Abstract
The dairy sector in India is the largest milk producer in the world. Substantial amounts of freshwater and energy are consumed during milk processing with concomitant impacts on sustainability. In this chapter, we study the energy efficiency at India’s largest milk processing plant and propose retrofits for improving the plant’s sustainability. Specifically, we report on exergy analysis of a milk powder manufacturing unit. Exergy of a system at a certain thermodynamic state is the maximum amount of work that can be obtained when the system moves from that state to one of equilibrium with its surroundings. In contrast to a conventional energy analysis, which maps the energy flows of the system and suggests opportunities for process integration, an exergy analysis pinpoints the locations, causes, and magnitudes of thermodynamic losses. The milk powder plant that is the focus of the current study consists of two sections—an evaporation section and a drying section. Our results reveal that exergy efficiency of certain units is very low (<20%). Significant improvements in energy efficiencies can be achieved through simple, low-cost retrofits to these units.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Increasing the energy efficiency of the industrial sector has the potential to significantly reduce the greenhouse gas emissions associated with the consumption of fossil fuels and the operating costs associated with energy use [1]. In the dairy processing industry, a very small amount of processing energy is actually stored in the final products and hence the vast majority of processing heat is eventually wasted. Since recovery of the waste heat and its subsequent utilization imply reduction in the net purchased energy, recovery of this waste heat is highly necessary [2].
India is now the largest producer of milk [3] owing to “Operation Flood” [4], a project launched by National Dairy Development Board (NDDB) in 1970 and led by late Dr. Verghese Kurien. India, with its enormous vegetarian population, is the largest consumer of its own dairy products such as butter, cheese, ghee, milk powder as these are the only acceptable sources of animal protein for the vegetarians [5]. Thus, dairying is a persistent source of income for the rural India. As per NDDB, the Indian dairy industry is all set to experience high growth rates in the next few years with demand likely to reach 200 million tonnes by 2022 [6].
The subject of this case study, Kaira District Co-operative Milk Producers’ Union Limited, widely known as Amul Dairy, is located in Anand, Gujarat. Amul is the largest food brand in India majoring in production of milk and milk products. Back to 1946, this dairy was the significant center of “white revolution.” It is run by collection of milk from around 700 thousand villagers [5]. With a daily milk handling capacity of 4.5 million liters, it manufactures ghee, butter, processed milk, milk powder, and flavored milk. Every unit has its own production capacity. This dairy processes 1800 m3 of raw milk per day. It produces 75 metric tonnes of milk powder, 75.5 metric tonnes of butter, 25 metric tonnes of flavored milk, 14 metric tonnes of ghee, and 55 metric tonnes of skim milk powder every day. The milk packaging section packs 90 m3 of processed milk every day.
The rest of the chapter is organized as follows: Sect. 2 gives a brief overview of exergy analysis. The milk powder production process is described in Sect. 3. Section 4 describes its exergy analysis. Two strategies are devised to make the overall plant more energy efficient. These are also discussed in this section.
2 Exergy Analysis
The term Exergy was first used by Rant [7] in 1956, and it refers to the Greek words ex meaning external and ergos meaning work. Szargut [8] defined exergy as: “Exergy is the amount of work obtainable when some matter is brought to a state of thermodynamic equilibrium with the common components of its surrounding nature by means of reversible processes, involving interaction only with the above-mentioned components of nature” [8]. Since the term reversible processes is involved, in other words, the exergy of a system at a certain thermodynamic state is the maximum amount of work that can be obtained when the system moves from particular state to a state of equilibrium with the surroundings. Thus, the basic difference between the first and second laws of thermodynamics is that the former states that energy is conserved but makes no distinction between energy forms, while the latter states that the energy transformation processes in a system can only proceed from a higher-quality form to a lower-quality form unless there is some net input of energy quality (such as work) from the surroundings, thereby inferring that different energy forms have different energy qualities.
Energy can be broadly classified into: (1) high-grade energy and (2) low-grade energy. High-grade form of energy is highly organized in nature, and conversion of such energy to some other high-grade form (W → W, such as electrical energy → thermal energy in electrical heater) is not dictated by the second law of thermodynamics. Conversion of high-grade energy to low-grade energy, although not desirable, takes place in our environment. This is because of dissipation of heat due to friction (e.g., mechanical work → electricity; some losses are there due to the friction in bearing of machineries). Thus, both the first and second laws of thermodynamics are to be considered for the analysis. Low-grade energy such as heat due to combustion, fission, fusion reactions and internal energies are highly random in nature, and their conversion to high-grade form (Q → W) has been of interest. But the second law of thermodynamics dictates that complete conversion of low-grade energy (Q) to high-grade energy (W) is never possible. That part of low-grade energy which is available for conversion is termed as available energy, availability, or exergy, and the part which gets rejected is known as unavailable energy or irreversibility [9]. Thus, the exergy analysis provides comprehensive and deeper insight into the process and new unforeseen ideas for improvements and hence is applicable for process evaluation and optimization purposes [10]. Not only does it help to determine the type, location, and magnitude of energy losses in a system, but by enabling the engineers to recognize the individual components efficiency it also helps to find means to reduce these losses to make the system more energy efficient [10, 11].
For the determination, quantification, and prioritization of possible energy savings in complex and large-scale industrial processes, including dairy production process, several scientific and engineering methods exist and are in continuous development [1]. One such method is the basic energy analysis which is a traditional approach to estimate various energy consumption processes [12]. The energy analysis is based on the first law of thermodynamics, which expressed the principle of conservation of energy. However, it provides no information about the irreversibility aspects of thermodynamic processes. The energy analysis is unable to distinguish the different qualities of energy such as heat quality which is dependent on the heat source temperature [13]. Due to these deficiencies and shortcomings of energy analysis, the exergy analysis which provides a more realistic view of the systems and processes has proved to be a more powerful tool for engineering evaluations. This is because exergy analysis considers the maximum amount of work obtainable from a stream of matter, heat, or work when some matter is brought to a state of thermodynamic equilibrium with the common components of natural surroundings by means of reversible processes, and is a measure of the potential of a stream to cause change, as a consequence of not being completely stable relative to the reference environment [14,15,16,17].
In a real processing operation, there are many product and waste streams—some requiring heating and others requiring cooling—and each stream may have different start and end temperatures, different flows and heat capacities. If a process stream needs to be cooled over a particular temperature range, it can take place against another process stream that needs to be heated over a similar temperature interval using a heat exchanger between the streams—and this is more attractive than using steam to heat one stream, and chilled water to cool the other. This situation presents a range of options for arranging heat transfer between these streams. This technique has been developed to formalize this design process: A process integration analysis of a processing operation will consider all the process streams that require heating collectively, and develop a representation of their total heat requirement in each temperature interval; the analysis will then do the same for all the process streams that require cooling [2].
Exergy analysis gained importance in chemical engineering after the year 1990. The current applications of exergy analysis in distillation operation, membrane technology, and CO2 capture are reviewed by Luis [18]. Review of exergy analysis of energy-intensive processes like cement, paper, iron, and steel is carried out by Luis and Bruggen [19]. Exergy analysis of methanol production shows that convective reforming process is 40% more exergy-efficient process than the steam reforming process [20]. The refrigeration cycle in the petrochemical plant that uses ethylene and propylene as refrigerant shows more losses in compressor and evaporator [21]. Energy efficiency, waste minimization, and effective raw material use can be measured and optimized by using utilizable exergy coefficient in chemical process design. Exergy load distribution analysis carried out for final optimal flow sheet topology shows an increase in exergy efficiency by 10% [22]. Second law concept is used to reduce energy consumption in the chemical industry by following some consequences suggested by Leites et al. [23]. Exergy analysis by Gao et al. shows that polygeneration systems for the production of energy and a chemical are promising in the future [24]. Thus, it can be seen that many design-related applications of exergy analysis are helpful to evaluate, compare, and improve the process. Bühler et al. [25] analyzed the energy and exergy efficiencies, as well as the destroyed and lost exergy, of 22 industrial sectors in Denmark for the years 2006 and 2012 [25]. The paper showed that the thermal process efficiencies range from 12 to 56% while industries with high-temperature processes such as cement and metal production achieve the highest efficiencies. The paper also gave a basis for future analyses toward undertaking energy efficiency measures in the industry by targeting the high-temperature processes, where large quantities of energy was found to be recoverable. Ertesvåg [26] compared the exergy scenario of different societies with the motivation that such studies may be able to create an awareness of the notion of energy quality and degradation of energy. His findings explain that societies with a large contribution from thermal power plants had relatively low total efficiencies while the industries generally had a higher efficiency [26].
The exergy-based performance evaluation and subsequent optimization of drying facilities have been a growing interest among the researchers in recent years. The main objective of exergy analysis of drying systems is to provide a clear picture of the process, to quantify the sources of inefficiency, to distinguish the quality of energy consumption, to select optimal drying conditions, and to reduce the environmental impact of drying systems [13]. Energy, exergy, and their impact on the environment are closely related as shown in Fig. 1. For instance, we all know that the environmental impact of emissions can be reduced by increasing the efficiency of resource utilization. However, increasing efficiency has sustainability implications as it lengthens the lives of existing resource reserves, but generally entails greater use of materials, labor, and more complex devices. Depending on the situation and the players involved, the additional cost may be justified by the added security associated with a decreased dependence on energy resources, by the reduced environmental impact, and by the social peace obtained through increased productive employment. Thus, it is suggested that the impact of energy resource utilization on the environment and the achievement of increased resource-utilization efficiency are best addressed by considering exergy. On the other hand, since these topics are critical elements in achieving sustainable development, exergy also appears to provide the basis for developing comprehensive methodologies for sustainability (shown in Fig. 2). Since the exergy of an energy form or a substance is a measure of its usefulness or quality to cause a change, it should be able to provide the basis for an effective measure of the potential of a substance or energy form to impact the environment [27].
A reversible device (module) for determining thermomechanical energy [28]
2.1 Methodology
A process stream with temperature T 1 and pressure P 1 with negligible kinetic and potential energies, undergoing reversible physical processes as shown in Fig. 3, is considered. The heat transfer to/from the ideal reversible module shown in Fig. 3 takes place at ambient temperature T 0, and the work produced is the maximum possible (the processes being reversible in nature) when the process stream at a state P 1, T 1 is brought to equilibrium with its natural surroundings (referred to as the environment), which is at the thermodynamic state of P 0, T 0. An energy balance for this system yields the following[28]:
Similarly, the entropy balance assuming no irreversibility yields the following [28]:
Equation (2) can then be solved to establish an expression for specific heat transfer [28]:
Equation (1) can then be solved with respect to specific work and rearranged to give the following[28]:
The specific exergy of the process stream entering the ideal device can be derived from Eq. (4) as follows [28]:
The change in specific exergy when a system undergoes a process from thermodynamic state 1 to state 2 is then given by the following expression (the enthalpy and entropy functions at the surrounding conditions (h 0, s 0) cancel due to subtraction) [28]:
This change in specific exergy, if negative, accounts for irreversibility, I.
A fundamental difference between energy and exergy is that while the former is conserved, the latter is destroyed in all real processes due to irreversibility. This exergy is lost in two different ways: internal losses caused by irreversibility in the process like chemical reaction, mixing, unrestricted expansion and external losses due to effluent streams like exhaust gases, purge, and bleed streams that are not utilized. Thus, it is common to define exergy efficiency as a ratio of useful exergy from the process to total exergy to the process and given by:
where the subscripts out and in denote the outgoing and incoming streams, respectively. The reversible device can be replaced with real industrial equipment like evaporator, dryer, heat exchanger.
For instance, in case of heaters and heat exchangers, the working fluid is to a higher temperature and hence the entropy change is given as follows:
Then, the irreversibility and efficiency are calculated with the help of the following equations:
where
- T0 :
-
Ambient temperature (298 K)
- Cp, milk :
-
3.93 kJ/kg K
- Cp, water :
-
4.18 kJ/kg K.
In pumps, compressors, and the thermal vapor recompression (TVR) unit, the work input which is the work done by electricity is to be considered in the calculation of irreversibility, I. The total exergy is calculated by taking into account the change in exergy caused by change in temperature as well as change in pressure in these devices. Thus, the exergy expression is given by the following equations:
From here on, irreversibility and efficiency are calculated with the help of Eqs. 14 and 15:
In evaporators, due to the heating potential of the generated vapors, the exergy of the vapors needs to be considered as a part of useful exergy obtained from the process and is given as follows:
where
- a1:
-
Heating medium (steam/vapor)
- a2:
-
Condensate
- b1:
-
Incoming working liquid
- b2:
-
Outgoing working liquid.
However, in dryers no such added exergy term is used in efficiency calculation due to the absence of any vapor. This is the basic difference between an evaporator and a dryer in terms of calculating exergy efficiency even though both the equipments are used to increase the concentration of the working fluid. The exergy efficiency of the dryer is given as follows:
Applying the above equations, irreversibility and exergy efficiencies can be obtained for various equipments in the process.
3 Milk Powder Production
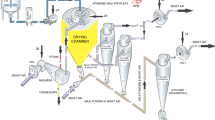
Milk drying is an energy-intensive process as it usually requires hot air as heating medium to allow simultaneous heat and mass transfer between the drying air and the milk. For dairy products, the most widely used technique for dehydration is spray drying after evaporation. These processes preserve food properties as they do not involve severe heat treatments and allow storage of powders at an ambient temperature [29]. The drying operation generally takes place in three sequential stages: (i) spray chamber, in which drying occurs within a few seconds; (ii) internal stationary fluid bed, added at the conical base of the spray chamber to better control particle agglomeration and drying [30]; and (iii) external fluid bed to fine-tune the powder moisture and to cool the out-feed product stream.
When the milk receives enough energy in the form of heat, its water molecules escape as vapor. The rate at which the water molecules escape depends on various factors, viz. temperature of milk, temperature of surroundings, the pressure above the milk surface, and the rate of heat transfer. Milk powder possesses various physio-chemical and functional properties which are important to both industrial and consumer uses.
3.1 Process Description
Milk powder manufacturing involves the gentle removal of water at the lowest possible cost under stringent hygiene conditions while retaining all the desirable natural properties of the milk—color, flavor, solubility, nutritional value [31]. During the process, the water present in the milk is removed by boiling the milk under reduced pressure at low temperature in a process known as evaporation. The resulting concentrated milk is then sprayed in a fine mist into hot air to remove further moisture, thereby producing the powder. Approximately 13 kg of whole milk powder (WMP) or 9 kg of skim milk powder (SMP) can be made from 100 L of whole milk [31]. The conventional process for the production of milk powder starts with taking the raw milk received at the dairy factory and pasteurizing and separating it into skim milk and cream using a centrifugal cream separator. If whole milk powder is to be manufactured, a portion of the cream is added back to the skim milk to produce milk with a standardized fat content.
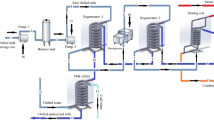
There are two sections in milk powder production: the evaporation section and the spray drying section. The pasteurized milk at 6–10 °C with around 13–14% solids is pumped into the five-effect evaporators, each accompanied with a preheater, where the water present in the milk gets vaporized with the help of vapor fed in the reverse direction. In Fig. 4, the hyphenated lines represent the flow direction of the pasteurized milk from left to right in the evaporation section, while the dotted lines represent the flow direction of the steam or vapor from right to left. Preheating causes a controlled denaturation of the whey proteins in the milk along with destroying bacteria, inactivating enzymes and generating natural antioxidants thereby imparting heat stability [31]. The steam which is fed in the first effect is generated by thermally compressing a part of the vapor generated in the third effect. The pressure in the five effects ranges from around 650 mmHg in first effect to around 500 mmHg in the last effect with a difference of about 30–50 mmHg between any two adjacent effects. The vapor generated in the evaporator-preheater assembly is split into three parts where first part is sent to the next effect, the second part to its accompanying preheater for heat exchange, and the third part for CIP water heating, thereby ensuring that no external source of heating is required (can be observed from Fig. 4). Condensate from the first effect, because of being in large amount as compared to other effects, gets collected in the pure condensate tank, while for all the other effects, the condensates get collected in the murky condensate tank as these condensates carry traces of solids present in the milk. The concentrated milk leaving the evaporation section has a temperature of around 92–93 °C and a solid content of around 30% [32].
A part of the milk leaving the five effects is flash cooled to around 58 °C and fed to the scraped surface heat exchanger where it is heated to 75 °C before being fed to the spray dryer (refer Fig. 4). Spray drying involves atomizing the milk concentrate from the evaporator into fine droplets. This is done inside a large drying chamber in a flow of hot air at a temperature of 180–200°C using either a spinning disk atomizer or a series of high-pressure nozzles. The milk droplets are cooled by evaporation, and they never reach the temperature of the air. The concentrate may be heated prior to atomization to reduce its viscosity and to increase the energy available for drying. The atomized particles come in contact with hot air, and water in it gets evaporated leaving a fine powder of around 4% moisture content with a mean particle size typically of <0.1 mm diameter which is collected in the silos [31]. Sometimes a secondary drying takes place in a fluidized bed through which hot air is blown to remove some more water to give a product with a moisture content of 2–4% [31]. Some amount of dried product may also get entrained with the exhaust air which is then separated in a high-efficiency cyclone separator and recycled back to the chamber. As shown in Fig. 4, the milk powder formed is then collected at the bottom of the dryer in bags or silos and sent for storage. More details about the process conditions with respect to the process flow diagram have been provided in Sect. 4.1 of the chapter.
3.2 Energy Efficiency Studies
There are a number of heat-recovery issues specific to the manufacturing dairy industry. For semi-batch operations, the availability or duty cycle of the source and target streams must be checked carefully. Heat exchangers can develop leaks, and heat recovery creates the possibility of contamination between streams. This is unacceptable in milk processing, and isolation by pressure differential double-plate heat exchangers or intermediate circuits is essential in such cases. Also many gas and liquid waste streams carry contaminants which may affect the surfaces of heat-recovery equipment. Good engineering solutions are available for most of these problems, but surface cleaning must be designed into the heat-recovery equipment. In the recovery of gas from waste gas streams, there is also the possibility of contaminants presenting a fire hazard. Many waste gas streams contain significant moisture that will condense if the temperature is lowered significantly. It is quite possible to design heat exchangers for operation with a gas stream below the dew point, and indeed such streams often represent large heat sources. There are significant problems of physically installing major heat-recovery equipment as dairy processing buildings are frequently crowded. Large heat exchangers impose back pressure on boilers and spray dryers. The ability of original equipment to tolerate this back pressure must be checked. In this context, the optimized use of the condensate should also be considered for the recovery of the condensate imparts some added benefits to the milk drying process such as savings of (i) water and water treatment costs, for replacing the lost condensate, (ii) heat, for prewarming new boiler feed water, and (iii) wastewater, because less condensate gets into the wastewater [2].
Reducing energy use associated with milk powder production has been the subject of numerous studies. Previous studies have mainly focused on three areas: (i) heat recovery from the spray dryer exhaust air, including source/sink integration options [33], industrial case studies [34, 35], and heat exchanger modeling [36], (ii) zonal and total site heat integration of dairy factories, including integration of the milk treatment process that occurs preceding the drying operations [37] and the application [38, 39] and design [40] of heat-recovery loops to semi-continuous processes, and (iii) an overview of good energy-efficient practices in spray drying [41]. More recent studies have looked heat-recovery targeting methods and heat exchange synthesis between the evaporator and spray dryer operations of a powder process.
Some energy analyses of the dairy industry in terms of exergy have been conducted in the last few decades. However, these have primarily analyzed the milk processing [42, 43] or the drying process [44]. Quijera et al. [42, 45] use the pinch and exergy methods to evaluate and optimize the integration of solar thermal technologies for the production of cheese and yoghurt. An exergy analysis of milk pasteurization was performed by Fang et al. [43], where the process was also optimized. The usefulness of exergy as a tool for the dairy industry was discussed by Vidal et al. [46]. A milk powder production facility in New Zealand was analyzed in this study, and product input/output states were studied on a component-wise basis. The exergy losses of flavored yoghurt production were determined by Sorgüvan and Özilgen [47]. The production of other ingredients than milk was also included from a cumulative exergy perspective. A study by Trägårdh [48] used exergy as a quality factor. Dincer and Sahin [49] proposed a new thermodynamic model for drying processes, based on exergy analysis. Evaporation in food processes was discussed in [50, 51], and different evaporator configurations for citrus processing were analyzed. Winchester [52] analyzed the modeling and operation of falling film evaporators at the example of a dairy plant. Choi in [53] focused on exergy analysis of thermal vapor recompression evaporators, suggesting new designs and operating parameters. From the above examples, it can be concluded that exergy analysis is a promising approach to study the energy efficiency for Amul’s milk powder production process.
4 Improving Energy Efficiency by Process Integration
In this section, the simulation of the normal operation of the milk powder plant which is the base scenario will be presented followed by its exergy analysis. With the exergy analysis, first the regions where the heat loss is comparatively more will be located which will be followed by developing strategies through process integration to make the entire plant much more energy efficient.
4.1 Simulation Results: Base Scenario
The process conditions of milk powder production are presented in Table 1. The model presented in Fig. 4 was simulated in CHEMCAD, and the simulation results are summarized in Table 2. These were validated with plant measurements. The drying of moisture from the milk takes place in two parts as discussed earlier—first in the evaporator and then in the dryer. Apart from these two sections, there is one more section where the regenerative heating and cooling of the milk takes place. The following gives a detailed picture of the simulation results in these three regions of the milk powder plant.
Evaporation: As shown in Fig. 4, the evaporator used in the plant is of backward feed type which means that the milk and vapor flow in opposite directions. The reason behind using backward effect evaporator in food processing industries like milk powder production is that here the milk of highest concentration will remain in the first, that is, the hottest effect. Therefore, the viscosity of the milk will remain low, leading to a high heat transfer coefficient which, in turn, will enhance the capacity of the system. So the pasteurized milk enters the evaporator at 6 °C where it gets concentrated from 14.9% solids to 30.03% solids. The pressure in the five effects ranges from 630 mmHg in first effect to 500 mmHg in fifth effect with a difference of about 30 mmHg between two adjacent effects. The partially concentrated milk leaving the evaporator section is at a temperature of 93.75 °C with an overall rise of 1.5–2 °C in the five effects. The extent of evaporation in the second and the third effects (21.44% in second and 25.61% in third) was found to be significantly more than the rest of the effects (<7%). This is due to the splitting of the vapor stream in the split fractions. To utilize the full potential of these two effects, the vapor splitters just preceding these two effects, that is, splitter first and second were given a fraction of 0.75 in favor of the vapors entering the second and third effects, respectively, while the remaining 0.25 was split between the vapors fed to the preheaters and that sent for CIP heating for each of these two effects. The water used for CIP cleaning is heated from 25 to 34.33 °C with a part of vapor generated in the evaporators. The amount of pure condensate and the amount of murky condensate collected are 400.9 kmol/h and 819.9 kmol/h, respectively. The pure condensate collected from first effect is sometimes used as CIP water or pumped back to boiler house depending on requirement.
Regenerative heating and cooling: The milk leaving the evaporator section (at 93.75 °C) is then sent to the regenerative heater where a part of the milk is cooled to 58.75 °C by flash cooling and the other part is heated to 94.21 °C (shown in Fig. 4). The latter is fed back for regenerative purpose. The part of the milk which is leaving at 58.75 °C is then fed to the scraped surface heat exchanger, where it is heated to 75.75 °C by steam at 3000 mmHg and fed to the spray dryer for removing the remaining moisture and concentrating it to the required solid concentration.
Spray drying: The milk leaving the scraped heat exchanger at 75.75 °C enters the spray dryer from the top where it gets atomized by a series of high-pressure nozzles. The dryer temperature is maintained at around 85 °C. The vaporization of moisture from the milk takes place due to the transfer of heat from the hot air to the milk. As a result, the atomized milk gets dried from 30.03% solids to 95.79% solids when it comes in contact with hot air blown from the bottom at a temperature of 185 °C and a pressure of 2000 mmHg (refer Fig. 4). The milk powder formed at a temperature of 40 °C and a moisture content of 4.21% is then collected at the bottom of the dryer and sent for storage. In this context, it can be mentioned that the final moisture content of the milk powder that is obtained is in sync with that of the actual process, which is around 4%.
4.2 Exergy Analysis of Base Scenario
To find the extent of heat loss in each section of the plant, exergy analysis of the base scenario was performed with the help of the equations discussed in Sect. 2 and the results are shown in (Table 3). From the results obtained, it could be concluded that some of the major equipments of the plant were running on significantly low energy efficiencies due to major heat losses which in turn bring down the energy efficiency of the entire plant. On close investigation, it was observed that major exergy destruction occurs in equipment which is a part of the evaporation section while the equipment present in the drying section shows relatively compatible efficiencies.
Preheaters, CIP heater, Regenerative heater, and Scraped heat exchanger: For equipment like preheaters, CIP heater, regenerative heater, and scraped surface heat exchanger, the method employed for calculating the exergy efficiency is same for all these four types of equipment, the working fluids (water for CIP heater and milk for the rest) do not exhibit any change of state. First, the change in entropy is calculated for both the two fluids between which the heat exchange is taking place. The net entropy change in both the fluids is then multiplied by the ambient temperature to obtain the irreversibility or net loss in heat in these equipments, which is nothing but the difference in exergy change of the cold stream and that of the hot stream. The exergy efficiency was then calculated by taking the ratio of the exergy gained by the cold stream (output exergy) to the exergy lost by the hot stream (input exergy). Compiling the results, it was observed that among the preheaters, only the ones belonging to the third (50.61%) and fifth (43.76%) effects showed decent efficiencies while the CIP heater, regenerative heater, and the scraped surface heat exchanger exhibited above par exergy efficiency values (55–70%); hence, they do not require any improvement as such.
TVR and pumps: For equipment like the pumps and the thermal vapor recompression unit, the work done by electricity also comes into the picture while doing the exergy analysis. An energy balance between the work done by electricity and the exergy content of the incoming and outgoing fluids gives us the value of the net irreversibility developed. One more key feature of this exergy analysis is the fact that here the exergy is contributed both by temperature and pressure because in these equipments the working fluid undergo change in temperature as well as change in pressure. The exergy efficiency was then calculated by dividing the work done by electricity by the net change in exergy of the working fluid.
The vapor from the third effect enters the TVR at 90.6 °C with a flow rate of 1754 kg/h, and the steam leaves at 271.66 °C. The enthalpy values corresponding to the inlet and outlet temperatures are −2.27 × 107 kJ/h and −1.89 × 107 kJ/h, respectively. The work done by electricity is 4.81 × 105 kJ/h. By using C p = 4.18 kJ/kg K, T o = 298 K, and R = 0.462 kJ/kg in Eqs. (11), (12), and (13) (along with the process conditions), e x1 and e x2 were found to be −20.68 kJ/kg and 279.83 kJ/kg, respectively. These details are used in Eqs. (14) and (15) to get the lost heat or irreversibility as 1.0053 × 106 kJ/h and efficiency as 32.36%.
Thus, the results obtained showed that the efficiency of TVR is below par considering the significance of the equipment in the entire process. Most of the pumps, on the other hand, exhibited above par efficiencies (>55–60%). Observing the result, the fact that the thermal vapor recompression unit, one of the most integral parts of the process, showed considerably low efficiency became prominent. Thus, it was determined that the primary approach toward devising a strategy to improve on the current scenario should first focus on reducing the exergy loss in this unit.
Evaporator and dryer: For the evaporator and the dryer, the methodology followed for doing the exergy analysis is more or less similar. The hot utility which is steam or vapor for the evaporators, is hot air for the dryer and the corresponding counterparts at the exit are condensates and exhaust for the evaporator and dryer, respectively. The only difference between these two equipments is that while calculating the exergy efficiency, the exergy content of the generated vapor must be included for the evaporators while no such exergy addition is needed for the dryer as no vapor is generated in this case. The reason being, the exergy of the vapor is considered as a part of exergy improvement as the energy content of this vapor is later utilized to vaporize the moisture content of the milk in the next effect or even CIP heating for that matter. It was observed that among the evaporators, the third effect, the one from which a part of the vapor is sent for thermal compression into steam, showed the least efficiency (17.17%). The dryer, on the other hand, turned out to be exhibiting one of the highest efficiency values (68.28%) in the whole analysis.
Thus, it is concluded that the exergy efficiency varies from a large extent in different sections of the milk powder plant and there is a wide scope in devising strategies to improve the situation. After verification of the above-mentioned exergy results, a couple of such strategies are discussed in the next chapter of the work where some of the flaws of the base scenario have been duly addressed.
4.3 Strategies to Improve Energy Efficiency
To reduce the amount of heat loss in the equipment and thereby improve the energy efficiency of the plant, different strategies that have the potential to improve the present situation were analyzed. As discussed earlier, it was identified that not only was the third effect showing the least efficiency among the evaporators, but also the thermal vapor recompression unit, where a part of the vapor generated in the third effect is thermally compressed into steam, also showed below par efficiency. This analysis presented us with a scope to devise a strategy to improve the exergy situation by keeping these two equipments at the focal point. Thus, there can be two options that can be pursued in this regard, which are to send either the vapor generated from second effect or that from fourth effect for thermal compression in place of that from the third effect.
First, the fourth effect was tried in the analysis. A part of the vapor generated in the fourth effect which was earlier being sent for CIP heating is now sent for thermal compression to steam which would then be fed to the first effect as the primary source of evaporation, while the part of vapor which was earlier sent for thermal compression is now sent to the CIP heater. Subsequent simulation of this new strategy followed by its exergy analysis yielded results which turned out to be below par even when compared to that of the base scenario. Earlier, the exergy efficiencies of TVR and third effect were 32.36 and 17.17%, respectively. After this new development, these values have further decreased to 28.96 and 15.72%, respectively. One reason of such an occurrence can be attributed to the fact that vapor sent to CIP heater from fourth effect is lesser than the vapor from third effect which is sent for thermal compression. Since these two have been interchanged in the new strategy, both TVR and third effect have suffered more losses. Hence, this strategy was dismissed.
Next, the second effect vapor is partly sent to TVR instead of the fourth effect and the portion which was earlier being sent to TVR is now sent to the CIP heater as before. Simulating this second model followed by its exergy analysis gave a glimpse of a better result with respect to the TVR and the third effect as compared to the base scenario. A detailed exergy analysis showed significant improvement in a few other major equipments, and this in turn formed the first strategy which could be proposed to Amul. The second strategy was developed by using the murky condensate as a hot utility to heat up the CIP water instead of the vapors, thereby utilizing the full potential of the vapors to evaporate the water present in the milk in all the five effects. These proposed strategies, believed to bring better results when implemented in the dairy, are discussed in the following.
4.3.1 Scenario 1
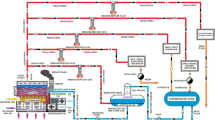
It is observed that in the base scenario, the part of the vapor leaving the third effect that is sent for CIP heating is more than the part sent for thermal compression into steam. Since, for the purpose of drying, the CIP heating is not of major priority, it can be concluded that there are scopes for the improvement in this section of the plant. Also, the amount of vapor sent for CIP heating from the second effect is much more than that from third effect (because of the split ratio at the splitters). Hence, the vapor from second effect which was earlier sent for CIP heating is now sent for thermal compression to steam and the vapor from third effect that was earlier sent for thermal compression is now sent for CIP heating in the new strategy (shown in Fig. 5). The following describes the simulation results obtained after implementing this new strategy:
Evaporation: With this new strategy now implemented in the existing plant operations, the milk leaving the evaporators now has a solid concentration of 31.4%, which earlier used to be 30.03%. The milk temperature has also risen a little to 93.87 °C. The pure condensate generated from first effect has increased from 400.9 to 438.6 kmol/h due to the fact that the amount of steam fed to first effect is more now, due to which the pure condensate obtained is also more. But the amount of murky condensate is reduced from 819.9 to 803.41 kmol/h due to the fact that now the vapor used for CIP heating is comparatively lesser which in turn results in lesser condensate generation (as shown in Table 4). At the exergy front, the efficiency of TVR in this scenario has showed a jump from 32.36% to 46.47% which is more than 10% while the efficiency of the third effect is at par with that of base scenario (shown in Table 5). Along with this, the first- and second-effect evaporators including their corresponding preheaters have showed a significant jump in efficiency (around 20%). The rest of the equipment has shown only minor variations.
Regenerative heating and cooling: The partially concentrated milk from the evaporators entered this section at 93.87 °C, and a part of it is again flash cooled to its design value of 58.75 °C. It is then heated to 75.75 °C in the scraped heat exchanger and fed to the spray dryer. This shows that there are not many changes in the process conditions involved at this section, and hence, the exergy values are more or less at par with that of base scenario. The regenerative heater now has an exergy efficiency of 69.7%, a minute drop from 69.94%, value in case of base scenario while the scraped surface heat exchanger shows a slight upward growth from 56.24% at base scenario to 56.98% in this new strategy.
Spray Drying: The fact that in the evaporators, milk already got concentrated to 31.4% (1.37% more than the base scenario) had a direct effect in the extent of drying as well. The final dried powder in this new strategy had an overall moisture content of 4.09%, which is 0.12% lesser than the base scenario (shown in Table 4). The final solid temperature was also maintained at around 40 °C in the presence of hot air blown from the bottom of the dryer at 185 °C and 2000 mmHg. As a result, the dryer efficiency rose a little from 68.28% in the base scenario to 69.1% in Scenario 1.
Thus, it is clear from Table 4 that in Scenario 1, due to improved efficiency of evaporation in first and second effects, the overall drying has improved. Thus, this strategy more or less addressed those equipments which were earlier exhibiting low exergy efficiency under base scenario and improved them to decent values. In the second scenario, another strategy is devised which when implemented with this Scenario 1 will show further improvement in the exergy results.
4.3.2 Scenario 2
In the previous model, vapor generated in the evaporators was used to heat the CIP water (for its 10 °C rise). But it was observed that instead of using the vapor if the condensate was used for CIP heating (since it is not a major priority in milk drying), then the energy efficiencies could be further improved. On implementing this change in Scenario 1, it showed significant rise in the efficiency values and reduction in the heat losses due to the fact that now more amount of vapor, which was earlier sent for CIP heating, was now used for the evaporation of the water present in the milk in the five effects. The modification made in Scenario 2 with respect to Scenario 1 is shown in Fig. 6. A detailed picture of the simulation results is presented in the following:
Evaporation: Adding this new strategy along with Scenario 1 has resulted in an increase in solid concentration in the milk leaving the evaporation effects from 31.4% solids in Scenario 1 to 32.3% solids under this combined strategy. The total water removal in all the five effects has also jumped further, from 51.77% in Scenario 1 to 52.54% under the present scenario. Since now more amount of vapor is being sent for evaporation, the condensates collected in both the pure and murky condensate tanks have increased (pure condensate: 453.5 kmol/h from 400.9 kmol/h in base scenario and murky condensate: 869.12 kmol/h from 819.9 kmol/h in base scenario). The final temperature of the CIP water, which is now 34.72 °C, is also found to have increased a little in this scenario (34.33 °C in base scenario and 34.41 °C in Scenario 1). From the exergy perspective, significant growth has been noticed in the third effect, both in the evaporator and in its corresponding preheater. Compared to Scenario 1, where the efficiency values of third evaporator and preheater were 16.59 and 47.37%, respectively, these values have now jumped to 21.75 and 50.02%, respectively. The detailed results are shown in Table 5. All the preheaters under this new strategy have shown decent improvement, while rest of the equipment exhibited minor variations.
Regenerative heating and cooling: The milk now leaves the evaporator at a temperature of 93.96 °C, which is a slight rise from Scenario 1 where the milk leaving the five effects and entering this section had a temperature of 93.87 °C. A part of the milk is cooled to its designed value of 58.75 °C and fed to the scraped surface heat exchanger where it is heated to 75.75 °C before being fed to the spray dryer. The exergy efficiency of both the regenerative heater and the scraped surface heat exchanger showed a slight drop, negligible enough to affect the overall exergy scenario of the whole plant.
Spray drying: The improvement in solid concentration in the evaporation section had a direct effect in the final moisture content of the milk powder as well. There had been a rise of 2.27% in the overall solid concentration in Scenario 2 from the base scenario, as a result of which the milk powder moisture content has reduced from 4.21% in base scenario to 4.013% in Scenario 2. These details are shown in Table 5. The final solid temperature remained within its designed limit of around 40 °C. All these have resulted in an improvement in the dryer efficiency, from 68.28% in base scenario and 69.1% in Scenario 1 to 69.78% in this combined strategy.
These two scenarios discussed above clearly depict how process integration helps to improve the overall efficiency of any process. A graphical representation depicting the comparative analysis of all the three scenarios for those equipments which show significant improvements has been presented in Fig. 7.
5 Summary
This work addresses opportunities to improve energy efficiency of a large-scale milk powder production unit through process integration based on exergy studies. A model of the milk powder plant of Amul Dairy was built and simulated in CHEMCAD to get an idea of all the equipment efficiencies under the normal operation of the plant. On the basis of exergy analysis, two strategies were proposed which if implemented can lead to significant improvement (more than 20% in some major equipment) in the exergy efficiencies.
The proposed approach for improving the energy efficiency can be extended to develop a holistic framework that can foster water and energy sustainability. This framework can be realized by an optimization approach that minimizes the energy and water usage using the proposed techniques while accounting for cost constraints and plant dynamics through appropriate models. The strategies suggested here are best applicable to milk powder plants worldwide and can be further generalized for various other industries to promote water and energy sustainability, respectively.
Abbreviations
- C p :
-
Specific heat capacity at constant pressure (in kJ/kg K)
- e x :
-
Specific exergy (in kJ/kg)
- h :
-
Specific enthalpy (in kJ/kg)
- \( {\dot{\text{m}}}\)̇:
-
Mass flow rate (in kg/s)
- p :
-
Pressure of a system (in bar)
- \({\dot{{Q}}}\) :
-
Heat flow (in kJ/s or kW)
- R :
-
Mass-based (substance dependent) gas constant (in kJ/kg K)
- s :
-
Specific entropy (in kJ/kg K)
- t :
-
Time (in s)
- T :
-
Temperature of a system (in °C or K)
- \( {\dot{{W}}}\) :
-
Power (or work per time) (in kW)
- Δ:
-
Change in property
- η :
-
Exergy efficiency
- P:
-
Pressure
- T:
-
Temperature
- 0:
-
Thermodynamic state with ambient conditions
- 1:
-
Thermodynamic state
- 2:
-
Thermodynamic state
- a:
-
Fluid a
- b:
-
Fluid b
- cv:
-
Control volume
- ex:
-
Exergy
- i:
-
Initial
- in:
-
Inlet stream
- f:
-
Final
- out:
-
Outlet stream
- p:
-
Constant pressure
- vap:
-
Vaporization
References
Bühler F, Nguyen T, Jensen JK, Elmegaard B (2016) Energy, exergy and advanced exergy analysis of a milk processing factory. In: Proceedings of ECOS 2016: 29th International conference on efficiency, cost, optimization, simulation and environmental impact of energy systems.
Bulletin of the International Dairy Federation 401/2005, 2005 Energy use in dairy processing
Deparment of Dairying Fisheries and Animal husbandary (2013) Annual Report—2012–13. Ministry of Agriculture, Government of India 15(1):1. https://doi.org/10.1016/j.parkreldis.2008.12.001
Banerjee A (2001) Dairying Systems in India. FAO Corporate Document Repository
Tiwari S, Srinivasan B (2017) Water conservation, reuse and challenges: a case study performed at Amul dairy. The water-food-energy nexus: processes, technologies and challenges, CRC Press ISBN: 9781498760836
National Dairy Development Board (2014) Annual Report, 2013–14
Rant Z (1956) Exergy, a new word for “technical available work”. Forschung auf dem Gebiete des Ingenieurwesens (in German) 22:36–37
Szargut J (1980) International progress in second law analysis. Energy 5(8–9):709–718
Chapter 3: Exergy analysis Available at: http://www.iitg.ernet.in/scifac/qip/public_html/cdcell/chapters/p_mahanta_adv_engg_thermo/Chapter-3.pdf
Kaushik SC, Siva RV, Tyagi SK (2011) Energy and exergy analyses of thermal power plants: a review. Renew Sustain Energy Rev 15:1857–1872
Siva RV, Kaushik SC, Tyagi SK (2012) Exergetic analysis of solar concentrator aided natural gas fired combined cycle power plant. Rene Energy 39:114–125
Nazghelichi T, Aghbashlo M, Kianmehr MH (2011) Optimization of an artificial neural network topology using coupled response surface methodology and genetic algorithm for fluidized bed drying. Comput Electron Agric 75(1):84–91
Aghbashlo M, Mobli H, Rafiee S, Madadlou A (2013) A review on exergy analysis of drying processes and systems. Renew Sustain Energy Rev 22:1–22
Dincer I (2002) On energetic, exergetic and environmental aspects of drying systems. Int J Energy Res 26:717–727
Dincer I, Rosen MA (2007) Exergy: energy, environment, and sustainable development. Elsevier, Amsterdam
Dincer I (2011) Exergy as a potential tool for sustainable drying systems. Sustain Cities Soc 1:91–96
Pandey AK, Tyagi VV, Park SR, Tyagi SK (2012) Comparative experimental study of solar cookers using exergy analysis. J Therm Anal Calorim 109:425–431
Luis P (2013) Exergy as a tool for measuring process intensification in chemical engineering. J Chem Technol Biotechnol 88(11):1951–1958
Luis P, Van der Bruggen B (2014) Exergy analysis of energy-intensive production processes: advancing towards a sustainable chemical industry. J Chem Technol Biotechnol 89:1288–1303
Rosen MA, Scott DS (1988) Energy and exergy analyses of a production process for methanol from natural gas. Int J Hydrogen Energy 13:617–623
Fábrega FM, Rossi JS, d’Angelo JVH (2010) Exergetic analysis of the refrigeration system in ethylene and propylene production process. Energy 35:1224–1231
Sorin M, Hammache A, Diallo O (2000) Exergy load distribution approach for multi-step process design. Appl Therm Eng 20:1365–1380
Leites IL, Sama DA, Lior N (2003) The theory and practice of energy saving in the chemical industry: some methods for reducing thermodynamic irreversibility in chemical technology processes. Energy 28:55–97
Gao L, Jin H, Liu Z, Zheng D (2004) Exergy analysis of coal-based polygeneration system for power and chemical production. Energy 29:2359–2371
Bühler F, Nguyen T, Elmegaard B (2015) Energy and exergy analysis of the danish industry sector. In: Proceedings of the 10th conference on sustainable development of energy, water and environment systems
Ertesvåg IS (2001) Society exergy analysis: a comparison of different societies. Energy 26:253–270
Rosen MA, Dincer I (2001) Exergy as the confluence of energy, environment and sustainable development. Exergy Int J 1(1):3–13
Gundersen T (2011) An introduction to the concept of exergy and energy quality. Energy Process Eng version 4
Schuck P, Dolivet A, Méjean S, Jeantet R (2008) Relative humidity of outlet air: the key parameter to optimize moisture content and water activity of dairy powders. Dairy Sci Technol 88:45–52
Birchal VS, Passos ML (2005) Modeling and simulation of milk emulsion drying in spray dryers. Braz J Chem Eng 22(2):293–302
Milk Powder—New Zealand Institute of Chemistry Available at: https://nzic.org.nz/ChemProcesses/dairy/3C.pdf
Training Manual—Amul 3 Powder Plant, Amul Dairy, Anand
Holland CR, McCann JB (1980) Heat recovery in spray drying systems. Int J Food Sci Technol 15:9–23
Mercer AC (1986) Improving the energy efficiency of industrial spray dryers. J Heat Recovery Syst 6:3–10
Miller J (1987) The use of recovered heat for preheating air to spray driers. New Zealand Energy Research and Development Committee Report. Palmerston North, New Zealand
Atkins MJ, Walmsley MRW, Neale JR (2011) Integrating heat recovery from milk powder spray dryer exhausts in the dairy industry. Appl Therm Eng 31:2101–2106
Robert C (2005) Bulletin of the IDF No. 401/2005- Energy use in dairy processing
Atkins MJ, Walmsley MRW, Neale JR (2010) The challenge of integrating non-continuous processes—milk powder plant case study. J Clean Prod 18:927–934
Atkins MJ, Walmsley MRW, Neale JR (2012) Process integration between individual plants at a large dairy factory by the application of heat recovery loops and transient stream analysis. J Clean Prod 34:21–28
Walmsley MRW, Walmsley TG, Atkins MJ, Neale JR (2012) Area targeting and storage temperature selection for heat recovery loops. Chem Eng Trans 29:1219–1224
Baker CGJ, McKenzie KA (2005) Energy consumption of industrial spray dryers. Drying Technol Int J 23:365–386
Quijera JA, Labidi J (2013) Pinch and exergy based thermosolar integration in a dairy process. Appl Therm Eng 50:464–474. https://doi.org/10.1016/j.applthermaleng.2012.06.044
Fang Z, Larson DL, Fleischmen G (1995) Exergy analysis of a pilot milk processing system. Trans ASAE 38:1825–1832
Erbay Z, Koca N (2012) Energetic, exergetic, and exergoeconomic analyses of spray-drying process during white cheese powder production. Dry Technol 30:435–444. https://doi.org/10.1080/07373937.2011.647183
Quijera JA, Alriols MG, Labidi J (2011) Integration of a solar thermal system in a dairy process. Renew Energy 36:1843–1853. https://doi.org/10.1016/j.renene.2010.11.029
Vidal M, Martin L, Martin M (2014) Can exergy be a useful tool for the dairy industry? In: 24th European Symposium Computer Aided Process Engineering—ESCAPE 24, vol 33, Elsevier, Amsterdam, pp 1603–1608. https://doi.org/10.1016/B978-0-444-63455-9.50102-1
Sorgüven E, Özilgen M (2012) Energy utilization, carbon dioxide emission, and exergy loss in flavored yoghurt production process. Energy 40:214–225. https://doi.org/10.1016/j.energy.2012.02.003
Traegardh C (1981) Energy and exergy analysis in some food processing industries. Leb Und—Technologie 14:213–217
Dincer I, Sahin AZ (2004) A new model of thermodynamic analysis of a drying process. Int J Heat Mass Transf 47:645–652. https://doi.org/10.1016./j.ijheatmasstransfer.2003.08.013
Leo MA (1982) Energy conservation in citrus processing. Food Technol 36:231–233
Balkan F, Colak N, Hepbasli A (2005) Performance evaluation of a triple-effect evaporator with forward feed using exergy analysis. Int J Energy Res 29:455–470. https://doi.org/10.1002/er.1074
Winchester J (2000) Model based analysis of the operation and control of falling film evaporators. Massey University
Choi HS, Lee TJ, Kim YG, Song SL (2005) Performance improvement of multiple-effect distiller with thermal vapor compression system by exergy analysis. Desalination 182:239–249. https://doi.org/10.1016/j.desal.2005.03.018
Bejan A, Dincer I, Lorente S, Reis AH, Miguel AF (2004) Porous media in modern technologies: energy, electronics. Biomedical and environmental engineering. Springer, New York p 396
Dincer I, Rosen MA (2005) Thermodynamic aspects of renewables and sustainable development. Renew Sustain Energy Rev 9:169–189
Acknowledgements
The authors gratefully acknowledge Mr. S.S. Sundaran (OSD, Public Relations) and Mr. Deepak R. Sharma (Deputy Manager, Production) of Amul Dairy, Anand, for their constant support in this project.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Srinivasan, B., Pal, J., Srinivasan, R. (2018). Enhancement of Energy Efficiency at an Indian Milk Processing Plant Using Exergy Analysis. In: De, S., Bandyopadhyay, S., Assadi, M., Mukherjee, D. (eds) Sustainable Energy Technology and Policies. Green Energy and Technology. Springer, Singapore. https://doi.org/10.1007/978-981-10-7188-1_19
Download citation
DOI: https://doi.org/10.1007/978-981-10-7188-1_19
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-7187-4
Online ISBN: 978-981-10-7188-1
eBook Packages: EnergyEnergy (R0)