Abstract
Microorganisms play an important niche in the control of soil populations producing a variety of bioactive compounds as an ecological strategy for competition for space and nutrients. Thus, the bioprospecting of microorganisms as potential antagonists for pathogen biocontrol, or obtaining their bioactive metabolites, is one of the alternatives currently studied for the control of diseases, especially in species of agronomic importance. In this chapter, we reviewed several microorganisms and how, in general, the products of their metabolism are obtained to be used in the control of pathogens.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
8.1 Introduction
Agricultural products are constantly under the pressure of phytopathogenic microorganisms, both during cultivation and after harvesting, causing significant economic losses. Fungi of several species, including Penicillium and Botrytis cinerea, besides bacteria such as Erwinia carotovora and Xanthomonas vesicatoria, have been mentioned as the main cause of diseases in plants (Trias et al. 2008). Currently, the control of these phytopathogens is carried out, almost exclusively, with bactericides, fungicides, and chemical insecticides, which can often cause serious environmental problems, besides selecting resistant strains due to unrestrained use. These problems, coupled with the society’s growing demand for new products that present lower risks to the environment, encourage the search for alternatives that are less damaging to the environment.
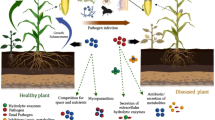
One of the most interesting proposed alternatives is biological control. It can be defined as the use of a living, nonpathogenic, preexisting organism, antagonistic to pathogenic microorganisms, which are used to eliminate or control the outcome of disease. Pantoea, Bacillus, and Pseudomonas are among the bacteria most used and described as biocontrol agents (Trias et al. 2008).
According to Borrero et al. (2006 and 2009), some mechanisms can be used in the biocontrol activity against pathogens: (A) competition for nutrients in the rhizosphere and deprived sites for colonization, (B) production of iron siderophores chelated by microorganisms (e.g., Pseudomonas spp.), (C) antimicrobial production, and (D) production of cell wall degrading enzymes (CWDEs).
As for antimicrobials, it is known that many substances produced by biocontrol agents have broad-spectrum activity against many pathogens. Many examples can be found in literature, such as the antifungal activity of pyrrolnitrin isolated from Pseudomonas and Burkholderia species and its antibacterial activity against Gram-positive bacteria (El-Banna and Winkelmann 1998, Ligon et al. 2000) and the antibiotic activity of phenols, produced and isolated from Pseudomonas aeruginosa, against Xanthomonas oryzae pv. oryzae (Shanmugaiah et al. 2010). Therefore, the selection, production, and use of antagonistic microorganisms, or antimicrobial molecules produced by them, are potential alternatives for the biological control of pathogens and pests, without damaging the development of plants and with reduced harm to the environment (Harman 2000).
In this chapter, we will discuss how interactions between biocontrol agents and the effect of natural compounds produced by the secondary metabolism of some bacteria occur against phytopathogenic microorganisms, as well as some commercial products currently used and future perspectives in the development of new antimicrobials.
8.2 Microorganisms and Biological Control
Phytopathogenic microorganisms are able to colonize the crop tissues through the natural openings of the plant or through wounds, causing serious diseases during handling and processing of agricultural products, leading to economic losses.
The integrated control used to minimize the damages related to these infections include the application of antibiotic compounds, copper-based compounds, cultural treatments, production of pathogen-free seeds or seedlings, and development of resistant varieties. These alternatives do not seem sufficient to minimize the impacts caused by pathogens, mainly because of the development of resistance by these microorganisms. The environmental damage caused by the application of chemical compounds is an even greater problem, once they have high toxicity.
There is a growing demand for an eco-friendly crop production, being necessary to find alternative control systems to replace the traditional chemical treatments in agriculture. Biological control, also called biocontrol, is defined “as the use of living organisms to suppress the population density or impact of a specific pest organism, making it less abundant or less damaging than it would otherwise be” (Eilenberg et al. 2001).
The use of microorganisms as biological control agents has been the focus of many researches in the last two decades, aiming to create more sustainable agriculture methods to control diseases caused by plant pathogens. These microorganisms are able to compete with the pathogens by different modes of action, producing antagonistic effects or inducing a plant defense mechanism. These microorganisms have a well-marked potential (Axel et al. 2012).
Many researches demonstrated the efficacy of biological control using microorganisms. On the control of phytopathogenic fungi, Banani et al. (2015) reported that the yeast Metschnikowia fructicola has been opportunely used in the control of a number of pathogens on fruits and vegetables, such as Penicillium expansum on apple and B. cinerea on grapes and on strawberries. Cordero-Ramírez et al. (2013) showed that strains of Bacillus subtilis and Bacillus cereus could control Fusarium oxysporum f. sp. radicis-lycopersici, a fungus that infects tomatoes and has the potential to reduce crop yield by 50%. Elkahouia et al. (2014) concluded that Bacillus sp. strain BCLRB2 produced various lipopeptides, with specific and broad spectrum of antifungal activities, presenting high antagonistic effect against Rhizoctonia solani, Sclerotinia sclerotiorum, Fusarium graminearum, F. oxysporum, and F. oxysporum cicero and a moderate antagonistic activity against F. culmorum. Ge et al. (2016) isolated a strain of Bacillus methylotrophicus able to inhibit mycelial growth and conidial germination of several plant pathogenic fungi in vitro and the growth of B. cinerea (the cause of gray tomato mold) by 60% in greenhouse conditions. Haidar et al. (2016) demonstrated the capability of two bacterial strains, B. pumilus and Paenibacillus sp., isolated from grapevine wood, to suppress Phaeomoniella chlamydospora via direct and/or indirect mechanisms.
The control of phytopathogenic bacteria is reported by Großkinsky et al. (2016). They identified the ability of Pseudomonas fluorescens G20-18 to efficiently control P. syringae infection in Arabidopsis sp., by producing microbial cytokinin and maintaining tissue integrity and, ultimately, biomass yield. Wu et al. (2016) showed the antagonistic effect of Bacillus amyloliquefaciens in the control of Ralstonia solanacearum, a well-known soilborne pathogen and causative agent of tobacco bacterial wilt.
Another strategy that has been studied is the use of bacterial strains that interrupt the quorum-sensing signaling by the pathogen. This mechanism of control is known as quorum quenching (QQ) and has been highlighted as a promising approach in biological control. Quorum-quenching bacteria (QBs) belong to various prokaryotic taxa and live in niches rich in nutrients, like rhizospheres and phyllospheres, and have been used to reduce the virulence factors of plant pathogenic bacteria (Alymanesh et al. 2016). Rhodococcus erythropolis and Bacillus sp. significantly reduced the pathogenicity of Pectobacterium carotovorum subsp. carotovorum and Erwinia amylovora, respectively. Pseudomonas was the most abundant and strongest QQ-based biocontrol agent in rhizospheres, and QBs with extracellular enzymatic QQ activities are among the best biocontrol agents (Alymanesh et al. 2016). P. aeruginosa, which produces enzymes with QQ capabilities, may be used to suppress the quorum-sensing apparatus of pathogens (Bokhove et al. 2010). Pseudomonas fluorescens strongly reduced symptom development of a soft-rot casual pathogen, E. carotovora, which is dependent on the quorum-sensing-mediated production of CWDEs by the QQ mechanism (Molina et al. 2003). Information found in literature support the hypothesis that QQ is a promising mechanism to control bacterial pathogens in agriculture (Zhang and Dong 2004).
These are only a few examples of the potential of microorganisms in the control of plant disease pathogens. Several other studies in the past years showed the use of bacteria, fungi, and actinomycetes as biocontrol agents. However, it is noticeable that the development of effective alternative products able to resist in inhospitable ecosystems is very difficult. Many experiments using biological control agents were conducted so far, but most of them were tested in in vitro and ex situ, with assays generally being carried under very simplified conditions (Axel et al. 2012). The application of microorganisms directly on plants or on the soil, as biological control agents, can fail because of their sensitivity to the new ecosystem. The isolation of their metabolites and its use in pathogen control can be a suitable alternative to overcome this problem.
8.3 Biocontrol by Secondary Metabolites
The search for new natural products increases daily, especially the ones looking for bioactive compounds from the secondary metabolism of microorganisms and plants (Bérdy 2005). Metabolism is a set of biochemical reactions occurring within the cells. In case of microorganisms, it can be divided into primary and secondary metabolism. In primary metabolism, components that are essential for cell survival during an exponential growth phase are produced. Secondary metabolites of microorganisms, produced for the final phase of exponential growth or near the beginning of the stationary phase, are complex organic molecules and require specific enzymatic reactions for their synthesis (Madigan et al. 2010).
Secondary metabolites are not essential to the growth and reproduction of the microorganism. Their synthesis depends almost exclusively on the conditions of cultivation, especially in relation to the composition of the environment and the environment (temperature, luminosity, agitation, among others). The vast majority of secondary metabolites are complex organic molecules and requires a large number of enzymatic reactions for their synthesis. These compounds are very important for survival. Even in small concentrations, they inhibit the growth of other microbial species, diminishing competition for nutrients and thus collaborating for species survival and selection (Madigan et al. 2010).
Among the groups of microorganisms studied in relation to the production of bioproducts, the group of filamentous actinomycetes has more than 10 thousand bioactive compounds, being considered the largest group of microbial metabolites ever studied (45%). In the group of bacteria, the genera that stand out are Pseudomonas and Bacillus, with about 3800 compounds studied, representing around 17% of all microbial metabolites with antibiotic activity (Bérdy 2012; Oliveira et al. 2016).
The search for bioactive compounds began with the discovery of lysozyme and penicillin by Alexander Fleming in the 1920s. Since then, new classes of compounds have been discovered, with antitumor, antiviral, and antiparasitic activities. Since the 1990s, there has been an exponential increase in the numbers of new metabolites discovered (mainly non-antibiotic compounds), but the occurrence of new chemical groups has decreased (de Oliveira et al. 2016).
Due to the growing problems with the arising new strains of multiresistant microorganisms and new pathologies, the need for new natural therapeutic agents is an emergency. New technologies promote rapid progress in phytopathological disease therapies, potentially renewing classical treatment methods and supplying the great demand for new products (Bérdy 2005).
Trichoderma used as controller agent of diseases in plant has great potential because of many factors: competitive activity for energy source, antibiotic metabolites that inhibited pathogen activity, and mycoparasitism (Chet 1987). These fungi also grow fast, with few nutritional requirements; produce CWDEs, factors that stimulate plant growth; make spores and chlamydospores; and acquire resistance to fungicides, and it is somewhat easy to obtain stable mutants (Melo 1991). During plant-fungus interaction, numerous elicitors released may induce signals transmitted in the plant, i.e., salicylic acid (SA), jasmonic acid (JA), and reactive oxygen species (ROS), triggering expression of defense proteins. Because of gene activation, the plant produces enzymes involved in direct suppression of pathogens and enhances the biochemical and structural barriers in plant organism. Depending on the Trichoderma strain, the defensive reactions activated by fungi may oscillate between the two types of systemic resistance, induced systemic resistance (ISR), and systemic acquired resistance (SAR) (Nawrocka and Małolepsza 2013).
Actinomycetes are gram-positive bacteria with typical filamentous growth. They are present in several environments, most frequently within soils, where they act as decomposers of organic matter. They are widely studied due to the bioactive metabolites they produce, like antimicrobials and enzymes, with biotechnological application.
Within this group, the genus Streptomyces sp. is one of the most studied producers of bioactive compounds, with a broad range of compounds from antimicrobial to antitumor activity. One of the antifungal compounds produced by this genus is the enzyme chitinase. It is a large and diverse group of glycosyl hydrolase enzymes ranging in size from 20 kDa to 90 kDa, and it is present in a broad spectrum of organisms (Kasprzewska 2003). Chitinase has the ability to degrade chitin in low molecular weight chitooligomers, and it is used as a source of energy for bacteria (Hamid et al. 2013). Chitin is present in the cell wall of fungi, algae, insect exoskeleton, and other invertebrates. As a result, the chitinase of some microorganisms has become an important tool in the biocontrol of pests in agriculture. In addition to the chitinases, Streptomyces genus also produces β-1,3-glucanase, which also acts on the degradation of fungal cell wall components (Singh et al. 1999).
Other compounds with antimicrobial activity, produced by Streptomyces sp., were the focus of studies of bioactive compounds, such as 3-phenulpropionic acid and 8-hydroxyquinoloine against Aspergillus flavus, Aspergillus niger, F. oxysporum, and Penicillium citrinum (Narayana et al. 2008), and chloroxaloterpin A and B (diterpenoids) against B. cinerea (Bi and Yu 2016) among others.
The genus Bacillus contains strict aerobic or facultative anaerobic Gram-positive bacteria. When under stress, they form an endospore, with the ability to survive and remain metabolically active under extreme conditions. Bacillus sp. present antagonistic properties and many species produce extracellular hydrophilic enzymes that break down polysaccharides, nucleic acids, and lipids, allowing the use of these products as carbon sources and electron donors. They also produce lipopeptides that act as biosurfactants and phosphate solubilizers. Bacillus spp. are good secretors of proteins and secondary metabolites with antimicrobial activity (bacitracin, polymyxin, thyrocytin, gramicidin, and circulin). Additionally, they are easy to grow and maintain and highly efficient for the biocontrol of pathogenesis (Han et al. 2015).
One of Bacillus mechanisms of action as an antagonist for fungi and bacteria is antibiosis. Isolated bacterial and fungal inhibitory compounds are very similar throughout the genus Bacillus, i.e., the three broad families of cyclic lipopeptides (CLPs), including zwittermicin, kanosamine bacillomycins, iturin, fengycins, and surfactins. The purified compounds suppressed the disease and inhibited development of oomycetes by stunting and deforming germ tubes of germinating cysts. They controlled damping-off disease of tomato seedlings, caused by R. solani, and presented an even higher inhibition activity against Plasmodiophora brassicae and Fusarium solani (Schneider et al. 1999, Suk et al. 1999, Yu et al. 2002).
The genus Pseudomonas is extensively studied in relation to the bioactive compounds that it produces in its secondary metabolism, for evidences of their application in biocontrol, plant growth promotion, bioremediation, and induction of resistance. Much alike the genus Streptomyces, they produce lytic enzymes, such as chitinases, β-1,3-glucanase, and proteases, which affect pathogenic fungi and bacteria (Gupta et al. 2006). In addition to these compounds, they produce pseudomonic acids, phenazines, indoles, pyrrolnitrins, and some peptides with diverse bioactivities.
Phenazines are a broad group of aromatic heterocyclic substances produced almost exclusively by bacteria, which can be easily extracted from the microbial culture, analyzed, and quantified by chromatographic methods. Their antifungal property has been well known and studied for a long time, but the mechanism of action is poorly understood. It is known that phenazine diffuses through or enters the membrane of the microorganism, acting as a reducing agent, resulting in the decoupling of oxidative phosphorylation and generating intracellular superoxide radicals and hydrogen peroxide that are fatal to the cell (Chin-a-Woeng et al. 2003). Phenazine compounds also have antibacterial action against Gram-positive bacteria, potentiated by the action of silver nanoparticles (Cardozo et al. 2013). Additionally, tumor cells are susceptible to respiratory interference and the generation of ROS by phenazine compounds (Pierson III and Pierson 2010). In plants, phenazines have been shown to induce systemic resistance against numerous pathogens and may influence growth. The main phenazines studied are phenazine-1-carboxylic acid (PCA), pyocyanine (PYA), and phenazine-1-carboxyamide (PCN).
Both PCA and PCN produced by Pseudomonas sp., have proved antifungal activity against several pathogenic fungi, such as B. cinerea (Zhang et al. 2015), R. solani (Olorunleke et al. 2015; Niu et al. 2016), Benjaminiella poitrasii (Tupe et al. 2014), Fusarium graminearum (Hu et al. 2014), among others.
The P. aeruginosa LV strain produced an unidentified organometallic compound with strong activity against various phytopathogens, such as Xanthomonas axonopodis (Lopes et al. 2012), Xanthomonas citri pv. citri (de Oliveira et al. 2011 and Oliveira et al. 2016), Xanthomonas arborícola pv. pruni (Vasconcellos et al. 2014), and S. sclerotiorum (Emiliano 2016). In scanning electron microscopy (SEM), it is possible to observe the population decline of X. citri pv. citri, morphological changes, and the reduction of extracellular polysaccharides when treated with the fraction called F3d (containing organometallic and phenazine compounds) (Fig. 8.1) (De Oliveira et al. 2016).
Scanning electron micrographs of orange leaf (Citrus sinensis cv. Valence) inoculated with X. citri pv citri (Xcc). (a) Control 24 h after inoculation; (b) Higher magnification of control, with extracellular polysaccharides (EPS) on the leaf surface; (c) Curative treatment 24h after F3d application; (d) Higher magnification of curative treatment, with morphological changes in bacterial shape and EPS absence. (VB, viable cell; NB, nonviable cell) (de Oliveira et al. 2016)
8.4 Obtaining Secondary Metabolites of Microorganisms for the Biological Control of Phytopathogens
In competitive terms, microorganisms that produce antimicrobial components are favored over nonproducers. These compounds have the advantage of species selection; they are very important for survival, because they can inhibit the growth of other microbial species even in small concentrations, reducing competition for nutrients.
Secondary metabolites are often produced after cell-associated growth processes, usually in the stationary phase. Secondary metabolism can be recognized as a general maintenance phenomenon for some species, and it is usually associated with plants and microorganisms. However, there is a variety of examples in the animal kingdom, such as the antibodies (Jung et al. 2008).
The following subsections will describe the main methodologies for the search, production, identification, and evaluation of natural compounds with antimicrobial properties.
8.4.1 Bioprospecting
The microbial metabolism has led to many studies with bioactive substances that can be used both in the control of diseases in agriculture and in therapeutic medicine, performing antimicrobial, antifungal, and antiviral functions among others (Lemire et al. 2013). The methodology for locating, evaluating, and systematically exploring microbial diversity in a given location, the main purpose of which is to search for genetic and biochemical resources for commercial purposes, is known as bioprospecting.
Bioprospecting mainly involves strategies for exploring the biodiversity of cultivable and noncultivable microorganisms, as well as genomic sequences already available in the database. All this has the purpose of identifying microorganisms, genes, enzymes, and/or metabolic pathways for subsequent strategic biotechnological applications in the industry or in the research itself.
Bioprospecting cultivable microorganisms require the cultivation and selection of microorganisms from existing microbial biodiversity in a given habitat or stored in collections for the application of a specific purpose. It is of great interest in the research to select microorganisms that grow specifically in a particular nutrient source. Less traditional and more efficient selection techniques, including mimicking of industrial conditions and automated process conditions, allowed the sterile cultivation of hundreds of microorganisms for desirable, even complex, characteristics in a single day. For example, miniaturized tests and online detection systems can be used to prospect large numbers of microorganisms in little time, producing bioactive compounds and identifying molecules of interest faster than before (Embrapa 2015). In addition, the cultivation of previously noncultivable microorganisms has been improved by increasing the knowledge on physiology, biochemistry, and microbial ecology, using large-scale phenotyping techniques, which permits the analysis of several characteristics simultaneously and facilitates the optimization of culture media.
Nonarable microorganisms account for approximately 99% of all species in outdoor environments and are an unexplored source of new antibiotics. Bioprospecting noncultivable microorganisms (microorganisms that cannot be cultivated with current techniques of microbiology) happened mainly due to the use of metagenomics. In this technique, the genetic material of microorganisms present in a given environment is collected, isolated, and amplified in DNA libraries. Metagenomic libraries allow not only the identification of the main microbial groups present in the sampled environment but also the genetic characterization (DNA sequence of the microbial pool) and its functional prospection. Microsystems have been constructed, and information on the microbiota of complex systems has been effectively obtained and used for biotechnological applications (Embrapa 2015). Several methods have also been developed to grow non-cultured in situ culture organisms such as diffusion chambers or the use of specific growth factors such as iron chelating siderophores (Losee et al. 2015).
Once colonies are produced and able to grow, a large number of substances can be obtained in the laboratory by in vitro culture.
8.4.2 Antimicrobial Natural Production
A bioreactor is basically a container that must be able to guarantee production under the desired conditions, to meet local containment regulations, and to monitor and control parameters such as pH, temperature, pressure, oxygen, and foam, among others. The three main fermentation techniques are batch, continuous, or fed-batch and continuous.
In industry, batch and fed fermentations have been used for the production of alcohol and fermented foods for 3000 years BC. At the beginning of the twentieth century, other applications have been popular, especially in World War II, with the production of antibiotics by culture submerging strains of bacteria and filamentous fungi. In human history, most fermentation processes were by batch. Feeding fermentation became common in antimicrobial production (Tempest and Wouters 1981). Products not associated with microbial growth, such as antibiotics, are not well produced by continuous fermentation.
Most antibiotics are products of secondary metabolism. The fermentation must be stopped at the stationary phase, just before the cells begin to die. The use of bioaccumulating microbial metabolites was investigated in order to evaluate the effect of microorganisms on the microbial activity of the actinomycete. Among fungi, ascomycetes, species of filamentous fungi and endophytes, are the most significant producers of bioactive compounds. Basidiomycetes are also frequently reported as good producers, while yeasts seldom produce these metabolites. The total number of bioactive fungal products is approximately 8.600, representing 38% of all microbial products. Of the approximately 22.500 antibiotics and bioactive microbial compounds, less than one percent, only about 150 compounds, is in direct use in human medicine, veterinary medicine, and/or agriculture (de Oliveira et al. 2016).
8.4.3 Characterization of Antimicrobials
One of the researcher’s tasks is to extract, isolate, and identify one or more pure substances from a crude extract. There are many processes and extraction systems described in the literature that can be used and adjusted if necessary. However, a trial and error approach is often necessary. The isolation of bioactive compounds is usually filled with difficulties and at every step requires judgment, improvisation, and new discoveries. The techniques most commonly used and described for extraction and/or pre-concentration of natural antimicrobial compounds or any bioactive compound are liquid phase extraction, solid phase extraction, supercritical fluid extraction, and solid membrane extraction (Gade et al. 2010).
The isolation of one or more substances from fractions of an extract can be a long and expensive process. Obtaining a pure compound many times requires several purification steps involving different techniques. This is particularly the case when it comes to bioactive metabolites wherein the target compound (e.g., natural antimicrobials) may be present only in trace amounts in a complex matrix of hundreds of other constituents or even have no standard for comparison. Chromatography is a physicochemical method of separating components from a mixture, performed by distributing these components between two phases, which are in close contact. One phase remains stationary while the other moves through it. During the passage of the mobile phase through the stationary phase, the components of the mixture are separated between the two phases, so that each component is selectively retained by the stationary phase, resulting in differential migration patterns of these components (Gade et al. 2010).
After the entire process of production, extraction, and purification of the natural antibiotic, it is possible to carry out the molecular identification. With the molecular structure and the functional groups that it possesses, it is possible to determine its physical properties and reactivity and to infer other biological activities and characteristics (Solomons and Fryhle 2000). One of the classic techniques for molecular determination is spectroscopy. When we apply an energy to matter, it can be absorbed and emitted and/or cause a chemical modification and be transmitted. Spectroscopy is the study of the interaction between energy and matter, and its results can provide detailed information on the molecular structure of the compound (Silvertein et al. 2005).
8.4.4 From Laboratory to Field
After the initial studies, the isolation of biological control from the laboratory phase to obtaining a commercial product is a difficult task. Information is needed regarding the efficacy, mode of action of the agent, survival, colonization, and toxicity potential for nontarget species. In addition, studies on formulation, stability, and shelf life are also needed (Mathre et al., 1999, Harman 2000).
The fact is that most agents selected for biological control, despite being antagonistic at in vitro stages, are not successful in in vivo or field conditions (Aysan et al. 2003). Therefore, in vitro antagonism should not be used as the sole criterion for the selection of potential biocontrol agents (Tani et al. 1990). A possible justification for the difference between in vitro and in vivo is that the success of the agents depends on controlled environmental conditions, such as greenhouses or seedlings, favoring their efficiency (Paulitz and Bélanger 2001).
Another problem is that the solid culture medium does not reflect the actual physical and chemical conditions of the environment (Rampazo 2004). One of the fundamental differences is that the leaf has two dimensions, with little vertical diffusion of solute occurring, in contrast to the high degree of nutrient diffusion that occurs in a solid medium (McCormack et al. 1994). In addition, the solid medium favors the action and development of the antagonist agent.
8.5 Commercial Products
Although the first report of the antagonistic interaction between microorganisms was carried out in 1874, when William Roberts demonstrated that the fungus Penicillium glaucum inhibited the growth of bacteria, the use of these agents as biocontrol in crop protection against diseases is relatively new and not yet consolidated, even more when compared to the use of chemical compounds. In 1979, just over 100 years after William’s discovery, the first commercial product containing an active bacterium, Agrobacterium radiobacter strain k 84, was registered in the United States, which was intended to control crown gall. As early as 1989, the use of Trichoderma harzianum ATCC 20476 was registered at the United States Environmental Protection Agency (EPA) for plant diseases control, originating the first commercial fungal product. According to the latest CPL survey in 2013, the biopesticide (products containing microorganism) market accounted for a total of $ 3 billion, representing only 5% of the total plant protection market. Also in the same year, approximately 2300 commercial products intended for plant protection contained microorganisms in their formulation, with a total registered species of 77 bacteria, 68 fungi and yeasts, 36 viruses, and 2 protozoa (Ravensberg 2015). The increase in the number of registered products that use microorganisms in their formulation is mainly due to the lower cost of developing when compared to the chemicals. While the cost of developing a chemical molecule since the discovery to commercialization is around $ 256 million and takes an average of 9 years, the same process for marketing a biological product ranges from $ 20 to $ 50 million and takes only 5 years (Olson 2015).
Among the species of bacteria commercially used in the biocontrol of plant diseases are those of the genus Bacillus, especially B. amyloliquefaciens, B. pumilus, and B. subtilis, widely used for the control of soilborne pathogens such as Fusarium, Rhizoctonia, and Alternaria. As previously mentioned, the genus Agrobacterium, mainly represented by A. radiobacter, is also used against soilborne pathogens, especially Agrobacterium tumefaciens, the causative agent of crown gall. Some products containing Pseudomonas strains are effective in controlling foliar diseases, especially those caused by bacteria, besides guaranteeing fruit sanity after harvest (Fravel 2005; Junaid et al. 2013).
The genus Trichoderma is the most registered genus of fungi for commercial use. Its use is based on the control of soil diseases as mentioned above; however, the genus Trichoderma is known as a generalist biocontrol, due to its action against a broad spectrum of pathogens such as B. cinerea, S. sclerotiorum, Sclerotium spp., Pythium ultimum, Phytophthora spp., Armillaria spp., Verticillium spp., Gaeumannomyces graminis, R. solani, and F. oxysporum. Approximately 250 products containing Trichoderma are registered worldwide (Woo et al. 2014). Other fungi are widely marketed, mainly to control soil diseases and even nematodes. Among them are A. flavus, Clonostachys rosea, Gliocladium spp., Paecilomyces spp., Pochonia, Ampelomyces quisqualis, and others (Bettiol et al. 2012). There are also products that use yeasts such as Aureobasidium pullulans and Candida oleophila, used to control some foliar diseases after harvest.
Despite being relatively recent, the market for microorganism-based products for plant protection presents a promising future with an annual growth rate of 15.6% (Marrone 2014) and some optimistic projections that, in the future, the biological products market will take the place of the chemical market (Olson 2015). Such projection does not appear to be so distant from reality when considering the advantages provided by the use of bioproducts in agriculture when compared to the use of chemicals. Bhattacharjee and Dey (2014) summarize these advantages: (1) less environmental pollution; (2) less impact on beneficial organisms; (3) lower production cost and less probability of resurgence; (4) several applications; (5) biopesticides are highly efficient in controlling soil pathogens, where chemical control is not as effective; and (6) they can induce systemic resistance in plants.
Table 8.1 presents part of the worldwide market for biocontrol agents of plant diseases available. The products are assembled according to the group of microorganisms to which they belong. For each product, the following are presented: commercial name, diseases and pathogens target, mode of action and specific characteristics of the strain, and the culture where it is commonly applied.
8.6 Future Perspectives
There are many challenges in biological control: the diversity of agents, the interaction with the host plant, the spectrum action of the metabolite produced by these agents, and the persistence of these metabolites in the environment, among other questions.
The use of live microorganisms as biocontrol agents is not a simple task. There are many environmental factors that make difficult the survival of these microorganisms in the environment, like climatic conditions and the interactions with the host and other microorganisms.
It is necessary to enlarge the range of biocontrol agents suitable for commercial use, either in the search for microorganisms that persist in the environment or in the isolation of secondary metabolites that can be applied to the crops.
Researches that focus on investigating the potential effects of these agents on the environment and human and animal health have to be improved. To combine biocontrol methods with other sustainable management techniques and to guide producers on the correct use of these agents is another challenge.
References
Alymanesh M, Taheri P, Tarighi S (2016) Pseudomonas as a frequent and important quorum quenching bacterium with biocontrol capability against many phytopathogens. Biocontrol Sci Tech 26:1719–1735
Axel C, Zannini E, Coffey A, Guo J, Waters DM, Arendt EK (2012) Ecofriendly control of potato late blight causative agent and the potential role of lactic acid bacteria: a review. Appl Microbiol Biotechnol 96:37–48
Aysan Y, Karatas A, Cinar O (2003) Biological control of bacterial stem rot caused by Erwinia chrysanthemi on tomato. Crop Prot 22:807–811
Banani H, Spadaro D, Zhang D, Matic S, Garibaldi A, Gullino ML (2015) Postharvest application of a novel chitinase cloned from Metschnikowia fructicola and overexpressed in Pichia pastoris to control brown rot of peaches. Int J Food Microbiol 199:54–61
Bérdy J (2005) Bioactive microbial metabolites. J Antibiot 58(1):1–26
Bérdy J (2012) Thoughts and facts about antibiotics: where we are now and where we are heading. J Antibiot 65:385–395
Bettiol W, Morandi MAB, Pinto ZV, Júnior TJP, Corrêa EB, MouraAB LCMM, Costa JCB, Bezerra JL (2012) Produtos Comerciais à base de agentes de biocontrole de doenças de plantas. Embrapa Meio Ambiente, Jaguariúna
Bhattacharjee R, Dey U (2014) An overview of fungal and bacterial biopesticides to control plant pathogens/diseases. Afr J Microbiol Res 8:1749–1762
Bi Y, Yu Z (2016) Diterpenoids from Streptomyces sp. SN194 and their antifungal activity against Botrytis cinerea. J Agric Food Chem 64:8525–8529
Bokhove M, Jimenez PN, Quax WJ, Dijkstra BW (2010) The quorum-quenching N-acyl homoserine lactone acylase PvdQ is an Ntn-hydrolase with an unusual substrate-binding pocket. Proc Natl Acad Sci 107:686–691
Borrero C, Ordovás J, Trillas MI, Avilés M (2006) Tomato fusarium wilt suppressiveness. The relationship between the organic plant growth media and their microbial communities as characterised by biolog. Soil Biol Biochem 38:1631–1637
Borrero C, Trillas MI, Avilés M (2009) Carnation Fusarium wilt suppression in four composts. Eur J Plant Pathol 123:425–433
Cardozo VF, Oliveira AG, Nishio EK, Perugini MRE, Andrade CGTJ, Silveira WD, Durán N, Andrade G, Kobayashi RKT, Nakazato G (2013) Antibacterial activity of extracellular compounds produced by a Pseudomonas strain against methicillin-resistant Staphylococcus aureus (MRSA) strains. Ann Clin Microbiol Antimicrob 12:1–8
Chet I (1987) Innovative approaches to plant disease control. 372 p
Chin-A-Woeng TFC, Bloemberg GV, Lugtenberg BJJ (2003) Phenazines and their role in biocontrol by Pseudomonas bacteria. New Phytol 157:503–523
Cordero-Ramírez JD, López-Rivera R, Figueroa-Lopez AM, Mancera-López ME, Martínez-Álvarez JC, Apodaca-Sánchez MA, Maldonado-MendozaI E (2013) Native soil bacteria isolates in Mexico exhibit a promising antagonistic effect against Fusarium oxysporum f sp radicis-lycopersici. J Basic Microbiol 53:838–847
De Oliveira AG, Murate LS, Spago FR, Lopes LP, Beranger JPO, San Martin JAB, Nogueira MA, Mello JCP, Andrade CGTJ, Andrade G (2011) Evaluation of the antibiotic activity of extracellular compounds produced by the Pseudomonas strain against the Xanthomonas citri pv. citri 306 strain. Biol Control 56:125–131
De Oliveira AG, Spago FR, Simionato AS, Navarro MO, Silva CS, Barazetti AR, Cely MV, Tischer CA, San Martin JA, Andrade CG, Novello CR, Mello JC, Andrade G (2016) Bioactive organocopper compound from Pseudomonas aeruginosa inhibits the growth of Xanthomonas citri subsp. citri. Front Microbiol 7:1–12
Eilenberg J, Hajek A, Lomer C (2001) Suggestions for unifying the terminology in biological control. BioControl 46:387–400
El-Banna N, Winkelmann G (1998) Pyrrolnitrin from Burkholderia cepacia: antibiotic activity against fungi and novel activities against streptomycetes. J Appl Microbiol 85:69–76
Elkahouia S, Djébali N, Karkouch I, Ibrahim AH, Kalai L, Bachkouel S, Tabbene O, Limam F (2014) Mass spectrometry identification of antifungal lipopeptides from Bacillus sp. BCLRB2 against Rhizoctonia Solani and Sclerotinia Sclerotiorum. Appl Biochem Microbiol 50:161–165
Embrapa (2015) Title of preprint. http://ainfo.cnptia.embrapa.br/digital/bitstream/item/137596/1/bioprospeccao-microbiana-web.pdf
Emiliano J (2016) Componentes do metabolismo secundário bacteriano com potencial inibitório sobre Sclerotinia sclerotiorum. Dissertation, Universidade Estadual de Ponta Grossa.
Fravel DR (2005) Commercialization and implementation of biocontrol. Annu Rev Phytopathol 43:337–359
Gade A, Ingle A, Whiteley C, Rai M (2010) Mycogenic metal nanoparticles: progress and applications. Biotechnol Lett 32:593–600
Ge B, Liu B, Nwet TT, Zhao W, Shi L, Zhang K (2016) Bacillus methylotrophicus Strain NKG-1, isolated from Changbai Mountain, China, has potential applications as a biofertilizer or biocontrol Agent. PLoS One 11:1–13
Großkinsky DK, Tafner R, Moreno MV, Stenglein SA, Salamone IEG, Nelson LM, Novák O, Strnad M, van der Graaff E, Roitsh T (2016) Cytokinin production by Pseudomonas fluorescens G20-18 determines biocontrol activity against Pseudomonas syringae in Arabidopsis. Sci Rep 6:1–11
Gupta CP, Kumar B, Dubey RC, Maheshwari DK (2006) Chitinase-mediated destructive antagonistic potential of Pseudomonas aeruginosa GRC1 against Sclerotinia sclerotiorum causing stem rot of peanut. BioControl 51:821–835
Haidar H, Roudeta J, Bonnarda O, Dufoura MC, Corio-Costeta MF, Ferta M, Gautiera T, Deschampsa A, Fermauda M (2016) Screening and modes of action of antagonistic bacteria to control the fungal pathogen Phaeomoniella chlamydospora involved in grapevine trunk diseases. Microbiol Res 192:172–184
Hamid R, Khan MA, Ahmad M, Ahmad MM (2013) Chitinases: An update. J Pharm Bioallied Sci 5:21–29
Han JH, Shim H, Shin JH, Kim KS (2015) Antagonistic activities of Bacillus spp. strains isolated from tidal flat sediment towards anthracnose pathogens Colletotrichum acutatum and C. gloeosporioides in South Korea. Plant Pathol J 31:165–175
Harman GE (2000) Myths and dogmas of biocontrol changes in perceptions derived from research on Trichoderma harzinum T-22. Plant Dis 84:377–393
Hu W, Gao Q, Hamada MS, Dawood DH, Zheng J, Chen Y, Ma Z (2014) Potential of Pseudomonas chlororaphis subsp. Aurantiaca Strain Pcho10 as a biocontrol agent against Fusarium graminearum. Phytopathology 104:1289–1297
Junaid JM, Dar NA, Bhat TA, Bhat AH, Bhat MA (2013) commercial biocontrol agents and their mechanism of action in the management of plant pathogens. Int J Mod Plant Anim Sci 1:39–57
Jung WK, Koo HC, Kim KW, Shin S, Kim SH, Park YH (2008) Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli. Appl Environ Microbiol 74:2171–2178
Kasprzewska A (2003) Plant chitinases-Regulation and function. Cell Mol Biol Lett 8:809–824
Lemire JA, Harrison JJ, Turner RJ (2013) Antimicrobial activity of metals: mechanisms, molecular targets and applications. Nat Rev Microbiol 11:371–384
Ligon JM, Hill DS, Hammer PE, Torkewitz NR, Hofmann D, Kempf HJ, van Pee KH (2000) Natural products with antifungal activity from Pseudomonas biocontrol bacteria. Pest Manage 56:688–695
Ling LL, Schneider T, Peoples AJ, Spoering AL, Engels I, Conlon BP, Mueller A, Schäberle TF, Hughes DE, Epstein S, Jones M, Lazarides L, Steadman VA, Cohen DR, Felix CR, Fetterman KA, Millett WP, Nitti AG, Zullo AM, Chen C, Lewis K (2015) A new antibiotic kills pathogens without detectable resistance. Nature 517(7535):455–459
Lopes LP, de Oliveira AG, Beranger JPO, Góis CG, Vasconcellos FCS, San Martin JAB, Andrade CGTJ, Mello JCP, Andrade G (2012) Activity of extracellular compounds of Pseudomonas sp. against Xanthomonas axonopodis in vitro and bacterial leaf blight in eucalyptus. Trop Plant Pathol 37:233–238
Madigan MT, Martinko JM, Dunlap PV, Clark DP (2010) Microbiologia Industrial. In: Madigan MT, Martinko JM, Dunlap PV, Clark DP Microbiologia de Brock. 12 edn. Porto Alegre: Artmed 734-760
Marrone PG (2014) The market and potential for biopesticides. In: Biopesticides: state of the art and future opportunities. American Chemical Society, Washington, DC, pp 245–258
Mathre DE, Cook RJ, Callan NW (1999) From discovery to use – traversing the world of commercializing biocontrol agents for plant disease control. Plant Dis 83:971–983
McCormack PJ, Bailey MJ, Jeffries P (1994) An artificial wax substrate for the in vitro study of phylloplane micro-organisms. J Microbiol Methods 19:19–28
Melo IS (1991) Potencialidades de utilização de Trichoderma spp. no controle biológico de doenças de plantas. In: Bettiol W (Org.) Controle biológico de doenças de plantas. EMBRAPA, Brasília, p 388
Molina L, Constantinescu F, Michel L, Reimmann C, Duffy B, Défago G (2003) Degradation of pathogen quorum-sensing molecules by soil bacteria: a preventive and curative biological control mechanism. FEMS Microbiol Ecol 45:71–81
Narayana KJP, Prabhakar P, Vijayalakshmi M, Venkateswarlu Y, Krishna PSJ (2008) Study on bioctive compounds from Streptomyces sp. ANU 6277. Polish J Microbiol 57:35–39
Niu J, Chen J, Xu Z, Zhu X, Wu Q, Li J (2016) Synthesis and bioactivities of amino acid ester conjugates of phenazine-1-carboxylic acid. Bioorg Med Chem Lett 26:5384–5386
Nawrocka J, Małolepsza U (2013) Diversity in plant systemic resistance induced by Trichoderma. Biol Control 67(2):149–156
Olorunleke FE, Hua GKH, Kieu NP, Ma Z, Höfte M (2015) Interplay between orfamides, sessilins and phenazines in the control of Rhizoctonia diseases by Pseudomonas sp. CRM12a. Environ Microbiol Rep 7:774–781
Olson S (2015) An analysis of the biopesticide market now and where it is going. Outlooks Pest Manag 26:203–206
Paulitz TC, Bélanger RR (2001) Biological control in greenhouse systems. Ann Rev Phytophatol 39:103–133
Pierson LS III, Pierson EA (2010) Metabolism and function of bacteria in the environment and biotechnological processes. Appl Microbiol Biotechnol 86:1659–1670
Rampazo LGL (2004) Avaliação de agentes biológicos e seus produtos na incidência de lesões foliares do Cancro Cítrico. Dissertation, Universidade Estadual de Londrina
Ravensberg JW (2015) Commercialisation of microbes: present situation and future prospects. In: Lugtenberg B (ed) Principles of plant-microbe interactions. Springer, Leiden, pp 309–317
Schneider JK, Taraz H, Budzikiewicz P, Jacques P, Thonart (1999) The structure of two fengycins from Bacillus subtilis S499. Z Naturforsch 54:859–865
Shanmugaiah V, Mathivanan N, Varghese B (2010) Purification, crystal structure and antimicrobial activity of phenazine-1-carboxamide produced by a growth-promoting biocontrol bacterium, Pseudomonas aeruginosa MML2212. J Appl Microbiol 108:703–711
Silvertein RM, Webster F, Kiemle D (2005) Spectrometric identification of organic compounds, 7th edn. Wiley, Hoboken
Singh PP, Shin YC, Park CS, Chung YR (1999) Biological control of Fusarium wilt of cucumber by chitinolytic bacteria. Biol Control 89:92–99
Solomons T, Fryhle CB (2000) Química Orgânica, 7th edn. LTC editora, Rio de Janeiro, p 1
Suk WS, Son HJ, Lee G, Lee SJ (1999) Purification and characterization of biosurfactants produced by Pseudomonas sp. SW 1. J Microbiol Biotechnol 9:56–61
Tani A, Takeuchi T, Horita H (1990) Biological control of scab, black scurf and soft roto f potato by seed tuber bacterization. In: Homey D (ed) Biological control of soil borne plant pathogens. CAB International, Wallingford, pp 143–164
Tempest DW, Wouters J (1981) Properties and performance of microorganisms in chemostat culture. Enzym Microb Technol 3:283–290
Trias R, Bañeras L, Montesinos E, Badosa E (2008) Lactic acid bacteria from fresh fruit and vegetables as biocontrol agents of phytopathogenic bacteria and fungi. Int Microbiol 11:231–236
Tupe SG, Kulkarni RR, Shirazi F, Sant DG, Joshi SP, Deshpande MV (2015) Possible mechanism of antifungal phenazine-1-carboxamide from sp. against dimorphic fungi and human pathogen. J Appl Microbiol 118(1):39–48
Vasconcellos FCS, de Oliveira AG, Lopes-Santos L, Beranger JPO, Cely MVT, Simionato AS, Pistori JF, Spago FR, Mello JCP, San Martin JAB, Andrade CGTJ, Andrade G (2014) Evaluation of antibiotic activity produced by Pseudomonas aeruginosa LV strain against Xanthomonas arborícola pv. pruni. Agric Sci 5:71–76
Woo SL, Ruocco M, Vinale F, Nigro M, Marra R, Lombardi N, Pascale A, Lanzuise S, Manganiello G, Lorito M (2014) Trichoderma-based products and their widespread use in agriculture. Open Mycol J 8:71–126
Wu B, Wang X, Yang L, Yang H, Zeng H, Qiu Y, Wang C, Yu J, Li J, Xu D, He Z, Chen S (2016) Effects of Bacillus amyloliquefaciensZM9 on bacterial wilt and rhizosphere microbial communities of tobacco. App Soil Ecol 103:1–12
Yu GY, Sinclair JB, Hartman GL, Bertagnolli BL (2002) Production of iturin A by Bacillus amyloliquefaciens suppressing Rhizoctonia solani. Soil Biol Biochem 34:955–963
Zhang LH, Dong YH (2004) Quorum sensing and signal interference: diverse implications. Mol Microbiol 53:1563–1571
Zhang Y, Wang C, Su P, Liao X (2015) Control effects and possible mechanism of the natural compound Phenazine-1-Carboxiamide against Botrytis cinerea. PLoS One 10:1–17
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Simionato, A.S. et al. (2017). Strategies for Biological Control and Antagonisms. In: Singh, D., Singh, H., Prabha, R. (eds) Plant-Microbe Interactions in Agro-Ecological Perspectives. Springer, Singapore. https://doi.org/10.1007/978-981-10-6593-4_8
Download citation
DOI: https://doi.org/10.1007/978-981-10-6593-4_8
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-6592-7
Online ISBN: 978-981-10-6593-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)