanism, these tests are chiefly based on concepts used for assessing low molecular weight substances. Some modifications have been done over the years for different environments in which plastics might degrade.
5.1 Test Methods for Study of Biodegradation
These tests assess the changes in the material properties of polymers. Multiple test measures are required to evaluate the biodegradability of polymer for the reasons that
-
Leaching of additives, plasticizers, etc., may also result in the observed weight loss and not only polymer degradation;
-
Very small change in chemical makeup of polymer could result in high reduction in material strength, e.g., 5% of mineralization can cause 90% decrease of polymer strength;
-
CO2 production may be a result of degradation of low molecular weight part of the polymer, with no degradation of longer chains (Mohan 2011).
The current test methods are not sufficient and offer a very little flexibility to take into account the wide assortment of advanced polymers (like the ones used in electronics or aviation applications) consumed in different environmental conditions (Gu and Gu 2005). In principle, these tests can be divided into three types (Fig. 4).
5.1.1 Field Test(s)
These tests are carried out by burying the plastic sample in the soil, placing them in water sources like rivers, lakes, or by performing complete composting. Although these tests are easy and widely used, there are some associated disadvantages as well such as (i) the environmental factors like temperature, humidity, pH cannot be well controlled; (ii) analytical prospects to monitor the degradation are limited. In such situations, only possibility is to evaluate disintegration via measuring weight loss or to estimate visible changes in the polymer sample. Complex and open-ended environmental conditions make study of residues, intermediates, degradation pathways, CO2 evolution, or O2 consumption complicated.
5.1.2 Simulation Test(s)
As a substitute to field tests, a range of simulation test has been developed in which degradation process is done in a real environment like soil, in H2O, or in compost, but the contact to the environment is provided in a laboratory reactor. The important abiotic factors such as temperature, pH, humidity that affect the degradation process can be controlled and adjusted. To speed up the degradation and thereby reducing the test durations, sometimes additional nutrients are added to the sample. Examples for such tests include test simulating landfills (McCartin et al. 1990; McCarthy et al. 1992; Smith et al. 1990), the controlled composting test (Pagga et al. 1995; Tosin et al. 1996; Degli-Innocenti et al. 1998; Ohtaki et al. 1998; Tuominen et al. 2002), the soil burial test (Pantke 1990), and aquarium tests (Püchner et al. 1995). The only disadvantage is that it lacks reproducibility because of varying microbial population.
5.1.3 Laboratory Tests
Most reproducible tests, here a pre decided growth media is used which is then inoculated either with individual microbial strains or with mixed microbial inoculums. Reaction conditions are tailored for the activity of a particular microbe. Polymers frequently show higher degradation rates in laboratory tests compared to natural conditions. Examples of such tests are petri dish screen, closed bottle test, rapid detection method, gravimetry, surface hydrolysis, respirometry, and measurement of biogas. These tests offer the advantage of systematic investigations into reaction mechanism but it is still not possible to prove biodegradation in terms of metabolization by microorganisms (Mohan 2011).
5.2 Standard Test Methods
5.2.1 Visual Observations
Visual observations can be made in almost all the tests. Effects used to express degradation comprise of changes in color, roughening of the surface, defragmentation, formation of holes or cracks, or formation of biofilms on the plastic surface. These parameters can be used as indications of microbial attack but they do not essentially prove the biodegradation in terms of metabolism. To get information about the reaction mechanism, more sophisticated observations can be made using various characterizations techniques including but not limited to scanning electron microscopy or atomic force microscopy (Ikada 1999).
5.2.2 Residual Polymer Determination by Weight Loss
The mass loss of test samples is commonly noted in degradation tests (mainly in field and simulation tests), although this also does not give any direct proof for biodegradation. By combining structural analysis of residual material and low molecular weight intermediates, detailed information concerning degradation process can be gathered, especially when using a defined synthetic test medium (Witt et al. 2001).
5.2.3 Change in Molecular Mass and Subsequently in Mechanical Properties
If only small changes in the mass of samples are noted, then the changes in mechanical properties are normally used. Some properties like tensile strength are very receptive to changes in the molecular mass of polymers, which is also an indicator of degradation (Erlandsson et al. 1997).
5.2.4 Clear Zone Formation
It is a simple semi-quantitative method. The polymer size is reduced to fine particles and spread onto an agar medium. Microbes are introduced to the medium. The formation of clear halo around microbial colony is taken as an indication of capability of microbe to depolymerize the polymer. Although this method is typically employed to screen microbes for degradation potential for certain polymers (Nishida and Tokiwa 1993; Abou-Zeid 2001), it can also be utilized to gather semi-quantitative results by analyzing the growth of clear zones (Augusta et al. 1993).
5.2.5 O2 Consumption or CO2 Evolution
In aerobic respiration, microbes utilize oxygen and form CO2. Consequently, the respirometric test, used for estimating the oxygen consumption (Hoffmann et al. 1997), and Sturm test, for estimating the formation of CO2, are often used as good evaluators to estimate polymer degradation in laboratory test with good accuracy.
5.2.6 Radio Labeling
Net CO2 and 14CO2 evolution measurements are simple, non-destructive and measure ultimate biodegradation. Using this system for biodegradation studies in varying microbial environments exhibits precision and consistency (Sharabi and Bartha 1993). However, labeled materials are quite expensive and not easily available. The licensing and related radioactive waste disposal problems present some major disadvantages.
5.2.7 Controlled Composting Test
In this process, a definite weight of dry plastic is mixed with fixed amount of mature compost and incubated at 58 °C with 65% moisture content. Biodegradation is examined by measuring the amount of material carbon changed into gaseous carbon oxide, i.e., the CO2 evolved from the polymer–compost mixture minus the CO2 evolved from the unamended compost (as blank) which is tested in a different reactor (Bellina et al. 1999).
6 Characterization Techniques Used to Study Microbial Depolymerization
The biodegradability of the plastic can be characterized using several parameters such as oxygen uptake rate, carbon dioxide evolution rate, accumulation of biomass, surface changes, and changes in physiochemical properties of plastics (Albertsson and Huang 1995). Numerous analytical techniques have been used to investigate the degree and character of degradation. The examination of changes in physical and chemical properties of plastic both before and after degradation helps to understand reaction mechanisms and/or degradation pathway.
6.1 Dynamic Mechanical Analysis (DMR) Using Universal Testing Systems
Study the mechanical properties of plastics such as elongation at fail, tensile strength, and modulus of the polymer. Physical properties observed for changes are morphology (microcracks), contact angle, density, viscosity, glass transition temperatures, melting temperatures, amorphous part and crystalline part of the polymer, etc.
6.2 Scanning Electron Microscopy (SEM)
SEM helps in studying the surface morphology of the polymer films. The samples are generally have to be sputter-coated with gold or some metal ion before examination. Variable-pressure SEM is helpful for direct observation without using any surface metallization at suitable magnification (Pinzari et al. 2006).
6.3 Thermogravimetric Analysis (TGA)
It observes the changes in mass of sample due to heating. Dynamic thermogravimetry can be used to study the activation energy, rate of decomposition reactions, and temperature at maximum decomposition rate (Carrasco and Pages 1996).
6.4 Fourier Transform Infrared Spectroscopy (FTIR)
Chemical changes produced in polymers during or after microbial degradation processes are analyzed by FTIR (Mohamed et al. 2007; Singh and Sharma 2008; Elashmawi et al. 2008; Milstein et al. 1994). It helps to explain physical and chemical structures, degradation reactions, end group detection, hydrogen bonding, cross-linking behavior of molecules, and copolymer composition in solid- and liquid-form polymers.
6.5 Nuclear Magnetic Resonance Spectroscopy (NMR)
NMR spectroscopy (both 1H and 13C) is the most versatile analytical tool used to understand degradation reaction pathways and formed intermediate compounds (Massardier-Nageotte et al. 2006; Schlemmer et al. 2009; Shah et al. 2008).
6.6 Gel Permeation Chromatography (GPC)
GPC also known as size-exclusion chromatography (SEC) is used to find out molecular mass distribution of polymers and changes in molecular weight after biodegradation (Peng and Shen 1999; Kale et al. 2006; Walter et al. 1995).
6.7 High-Pressure Liquid Chromatography (HPLC)
HPLC is extensively used to separate molecules on the basis of their size. It helps to analyze the metabolic degradation products of xenobiotics, e.g., biotransformation products of styrene, epoxy styrene, phenylacetaldehyde, and 2-phenylethanol were detected by using reverse-phase high-pressure liquid chromatography (Beltrametti et al. 1997; Marconi et al. 1996).
6.8 Gas Chromatography–Mass Spectrometry (GC/MS)
The GC/MS technique comprises a gas chromatograph coupled to a mass spectrometer and is used to identify and quantify residual monomers, trace contaminants, analyzing volatile components, gas mixtures, solvent in resins and coatings, verifying additive levels in polymers and for on-line or at-line monitoring of reactions.
6.9 Biochemical Analysis
The metabolic activity of the cells in the culture and in the biofilm can be determined by various biochemical assays, e.g., by adenosine triphosphate assays, by fluorescein diacetate analysis, and by protein analysis (Orr et al. 2004; Koutny et al. 2006a, b).
6.10 Molecular Techniques
Microbial depolymerization and degradation usually take longer time periods. Once the degradation pathway is known, genetic engineering allows us to (i) reduce the reaction time, (ii) make the plastic more susceptible for microbial attack, (iii) modify regulatory mechanisms, (iv) alter the properties of degradative enzymes, and (v) assemble degradative enzymes from different organisms within a single organism. This can be done by exploiting genetic engineering techniques such as DNA and protein sequencing and production of recombinant organisms, etc.
Some biosynthetic genes, e.g., phbA (for 3-ketothiolase), phbB (NADPH-dependent acetoacetyl-CoAreductase), and phbC (PHB synthase) from acetyl-CoA have been cloned to produce PHA and PHB acids (Slater et al. 1988). These genes are clustered, presumably organized in one operon, and expressed in Escherichia coli and in also in different species of Pseudomonas. The enzymes implicated in plastic degradation should be characterized, and further genes liable for those enzymes should be worked out. Once these genes are identified, they can be modified to boost their degrading capacity (Aswale and Ade 2008). Efficient degrading microbes should be multiplied at larger scale to decompose the plastic at commercial level.
7 Significance
Efficient technology, low cost, and relatively eco-friendly treatment capable of minimizing and even eliminating synthetic polymers hold a great environmental interest. Microbial population is now being extensively studied for biodegradation of synthetic polymers. Biodegradability tests are essential to assess and minimize the environment impacts and accumulation of plastics. If plastics can be made to degrade more effectively by microbes, it will reduce solid waste problems which raise a plethora of environmental concerns. Microbial enzymes are crucial to biodegradation of plastics. There is a great potential and scope in exploring these microbes and tailoring according to specific needs like to grow in varied environmental conditions, for better utilization of plastics carbon for their energy needs.
8 Future Perspective
The microbial depolymerization of synthetic polymers is a multifaceted phenomenon. Strict nature-like experiments are difficult to carry out in laboratory settings because of number of parameters occurring during the biogeochemical recycling which cannot be entirely replicated and directed in vitro. Information available so far regarding microbial ability for degrading synthetic plastics is build on a few bacteria (representing < 0.1% of total bacteria) that could grow on culture media. Thus, high diversity of microorganisms present in nature has not been even partially explored yet. Making use of molecular technologies such as genomics, proteomics, transcriptomics, metabolomics makes it feasible to discover novel microorganisms (even non-culturable ones) involved in plastic degradation and investigate properties of microorganisms arising from the interplay of proteins, genes, other macro–micro molecules and the environment.
9 Conclusion
Polymers are generally resistant toward biodegradation. Physicochemical methods of degradation have been more successful than biological ones. However, exploration of microbial depolymerization is a step toward plastics management by sustainable method.
References
Abou-Zeid DM, Müller RJ, Deckwer WD (2001) Degradation of natural and synthetic polyesters under anaerobic conditions. J Biotechnol 86(2):113–126
Abrusci C, Pablos JL, Corrales T, López-Marín J, Marín I, Catalina F (2011) Biodegradation of photo-degraded mulching films based on polyethylenes and stearates of calcium and iron as pro-oxidant additives. Int Biodeterior Biodegradation 65(3):451–459
Akutsu Y, Nakajima-Kambe T, Nomura N, Nakahara T (1998) Purification and properties of a polyester polyurethane-degrading enzyme from Comamonas acidovorans TB-35. Appl Environ Microbiol 64(1):62–67
Alariqi SA, Kumar AP, Rao BSM, Singh RP (2006) Biodegradation of γ-sterilised biomedical polyolefins under composting and fungal culture environments. Polym Degrad Stab 91(5):1105–1116
Albertsson AC, Huang SJ (eds) (1995) Degradable polymers, recycling, and plastics waste management. Marcel Dekker, New york
Albertsson AC, Andersson SO, Karlsson S (1987) The mechanism of biodegradation of polyethylene. Polym Degrad Stab 18(1):73–87
Alexander M (1977) Introduction to soil microbiology 2edn. Wiley, US
Ali MI, Ahmed S, Robson G, Javed I, Ali N, Atiq N, Hameed A (2014) Isolation and molecular characterization of polyvinyl chloride (PVC) plastic degrading fungal isolates. J Basic Microbiol 54(1):18–27
Allen NS, Edge M, Mourelatou D, Wilkinson A, Liauw CM, Parellada MD, Barrio JA, Santa Quiteria VR (2003) Influence of ozone on styrene–ethylene–butylene–styrene (SEBS) copolymer. Polym Degrad Stab 79(2):297–307
Arutchelvi J, Sudhakar M, Arkatkar A, Doble M, Bhaduri S, Uppara PV (2008) Biodegradation of polyethylene and polypropylene. Indian J Biotechnol 7:9–22
Arvanitoyannis I, Biliaderis CG, Ogawa H, Kawasaki N (1998) Biodegradable films made from low-density polyethylene (LDPE), rice starch and potato starch for food packaging applications: Part 1. Carbohyd Polym 36(2–3):89–104
Aswale P, Ade A (2008) Assessment of the biodegradation of polythene. Bioinfolet 5:239
Augusta J, Müller RJ, Widdecke H (1993) A rapid evaluation plate-test for the biodegradability of plastics. Appl Microbiol Biotechnol 39(4–5):673–678
Babu RP, O’connor K, Seeram R (2013) Current progress on bio-based polymers and their future trends. Prog Biomater 2(1):8
Barkay T, Navon-Venezia S, Ron EZ, Rosenberg E (1999) Enhancement of solubilization and biodegradation of polyaromatic hydrocarbons by the bioemulsifier alasan. Appl Environ Microbiol 65(6):2697–2702
Basnett P, Knowles JC, Pishbin F, Smith C, Keshavarz T, Boccaccini AR, Roy I (2012) Novel biodegradable and biocompatible Poly (3-hydroxyoctanoate)/bacterial cellulose composites. Adv Eng Mater 14(6):B330–B343
Bellia G, Tosin M, Floridi G, Degli-Innocenti F (1999) Activated vermiculite, a solid bed for testing biodegradability under composting conditions. Polym Degrad Stab 66(1):65–79
Beltrametti F, Marconi AM, Bestetti G, Colombo C, Galli E, Ruzzi M, Zennaro E (1997) Sequencing and functional analysis of styrene catabolism genes from Pseudomonas fluorescens ST. Appl Environ Microbiol 63(6):2232–2239
Benedict CV, Cameron JA, Huang SJ (1983) Polycaprolactone degradation by mixed and pure cultures of bacteria and a yeast. J Appl Polym Sci 28(1):335–342
Bhardwaj H, Gupta R, Tiwari A (2013) Communities of microbial enzymes associated with biodegradation of plastics. J Polym Environ 21(2):575–579
Bonhomme S, Cuer A, Delort AM, Lemaire J, Sancelme M, Scott G (2003) Environmental biodegradation of polyethylene. Polym Degrad Stab 81(3):441–452
Bovey FA, Winslow FH (1979) Macromolecules: an introduction to polymer science. Academic, New York
Calabia BP, Tokiwa Y (2004) Microbial degradation of poly (D-3-hydroxybutyrate) by a new thermophilic Streptomyces isolate. Biotech Lett 26(1):15–19
Carrasco F (1996) Thermogravimetric analysis of polystyrene: influence of sample weight and heating rate on thermal and kinetic parameters. J Appl Polym Sci 61(1):187–197
Caruso G (2015) Plastic degrading microorganisms as a tool for bioremediation of plastic contamination in aquatic environments. J. Pollut Eff Cont 3:112
Chaisu K, Siripholvat V, Chiu CH (2015) New method of rapid and simple colorimetric assay for detecting the enzymatic degradation of poly lactic acid plastic films. Int J Life Sci Biotechnol Pharm 4(1):57–61
Chambliss WG (1983) The forgotten dosage form: enteric-coated tablets. Pharm Technol 7(9):124–140
Chandra R, Rustgi R (1997) Biodegradation of maleated linear low-density polyethylene and starch blends. Polym Degrad Stab 56(2):185–202
Chandra R, Rustgi R (1998) Biodegradable polymers. Prog Polym Sci 23(7):1273–1335
Chowdhury AA (1963) Poly-β-hydroxybuttersäure Abbauende Bakterien und Exoenzym. Arch Microbiol 47(2):167–200
Das MP, Kumar S (2015) An approach to low-density polyethylene biodegradation by Bacillus amyloliquefaciens. 3 Biotech 5(1):81–86
David C, De Kesel C, Lefebvre F, Weiland M (1994) The biodegradation of polymers: recent results. Macromol Mater Eng 216(1):21–35
Degli-Innocenti F, Tosin M, Bastioli C (1998) Evaluation of the biodegradation of starch and cellulose under controlled composting conditions. J Polym Environ 6(4):197–202
Deguchi T, Kakezawa M, Nishida T (1997) Nylon biodegradation by lignin-degrading fungi. Appl Environ Microbiol 63(1):329–331
Deguchi T, Kitaoka Y, Kakezawa M, Nishida T (1998) Purification and characterization of a nylon-degrading enzyme. Appl Environ Microbiol 64(4):1366–1371
Devi RS, Kannan VR, Nivas D, Kannan K, Chandru S, Antony AR (2015) Biodegradation of HDPE by Aspergillus spp. from marine ecosystem of Gulf of Mannar, India. Mar Pollut Bull 96(1):32–40
Donald V, Voet Judith G, Pratt Charlotte W (2006) Fundamentals of Biochemistry Life at the Molecular Level. Wiley, New york
Donelli I, Freddi G, Nierstrasz VA, Taddei P (2010) Surface structure and properties of poly-(ethylene terephthalate) hydrolyzed by alkali and cutinase. Polym Degrad Stab 95(9):1542–1550
Duddu MK, Tripura KL, Guntuku G, Divya DS (2015) Biodegradation of low density polyethylene (LDPE) by a new biosurfactant-producing thermophilic Streptomyces coelicoflavus NBRC 15399T. Afr J Biotech 14(4):327–340
Edmonds P, Cooney JJ (1968) Microbial growth in a fuel-water system containing polyesterurethane foam. Appl Microbiol 16(2):426
Elashmawi IS, Hakeem NA, Abdelrazek EM (2008) Spectroscopic and thermal studies of PS/PVAc blends. Physica B 403(19):3547–3552
Erlandsson B, Karlsson S, Albertsson AC (1997) The mode of action of corn starch and a pro-oxidant system in LDPE: influence of thermo-oxidation and UV-irradiation on the molecular weight changes. Polym Degrad Stab 55(2):237–245
Expandable Polystyrene Molders Association (2004) Test initiatives highlights EPS performance. EPS Newsline 8:1–8
Flavel BS, Shapter JG, Quinton JS (2006, July). Nanosphere lithography using thermal evaporation of gold. In: International Conference on IEEE Nanoscience and Nanotechnology (ICONN’06)
Flemming HC (1998) Relevance of biofilms for the biodeterioration of surfaces of polymeric materials. Polym Degrad Stab 59(1–3):309–315
Fuhs GW (1961) Der mikrobielle Abbau von Kohlenwasserstoffen. Arch Microbiol 39(4):374–422
Gajanand E, Soni LK, Dixit VK (2014) Biodegradable polymers: a smart strategy for today’s crucial needs. Crit Rev Environ Sci Technol 3
Gajendiran A, Krishnamoorthy S, Abraham J (2016) Microbial degradation of low-density polyethylene (LDPE) by Aspergillus clavatus strain JASK1 isolated from landfill soil. 3 Biotech 6(1):1–6
Garlotta D (2001) J Polym Environ 9:63
Gautam R, Bassi AS, Yanful EK (2007) A review of biodegradation of synthetic plastic and foams. Appl Biochem Biotechnol 141(1):85–108
Ghosh RN, Ray BC (2004) Optimized print properties on starch blended and surface grafted polyethylene films for biodegradable packaging. J Polym Mater 21(4):425–437
Gimouhopoulos K, Doulia D, Vlyssides A, Georgiou D (2000) Optimization studies of waste plastics-lignite catalytic coliquefaction. Waste Manage Res 18(4):352–357
Gowariker VR, Viswanathan NV, Sreedhar J (2000) Polymer science. New Age International (P) Limited Publishers, New Delhi, India, pp 263–290
Gross RA (1993) Cellulose acetate biodegradability in simulated aerobic composting and anaerobic bioreactors as well as by a bacterial isolate derived from compost. In: Fundamentals of biodegradable materials and packaging, pp 257–279
Gross RA, Gu JD, Eberiel D, McCarthy SP (1995) Laboratory-scale composting test methods to determine polymer biodegradability: model studies on cellulose acetate. J Macromol Sci Part A Pure Appl Chem 32(4):613–628
Grover A, Gupta A, Chandra S, Kumari A, Khurana SP (2015) Polythene and environment. Int J Environ Sci 5(6):1091
Gu JD (2003) Microbiological deterioration and degradation of synthetic polymeric materials: recent research advances. Int Biodeterior Biodegradation 52(2):69–91
Gu JG, Gu JD (2005) Methods currently used in testing microbiological degradation and deterioration of a wide range of polymeric materials with various degree of degradability: a review. J Polym Environ 13(1):65–74
Gu JD, Ford TE, Mitton DB, Mitchell R (2000) Microbial degradation and deterioration of polymeric materials. In: Revie W (ed) The Uhlig Corrosion Handbook, vol 2. Wiley, New York, pp 439–460
Hadad D, Geresh S, Sivan A (2005) Biodegradation of polyethylene by the thermophilic bacterium Brevibacillus borstelensis. J Appl Microbiol 98(5):1093–1100
Hazen TC, Dubinsky EA, DeSantis TZ, Andersen GL, Piceno YM, Singh N, Jansson JK, Probst A, Borglin SE, Fortney JL, Stringfellow WT (2010) Deep-sea oil plume enriches indigenous oil-degrading bacteria. Science 330(6001):204–208
Hendrickson L, Connole KB (1995) Review of stabilization of polyolefin insulated conductors. Part I: theory and factors affecting stability. Polym Eng Sci 35(2):211–217
Hermanová S, Šmejkalová P, Merna J, Zarevúcka M (2015) Biodegradation of waste PET based copolyesters in thermophilic anaerobic sludge. Polym Degrad Stab 111:176–184
Heumann S, Eberl A, Pobeheim H, Liebminger S, Fischer-Colbrie G, Almansa E, Cavaco-Paulo A, Gübitz GM (2006) New model substrates for enzymes hydrolysing polyethyleneterephthalate and polyamide fibres. J Biochem Biophys Methods 69(1):89–99
Ho KLG, Pometto AL, Gadea-Rivas A, Briceño JA, Rojas A (1999) Degradation of polylactic acid (PLA) plastic in Costa Rican soil and Iowa state university compost rows. J Polym Environ 7(4):173–177
Hoang KC, Tseng M, Shu WJ (2007) Degradation of polyethylene succinate (PES) by a new thermophilic Microbispora strain. Biodegradation 18(3):333–342
Hoffmann J, Řezníčèková I, Vaňòková S, Kupec J (1997) Manometric determination of biological degradability of substances poorly soluble in aqueous environments. Int Biodeterior Biodegradation 39(4):327–332
Holland KA, Rae ID (1987) Thermal degradation of polymers. III. Thermal degradation of a compound which models the head-to-head linkage in poly (methyl methacrylate). Aust J Chem 40(4):687–692
Howard GT, Mackie RI, Cann IKO, Ohene-Adjei S, Aboudehen KS, Duos BG, Childers GW (2007) Effect of insertional mutations in the pueA and pueB genes encoding two polyurethanases in Pseudomonas chlororaphis contained within a gene cluster. J Appl Microbiol 103(6):2074–2083
Howard GT (2012) Polyurethane biodegradation. In: Microbial degradation of xenobiotics. Berlin Heidelberg, Springer, pp 371–394
Huang JC, Shetty AS, Wang MS (1990) Biodegradable plastics: a review. Adv Polym Technol 10(1):23–30
Ikada E (1999) Electron microscope observation of biodegradation of polymers. J Environ Polym Degrad 7(4):197–201
Inoue T, Miyazaki M, Kamitani M, Kano J, Saito F (2004) Mechanochemicaldechlorination of polyvinyl chloride by co-grinding with various metal oxides. Adv Powder Technol 15(2):215–225
Ishigaki T, Kawagoshi Y, Ike M, Fujita M (1999) Biodegradation of a polyvinyl alcohol-starch blend plastic film. World J Microbiol Biotechnol 15(3):321–327
Jakubowicz I (2003) Evaluation of degradability of biodegradable polyethylene (PE). Polym Degrad Stab 80(1):39–43
Jarerat A, Tokiwa Y (2001) Degradation of poly (tetramethylene succinate) by thermophilic actinomycetes. Biotech Lett 23(8):647–651
Jen-hou L, Schwartz A (1961) Zum Verhalten von bakteriengemischen gegentiber polyfithylen verschiedenen mittleren Molekulargewichts. Kunststoffe 51:317–320
Kakudo SHINJI, Negoro SEIJI, Urabe ITARU, Okada HIROSUKE (1993) Nylon oligomer degradation gene, nylC, on plasmid pOAD2 from a Flavobacterium strain encodes endo-type 6-aminohexanoate oligomer hydrolase: purification and characterization of the nylC gene product. Appl Environ Microbiol 59(11):3978–3980
Kale G, Auras R, Singh SP (2006) Degradation of commercial biodegradable packages under real composting and ambient exposure conditions. J Polym Environ 14(3):317–334
Kanagawa K, Negoro S, Takada N, Okada H (1989) Plasmid dependence of Pseudomonas sp. strain NK87 enzymes that degrade 6-aminohexanoate-cyclic dimer. J Bacteriol 171(6):3181–3186
Kanagawa K, Oishi M, Negoro S, Urabe I, Okada H (1993) Characterization of the 6-aminohexanoate-dimer hydrolase from Pseudomonas sp. NK87. Microbiology 139(4):787–795
Kefeli AA, Razumovskii SD, Zaikov GY (1971) Interaction of polyethylene with ozone. Polym Sci USSR 13(4):904–911
Kinoshita S, Negoro S, Muramatsu M, Bisaria VS, Sawada S, Okada H (1977) 6‐Aminohexanoic acid cyclic dimer hydrolase. A new cyclic amide hydrolase produced by Acromobacter guttatus KI72. Eur J Biochem 80(2):489–495
Kinoshita S, Terada T, Taniguchi T, Takene Y, Masuda S, Matsunaga N, Okada H (1981) Purification and characterization of 6-Aminohexanoic-Acid-Oligomer Hydrolase of Flavobacterium sp. KI72. Eur J Biochem 116(3):547–551
Kırbaş Z, Keskin N, Güner A (1999) Biodegradation of polyvinylchloride (PVC) by white rot fungi. Bull Environ Contam Toxicol 63(3):335–342
Kleeberg I, Hetz C, Kroppenstedt RM, Müller RJ, Deckwer WD (1998) Biodegradation of aliphatic-aromatic copolyesters by Thermomonospora fusca and other thermophilic compost isolates. Appl Environ Microbiol 64(5):1731–1735
Kleeberg I, Welzel K, VandenHeuvel J, Müller RJ, Deckwer WD (2005) Characterization of a new extracellular hydrolase from Thermobifida fusca degrading aliphatic–aromatic copolyesters. Biomacromolecules 6(1):262–270
Koshland DE (1995) The key–lock theory and the induced fit theory. Angew Chem Int Ed Engl 33(23–24):2375–2378
Koutny M, Lemaire J, Delort AM (2006a) Biodegradation of polyethylene films with prooxidant additives. Chemosphere 64(8):1243–1252
Koutny M, Sancelme M, Dabin C, Pichon N, Delort AM, Lemaire J (2006b) Acquired biodegradability of polyethylenes containing pro-oxidant additives. Polym Degrad Stab 91(7):1495–1503
Kyrikou I, Briassoulis D (2007) Biodegradation of agricultural plastic films: a critical review. J Polym Environ 15(2):125–150
Lee SY (1996) Bacterial polyhydroxyalkanoates. Biotechnol Bioeng 49(1):1–14
Lenz R (1993) Biodegradable polymers. Biopolymers I 1–40
Liang TW, Jen SN, Nguyen AD, Wang SL (2016) Application of Chitinous materials in production and purification of a Poly (L-lactic acid) depolymerase from Pseudomonas tamsuii TKU015. Polymers 8(3):98
Lim HA, Raku T, Tokiwa Y (2005) Hydrolysis of polyesters by serine proteases. Biotech Lett 27(7):459–464
Lin YH, Yen HY (2005) Fluidised bed pyrolysis of polypropylene over cracking catalysts for producing hydrocarbons. Polym Degrad Stab 89(1):101–108
Liu C, Zeng JB, Li SL, He YS, Wang YZ (2012) Improvement of biocompatibility and biodegradability of poly (ethylene succinate) by incorporation of poly (ethylene glycol) segments. Polymer 53(2):481–489
Loredo-Treviño A, Gutiérrez-Sánchez G, Rodríguez-Herrera R, Aguilar CN (2012) Microbial enzymes involved in polyurethane biodegradation: a review. J Polym Environ 20(1):258–265
Lugauskas A, Levinskait L, Pečiulyt D (2003) Micromycetes as deterioration agents of polymeric materials. Int Biodeterior Biodegradation 52(4):233–242
Ma A, Wong Q (2013) Identification of esterase in Aspergillus flavus during degradation of polyester polyurethane 1. Can Young Sci J 2013(2):24–31
Mahalakshmi V, Siddiq A, Andrew SN (2012) Analysis of polyethylene degrading potentials of microorganisms isolated from compost soil. Int J Pharm Biol Arch 3(5):1190–1196
Marcilla A, Gómez A, Menargues S, Garcı́a-Martı́nez J, Cazorla-Amorós D (2003) Catalytic cracking of ethylene-vinyl acetate copolymers: comparison of different zeolites. J Anal Appl Pyrol 68:495–506
Marconi AM, Beltrametti F, Bestetti G, Solinas F, Ruzzi M, Galli E, Zennaro E (1996) Cloning and characterization of styrene catabolism genes from Pseudomonas fluorescens ST. Appl Environ Microbiol 62(1):121–127
Masaki K, Kamini NR, Ikeda H, Iefuji H (2005) Cutinase-like enzyme from the yeast Cryptococcus sp. strain S-2 hydrolyzes polylactic acid and other biodegradable plastics. Appl Envir Microbiol 71(11):7548–7550
Massardier-Nageotte V, Pestre C, Cruard-Pradet T, Bayard R (2006) Aerobic and anaerobic biodegradability of polymer films and physico-chemical characterization. Polym Degrad Stab 91(3):620–627
McCarthy SP, Gada M, Smith GP, Tolland V, Press B, Eberiel D, Bruell C, Gross R (1992) The accelerated biodegradability of plastic materials in simulated compost and landfill environments. ANTEC 92–Shaping Future 1:816–818
McCartin SM, Press B, Eberiel D, McCarthy SP, Kaplan D, Meyer J (1990) Simulated landfill study on the accelerated biodegradability of plastics materials. Polym Prepr Div Polym Chem Am Chem Soc 440
Mehmood CT, Qazi IA, Hashmi I, Bhargava S, Deepa S (2016) Biodegradation of low density polyethylene (LDPE) modified with dye sensitized titania and starch blend using Stenotrophomonas pavanii. Int Biodeterior Biodegradation 113:276–286
Meyer TJ, Caspar JV (1985) Photochemistry of metal-metal bonds. Chem Rev Washington, DC, United States, p 187–218
Milstein O, Gersonde R, Huttermann A, Frund R, Feine HJ, Ludermann HD, Chen MJ, Meister JJ (1994) Infrared and nuclear magnetic resonance evidence of degradation in thermoplastics based on forest products. J Polym Environ 2(2):137–152
Mitchell R (1996) Hazards to space missions from microbial biofilms. Biodeterior Biodegradation 133:3–16
Mohamed A, Gordon SH, Biresaw G (2007) Polycaprolactone/polystyrene bioblends characterized by thermogravimetry, modulated differential scanning calorimetry and infrared photoacoustic spectroscopy. Polym Degrad Stab 92(7):1177–1185
Mohan K (2011) Microbial deterioration and degradation of polymeric materials. J Biochem Technol 2(4):210–215
Mohanty AK, Misra M, Hinrichsen G (2000) Biofibres, biodegradable polymers and biocomposites: an overview. Macromol Mater Eng 276(1):1–24
Motiwalla MJ, Punyarthi PP, Mehta MK, D’Souza JS, Kelkar-Mane V (2013) Studies on degradation efficiency of polycaprolactone by a naturally-occurring bacterium. J Env Bio 34:43–49
Muenmee S, Chiemchaisri W, Chiemchaisri C (2015) Microbial consortium involving biological methane oxidation in relation to the biodegradation of waste plastics in a solid waste disposal open dump site. Int Biodeterior Biodegradation 102:172–181
Muenmee S, Chiemchaisri W, Chiemchaisri C (2016) Enhancement of biodegradation of plastic wastes via methane oxidation in semi-aerobic landfill. Int Biodeterior Biodegradation 113:244–255
Murphy CA, Cameron JA, Huang SJ, Vinopal RT (1996) Fusariumpolycaprolactonedepolymerase is cutinase. Appl Environ Microbiol 62(2):456–460
Murthy KS, Enders NA, Mahjour M, Fawzi MB (1986) A comparative evaluation of aqueous enteric polymers in capsule coating. Pharm Technol 10(10):36–46
Nagai Y, Nakamura D, Miyake T, Ueno H, Matsumoto N, Kaji A, Ohishi F (2005) Photodegradation mechanisms in poly (2, 6-butylenenaphthalate-co-tetramethyleneglycol)(PBN–PTMG). I: influence of the PTMG content. Polym Degrad Stab 88(2):251–255
Nagarajan V, Singh M, Kane H, Khalili M, Bramucci M (2006) Degradation of a terephthalate-containing polyester by a thermophilic microbial consortium. J Polym Environ 14(3):281–287
Nakajima-Kambe T, Shigeno-Akutsu Y, Nomura N, Onuma F, Nakahara T (1999) Microbial degradation of polyurethane, polyester polyurethanes and polyether polyurethanes. Appl Microbiol Biotechnol 51(2):134–140
Nakamiya K, Ooi T, Kinoshita S (1997) Non-heme hydroquinone peroxidase from Azotobacter beijerinckii HM121. J Ferment Bioeng 84(1):14–21
Narayan R (1993) Biodegradation of polymeric materials (anthropogenic macromolecules) during composting. In: Science and engineering of composting: design, environmental, microbiological and utilization aspects. Washington, OH, Renaissance Publishers, pp 339–62
National Research Council (1993) In Situ bioremediation when does it work?. National Academy Press, Washington, DC, p 224
Negoro SEIJI, Kakudo SHINJI, Urabe I, Okada HIROSUKE (1992) A new nylon oligomer degradation gene (nylC) on plasmid pOAD2 from a Flavobacterium sp. J Bacteriol 174(24):7948–7953
Nishida H, Tokiwa Y (1993) Distribution of poly (β-hydroxybutyrate) and poly (ε-caprolactone) aerobic degrading microorganisms in different environments. J Polym Environ 1(3):227–233
Nishida H, Suzuki S, Tokiwa Y (1998) Distribution of poly (β-propiolactone) aerobic degrading microorganisms in different environments. J Environ Polym Degrad 6(1):43–58
Nomura N, Deguchi T, Shigeno-Akutsu Y, Nakajima-Kambe T, Nakahara T (2001) Gene Structures and Catalytic Mechanisms of Microbial Enzymes Able to Blodegrade the Synthetic Solid Polymers Nylon and Polyester Polyurethaoe. Biotech Gene Eng Rev 18(1):125–147
Nowak B, Pająk J, Łabużek S, Rymarz G, Talik E (2011) Biodegradation of poly (ethylene terephthalate) modified with polyester “Bionolle®” by Penicillium funiculosum. Polimery 56(1):35–44
Nwachukwu S, Obidi O, Odocha C (2010) Occurrence and recalcitrance of polyethylene bag waste in Nigerian soils. Afr J Biotech 9(37):6096–6104
Oda Y, Asari H, Urakami T, Tonomura K (1995) Microbial degradation of poly (3-hydroxybutyrate) and polycaprolactone by filamentous fungi. J Ferment Bioeng 80(3):265–269
Odusanya SA, Nkwogu JV, Alu N, Udo GE, Ajao JA, Osinkolu GA, Uzomah AC (2013) Preliminary studies on microbial degradation of plastics used in packaging potable water in Nigeria. Niger Food J 31(2):63–72
Ohtaki A, Sato N, Nakasaki K (1998) Biodegradation of poly-ε-caprolactone under controlled composting conditions. Polym Degrad Stab 61(3):499–505
Omichi H (1992) Handbook of Polymer Degradation. In: Hamid SH, Amin MB, Maadhah AG (eds) Marcel Dekker, New york, pp 335–344
Orhan Y, Hrenovic J, Buyukgungor H (2004) Biodegradation of plastic compost bags under controlled soil conditions. Acta Chimica Slovenica 51(3):579–588
Orr IG, Hadar Y, Sivan A (2004) Colonization, biofilm formation and biodegradation of polyethylene by a strain of Rhodococcus ruber. Appl Microbiol Biotechnol 65(1):97–104
Pagga U, Beimborn DB, Boelens J, De Wilde B (1995) Determination of the aerobic biodegradability of polymeric material in a laboratory controlled composting test. Chemosphere 31(11–12):4475–4487
Pantke M, Seal K (1990) An interlaboratory investigation into the biodeterioration testing of plastics, with special reference to polyurethanes. Part 2: soil burial experiments. Material und Organismen 25(2):87–98
Park H, Park K, Shalaby WS (2011) Biodegradable hydrogels for drug delivery. CRC Press
Peng X, Shen J (1999) Preparation and biodegradability of polystyrene having pyridinium group in the main chain. Eur Polymer J 35(9):1599–1605
Pinzari F, Pasquariello G, De Mico A (2006 April) Biodeterioration of paper: a SEM study of fungal spoilage reproduced under controlled conditions. Macromolecular Symposia 238(1):57–66, WILEY‐VCH Verlag
Pometto AL, Lee BT, Johnson KE (1992) Production of an extracellular polyethylene-degrading enzyme (s) by Streptomyces species. Appl Environ Microbiol 58(2):731–733
Pospíšil J, Pilař J, Billingham NC, Marek A, Horak Z, Nešpůrek S (2006) Factors affecting accelerated testing of polymer photostability. Polym Degrad Stab 91(3):417–422
Potts JE (1978) In: Grayson M (ed) Kirk-Othmer, Encyclopedia of Chemical Technology, Wiley-Interscience, New York, pp 626–668
Pramila R, Padmavathy K, Ramesh KV, Mahalakshmi K (2012) Brevibacillus parabrevis, Acinetobacter baumannii and Pseudomonas citronellolis-Potential candidates for biodegradation of low density polyethylene (LDPE). Afr J Bacteriol Res 4(1):9–14
Pranamuda H, Tokiwa Y, Tanaka H (1995) Microbial degradation of an aliphatic polyester with a high melting point, poly (tetramethylene succinate). Appl Environ Microbiol 61(5):1828–1832
Pranamuda H, Tokiwa Y, Tanaka H (1997) Polylactide degradation by an Amycolatopsis sp. Appl Environ Microbiol 63(4):1637–1640
Prema S, Uma MDP (2013) Degradation of poly lactide plastic by mesophilic bacteria isolated from compost. Int J Res Purif Appl Microbiol 3(4):121–126
Puechner P, Mueller WR, Bardtke D (1995) Assessing the biodegradation potential of polymers in screening-and long-term test systems. J Environ Polym Degrad 3(3):133–143
Qiu Z, Ikehara T, Nishi T (2003) Crystallization behaviour of biodegradable poly (ethylene succinate) from the amorphous state. Polymer 44(18):5429–5437
Ramis X, Cadenato A, Salla JM, Morancho JM, Valles A, Contat L, Ribes A (2004) Thermal degradation of polypropylene/starch-based materials with enhanced biodegradability. Polym Degrad Stab 86(3):483–491
Restrepo-Flórez JM, Bassi A, Thompson MR (2014) Microbial degradation and deterioration of polyethylene—a review. Int Biodeterior Biodegradation 88:83–90
Ronkvist ÅM, Xie W, Lu W, Gross RA (2009) Cutinase-catalyzed hydrolysis of poly (ethylene terephthalate). Macromolecules 42(14):5128–5138
Rutkowska M, Heimowska A, Krasowska K, Janik H (2002) Biodegradability of polyethylene starch blends in sea water. Pol J Environ Stud 11(3):267–272
Salgado CL, Sanchez E, Zavaglia CA, Granja PL (2012) Biocompatibility and biodegradation of polycaprolactone-sebacic acid blended gels. J Biomed Mater Res, Part A 100(1):243–251
Sanchez JG, Tsuchii A, Tokiwa Y (2000) Degradation of polycaprolactone at 50 °C by a thermotolerant Aspergillus sp. Biotech Lett 22(10):849–853
Sand W (2003) Microbial life in geothermal waters. Geothermics 32(4):655–667
Santo M, Weitsman R, Sivan A (2013) The role of the copper-binding enzyme–laccase–in the biodegradation of polyethylene by the actinomycete Rhodococcus ruber. Int Biodeterior Biodegradation 84:204–210
Schink B, Janssen PH, Frings J (1992) Microbial degradation of natural and of new synthetic polymers. FEMS Microbiol Rev 9(2–4):311–316
Schlemmer D, Sales MJ, Resck IS (2009) Degradation of different polystyrene/thermoplastic starch blends buried in soil. Carbohyd Polym 75(1):58–62
Schmidt-Naake G, Drache M, Weber M (2002) Combination of mechanochemical degradation of polymers with controlled free-radical polymerization. Macromol Chem Phys 203(15):2232–2238
Scott G (1984) Mechano-chemical degradation and stabilization of polymers. Polym Eng Sci 24(13):1007–1020
Sekiguchi T, Ebisui A, Nomura K, Watanabe T, Enoki M, Kanehiro H (2009) Biodegradability evaluation of several fibers soaked into deep sea and isolation of the biodegradable plastic degrading bacteria from deepocean water. Nippon Suisan Gakkaishi 75:1011–1018
Seretoudi G, Bikiaris D, Panayiotou C (2002) Synthesis, characterization and biodegradability of poly (ethylene succinate)/poly (ε-caprolactone) block copolymers. Polymer 43(20):5405–5415
Seymour Raymond B (1971) Additives for polymers, introduction to polymer chemistry. McGraw-Hill Book Company, New York, pp 268–271
Shah AA, Hasan F, Hameed A, Ahmed S (2008) Biological degradation of plastics: a comprehensive review. Biotechnol Adv 26(3):246–265
Shahnawaz M, Sangale MK, Ade AB (2016) Rhizosphere of Avicennia marina. Environ Sci Pollut Res 23(14):14621–14635
Sharabi NED, Bartha RICHARD (1993) Testing of some assumptions about biodegradability in soil as measured by carbon dioxide evolution. Appl Environ Microbiol 59(4):1201–1205
Sharon C, Sharon M (2017) Studies on biodegradation of polyethylene terephthalate: a synthetic polymer. J Microbiol Biotechnol Res 2(2):248–257
Sheik S, Chandrashekar KR, Swaroop K, Somashekarappa HM (2015) Biodegradation of gamma irradiated low density polyethylene and polypropylene by endophytic fungi. Int Biodeterior Biodegradation 105:21–29
Shigeno-Akutsu Y, Nakajima-Kambe T, Nomura N, Nakahara T (1999) Purification and properties of culture-broth-secreted esterase from the polyurethane degrader Comamonas acidovorans TB-35. J Biosci Bioeng 88(5):484–487
Shimao M (2001) Biodegradation of plastics. Curr Opin Biotechnol 12(3):242–247
Singh B, Sharma N (2008) Mechanistic implications of plastic degradation. Polym Degrad Stab 93(3):561–584
Slater SC, Voige WH, Dennis DE (1988) Cloning and expression in Escherichia coli of the Alcaligenes eutrophus H16 poly-beta-hydroxybutyrate biosynthetic pathway. J Bacteriol 170(10):4431–4436
Smith GP, Press B, Eberiel D, McCarthy SP, Gross RA, Kaplan DL (1990) Accelerated in-laboratory test to evaluate the degradation of plastics in landfill environments. In: Polymeric Materials Science and Engineering, Proceedings of the ACS Division of Polymeric Materials Science and Engineering, vol 63, pp 862–866
Stevens ES (2003) What makes green plastics green? Biocycle 44(3):24–27
Sukhumaporn S, Tokuyama S, Kitpreechavanich V (2012) Poly (L-Lactide)- degrading enzyme production by Actinomadura keratinilyticaT16-1 in 3 L airlift bioreactor and its degradation ability for biological. J Microbiol Biotechnol 22(1):92–99
Suzuki J, Hukushima K, Suzuki S (1978) Effect of ozone treatment upon biodegradability of water-soluble polymers. Environ Sci Technol 12(10):1180–1183
Takamoto T, Uyama H, Kobayashi S (2001) Lipase-catalyzed degradation of polyester in supercritical carbon dioxide. Macromol Biosci 1(5):215–218
Tang L, Wu Q, Qu B (2005) The effects of chemical structure and synthesis method on photodegradation of polypropylene. J Appl Polym Sci 95(2):270–279
Tezuka Y, Ishii N, Kasuya KI, Mitomo H (2004) Degradation of poly (ethylene succinate) by mesophilic bacteria. Polym Degrad Stab 84(1):115–121
Thomas BT, Olanrewaju-Kehinde DSK, Popoola OD, James ES (2015) Degradation of plastic and polythene materials by some selected microorganisms isolated from soil. World Appl Sci J 33(12):1888–1891
Tokiwa Y, Calabia BP (2006) Biodegradability and biodegradation of poly (lactide). Appl Microbiol Biotechnol 72(2):244–251
Tokiwa Y, Suzuki T (1974) Degradation of poly-ethylene glycol adipate by a fungus. J Ferment Technol
Tokiwa Y, Suzuki T (1977a) Purification and some properties of polyethylene adipate-degrading enzyme produced by Penicillium sp. Strain 14–3. Agric Biol Chem 41(2):265–274
Tokiwa Y, Suzuki T (1977b) Hydrolysis of polyesters by lipases. Nature 270(5632):76–78
Tokiwa Y, Suzuki T (1978) Hydrolysis of polyesters by Rhizopus delemar lipase. Agric Biol Chem 42(5):1071–1072
Tokiwa Y, Suzuki T (1981) Hydrolysis of copolyesters containing aromatic and aliphatic ester blocks by lipase. J Appl Polym Sci 26(2):441–448
Tokiwa Y, Suzuki T, Ando T (1979) Synthesis of copolyamide–esters and some aspects involved in their hydrolysis by lipase. J Appl Polym Sci 24(7):1701–1711
Tokiwa Y, Calabia BP, Ugwu CU, Aiba S (2009) Biodegradability of plastics. Int J Mol Sci 10(9):3722–3742
Tomita K, Ikeda N, Ueno A (2003) Isolation and characterization of a thermophilic bacterium, Geobacillus thermocatenulatus, degrading nylon 12 and nylon 66. Biotech Lett 25(20):1743–1746
Tosin M, Degli-Innocenti F, Bastioli C (1996) Effect of the composting substrate on biodegradation of solid materials under controlled composting conditions. J Polym Environ 4(1):55–63
Tribedi P, Sil AK (2013) Founder effect uncovers a new axis in polyethylene succinate bioremediation during biostimulation. FEMS Microbiol Lett 346(2):113–120
Tribedi P, Sil AK (2014) Cell surface hydrophobicity: a key component in the degradation of polyethylene succinate by Pseudomonas sp. AKS2. J Appl Microbiol 116(2):295–303
Tribedi P, Gupta AD, Sil AK (2015) Adaptation of Pseudomonas sp. AKS2 in biofilm on low-density polyethylene surface: an effective strategy for efficient survival and polymer degradation. Bioresources Bioprocess 2(1):14
Tseng M, Hoang KC, Yang MK, Yang SF, Chu WS (2007) Polyester-degrading thermophilicactinomycetes isolated from different environment in Taiwan. Biodegradation 18(5):579–583
Tsuji H, Miyauchi S (2001) Poly (L-lactide): VI Effects of crystallinity on enzymatic hydrolysis of poly (L-lactide) without free amorphous region. Polym Degrad Stab 71(3):415–424
Tuominen J, Kylmä J, Kapanen A, Venelampi O, Itävaara M, Seppälä J (2002) Biodegradation of lactic acid based polymers under controlled composting conditions and evaluation of the ecotoxicological impact. Biomacromol 3(3):445–455
Walter T, Augusta J, Müller RJ, Widdecke H, Klein J (1995) Enzymatic degradation of a model polyester by lipase from Rhizopus delemar. Enzym Microb Technol 17(3):218–224
Webb HK, Crawford RJ, Sawabe T, Ivanova EP (2009) Poly (ethylene terephthalate) polymer surfaces as a substrate for bacterial attachment and biofilm formation. Microbes Environ 24(1):39–42
Webb HK, Arnott J, Crawford RJ, Ivanova EP (2012) Plastic degradation and its environmental implications with special reference to poly (ethylene terephthalate). Polymers 5(1):1–18
Wei L, Liang S, McDonald AG (2015) Thermophysical properties and biodegradation behavior of green composites made from polyhydroxybutyrate and potato peel waste fermentation residue. Ind Crops Prod 69:91–103
Williams PT, Bagri R (2004) Hydrocarbon gases and oils from the recycling of polystyrene waste by catalytic pyrolysis. Int J Energy Res 28(1):31–44
Witt U, Einig T, Yamamoto M, Kleeberg I, Deckwer WD, Müller RJ (2001) Biodegradation of aliphatic–aromatic copolyesters: evaluation of the final biodegradability and ecotoxicological impact of degradation intermediates. Chemosphere 44(2):289–299
Woodruff MA, Hutmacher DW (2010) The return of a forgotten polymer—polycaprolactone in the 21st century. Prog Polym Sci 35(10):1217–1256
Yagi H, Ninomiya F, Funabashi M, Kunioka M (2009) Anaerobic biodegradation tests of poly (lactic acid) and polycaprolactone using new evaluation system for methane fermentation in anaerobic sludge. Polym Degrad Stab 94(9):1397–1404
Yoon MG, Jeon HJ, Kim MN (2012) Biodegradation of polyethylene by a soil bacterium and AlkB cloned recombinant cell. J Bioremed Biodegrad 3(4):1–8
Zafar U (2013) Ph.D. Thesis. The University of Manchester, Manchester
Zafar U, Houlden A, Robson GD (2013) Fungal communities associated with the biodegradation of polyester polyurethane buried under compost at different temperatures. Appl Environ Microbiol 79(23):7313–7324
Zhang M, Thomas NL (2011) Blending polylactic acid with polyhydroxybutyrate: the effect on thermal, mechanical, and biodegradation properties. Adv Polym Technol 30(2):67–79
Zhang J, Wang X, Gong J, Gu Z (2004) A study on the biodegradability of polyethylene terephthalate fiber and diethylene glycol terephthalate. J Appl Polym Sci 93(3):1089–1096
Zheng Y, Yanful EK, Bassi AS (2005) A review of plastic waste biodegradation. Crit Rev Biotechnol 25(4):243–250
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Sheel, A., Pant, D. (2018). Microbial Depolymerization. In: Varjani, S., Gnansounou, E., Gurunathan, B., Pant, D., Zakaria, Z. (eds) Waste Bioremediation. Energy, Environment, and Sustainability. Springer, Singapore. https://doi.org/10.1007/978-981-10-7413-4_4
Download citation
DOI: https://doi.org/10.1007/978-981-10-7413-4_4
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-7412-7
Online ISBN: 978-981-10-7413-4
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)